Abstract
To determine the efficacy and specific mechanism of vitamin D on intervertebral disc degeneration. The model of intervertebral disc degeneration was established in 3-month-old mice. Furthermore, the levels of intervertebral disc degeneration in the vitamin D group and the control group were detected one month later by X-ray, Western boltting, quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) et al. In addition, in vitro, we cultured mouse intervertebral disc nucleus pulposus cells to verify the effect of vitamin D on nucleus pulposus cells and study its mechanism. In vivo, compared with the control group, mice in the vitamin D group showed dose-dependent retardation of intervertebral disc degeneration in terms of reducing inflammatory responses, antioxidant stress, inhibiting apoptosis and delaying cell aging. In vitro, compared with the control group, collagen II was increased and collagen X was decreased in mice treated with vitamin D. In vivo and in vitro experiments, the expression of p65 and IκB kinase α was decreased and the expression of inhibitor of NF-κB was increased in the intervertebral disc tissue or nucleus pulposus cells of the vitamin D treatment group, indicating that vitamin D could suppress the NF-κB pathway. Vitamin D retarded intervertebral disc degeneration by inhibiting NF-κB pathway, which may relieve inflammatory reactions, resist oxidative stress, inhibit apoptosis and delay cell senescence.
Keywords: Intervertebral disc degeneration, vitamin D, NF-κB pathway
Introduction
Low back pain (LBP) has become a common disease in modern society and intervertebral disc degeneration (IVDD) is the main factor [1]. IVDD refers to the physiological and pathological process of natural aging and degradation of intervertebral disc [1]. It is a series of pathologic foundation of spinal degenerative diseases such as intervertebral disc herniation, spinal canal stenosis, spinal segmental instability and osteophyte formation and can cause neck and shoulder pain, waist and leg pain [2]. Studies show that 80% of adults have LBP at different ages [3]. With the advent of aging society, cervical and lumbar spine diseases caused by IVDD increasingly pose a threat to people’s health and seriously affect the quality of life of patients, especially patients over 50 years old [3]. It causes very heavy economic burden and social burden. Therefore, it is vital to find a new method to treat intervertebral disc degeneration.
Intervertebral disc consists of the nucleus pulposus at the center, the annulus fibrosus at the periphery and the endplate cartilage at the top and bottom [1]. During IVDD, inflammation, oxidative stress, apoptosis, senescence and other pathological factors are involved [4]. Current studies have shown that IL-1β and TNF-α are inflammatory mediators that play a major role in the process of intervertebral disc degeneration, which can accelerate the apoptosis of intervertebral disc cells and induce the increase of matrix metalloproteinases (MMPs) in the nucleus pulposus [5]. Furthermore, there is NO and its synthase in degenerative intervertebral disc tissue. Intervertebral disc tissue can release NO and degenerative intervertebral disc cells themselves produce NO at the same time to accelerate their own apoptosis, resulting in disc degeneration [6]. In addition, the decrease of active cells in intervertebral disc and the subsequent decrease of extracellular matrix synthesis and composition are one of the factors leading to the degeneration of intervertebral disc, and the excessive apoptosis of intervertebral disc cells is the direct cause of the decrease of the number of active cells. Gruber first examined the apoptosis of human intervertebral disc tissue. In the degenerative intervertebral disc specimens, the apoptosis rate of intervertebral disc cells was up to 53%~73% [7]. Wang et al. [8] found that, with the increase of apoptosis, the activity of intervertebral disc cartilage endplate cells decreased and the amount of collagen II synthesized by cells decreased. Gruber et al. [9] confirmed the close relationship between cellular aging and disc degeneration by observing the corresponding increase in senile beta galactosidase positive cells in degenerative discs. In aging intervertebral discs, the decrease in the number of nucleus pulposus cells is common and the residual nucleus pulposus cells are in a state of reduced function [10]. The failure of aging nucleus pulposus cells to continue dividing will prevent the disc from producing enough new nucleus pulposus cells to replace those lost due to necrosis and apoptosis [11]. The accumulation of senescent cells may contribute to the pathological changes in the intervertebral disc during aging and degeneration. Aging nucleus pulposus cells express a large number of inflammatory factors and matrix degradation enzymes, which deteriorate the living environment of surrounding cells, form a vicious cycle and produce a huge destructive effect [12].
Vitamin D is traditionally considered to be a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and multiple other biological effects [13,14]. However, recent studies have found that vitamin D plays an important role in antioxidant stress and anti-aging. In the skin study of 1,25(OH)2D3 deficient mice, it was found that the level of reactive oxygen species was significantly increased, the expression and activity of antioxidant enzymes in the skin tissue were decreased, and malondialdehyde, a lipid peroxidation metabolite, was significantly increased [15]. The results showed that 1,25(OH)2D3 can up-regulate the antioxidant system, reduce the level of oxidative stress in the body, and thus play a role in delaying skin aging. However, whether exogenous vitamin D supplementation improves disc degeneration is unclear. Considering the important role of the oxidative stress and senescence in IVDD, we hypothesized that PRL might also play a role in this condition.
In this study, we determined if vitamin D could improve intervertebral disc degeneration. We use tail-suspension mice as a model of mice with intervertebral disc degeneration and mice were injected with activated vitamin D intraperitoneally. Finally, we explored the effect of vitamin D on intervertebral disc degeneration.
