Abstract
Much is known about the structure, function, and stability of the SecA motor ATPase that powers the secretion of periplasmic proteins across the inner membrane of Escherichia coli. Most studies of SecA are carried out in buffered sodium or potassium chloride salt solutions. However, the principal intracellular salt of E. coli is potassium glutamate (KGlu), which is known to stabilize folded proteins and protein‐nucleic acid complexes. Here we report that KGlu stabilizes SecA, including its dimeric state, and increases its ATPase activity, suggesting that SecA is likely fully folded, stable, and active in vivo at 37°C. Furthermore, KGlu also stabilizes a precursor form of the secreted maltose‐binding protein.
Keywords: protein secretion, protein thermal stability, ATPase activity, SecYEG
SecA ATPase is an essential 102 kDa motor protein required for the secretion of periplasmic proteins across the plasma membrane of Escherichia coli via the SecYEG translocon complex. Proteins synthesized in the cytoplasm that are destined for export carry a cleavable N‐terminal signal sequence that is recognized by SecA but subsequently cleaved by membrane‐embedded signal peptidase.1 Since its discovery2, 3 in 1982, SecA has been intensively studied in many laboratories. Exhaustive biochemical and structural studies from numerous laboratories have revealed the essential features of SecA structure and function at the molecular level (see review by Crane and Randall4), although the structural details of its interactions with the SecYEG translocase are just now beginning to emerge.5 Since the first structure of SecA from Bacillus subtilis,6 structures of SecA from several species have been determined (see reviews7, 8) and suggest, along with biochemical studies,9, 10 that SecA is dimeric in the cytoplasm. However, little is known about SecA conformational states in the cytoplasm of E. coli, which are important for understanding in vivo conformational transitions that occur during secretion of preproteins across the inner membrane.11
As far as we can establish, most physicochemical studies of SecA are carried out in buffered KCl or NaCl solutions. Analytical ultracentrifuge studies of SecA show that dimerization depends strongly on ionic strength.10 For example, in 100 mM KCl about 90% of SecA is dimeric while in 500 mM KCl, about 95% is monomeric.12 Although there are wide variations in buffer compositions reported in the literature, SecA is typically studied as a dimer in buffered chloride solutions. Using 50 mM KCl and SecA extracted from E. coli, Song and Kim13 observed through tryptophan fluorescence measurements that SecA tertiary structure was 50% destabilized at 37°C while CD spectroscopy revealed a complete unfolding transition with a midpoint of about 45°C. Similar unfolding temperatures had been observed earlier by Ulbrandt et al.14 Den Blaauwen et al.15 found similar transition temperatures by calorimetry for B. subtilis SecA.
We found it interesting that SecA is apparently destabilized at the typical E. coli growth temperature of 37°C. This suggested either that destabilization might be important for function or that 50 mM KCl was a poor representative of the E. coli intracellular environment. Looking more closely at the latter possibility, we learned that when E. coli are grown in glucose media, their intracellular metabolome is dominated, on a molar basis, by amino acids (49%) and that glutamate is the most abundant with concentrations ranging from ≈100 mM under normal conditions16 and up to 300 mM in response to osmotic stress.17, 18 Together, these data indicate that potassium glutamate (KGlu) is the primary cytoplasmic salt in E. coli. Furthermore, we learned that KGlu can stabilize soluble proteins, because Glu− interacts unfavorably with apolar side‐chains and amide groups.19, 20, 21 Given these facts, we examined the stability of SecA in KGlu solutions. We report here that KGlu significantly stabilizes SecA, including its dimeric state, and increases its ATPase activity.
We first confirmed the findings of Song and Kim,13 as shown in Figure 1 and Table S1. Panels a and c show thermal denaturation curves determined using Trp fluorescence and CD spectroscopy, respectively. In buffered 50 mM KCl or KF (black curves), the denaturation midpoints are entirely consistent with Song and Kim's observations (37.7 ± 0.4°C by Trp fluorescence and 43.6 ± 0.5°C by CD spectroscopy). In both cases, unfolding was irreversible if the denaturation midpoints were exceeded. We interpret these results to mean that as temperature is raised, tertiary structure is perturbed causing increased aqueous exposure of Trp residues followed by loss of secondary structure. We found negligible changes in the thermal transition midpoints for KCl concentrations as high as 750 mM, indicating that ionic strength had little effect on SecA stability (Fig. S1). In contrast, replacement of Cl− by Glu− caused dramatic increases in stability as judged by both Trp fluorescence and CD spectroscopy [Fig. 1(a, c), Table S1). Stability progressively increased with increasing Glu− concentration up to about 400 mM and then began to level off at about 42°C by Trp fluorescence and 48°C by CD spectroscopy [Fig. 1(b, d)]. These results indicate that SecA is likely to be stable at E. coli growth temperatures well beyond 37°C.
Figure 1.
