Abstract
Mukesh Kumar, Yuvana S. Priya, Virendra Mathur, Harendra Kumar, and Vadamalai Elangovan (2016) The ultrastructural hair morphology of 09 insectivorous bats such as Pipistrellus coromandra, P. ceylonicus, Scotophilus kuhlii, S. heathii, Hipposideros fulvus, H. lankadiva, Megaderma lyra, Rhinopoma micorphyllum and R. hardwickii were examined through scanning electron microscope to validate the use of hair characteristics as supplemental taxonomic tools for species recognition. The results suggest that the hair characteristics such as scale cuticle, divergence from the shaft and degree of hastateness varied among different species of bats. The coronal divergent scale was found in P. coromandra, P. ceylonicus, H. fulvus, and H. lankadiva while coronal divaricate scale was found in R. micorphyllum and R. hardwickii. However, imbricate type of scale was found in S. kuhlii, S. heathii and M. lyra with different degree of hastateness among them. The different types of hastateness found among these insectivorous bats include unequal hastate, equal hastate, alternate, elongate, rounded, simple, denticulate, acuminate and cusped. The hair characteristics such as hair length, scale length, scale width, scale index and width index differed among different species. However, there was no difference in the structure of scales among dorsal, ventral and neck hairs. The ultrastructural diverseness in the hair morphology of different insectivorous species suggests that the structural features of hairs could be used for species recognition.
Keywords: Hair cuticle, Guard hairs, Microchiroptera, Scanning electron microscope, Taxonomy
BACKGROUND
Bats are widely distributed group of mammals, second only to the ubiquitous rodents (Hill and Smith 1984; Churchill 1998). The identification of many bat species is a difficult task due to their cryptic nature. The scanning electron microscopy is a tool to study the ultrastructural variations in the hair morphology of different species of bats. Hair is a special epidermal characteristic of mammals. It normally consists of scales or cuticle, cortex and medulla. The shape and arrangement of these three layers are considered to be important in identification (Brunner and Coman 1974). It has been used in food habit studies of predators, forensic sciences, archeological studies, and mammalian identification (Mayer 1952; McFadden 1968; Brunner and Coman 1974; Appleyard 1978; Kennedy 1982; Valente 1983; Oli 1993; Wallis 1993; Dagnall et al. 1995). The cuticular scale patterns are useful in distinguishing hair from diverse mammalian faunas (Mathiak 1938; Mayer 1952; Appleyard 1960; Adorjan and Kolenosky 1969). The hair morphology studies enabled to use hair characteristics to separate families, genera and species of bats (Quay 1970). Moore and Braun (1983) developed a key to Tennessee bats in which they were able to discriminate among most of the 13 species (of seven genera) that occur in that state.
The major types of scales in bats are termed coronal and imbricate. Coronal scales, in contrast to imbricate, form a complete or cleft cylinder around the shaft, with successive coronal scales nested inside each other. Imbricate scales have two or more overlapping scales encircling the shaft without divergence from the shaft. Alternate describes coronal scales that have one side significantly taller than the other, with enlarged half is positioned opposite that of adjacent scales. Coronal scales of different species differ in the degree of distal edge diverges from the shaft, from little or no divergence (appressed) like a coffee mug, to moderate flaring (divergent) similar to many tumbler, to extreme separation (divaricate) reminiscent of a goblet in side view. A smooth margin is termed entire, whereas a cleft edge has a narrow V-shaped notch, otherwise entire border. Tri-cusp refers to a particular type of alternate scale that, in side view, has three peaks separated by rounded troughs (Nason 1948; Benedict 1957).
There are four types of mammalian hairs i.e. vibrissae, overhair, guard hair, and under hair. Vibrissae hairs are commonly grow around the nostrils, above the lips, and on other parts of the face of most mammals, as well as on the forelegs and feet of some animals. Vibrissae are usually thicker and stiffer than other types of hair. Over hairs are the longest hairs of a mammal’s coat and sparsely distributed. Guard hairs are long and the coarsest hairs in a mammal’s coat, forming the top coat (or outer coat). Guard hairs add the sheen to the coat of an animal. Williams (1938) reported that cuticular scale structure varied at different positions along the hair shaft and found that the scale structure was useful in distinguishing the hair of bats from that of insectivores and rodents. Benedict (1957) initially concluded that the structure of hairs from bats, as seen under a light microscope, provided reliable identification only for categories above the level of species. Scanning Electron Microscope has made possible a detailed examination of cuticular scales of hair. It offers the advantage of allowing direct observations of scale patterns, high resolution, and great magnification and has received attention in forensic sciences and diagnostic research. Though, a range of morphological characteristics and molecular techniques are being used for taxonomy and species recognition of bats, the ultrastructural hair morphology will be an additional taxonomic key for species recognition in bats. Therefore, we hypothesized that the hair morphology vary among bat species adequately and if so, can it be used for taxonomic purpose? To test the hypothesis, we examined the ultrastructural hair characteristics of 09 species of insectivorous bats and validated the use of hair characteristics as supplemental taxonomic tools for species recognition.
MATERIALS AND METHODS
Sample collection
Bats were captured by standard mist netting methods (Kunz and Kurta 1988), using 3 meter nylon mist nets (AVINET, USA) / Hoop net at different locations of Uttar Pradesh for species recognition and collection of hair samples. The hair samples were collected by pinching a small tuft between the thumb and forefinger, or plucking the hairs with the help of a fine forceps from the dorsal (body), ventral (abdomen) and neck regions of bats. Care was taken while plucking the hair close to the skin to ensure that the base of each hair was included in the sample and thereafter the bat was released at the site of capture. Hair samples were labeled and stored in sample vials for further process.