Materials and methods
Animals and grouping
A total of 60 male C57BL/6 mice were devoted to the intervertebral disc degeneration model. The mice were 3 months and weight of 25-35 g. Mice were bred in a cage under normal environmental conditions (conventional food and drinking water, room temperature of 24°C, 12-hour artificial circadian cycle). The experiment mice were randomly divided in four groups (control group, vehicle group, low dose group and high dose group). The control group was mice without suspended tails. The vehicle group was mice with suspended tails and injected with the same amount of normal saline intraperitoneally. The low dose group was mice with suspended tails and injected with low doses of vitamin D intraperitoneally. The high dose group was mice with suspended tails and injected with high doses of vitamin D intraperitoneally.
Operative procedure and treatment
First, the mice were anesthetized with 2 percent paraformaldehyde at a dose of 0.2 mL/g. Then, the tail of the mouse was fixed with medical adhesive tape and the pulley on the top of the cage was suspended from the tail of the mouse. The height was controlled so that the hind legs of the mouse were just suspended from the ground [16]. Hang tail for one month. The vehicle group mice were injected daily to 100 microns of normal saline. The low dose group mice were injected daily to 100 μL of low concentration of vitamin D solution (500 IU/kg), and the high dose group mice were injected daily to 100 μl of high concentration of vitamin D solution (2000 IU/kg).
Cell culture and treatment
The mouse nucleus pulposus cells (NPCs) were purchased from Saibaikang Biotechnology. The cells were cultured in dulbecco’s modified eagle medium/F12 (DMEM/F12) medium (Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and 1% penicillin/streptomycin. When the cells grow to the right density, they were treated with different concentrations of vitamin D. In addition, recombinant mouse IL-1β was used to stimulate NPCs degeneration. Then, the treated cells were used for subsequent experiments.
X-ray examination
After 4 weeks of mouse bred, 3 mice were randomly selected from each group for X-ray detection. The mice were anesthetized and placed in a lateral position with their tails straight in an X-ray irradiometer. Radiographs were taken at a collimator-to-film distance of 30 cm, an exposure of 25 mAs and a penetration power of 160 kV. Disc Height Index (DHI) is used to evaluate the degree of disc degeneration. DHI is the ratio of the height of intervertebral height to the sum of the height of adjacent two vertebral bodies.
Western blotting technology
Intervertebral disc tissue or NPCs treated differently were transformed into proteins on ice using a total protein extraction kit containing protease inhibitors and phosphatase inhibitors. Protein containing compounds was centrifuged by high speed centrifuge (13000 rpm, 15 minutes) in low temperature (4°C) for the purpose of supernatant fluid. The concentration of protein solution was determined by double bicinchoninic Acid (BCA) method (Pierce, Rockford, IL, USA). After the concentration of protein in each group was stabilized, protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel. Then transfer the dispersed proteins to polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA) at 4°C for 2 h. Five percent non-fatty milk was prepared with Tris-buffered saline with Tween-20 (TBS-T) to block the aspecific antigen for 1 h. After washed 3 times with TBST, the membrane was incubated with primary antibody (Collagen II, Abcam, Cambridge, MA, USA, Rabbit, 1:5000; Collagen X, Abcam, Cambridge, MA, USA, Rabbit, 1:300; Aggrecan, Abcam, Cambridge, MA, USA, Rabbit, 1:3000; SOD-1, Abcam, Cambridge, MA, USA, Rabbit, 1:3000; SOD-2, Abcam, Cambridge, MA, USA, Rabbit, 1:3000; Caspase3, Cell Signaling Technology, Danvers, MA, USA, Rabbit, 1:1000; Caspase8, Cell Signaling Technology, Danvers, MA, USA, Rabbit, 1:1000; Caspase9, Cell Signaling Technology, Danvers, MA, USA, Rabbit, 1:1000; bcl-2, Abcam, Cambridge, MA, USA, Rabbit, 1:2000; IL-1β, Abcam, Cambridge, MA, USA, Rabbit, 1:1000, TNF-α, Abcam, Cambridge, MA, USA, Rabbit, 1:1000; p16, Abcam, Cambridge, MA, USA, Rabbit, 1:1000; p19, Abcam, Cambridge, MA, USA, Rabbit, 1:1000; p53 Abcam, Cambridge, MA, USA, Rabbit, 1:1000; BMI-1, Abcam, Cambridge, MA, USA, Rabbit, 1:3000; GAPDH, Proteintech, Rosemont, IL, USA, 1:10000) at 4°C overnight. The membrane was separated from the primary antibody and washed 3 times. Specifically combined with the second antibody (Goat Anti-Rabbit IgG, YiFeiXue Biotechnology, Nanjing, China, 1:3000), it was incubated with the membrane at room temperature for 2 h and washed again for 3 times. Electrochemical luminescence (ECL) was used to display the target protein on the exposure machine.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
1 mL TRIzol (Invitrogen, Carlsbad, CA, USA) was added to intervertebral disc tissue and homogenized after shearing. The nucleic acid protein protein complex was completely separated after 5 minutes at room temperature. For cells, 0.5 mL TRIzol was added to each of the six orifice plates, and the ice shaker was used for 10 minutes. 0.2 mL chloroform was added into every 1 mL TRIzol, and the tubes were violently shaken for 15 seconds and then left at room temperature for 3 minutes. The mixture was centrifuged for 15 minutes (10000 RPM, 4°C) and then the upper water phase was sucked out to join the isopropyl alcohol. The mixture was vibrated and placed at room temperature for 10 minutes. After the mixture has been centrifuged for 10 minutes (10000 RPM, 4°C), we get the RNA precipitation and discard the supernatant. After washing RNA precipitation with 75% ethanol, the mixture was centrifuged (10000 RPM, 4°C) for 5 minutes. The supernatant was discard and then we add 30 μL RNase free water to dissolve it. RNA concentrations were measured on Nano Drop to determine absorbance at 260 nm, 230 nm and 280 nm. If A260/A280 were between 1.8 and 2.0, the RNA quality was considered to be up to standard and could be used in subsequent experiments.