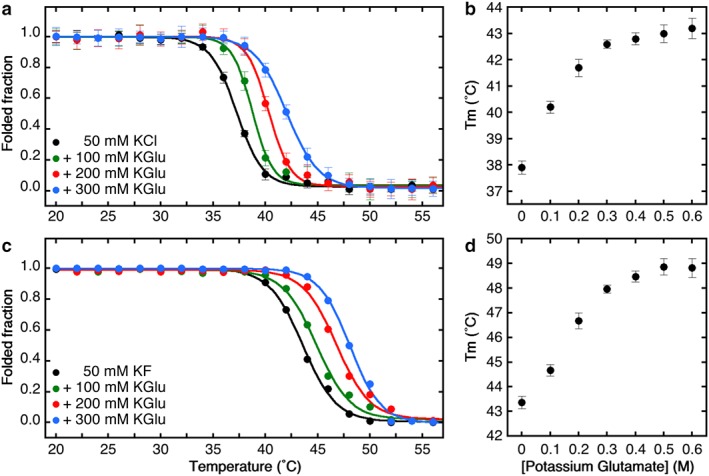
Cytoplasmic osmolytes stabilize SecA. (a) Changes in tertiary structure monitored by tryptophan fluorescence as a function of the temperature. The folded fraction was determined from the Trp fluorescence at 340 nm, normalized by setting the intensities of the native (20°C) and fully unfolded SecA (60°C) to be 1 and 0, respectively, after proper correction for the temperature dependence of the fluorescence intensity. (b) Effect of cytoplasmic osmolytes on the melting temperature of SecA determined by Trp fluorescence. (c) Changes in secondary structure monitored by circular dichroism as a function of the temperature and normalized using the molar ellipticity of native (20°C) and unfolded (60°C) SecA to be 1 and 0, respectively. (d) Effect of potassium glutamate on the stability of the secondary structure.
As noted earlier, high KCl concentrations are known to destabilize SecA dimers. We verified this well‐established finding using size‐exclusion chromatography for 5 μM SecA at 22 and 37°C (Fig. 2, black curves). In 500 mM KCl at 22°C, SecA exists mostly (90%) as monomers, as expected from the analytical ultracentrifuge measurements of Wowor et al.12 But at 37°, the equilibrium shifts toward dimers, suggesting that the dimerization interface involves hydrophobic as well as electrostatic interactions. Results from measurements in KGlu (Fig. 2, red curves) were quite similar to the KCl results up to 300 mM. In 500 mM KGlu, however, dimers started to appear at 22°, becoming highly favored at 37°C. We conclude that the salt dependence of SecA dimerization is generally similar for both KCl and KGlu up to 300 mM, although close examination of the curves reveals a slight preference for dimer in KGlu compared to KCl. At 500 mM salt concentrations, the dimeric form of SecA is strongly favored in KGlu. Broadly speaking, high KGlu concentrations stabilize SecA dimers.
Figure 2.
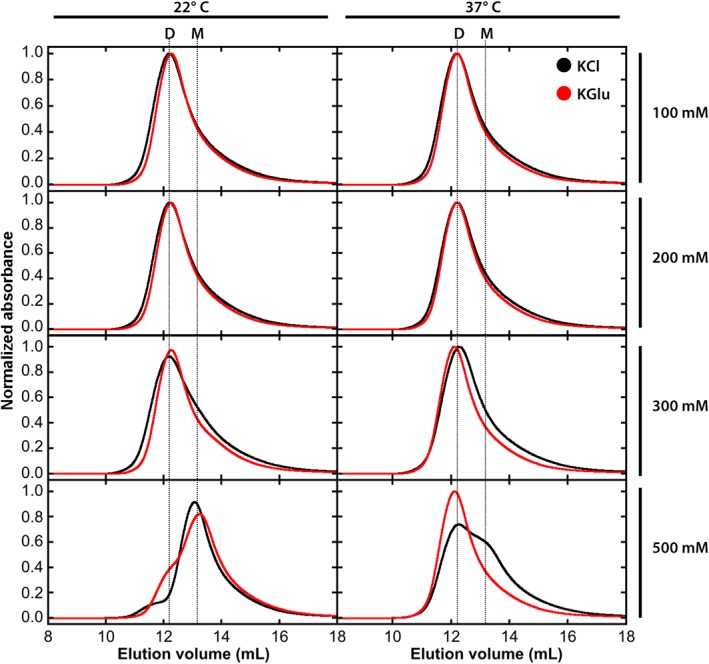
SecA is stabilized as a dimer at 37°C in the presence of potassium glutamate. The oligomeric state of 5 μM SecA protein was determined by size exclusion chromatography at 22°C (left‐hand panels) or 37°C (right‐hand panels) in KCl and KGlu concentrations ranging from 100 mM to 500 mM. KCl results are indicated by black curves and KGlu results by red curves.