Hair samples of bat species namely Pipistrellus coromandra (Kaisarganj: 27°15'05"N 81°32'50"E, and Shikohabad: 27°06'54"N 78°34'26"E), P. ceylonicus (Lucknow: 26°46'09"N 80°55'45"E, and Raebarelly: 26°17'37"N 81°12'18"E), Scotophilus kuhlii (Lucknow: 26°37'36"N 80°55'16"E, and Purwa: 26°27'20"N 80°46'11"E), S. heathii (Jaunpur: 25°45'32"N 82°41'06"E, and Hardoi: 27°23'57"N 80°08'53"E), Hipposideros fulvus (Sultanpur: 26°13'31"N 82°17'02"E, and Jhansi: 25°44'53"N 78°55'28"E), H. lankadiva (Karwi: 25°12'59"N 80°55'03"E, and Chitrakot: 25°13'06"N 80°46'11"E), Megaderma lyra (Shikohabad: 27°06'01"N 78°36'01"E, and Agra: 26°56'06"N 78°32'31"E), Rhinopoma microphyllum (Chunar: 25°07'30"N 82°52'33"E, and Mathura: 27°26'18"N 77°43'09"E), R. hardwickii (Allahabad: 25°26'32"N 81°49'15"E, and Fatehpur Sikri: 27°05'50"N 77°39'47"E) were collected at different locations of Uttar Pradesh.
Sample preparation
For scanning electron microscopy, the hair samples were cleaned and fixed in 2.5% glutaraldehyde fixative for 2 to 6 hours at 4°C. After the primary fixation, the samples were washed with 0.1 M phosphate buffer for 3 changes each of 15 min and fixed in 1% osmium tetroxide as a post fixation for 2 hours and washed with 0.1 M phosphate buffer for 3 changes each of 15 min at 4°C. Thereafter, the samples were dehydrated with increasing concentration such as 30%, 50%, 70%, 90%, 95% acetone and 100% dry acetone for 30 min. All steps were carried out at 4°C. Hairs were mounted with double-sided carbon tape on aluminium stubs, sputter-coated with palladium coater (Auto Fine Coater JFC- 1600 JEOL, Japan). Each sample was examined by JEOL JSM 6490 LV (Tokyo, Japan) scanning electron microscope at different magnifications and accelerating voltages.
Analysis
The hair shaft was divided into three regions, the proximal region near the root, the medial region in the middle and the distal region near the tip of hair. For comparison purpose, only the midsection of each hair was used in this study. According to Benedict (1957), the scales in the mid-region of a hair shaft are mature and uniform. The scale length (from the free distal edge of one scale to that of the next) and scale width were measured from each micrograph using measuring tools of the JEOL software. Scale index was calculated by dividing the greatest diameter of the hair into the greatest length of a scale of the hair. Width index was calculated by dividing the greatest diameter of the hair into the lowest diameter of the hair. The type of scale hastate (i.e. scale margin) was determined based on previous studies (Brown 1942; Nason 1948; Benedict 1957). The terminology used to describe the scales in this study was adapted from previous studies on chiropteran hair morphology (Brown 1942; Nason 1948; Benedict 1957; Schaetz et al. 2009). One-way analysis of variance (ANOVA) was performed to determine the differences among different regions (dorsal, ventral and neck) of hairs. The sexual differences on hair characteristics were compared using T-test (KyPlot Software). The study was carried out as per existing procedure of the Babasaheb Bhimrao Ambedkar University, Lucknow, India.
RESULTS
The ultrastructural hair morphology of 09 insectivorous bats examined in this study differed in their cuticular pattern, divergence from hair shaft and degree of hastateness (Table 1). The coronal scale which completely encircled the hair shaft was observed predominantly in many species compared to the imbricate scale. In case of imbricate scale, two or more overlapping scales encircled the hair shaft with divergent scale margin whereas an individual coronal scale completely encircled the hair shaft. The degree of hastateness observed in the insectivore bats include simple, equal hastate, unequal hastate, alternate, elongate, rounded, acuminate and cusped (Table 1). There was no change in the scale characteristics among dorsal, ventral and neck regions of different species of bats. The mean hair length, scale length and scale width of dorsal, ventral and neck hairs of male and female Pipistrellus coromandra, P. ceylonicus, Scotophilus kuhlii, S. heathii, Hipposideros fulvus, H. lankadiva, Megaderma lyra, R. micorphyllum and R. hardwickii given in table 2 and the scale index, width index and angle of divergence from hair shaft of dorsal, ventral and neck hairs given in table 3.
Table 1. Summary of hair scale characteristics of dorsal, ventral and neck regions of microchiropteran bats.
| Family | Name of species | Dorsal region | Ventral region | Neck region | ||||||
| Scale type | Divergence from shaft | Degree of hastateness | Scale type | Divergence from shaft | Degree of hastateness | Scale type | Divergence from shaft | Degree of hastateness | ||
| Vespertilionidae | P. coromandra | Coronal | Divergent | Unequal hastate | Coronal | Divergent | Unequal hastate | Coronal | Divergent | Unequal hastate |
| P. ceylonicus | Coronal | Divergent | Alternate | Coronal | Divergent | Alternate | Coronal | Divergent | Alternate | |
| S. kuhlii | Imbricate | Divergent | Elongate | Imbricate | Divergent | Elongate | Imbricate | Divergent | Elongate | |
| S. heathii | Imbricate | Divergent | Rounded | Imbricate | Divergent | Rounded | Imbricate | Divergent | Rounded | |
| Hipposiderdae | H. fulvus | Coronal | Divergent | Equal hastate | Coronal | Divergent | Equal hastate | Coronal | Divergent | Unequal hastate |
| H. lankadiva | Coronal | Divergent | Simple | Coronal | Divergent | Simple | Coronal | Divergent | Simple | |
| Megadermatidae | M. lyra | Imbricate | Divergent | Acuminate | Imbricate | Divergent | Acuminate | Imbricate | Divergent | Acuminate |
| Rhinopomatidae | R. microphyllum | Coronal | Divaricate | Cusped | Coronal | Divaricate | Cusped | Coronal | Divaricate | Cusped |
| R. hardwickii | Coronal | Divaricate | Cusped | Coronal | Divaricate | Cusped | Coronal | Divaricate | Cusped | |
Table 2. Hair length (mm), scale length (µm) and scale width (µm) of dorsal, ventral and neck hairs of microchiropteran bats.