mRNA quantitative analysis is achieved using Prism 7300 Sequence Detection System, 25 μL reaction System is used including SYBR Green (12.5 μL), 10 Mm of primers (0.5 mL each from the stock), 10.5 μL of water and 0.5 μL of template. The PCR conditions were as follows: 10 min denaturation at 95°C; 40 cycles of denaturation at 95°C for 15 s; 60°C annealing for 30s and 72°C extension for 30 s. The data is analyzed by SDS software and the results are then output to EXCEL for further analysis. Endogenous GAPDH was used to standardize the data. The comparative threshold cycle (Ct) method, that is, the 2-ΔΔCt method was used to calculate fold amplification.
Enzyme linked immunosorbent assay
Serum and intervertebral disc samples were taken from mice. The serum was centrifuged for 5 minutes and supernatant was collected. After adding appropriate amount of PBS to the intervertebral disc tissue, the mixture was homogenized and centrifuged for 10 minutes to collect supernatant. Standard product wells are set on the 96-well plate and standard products of different concentrations are added successively. The samples to be tested were added into the corresponding wells, and then the plates were sealed with the sealing film and incubated at room temperature for 30 minutes. Then we discarded the liquid and filled each well with washing solution for 30 seconds, and repeat 5 times. Then the enzyme standard reagent was added into each well, except blank wells. Then, the colorant was added into each well and we made it avoid light for 15 minutes. The termination solution was added to terminate the reaction and the absorbance (OD value) of each well was measured sequentially at the wavelength of 450 nm by zeroing in the blank hole. With the concentration of standard product as the abscissa and OD value as the ordinate, the standard curve was drawn to calculate the sample concentration.
Cell counting Kit-8 assay
Cell Counting Kit-8 (CCK8) assay (Dojindo, Kumamoto, Japan) was used to determine the optimal concentration and time of vitamin D. NPCs was inoculated in 96-well plates with 3000 per well, and medium containing different concentrations of vitamin D was added the next day. Blank control group was established: equal amount of medium was added without cells. CCK8 solution was added into each group for dark incubation for 1 h at 6 h, 12 h, 24 h, and 48 h of culturing, respectively, and then the absorbance value at the wavelength of 450 nm was determined by enzyme labelling instrument.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 21.0 statistical software (IBM, Armonk, NY, USA) was used to analyze the experimental data. Measurement data is expressed as χ±s; t-test was used for comparisons between the two groups. Single-factor anova was used for the comparison between groups with different concentrations. LSD test or SNK test was used for pairwise comparison under the condition of homogeneity of variance. Test level α = 0.05. All experiments were repeated 3 times.
Result
Vitamin D retards IVDD in a mouse model
To determine the efficacy of vitamin D in the treatment of intervertebral disc degeneration, we conducted X-ray examination on four groups of mice and evaluated the status of the intervertebral disc in mice by DHI according to the imaging results (Figure 1A). The results showed that the intervertebral disc index of the vehicle group was significantly lower than that of the control group. After the administration of vitamin D, the DHI of mice showed a significant increase. In the Western Blotting experiment (Figure 1B), it was found that the collagen II and aggrecan of the intervertebral disc of mice in the vehicle group were significantly decreased and the expression levels of these two proteins were significantly increased and showed dose-dependent after the treatment of vitamin D. On the contrary, the expression of collagen X was significantly increased in the vehicle group and decreased in the vitamin D group. Similar results were found at mRNA levels (Figure 1C). This indicated that the degree of intervertebral disc degeneration was significantly increased in mice with suspended tail, and vitamin D can retard this process of degeneration.
Figure 1.