| Name of species | Sex | Hair length (mm) | Scale length (µm) | Scale width (µm) | ||||||
| Dorsal | Ventral | Neck | Dorsal | Ventral | Neck | Dorsal | Ventral | Neck | ||
| P. coromandra | ♂ (n = 4) | 5.24 ± 1.55 | 4.26 ± 0.48 | 4.39 ± 0.82 | 12.63 ± 1.47 | 12.27 ± 1.41 | 11.24 ± 1.58 | 12.03 ± 1.17 | 11.77 ± 1.13 | 11.22 ± 0.91 |
| ♀ (n = 2) | 4.84 ± 0.44 | 4.94 ± 0.50 | 4.44 ± 0.52 | 15.88 ± 1.00 | 13.96 ± 1.85 | 11.99 ± 1.34 | 12.67 ± 1.02 | 12.69 ± 0.78 | 11.61 ± 0.41 | |
| P. ceylonicus | ♂ (n = 2) | 4.82 ± 0.24 | 4.38 ± 0.27 | 4.05 ± 0.38 | 11.34 ± 1.44 | 10.39 ± 1.38 | 7.80 ± 0.91 | 14.54 ± 0.21 | 10.19 ± 0.63 | 8.51 ± 0.24 |
| ♀ (n = 2) | 4.61 ± 0.46 | 4.18 ± 0.19 | 4.04 ± 0.32 | 14.00 ± 1.54 | 14.19 ± 0.90 | 9.49 ± 1.46 | 14.40 ± 0.34 | 11.99 ± 0.52 | 11.84 ± 0.39 | |
| S. kuhlii | ♂ (n = 3) | 4.70 ± 0.96 | 5.36 ± 0.49 | 4.93 ± 0.50 | 17.49 ± 2.55 | 17.17 ± 2.79 | 18.21 ± 2.63 | 15.14 ± 1.17 | 14.01 ± 0.85 | 13.74 ± 0.75 |
| ♀ (n = 2) | 5.10 ± 0.93 | 5.19 ± 0.50 | 4.86 ± 0.28 | 18.93 ± 2.27 | 16.96 ± 2.68 | 18.40 ± 2.40 | 15.07 ± 0.98 | 15.44 ± 1.03 | 12.99 ± 2.06 | |
| S. heathii | ♂ (n = 6) | 5.34 ± 0.59 | 5.29 ± 0.78 | 6.28 ± 0.91 | 15.58 ± 2.47 | 16.42 ± 1.98 | 15.58 ± 2.53 | 14.29 ± 1.72 | 13.66 ± 1.67 | 12.74 ± 1.53 |
| ♀ (n = 2) | 6.76 ± 0.48 | 5.29 ± 0.85 | 6.19 ± 0.47 | 18.06 ± 2.49 | 15.14 ± 2.51 | 13.11 ± 2.38 | 17.51 ± 1.56 | 15.83 ± 1.57 | 13.32 ± 0.65 | |
| H. fulvus | ♂ (n = 2) | 6.97 ± 0.69 | 6.54 ± 0.50 | 7.05 ± 0.53 | 15.27 ± 1.70 | 13.32 ± 2.44 | 12.74 ± 1.71 | 13.31 ± 0.80 | 14.40 ± 2.54 | 13.45 ± 0.83 |
| ♀ (n = 2) | 6.07 ± 0.53 | 5.80 ± 0.63 | 6.31 ± 1.07 | 11.53 ± 0.98 | 14.70 ± 2.34 | 15.86 ± 0.50 | 13.60 ± 0.43 | 13.54 ± 0.77 | 11.21 ± 0.37 | |
| H. lankadiva | ♂ (n = 3) | 7.95 ± 0.41 | 7.04 ± 0.94 | 9.53 ± 0.61 | 15.91 ± 2.59 | 12.97 ± 1.89 | 13.68 ± 2.51 | 18.12 ± 2.10 | 14.32 ± 1.40 | 15.64 ± 2.57 |
| ♀ (n = 3) | 7.39 ± 0.54 | 6.94 ± 0.41 | 8.76 ± 0.99 | 15.75 ± 2.55 | 13.02 ± 1.96 | 11.90 ± 1.74 | 18.13 ± 0.62 | 15.79 ± 0.62 | 12.65 ± 0.96 | |
| M. lyra | ♂ (n = 4) | 10.20 ± 1.52 | 7.61 ± 1.43 | 7.80 ± 1.48 | 14.29 ± 2.70 | 14.20 ± 2.76 | 13.64 ± 2.23 | 14.92 ± 1.95 | 12.67 ± 1.26 | 13.23 ± 1.88 |
| ♀ (n = 3) | 10.15 ± 1.83 | 7.56 ± 1.29 | 7.58 ± 0.60 | 14.08 ± 2.51 | 13.44 ± 2.29 | 13.95 ± 2.64 | 15.41 ± 0.93 | 13.66 ± 1.42 | 14.32 ± 0.87 | |
| R. microphyllum | ♂ (n = 5) | 5.57 ± 1.24 | 4.93 ± 0.34 | 5.57 ± 0.66 | 15.45 ± 2.18 | 15.22 ± 2.40 | 16.80 ± 2.59 | 17.00 ± 1.40 | 18.04 ± 2.24 | 17.73 ± 2.27 |
| ♀ (n = 2) | 5.40 ± 0.60 | 4.83 ± 0.48 | 5.01 ± 0.29 | 15.98 ± 2.01 | 16.20 ± 2.27 | 16.93 ± 2.93 | 18.20 ± 1.45 | 17.06 ± 0.81 | 17.58 ± 0.71 | |
| R. hardwickii | ♂ (n = 5) | 5.76 ± 1.08 | 5.64 ± 1.14 | 5.68 ± 0.43 | 15.68 ± 2.91 | 15.05 ± 2.56 | 15.25 ± 2.61 | 17.61 ± 1.75 | 18.17 ± 2.70 | 15.36 ± 1.63 |
| ♀ (n = 3) | 5.83 ± 0.70 | 4.99 ± 0.54 | 5.67 ± 0.62 | 15.46 ± 2.29 | 16.89 ± 1.67 | 15.01 ± 3.27 | 17.17 ± 0.87 | 17.39 ± 2.42 | 16.00 ± 1.81 | |
Table 3. Scale index (µm), width index (µm) and angle of divergence from the shaft (°) of microchiropteran bats.
| Name of species | Sex | Scale index (µm) | Width index (µm) | Angle of divergence (°) | |||||||||
| Dorsal | Ventral | Neck | Dorsal | Ventral | Neck | Dorsal | Ventral | Neck | |||||
| P. coromandra | ♂ (n = 4) | 0.94 ± 0.33 | 0.89 ± 0.21 | 0.75 ± 0.06 | 1.14 ± 0.10 | 1.21 ± 0.09 | 1.36 ± 0.26 | 37.20 ± 3.50 | 39.60 ± 4.78 | 38.22 ± 5.66 | |||
| ♀ (n = 2) | 0.71 ± 0.11 | 0.91 ± 0.18 | 0.88 ± 0.11 | 1.16 ± 0.05 | 1.19 ± 0.08 | 1.08 ± 0.06 | 39.37 ± 2.69 | 39.39 ± 3.22 | 40.47 ± 2.60 | ||||
| P. ceylonicus | ♂ (n = 2) | 1.13 ± 0.03 | 0.92 ± 0.04 | 0.97 ± 0.01 | 1.04 ± 0.02 | 1.16 ± 0.02 | 1.05 ± 0.02 | 20.72 ± 2.75 | 26.41 ± 3.89 | 22.65 ± 3.55 | |||
| ♀ (n = 2) | 0.91 ± 0.01 | 0.83 ± 0.01 | 1.10 ± 0.03 | 1.04 ± 0.02 | 1.09 ± 0.06 | 1.08 ± 0.03 | 22.22 ± 1.42 | 28.53 ± 3.54 | 19.11 ± 1.47 | ||||
| S. kuhlii | ♂ (n = 3) | 0.71 ± 0.16 | 0.79 ± 0.44 | 0.59 ± 0.05 | 1.27 ± 0.06 | 1.17 ± 0.09 | 1.17 ± 0.02 | 40.41 ± 4.46 | 50.36 ± 3.92 | 47.69 ± 5.39 | |||
| ♀ (n = 2) | 0.86 ± 0.19 | 0.79 ± 0.10 | 0.67 ± 0.18 | 1.10 ± 0.09 | 1.13 ± 0.02 | 1.15 ± 0.14 | 45.56 ± 3.24 | 49.61 ± 2.94 | 48.93 ± 2.21 | ||||
| S. heathii | ♂ (n = 6) | 0.82 ± 0.28 | 0.70 ± 0.08 | 0.81 ± 0.21 | 1.25 ± 0.07 | 1.31 ± 0.19 | 1.33 ± 0.27 | 46.24 ± 3.72 | 47.90 ± 3.46 | 50.86 ± 2.38 | |||
| ♀ (n = 2) | 0.74 ± 0.05 | 0.86 ± 0.04 | 0.70 ± 0.09 | 1.22 ± 0.05 | 1.18 ± 0.12 | 1.11 ± 0.08 | 49.78 ± 3.57 | 48.72 ± 4.15 | 50.57 ± 0.76 | ||||
| H. fulvus | ♂ (n = 2) | 0.82 ± 0.05 | 0.86 ± 0.21 | 0.82 ± 0.05 | 1.15 ± 0.04 | 1.09 ± 0.06 | 1.10 ± 0.10 | 25.87 ± 2.22 | 43.37 ± 3.16 | 25.49 ± 3.64 | |||
| ♀ (n = 2) | 1.08 ± 0.02 | 0.80 ± 0.05 | 0.58 ± 0.01 | 1.06 ± 0.03 | 1.14 ± 0.06 | 1.20 ± 0.06 | 24.98 ± 1.40 | 40.81 ± 2.16 | 27.69 ± 2.55 | ||||
| H. lankadiva | ♂ (n = 3) | 0.90 ± 0.13 | 0.93 ± 0.06 | 0.86 ± 0.21 | 1.11 ± 0.03 | 1.09 ± 0.02 | 1.11 ± 0.04 | 20.66 ± 1.75 | 22.26 ± 1.13 | 20.68 ± 1.86 | |||
| ♀ (n = 3) | 0.94 ± 0.03 | 1.04 ± 0.01 | 1.20 ± 0.39 | 1.09 ± 0.02 | 1.09 ± 0.02 | 1.18 ± 0.05 | 22.41 ± 1.27 | 20.80 ± 1.28 | 20.99 ± 1.35 | ||||
| M. lyra | ♂ (n = 4) | 0.88 ± 0.07 | 0.70 ± 0.07 | 0.79 ± 0.16 | 1.17 ± 0.07 | 1.23 ± 0.02 | 1.20 ± 0.11 | 26.06 ± 1.86 | 25.46 ± 0.87 | 26.43 ± 2.64 | |||
| ♀ (n = 3) | 0.92 ± 0.05 | 0.90 ± 0.07 | 0.91 ± 0.22 | 1.14 ± 0.07 | 1.22 ± 0.02 | 1.22 ± 0.03 | 26.49 ± 2.41 | 28.31 ± 2.72 | 28.13 ± 2.04 | ||||