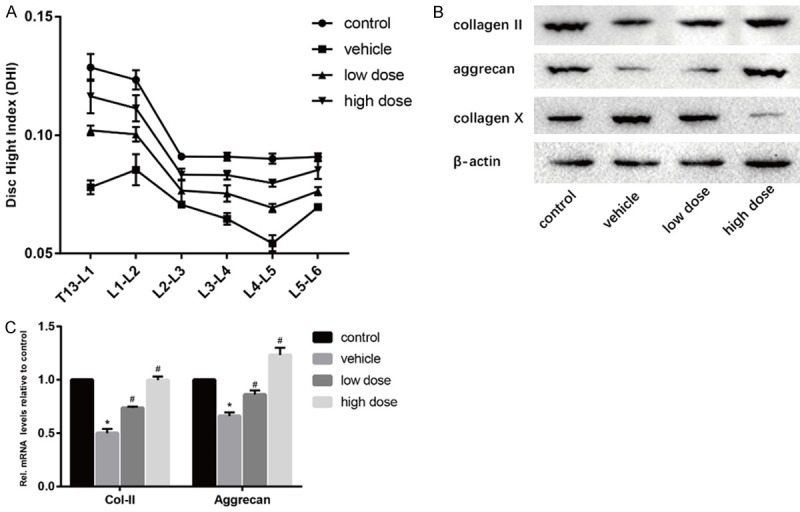
Effect of vitamin D on intervertebral disc degeneration. A. Results of the difference of DHI in four groups were calculated. B. Results of protein expression of collagen II, aggrecan and collagen X in four groups were determined by Western blotting. β-Actin was used as an internal control. C. Results of mRNA expression of collagen II and aggrecan in four groups were determined by real time PCR. (“*” means there is a statistical difference with the control group and “#” means there is a statistical difference with the vehicle group. N number is all 3. Collagen-II: vehicle-control. P = 0.00002; low dose-vehicle, P = 0.0005; high dose-vehicle, P = 0.00008. Aggrecan, vehicle-control. P = 0.00006; low dose-vehicle, P = 0.0024; high dose-vehicle, P = 0.00019).
Vitamin D inhibits inflammation of the intervertebral disc
Expression levels of IL-1β and TNF-α were detected by Western Blotting (Figure 2A). The results showed that the expression of IL-1β and TNF-α in the intervertebral disc of the vehicle group mice was significantly increased and inflammatory factors were involved in the degeneration of the intervertebral disc. After the intervention of vitamin D, the expression levels of IL-1β and TNF-α decreased significantly. Similar results were found for mRNA levels (Figure 2B). Elisa showed that vitamin D significantly reduced the expression levels of IL-1β and TNF-α in intervertebral disc tissue, but there was no significant difference in blood serum (Figure 2C and 2D). This suggested that vitamin D may retard disc degeneration by inhibiting inflammation.
Figure 2.
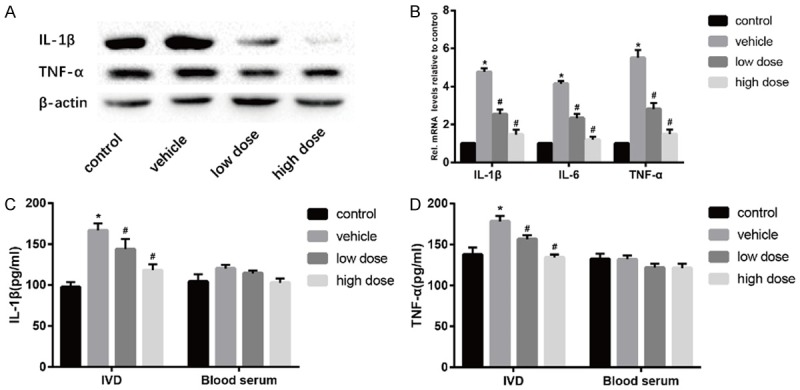
Vitamin D reduces inflammation in the intervertebral disc. (A) Results of protein expression of IL-1β and TNF-α in four groups was determined by Western blotting. β-Actin was used as an internal control. (B) Results of mRNA expression of IL-1β, IL-6 and TNF-α in four groups were determined by real time PCR. In addition, The protein expression of IL-1β (C) and TNF-α (D) was measured by Elisa. (“*” means there is a statistical difference with the control group and “#” means there is a statistical difference with the vehicle group. N number is all 3. IL-1β: vehicle-control. P = 0.0003; low dose-vehicle, P = 0.05; high dose-vehicle, P = 0.00156. TNF-α: vehicle-control. P = 0.00285; low dose-vehicle, P = 0.01023; high dose-vehicle, P = 0.00055).
Vitamin D inhibits oxidative stress of the intervertebral disc
Oxidative stress plays an important role in intervertebral disc degeneration. In the process of oxidative stress, SOD-1 and SOD-2 are important indicators of antioxidant stress. In the Western blotting and real time PCR (Figure 3A and 3B), we found that the oxidative stress level of mice in the vehicle group was significantly reduced, and the level of SOD-1 and SOD-2 could be significantly increased by the treatment of vitamin D. These data suggested that vitamin D slowed disc degeneration through antioxidant stress.
Figure 3.
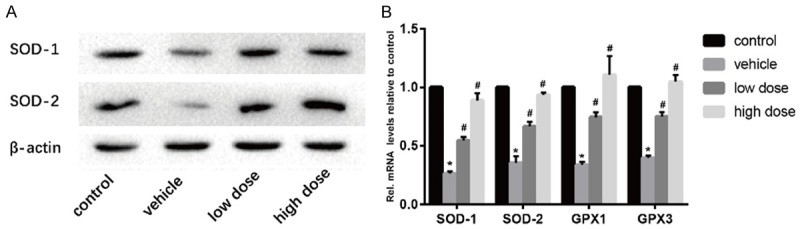
Vitamin D reduces oxidative stress in intervertebral discs. A. Results of protein expression of SOD-1 and SOD-2 in four groups was determined by Western blotting. β-Actin was used as an internal control. B. Results of mRNA expression of SOD-1, SOD-2, GPX1 and GPX3 in four groups were determined by real time PCR. (“*” means there is a statistical difference with the control group and “#” means there is a statistical difference with the vehicle group. N number is all 3. SOD-1: vehicle-control. P = 0.0000007; low dose-vehicle, P = 0.00017; high dose-vehicle, P = 0.00008. SOD-2: vehicle-control. P = 0.00004; low dose-vehicle, P = 0.0014; high dose-vehicle, P = 0.00007. GPX1: vehicle-control. P = 0.0000017; low dose-vehicle, P = 0.00015; high dose-vehicle, P = 0.00124. GPX3: vehicle-control. P = 0.0000007; low dose-vehicle, P = 0.00014; high dose-vehicle, P = 0.00005).