| R. microphyllum | ♂ (n = 5) | 0.97 ± 0.09 | 1.08 ± 0.20 | 0.93 ± 0.17 | 1.21 ± 0.08 | 1.18 ± 0.04 | 1.20 ± 0.21 | 52.25 ± 3.87 | 45.84 ± 2.33 | 45.35 ± 4.39 | |||
| ♀ (n = 2) | 1.12 ± 0.16 | 0.87 ± 0.06 | 0.91 ± 0.06 | 1.14 ± 0.06 | 1.14 ± 0.07 | 1.11 ± 0.06 | 44.60 ± 2.21 | 42.89 ± 2.08 | 44.71 ± 2.10 | ||||
| R. hardwickii | ♂ (n = 5) | 1.09 ± 0.24 | 1.09 ± 0.25 | 1.05 ± 0.29 | 1.20 ± 0.10 | 1.20 ± 0.05 | 1.18 ± 0.06 | 49.36 ± 3.00 | 50.71 ± 2.43 | 50.88 ± 3.90 | |||
| ♀ (n = 3) | 1.00 ± 0.07 | 0.89 ± 0.18 | 0.90 ± 0.07 | 1.11 ± 0.05 | 1.16 ± 0.07 | 1.25 ± 0.12 | 44.04 ± 1.74 | 4.37 ± 2.27 | 45.48 ± 2.58 | ||||
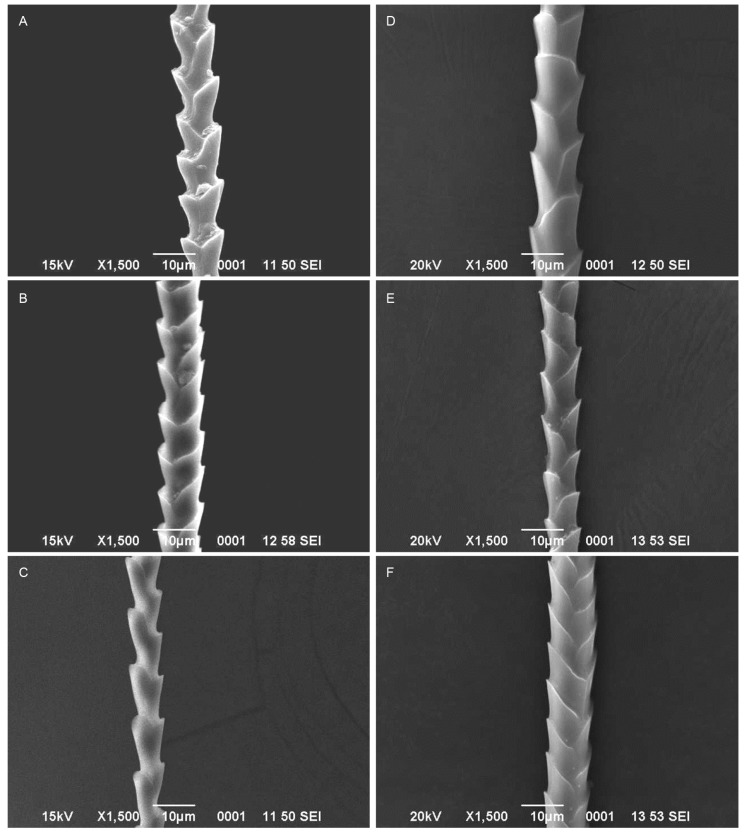
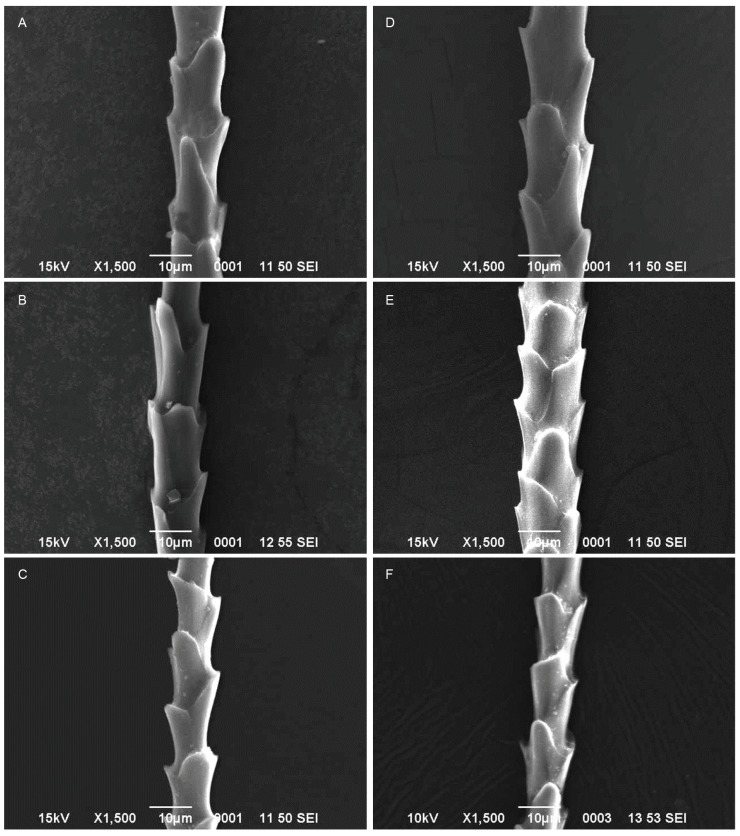
The vespertilionid bats such as P. coromandra and P. ceylonicus had coronal scales with divergence from the hair shaft in dorsal, ventral and neck regions. The degree of hastateness was unequal in case of P. coromandra (Figs. 1A, B, C), while it was alternate in P. ceylonicus (Figs. 1D, E, F; Table 1). Imbricate scales with divergence from shaft were observed at dorsal, ventral and and P. ceylonicus had coronal scales with divergence from the hair shaft in dorsal, ventral and neck regions. The degree of hastateness was unequal in case of P. coromandra (Figs. 1A, B, C), while it was alternate in P. ceylonicus (Figs. 1D, E, F; Table 1). Imbricate scales with divergence from shaft were observed at dorsal, ventral and neck hairs of S. kuhlii (Figs. 2A, B, C) and S. heathii (Figs. 2D, E, F). The degree of hastateness of S. kuhlii was elongate and it was rounded in S. heathii (Table 1). One way ANOVA showed significant difference in the hair length (F3,176 = 28.96; p < 0.001), scale length (F3,489 = 135.71; p < 0.001) and scale width (F3,502 = 67.66; p < 0.001), scale index (F3,80 = 7.00; p < 0.001), width index (F3,80 = 6.98; p < 0.001) and angle of divergence from hair shaft (F3,224 = 3.61; p < 0.001) among P. coromandra, P. ceylonicus, S. kuhlii and S. heathii. However, there was no significant difference in the hair length of male and female of P. coromandra (t = 0.59; d.f. = 76; p > 0.05), P. ceylonicus (t = 0.87; d.f.=23;p>0.05),S.kuhlii(t=0.25;d.f.=43;p> 0.05) and S. heathii (t = 0.55; d.f. = 51; p > 0.05).