Vitamin D prevents the apoptosis of NPCs
We detected the expression level of caspase 3, caspase 8 and bcl-2, which involves the process of apoptosis by Western blotting (Figure 4A). The results showed that the expression level of caspase3 and caspase8 in mice in the vehicle group was higher than the control group and the bcl-2, an antiapoptotic cytokine was opposite. However, after the administration of vitamin D, the apoptosis of NPCs was prevented significantly. Furthermore, real-time PCR showed similar results (Figure 4B). These data indicated that vitamin D can retard IVDD by preventing the apoptosis of NPCs.
Figure 4.
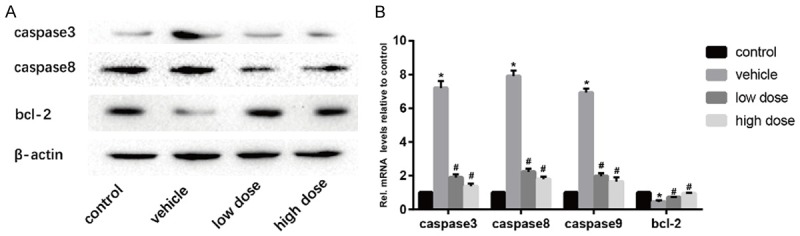
Vitamin D inhibits apoptosis of intervertebral disc cells. A. Results of protein expression of caspase 3, caspase8 and bcl-2 in four groups was determined by Western blotting. β-Actin was used as an internal control. B. Results of mRNA expression of caspase 3, caspase8, caspase9 and bcl-2 in four groups were determined by real time PCR. (“*” means there is a statistical difference with the control group and “#” means there is a statistical difference with the vehicle group. N number is all 3. Caspase3: vehicle-control. P = 0.00001; low dose-vehicle, P = 0.00003; high dose-vehicle, P = 0.00002. Caspase8: vehicle-control. P = 0.000003; low dose-vehicle, P = 0.00001; high dose-vehicle, P = 0.000007. Caspase9: vehicle-control. P = 0.000002; low dose-vehicle, P = 0.000009; high dose-vehicle, P = 0.00001. Bcl-2: vehicle-control. P = 0.00039; low dose-vehicle, P = 0.00568; high dose-vehicle, P = 0.0008).
Vitamin D delays the senescence of NPCs
Western blotting results showed that a large amount of p16 and p19 was expressed in the intervertebral discs of mice in the vehicle group. After the intervention of vitamin D, these senescence related molecules were significantly reduced. Bmi-1, as an anti-aging molecule, showed an opposite trend (Figure 5A). Similar results were found in real-time PCR (Figure 5B). These results suggested that vitamin D can effectively delay the aging of nucleus pulposus cells in intervertebral disc, thereby improving the degeneration of intervertebral disc.
Figure 5.
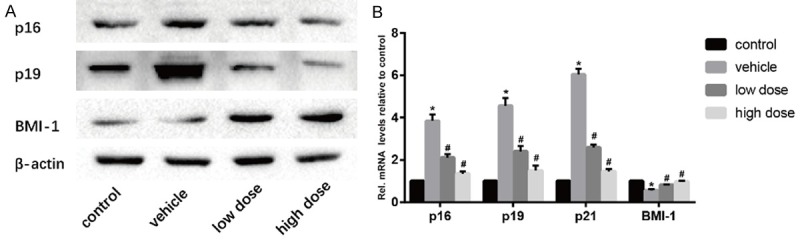
Vitamin D delays the aging of intervertebral disc cells. A. Results of protein expression of p16, p19, and BMI-1 in four groups was determined by Western blotting. β-Actin was used as an internal control. B. Results of mRNA expression of p16, p19, p21 and BMI-1 in four groups were determined by real time PCR. (“*” means there is a statistical difference with the control group and “#” means there is a statistical difference with the vehicle group. N number is all 3. p16: vehicle-control. P = 0.00009; low dose-vehicle, P = 0.00099; high dose-vehicle, P = 0.00019. p19: vehicle-control. P = 0.00007; low dose-vehicle, P = 0.00113; high dose-vehicle, P = 0.00026. p21: vehicle-control. P = 0.000005; low dose-vehicle, P = 0.000039; high dose-vehicle, P = 0.00001. BMI-1: vehicle-control. P = 0.00017; low dose-vehicle, P = 0.00179; high dose-vehicle, P = 0.00064).
Vitamin D retards NPCs degeneration in vitro
We detected the optimal concentration and the optimal time of cultured NPCs with vitamin D through CCK8 experiment and found that the highest cell viability was reached when culturing NPCs with vitamin D at 10-8 M for 24 hours (Figure 6A). Recombinant mouse IL-1β (10 ng/mL) was used to stimulate degeneration of NPCs. Western blotting and Real Time PCR showed that both with and without stimulation of IL-1β, vitamin D can effectively increase the level of type collagen II and aggrecan, and decrease the level of type collagen X (Figure 6B and 6C). This suggested that vitamin D may act directly on NPCs to retard disc degeneration.