Fig. 1.
Fig. 1. Scanning electron micrographs of guard hairs of Pipistrellus coromandra (A: Dorsal, B: Ventral, C: Neck hairs). P. ceylonicus (D: Dorsal, E: Ventral, F: Neck hairs).
Fig. 2.
Fig. 2. Scanning electron micrographs of guard hairs of Scotophilus kuhlii (A: Dorsal, B: Ventral, C: Neck hairs). S. heathii (D: Dorsal, E: Ventral, F: Neck hairs).
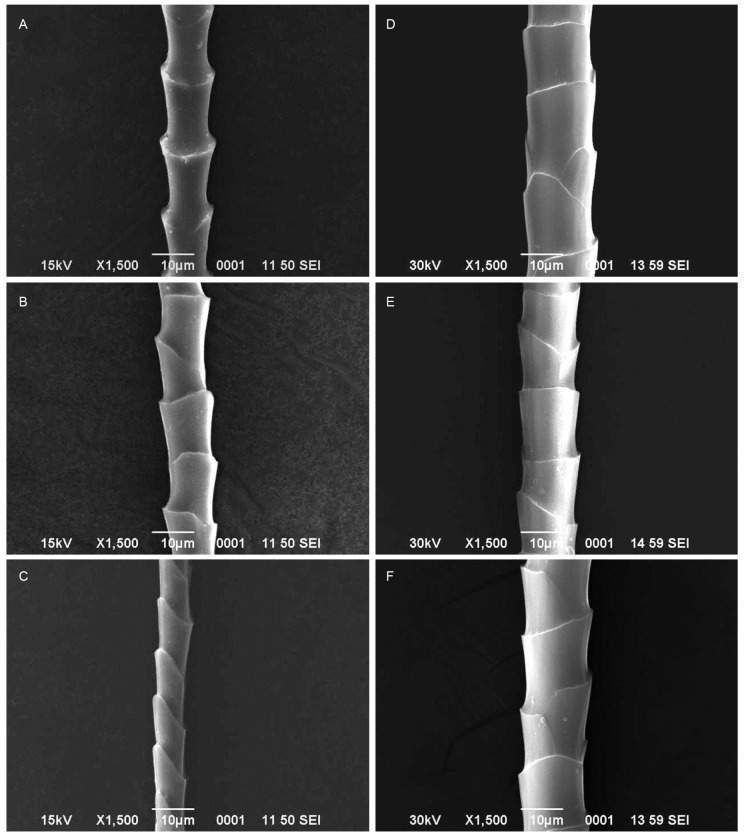
The hipposideros bats such as H. fulvus (Figs. 3A, B, C) and H. lankadiva (Figs. 3D, E, F) had coronal scales (Table 1). The scale of H. fulvus and H. lankadiva relatively less diverged from the shaft compared to the bats of other families. The dorsal and ventral hairs of H. fulvus showed equal hastateness while unequal hastateness was observed in the neck hairs and simple type of hastateness was observed in dorsal, ventral and neck hairs of H. lankadiva. There was a significant difference in the hair characteristics such as hair length (t = 5.98; d.f. = 57; p < 0.001), scale width (t = 8.25; d.f. = 181; p < 0.001) and angle of divergence (t = 9.52; d.f. = 102; p < 0.001) among H. fulvus and H. lankadiva. However, there was no intersexual variations and thus showed non- significant difference in the hair length (t = 1.66; d.f. = 58; p > 0.05), scale length (t = 0.71; d.f. = 139; p > 0.05) and scale width (t = 1.41; d.f. = 193; p > 0.05) of male and female H. lankadiva, while the hair length (t = 3.11; d.f. = 26; p < 0.01) and scale width (t = 2.55; d.f. = 85; p < 0.05) of male and female H. fulvus differed significantly. The megadermatid bat M. lyra had imbricate scales (Figs. 4A, B, C; Table 1) that formed by overlapping of two or more scales around the hair shaft and the scale margin had less diverged from the hair shaft. Acuminate type of hastateness was observed in the scales of M. lyra. There was no significant difference in the hair length (t = 0.25; d.f. = 67; p > 0.05) and scale length (t = 0.55; d.f. = 172; p > 0.05) of male and female M. lyra. However, the scale width (t = 2.96; d.f. = 206; p < 0.01) and angle of divergence from the shaft (t = 2.69; d.f. = 29; p < 0.05) of male and female bats showed significant difference.
Fig. 3.
Fig. 3. Scanning electron micrographs of guard hairs of Hipposideros fulvus (A: Dorsal, B: Ventral, C: Neck hairs). H. lankadiva (D: Dorsal, E: Ventral, F: Neck hairs).
Fig. 4.