Figure 6.
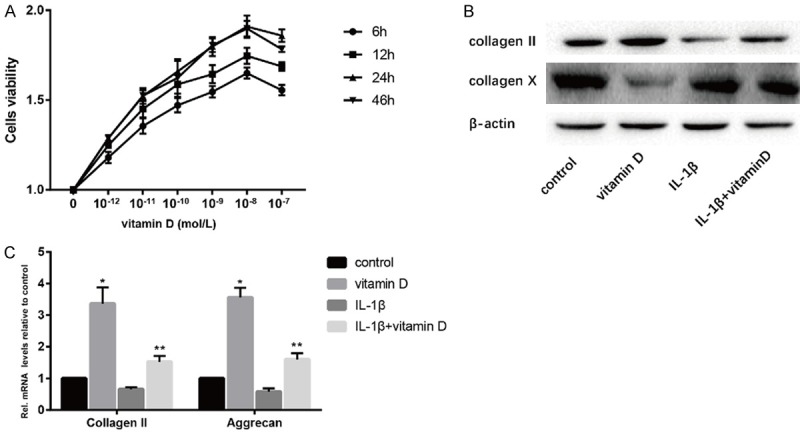
Vitamin D retarded NPCs degeneration in vitro. IL-1β (10 ng/ml) was uesd to promote NPCs degeneration. A. The optimal concentration and time of vitamin D were determined by CCK8 assay. B. Results of protein expression of collagen II and collagen X were determined by Western blotting. C. Results of mRNA expression of collagen II and aggrecan were determined by real time PCR. (“*” means there is a statistical difference with the control group and “**” means there is a statistical difference with the IL-1β group. N number is all 3. Collagen II: vitamin D-control. P = 0.00125; IL-1β+vitamin D-IL-1β, P = 0.0015. Aggrecan: vitamin D-control. P = 0.00012; IL-1β+vitamin D-IL-1β, P = 0.00129).
Vitamin D represses the NF-κB pathway
NF-κB pathway plays an important role in IVDD, so we determined the activity of NF-κB pathway in mice intervertebral discs of the four groups and NPCs. The results showed that the expression of p65 and IκB kinase α (IKKα) were significantly increased and the expressions of inhibitor of NF-κBα (IκBα) were significantly decreased in the vehicle group compared with the control group. After the administration of vitamin D, the expressions of p65 and IKKα were decreased and the expressions of IκBα were increased (Figure 7A and 7B). In vitro experiments, the expressions of p65 were lower in the vitamin D group than those in the control group and were decreased when stimulated with vitamin D (Figure 7C and 7D). This suggested that vitamin D inhibited the NF-κB pathway.
Figure 7.
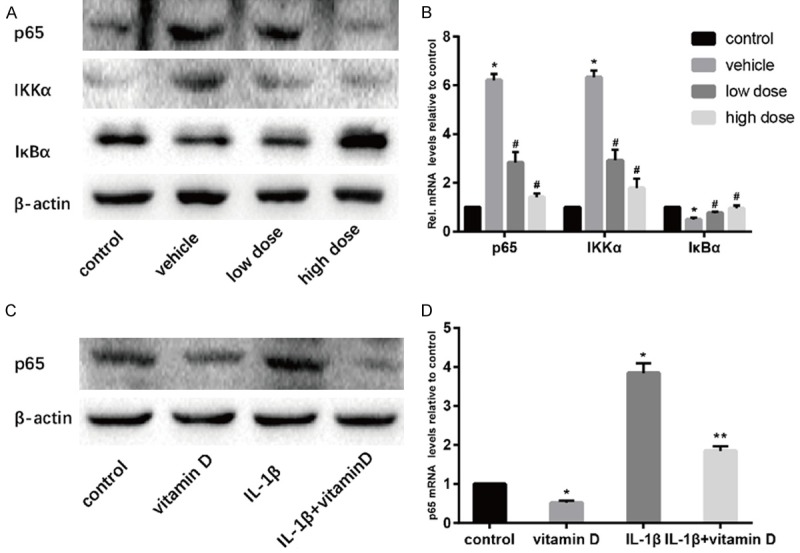
Vitamin D retarded IVDD by inhibiting NF-κB pathway. Results of protein expression of p65, IKKα and IκBα in vivo was determined by Western blotting (A) and real time PCR (B). Results of expression of p65 in vitro was determined by Western blotting (C) and real time PCR (D). (“*” means there is a statistical difference with the control group, “#” means there is a statistical difference with the vehicle group and “**” means there is a statistical difference with the IL-1β group. N number is all 3. p65: vehicle-control. P = 0.000002; low dose-vehicle, P = 0.00032; high dose-vehicle, P = 0.00001. IKKα: vehicle-control. P = 0.000004; low dose-vehicle, P = 0.00032; high dose-vehicle, P = 0.00008. IκBα: vehicle-control. P = 0.00041; low dose-vehicle, P = 0.01042; high dose-vehicle, P = 0.00526. p65: vitamin D-control. P = 0.0001; IL-1β+vitamin D-IL-1β, P = 0.00024).
Discussion
Traditional treatments for disc degeneration include operative treatments and expectant treatments. Non-steroidal anti-inflammatory drugs are the most commonly used drugs [4,17]. However, these drugs do not have the effect of radical cure or prevention. Our study is the first to demonstrate the protective effect of vitamin D on intervertebral disc degeneration. Furthermore, vitamin D is commonly used in clinical medicine and has few side effects, so it is a promising and safe drug [18].
Vitamin D is a derivative of steroids. In human skin, cholesterol dehydrogenation can produce 7-dehydrocholesterol, namely vitamin D progenitor, which is isomerized into vitamin D under ultraviolet radiation [19]. Vitamin D was firstly discovered due to its function on regulating calcium and phosphorus metabolism and maintaining normal blood calcium level [20]. In recent years, more and more studies have found that vitamin D has functions other than calcium and phosphorus regulation [21]. Grunwald et al. [22] demonstrated a cause-effect relationship between vitamin D deficiency and oxidative stress. They found a differential effect of vitamin D on cardiovascular risk factors such as oxidative stress and insulin resistance. In addition, vitamin D regulates a large number of cyclin-related proteins such as cyclines and cyclin-dependent kinases. Senescence associated genes such as p16, p19, p21 and p27 are also regulated by vitamin D [23]. Therefore, antioxidant stress and anti-aging may be the main aspects of vitamin D in the treatment of intervertebral disc degeneration. In our study, vitamin D also reduced levels of inflammation and apoptosis in the intervertebral disc. This revealed that vitamin D improves disc degeneration in many ways and factors influence each other in the process of disc degeneration.
Multiple signaling pathways are involved in disc degeneration such as Wnt/β-catenin signaling pathway, MAPK signaling pathway, JNK signaling pathway, NF-κB signaling pathway, Notch signaling pathway and so on [24]. Among them, NF-κB signaling pathway is a very classical signal pathway involving intervertebral disc degeneration. Degenerative intervertebral discs are characterized by increased levels of inflammatory factors and matrix metallase, suggesting that the regulation of inflammatory responses plays a key role in the degenerative process of intervertebral discs [25]. Studies have confirmed that inflammatory factors closely related to the occurrence and development of intervertebral disc degeneration mainly include IL-1β, IL-6, IL-8 and IL-10, and NF-κB is an important transcription factor in the inflammatory response [26]. NF-κB can be activated by different exogenous and endogenous stimuli in degenerative intervertebral discs [27]. At rest, NF-κB binds to the inhibitory protein IκBα in an inactive state in the cytoplasm, preventing NF-κB from entering the nucleus. After being stimulated by many inductive factors or IκBα is phosphorylated, the subunit of NF-κB p65 translocates into the nucleus and binds to the MMP promoter region containing the binding site of NF-κB, thereby exerting transcriptional regulation. Activation of NF-κB induces up-regulation of MMPs and inflammatory factors, which in turn can further activate and up-regulate MMPs, thereby forming a positive feedback cascade signaling pathway and aggravating the inflammatory reaction [26]. Therefore, the inflammatory response of the body depends to a large extent on the activation of NF-κB in cells. In our study, the expression of NF-κB signaling pathway related molecules in mice treated with vitamin D was significantly decreased and similar results were obtained in vitro, suggesting that vitamin D can inhibit NF-κB signaling pathway.
In summary, our study demonstrated the effect of vitamin D on intervertebral disc degeneration and this is mainly due to the inhibitory effect of vitamin D on NF-κB signaling pathways (Figure 8). This finding could be of great help in the clinical treatment of intervertebral disc degeneration.
Figure 8.
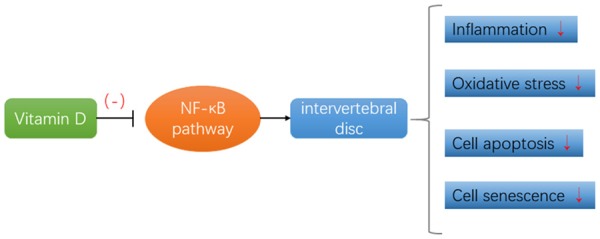
Vitamin D inhibits the NF-κB signaling pathway and thus retards IVDD. This role mainly includes relieving inflammatory reactions, resisting oxidative stress, inhibiting apoptosis and delaying cell senescence.
Conclusions
Vitamin D inhibits NF-κB signaling pathways, reduces the level of inflammation and oxidative stress in the intervertebral disc, delays cell aging, and inhibits apoptosis. Therefore, vitamin D can greatly improve intervertebral disc degeneration.
Disclosure of conflict of interest
None.