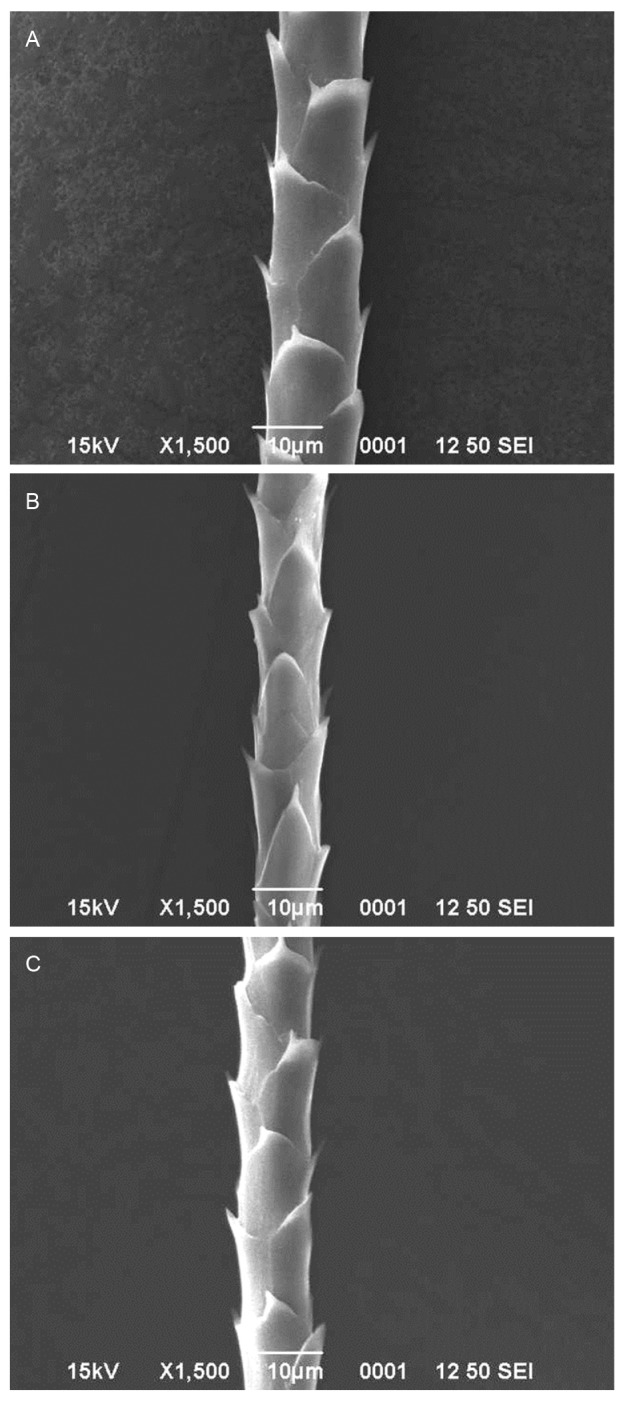
Fig. 4. Scanning electron micrographs of guard hairs of Megaderma lyra (A: Dorsal, B: Ventral, C: Neck hairs).
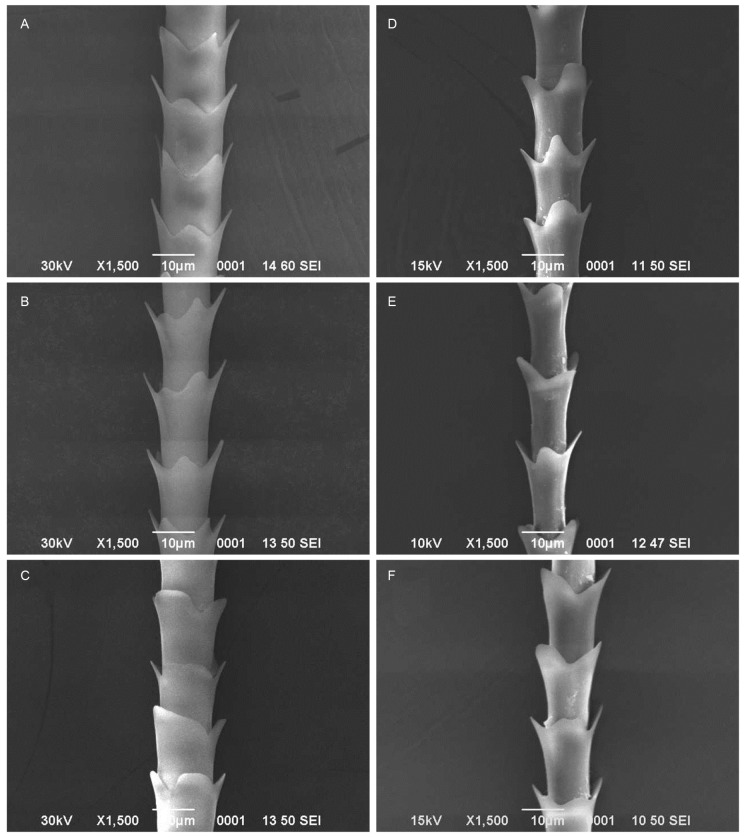
The rhinopomatid bats such as R. microphyllum (Figs. 5A, B, C) and R. hardwickii (Figs. 5D, E, F) had coronal divaricate scales with cusped hastateness (Table 1). There was a significant difference in the hair length (F2,228 = 26.74; p < 0.001), scale width (F2,523 = 8.29; p < 0.001) and angle of divergence (F2,192 = 5.68; p < 0.01) between R. microphyllum and R. hardwickii. There was no intersexual difference in the hair length of R. microphyllum (t = 1.40; d.f. = 87; p > 0.05) and R. hardwickii (t = 1.14; d.f. = 71; p > 0.05). In consistent to the hair length, the scale length (t = 1.45; d.f. = 202; p > 0.05) and scale width (t = 1.15; d.f. = 213; p > 0.05) of male and female of R. microphyllum and scale length (t = 1.31; d.f. = 153; p > 0.05) and scale width (t = 1.79; d.f. = 194; p > 0.05) of male and female R. hardwickii did not differ significantly.
Fig. 5.
Fig. 5. Scanning electron micrographs of guard hairs of Rhinopoma microphyllum (A: Dorsal, B: Ventral, C: Neck hairs). R. hardwickii (D: Dorsal, E: Ventral, F: Neck hairs).
DISCUSSION
The results of this study showed a distinct difference in the hair morphology of different species of insectivorous bats. It is evident that the hair characteristics differed among the bat species of a family, e.g. the vespertilionid bats had coronal as well as imbricate scale types with different degrees of hastateness such as unequal, alternate, elongate and rounded. Though, the hipposiderid bats had coronal divergent scales but the hastateness differed among them like equal, unequal and simple. The lone species of family Megadermatidae, Megaderma lyra had imbricate scale with acuminate hastateness while the rhinopomatid bats had coronal divaricate scales with cusped hastateness.
The hair morphological characters such as hair length, scale length, scale width, scale index, width index and angle of divergence from the hair shaft of verspertilionid bats such as Pipistrellus coromandra, P. ceylonicus, Scotophilus kuhlii and S. heathii differed significantly which showed the suitability of hair characteristics for taxonomical usage. Similar differences were observed among hipposiderid bats such as Hipposideros fulvus and H. lankadiva, and rhinopomatid bats such as Rhinopoma microphyllum and R. hardwickii. The structural analysis of the hair cuticle pattern reveals the systematic relationships between and within different mammalian groups. The results of present investigation clearly showed the species specific structural hair characteristics in insectivorous bats. However, the hair morphology of the species examined in this study did not show structural differences among sex as well as different regions such as dorsal, ventral and neck.