References
- 1.Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK. Intervertebral disk degeneration and repair. Neurosurgery. 2017;80:S46–S54. doi: 10.1093/neuros/nyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji ML, Lu J, Shi PL, Zhang XJ, Wang SZ, Chang Q, Chen H, Wang C. Dysregulated miR-98 contributes to extracellular matrix degradation by targeting IL-6/STAT3 signaling pathway in human intervertebral disc degeneration. J Bone Miner Res. 2016;31:900–909. doi: 10.1002/jbmr.2753. [DOI] [PubMed] [Google Scholar]
- 3.Shelerud RA. Epidemiology of occupational low back pain. Clin Occup Environ Med. 2006;5:501–528. doi: 10.1016/j.coem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Xu TT, Liao F, Jin HT, Tong PJ, Xiao LW, Wu CL. Research advance on intervertebral disc degeneration and cell death. Zhongguo Gu Shang. 2015;28:673–678. [PubMed] [Google Scholar]
- 5.Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy-le-grand) 2007;53:4–18. [PubMed] [Google Scholar]
- 6.Jiang L, Yuan F, Dong J. Comment on Park et al.: high glucose-induced oxidative stress promotes autophagy through mitochondrial damage in rat notochordal cells. Int Orthop. 2014;38:675–676. doi: 10.1007/s00264-013-2223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JW, Ni BB, Li B, Yang YH, Jiang SD, Jiang LS. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem. 2014;34:1175–1189. doi: 10.1159/000366330. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Jiang JM, Wang FL, Fu ZZ, Zhang ZF, Qu DB. Biological characteristics of human degenerative vertebral endplate cells. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:871–874. [PubMed] [Google Scholar]
- 9.Gruber HE, Gordon B, Williams C, Norton HJ, Hanley EJ. Vertebral endplate and disc changes in the aging sand rat lumbar spine: cross-sectional analyses of a large male and female population. Spine (Phila Pa 1976) 2007;32:2529–2536. doi: 10.1097/BRS.0b013e318158cd69. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24:398–408. doi: 10.1016/j.joca.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Shu CC, Smith MM, Smith SM, Dart AJ, Little CB, Melrose J. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab W, Schulze-Tanzil G, Mobasheri A, Dressler J, Kotzsch M, Shakibaei M. Interleukin-1beta-induced expression of the urokinase-type plasminogen activator receptor and its co-localization with MMPs in human articular chondrocytes. Histol Histopathol. 2004;19:105–112. doi: 10.14670/HH-19.105. [DOI] [PubMed] [Google Scholar]
- 13.Jean G, Souberbielle JC, Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients. 2017;9 doi: 10.3390/nu9040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pincikova T, Paquin-Proulx D, Sandberg JK, Flodstrom-Tullberg M, Hjelte L. Vitamin D treatment modulates immune activation in cystic fibrosis. Clin Exp Immunol. 2017;189:359–371. doi: 10.1111/cei.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, Yao X, Chen ZY, Vanhoutte PM, Huang Y. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33:2980–2990. doi: 10.1093/eurheartj/ehr459. [DOI] [PubMed] [Google Scholar]
- 16.Yasuoka H, Asazuma T, Nakanishi K, Yoshihara Y, Sugihara A, Tomiya M, Okabayashi T, Nemoto K. Effects of reloading after simulated microgravity on proteoglycan metabolism in the nucleus pulposus and anulus fibrosus of the lumbar intervertebral disc: an experimental study using a rat tail suspension model. Spine (Phila Pa 1976) 2007;32:E734–E740. doi: 10.1097/BRS.0b013e31815b7e51. [DOI] [PubMed] [Google Scholar]
- 17.Jiang JY, Lu XH. Biological treatment for intervertebral disc degeneration. Zhongguo Gu Shang. 2016;29:576–580. [PubMed] [Google Scholar]
- 18.Hussain S, Singh A, Akhtar M, Najmi AK. Vitamin D supplementation for the management of knee osteoarthritis: a systematic review of randomized controlled trials. Rheumatol Int. 2017;37:1489–1498. doi: 10.1007/s00296-017-3719-0. [DOI] [PubMed] [Google Scholar]
- 19.Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc. 2017;76:392–399. doi: 10.1017/S0029665117000349. [DOI] [PubMed] [Google Scholar]
- 20.Dereje S, Muradov I, Nazzal S, Nguyen T. Cholecalciferol (D(3)) versus ergocalciferol (D(2)) in older adults. Consult Pharm. 2017;32:337–339. doi: 10.4140/TCP.n.2017.337. [DOI] [PubMed] [Google Scholar]
- 21.Kattner L, Bernardi D. An efficient synthesis of 1α,25-dihydroxyvitamin D3 LC-biotin. J Steroid Biochem Mol Biol. 2017;173:89–92. doi: 10.1016/j.jsbmb.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald T, Fadia S, Bernstein B, Naliborski M, Wu S, Luca F. Vitamin D supplementation, the metabolic syndrome and oxidative stress in obese children. J Pediatr Endocrinol Metab. 2017;30:383–388. doi: 10.1515/jpem-2016-0211. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappe DL, Muhlestein JB. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 24.DiPaola CP, Farmer JC, Manova K, Niswander LA. Molecular signaling in intervertebral disk development. J Orthop Res. 2005;23:1112–1119. doi: 10.1016/j.orthres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhongyi S, Sai Z, Chao L, Jiwei T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine (Phila Pa 1976) 2015;40:224–232. doi: 10.1097/BRS.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 26.Wang XF, Zhang AP, Sun ZY, Liu C, Kuang LH, Tian JW. Expression of NF-kappaB in a degenerative human intervertebral disc model. Zhonghua Yi Xue Za Zhi. 2017;97:1324–1329. doi: 10.3760/cma.j.issn.0376-2491.2017.17.011. [DOI] [PubMed] [Google Scholar]
- 27.Han Y, Li X, Yan M, Yang M, Wang S, Pan J, Li L, Tan J. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-kappaB pathway: implications for disc degeneration. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.03.111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