The results of present study were consistent with earlier studies which carried out using scanning as well as light microscopes (Benedict 1957; Nursel and Irfan 2007). The hair morphology of molossid bats were correlated with their life history strategy (Nason 1948). Benedict (1957) and Moore and Braun (1983) used the hair morphology for taxonomic investigation. The hair morphology study in bats may increase the opportunity to investigate the food habit, prey predator relationships in bats and forensic studies. Though, the environmental factors do influence hair structure but not to the extent of entirely altering the basic characters. Therefore, the hair morphology could be used for species recognition of bats. The outcome of this study is an addition to the taxonomy of chiropteran fauna as well as it provides the species specific ultrastructural hair characteristics of insectivorous bats.
CONCLUSIONS
The ultrastructural hair morphology of insectivorous bats such as Pipistrellus coromandra, P. ceylonicus, Scotophilus kuhlii, S. heathii, Hipposideros lankadiva, H. fulvus, Megaderma lyra, Rhinopoma microphyllum and R. hardwickii was investigated using scanning electron microscope. The scale cuticle, divergence from the shaft and degree of hastateness varied among different species. The hair characteristics such as hair length, scale length, scale width, scale index and width index also differed among different species and it suggested that the structural features of hairs could be used for taxonomic purpose.
Acknowledgments
Acknowledgments: The authors thank the University Science Instrumentation Centre for extending scanning electron microscope facility and the Archaeological Survey of India for permitting us to collect bat hair samples. The financial assistance of Uttar Pradesh State Biodiversity Board, Uttar Pradesh and University Grants Commission, New Delhi through research projects (No. 493/3-4- 48/2013) and (No. 42-530/2013(SR)), respectively to VE is acknowledged. MK is a University Grants Commission-Rajeev Gandhi National fellowship holder.
Abbreviations
- %
Percentage
- °C
Degree Celsius
- °
Degree
- M
Molarities
- μm
Micrometer
- d.f.
Degree of freedom
- min
Minutes
- mm
Millimeter
- e.g.
Example
- ♂
Male
- ♀
Female
References
- Adorjan AS, Kolenosky GB. 1969. A manual for the identification of hairs of selected Ontario mammals. Ontario Department of Lands and Forests Research Report (Wildlife). Publi- cation 90, Ontario, Canada.
- Appleyard HM. 1960. Guide to the identification of animal fibers. Wool Industries Research Association, Leeds, United Kingdom.
- Appleyard HM. 1978. Guide to the identification of animal fibers. Wool Industries Research Association, Leeds, United Kingdom.
- Benedict FA. 1957. Hair structure as a generic character in bats. Univ Calif Publ Zool 59:258-548.
- Brown FM. 1942. The microscopy of mammalian hair for anthropologists. Univ Calif Publ Zool 85:250-274.
- Brunner H, Coman B. 1974. The Identification of Mammalian Hair. Inkata Press, Melbourne, Victoria.
- Churchill SK. 1998. A comprehensive guide to all of Australia’s bats, with photos, detailed information and a key. Australian Bats, New Holland Publishers, Sydney.
- Dagnall JL, Duckett JG, Gurnell J. 1995. A simple negative staining technique for the identification of mammal hairs. J Zool (London) 237:670-675. doi:10.1111/j.1469- 7998.1995.tb05025.x.
- Hill JE, Smith JD. 1984. Bats: A Natural History. Univ of Texas Pr, Austin.
- Kennedy AJ. 1982. Distinguishing characteristics of the hair of wild and domestic canids from Alberta. Can J Zool 60(4):536-541. doi:10.1139/z82-080.
- Kunz TH, Kurta A. 1988. Capture methods and holding devices. In: Kunz TH (ed) Ecological and Behavioral Methods for the Study of Bats. Smithsonian Institution, Washington DC, pp. 1-29.
- Mathiak HA. 1938. A key to hairs of the mammals of southern Michigan. J Wildl Manage 2(4):251-268.
- Mayer WV. 1952. The hair of California mammals with keys to the dorsal guard hairs of California mammals. Am Midl Nat 48(2):480-512.
- McFadden WD. 1968. A rapid method for studying the surface structure of mammalian hair fibers. Research report 145, New Mexico State University, Agricultural Experiment Station. Las Cruces, New Mexico.
- Moore DW, Braun JK. 1983. Keys to the hairs of the families Soricidae, Vespertilionidae, and Muridae within Tenne- ssee. J Tennessee Acad Sci 58(3&4):40-43.
- Nason ES. 1948. Morphology of hair of eastern North American bats. Am Midl Nat 39(2):345-361.
- Nursel A, Irfan A. 2007. Some Taxonomic Features of Taphozous nudiventris Cretzschmar, 1830-31 from Turkey (Chiroptera: Emballonuridae). Turk J Zool 31:165-170.
- Oli MK. 1993. A key for the identification of the hair of mammals of a snow leopard (Panthera uncia) habitat in Nepal. J Zool (London) 231:71-93. doi:10.1111/j.1469-7998.1993. tb05354.x.
- Quay WB. 1970. Integument and derivatives. In: Wimsatt WA (ed) Biology of bats. Academic Press, New York, pp. 1-56.
- Schaetz BA, Kurta A, Rodriguez-Dsuran A, Munzer OM, Foster R. 2009. Identification of bats in Puerto Rico using the scanning electron microscope to examine body hairs. Caribb J Sci 45:125-130.
- Valente A. 1983. Hair Structure of the woolly mammoth, Mammuthus primigenius and the modern elephants, Elphas maximus and Loxodonta Africana. J Zool (London) 199:271-274. doi:10.1111/j.1469-7998.1983.tb02095.x.
- Wallis RL. 1993. A key for the identification of guard hairs of some Ontario mammals. Can J Zool 71(3):587-591. doi:10.1139/z93-080.
- Williams CS. 1938. Aids to the identification of mole and shrew hairs with general comments on hair structure and hair determination. J Wildl Manage 2:239-250. doi:10.2307/3795672.