Given that Glu− stabilizes SecA, what are the consequences for its ATPase activity? To answer this question, we measured the dependence of endogenous ATP hydrolysis on temperature using Glu− concentrations ranging from 100 to 300 mM (Fig. 3). At each concentration, we measured ATPase activities (nmol P i/μg SecA/min) at 17 temperatures between 22°C and 60°C (i.e., 2.5° steps). As expected, rates increased with temperature until the beginning of the thermal transition and then declined as SecA unfolded. The rate observed for 50 mM KCl at 22°C (0.038 ± 0.007 nmol P i/μg SecA/min) is similar to values obtained earlier by other investigators,22, 23, 24 as is the increase in rate with temperature. For 50 mM KCl, the rate was maximum at 37°C, declining to background values at about 50°C (panel a). Panels b, c, and d of Figure 3 reveal modest activity increases at 22°C as Glu− was increased from 100 to 300 mM. For all concentrations, activities increased with temperature reaching a maximum of about 0.11 nmol P i/μg SecA/min at 42°C in 300 mM KGlu−. These data are consistent with the thermal denaturation data (Fig. 1) and show that stabilization of SecA by Glu− improves ATPase activity. KGlu therefore both stabilizes SecA tertiary structure and enhances its activity in solution.
Figure 3.
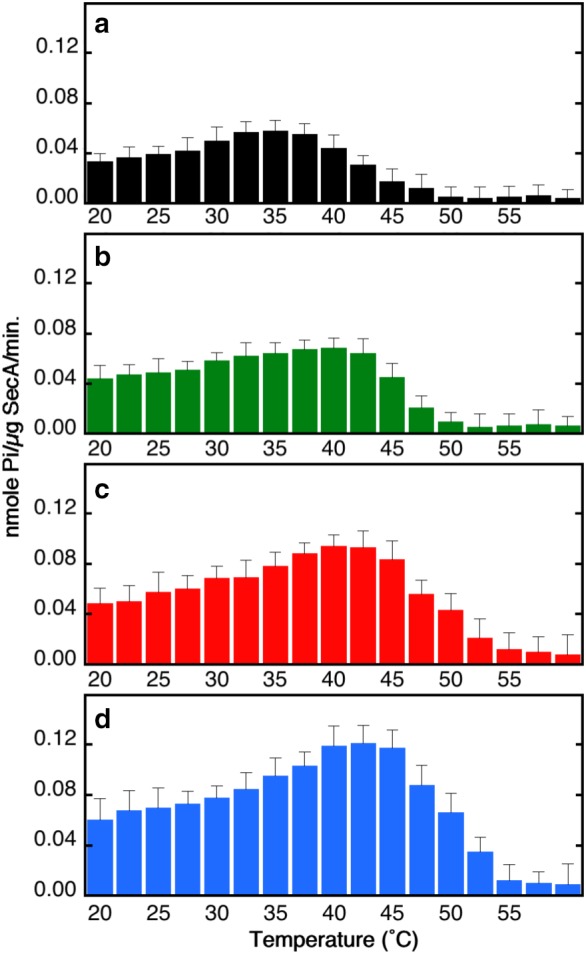
Potassium glutamate enhances the endogenous ATPase activity of SecA. The ATPase activities of SecA were determined in (a) 20 mM phosphate buffer pH 7.4, 50 mM KCl, 1 mM DTT, 5 mM MgCl2, 2 mM ATP, and (b) 100, (c) 200, or (d) 300 mM potassium glutamate pH 7.4.
Other investigators have shown that the conformation of SecA changes when it binds to the membrane25 or the translocon SecYEG,26, 27 resulting in a more open conformation. Solution studies involving low concentration of denaturant appear to mimic this “translocation‐active SecA” and have shown that the destabilization of the tertiary structure by urea stimulates the ATPase activity of SecA.11, 13 How the stabilization of SecA by KGlu in the cytoplasm will affect its binding and conformation when bound to the membrane or translocon remains to be determined.
Escherichia coli contains a complex mixture of osmolytes in its cytoplasm. Although glutamate is the major component, several others osmolytes can also be present that also affect SecA stability and activity. For example, under anaerobic glucose‐limiting conditions, as found in the digestive track when bacteria are passing through the colon, acetate can replace glutamate and accumulate in the cytoplasm at concentration up to 500 mM.28, 29 Woodbury et al.30 have shown that potassium acetate can shift the monomer–dimer equilibria of SecA toward the dimer. We found that potassium acetate also stabilizes SecA (Fig. S2, red line), suggesting that the protein is likely folded at physiologically relevant temperatures even when acetate replaces glutamate.
The above results suggest that other proteins within the cytoplasm might also be stabilized by primary cytoplasmic salts, as suggested by studies of the NTL9 ribosomal protein domain.19 A question of particular interest, of course, is how Glu− might affect the cytoplasmic stability of proteins destined for secretion. The stability and folding of the E. coli periplasmic maltose‐binding protein (MalE) and its cytoplasmic precursor (preMalE) have been studied extensively in the laboratories of Linda Randall31 –33 and Raghavan Varadarajan.34,35 PreMalE is a 396‐residue protein carrying a 26‐residue presequence (MKIKTGARILALSA14LTTMMFSASALA–). Park et al.31 showed via guanidine hydrochloride denaturation and renaturation that the A14E signal sequence had no effect on the unfolding rate of the protein but dramatically slowed the refolding rate. This suggested that a key role for the signal sequence is to slow down intracellular folding as means of keeping preMalE competent for export and for the binding of the SecB chaperone that generally works in concert with SecA to facilitate export of preMalE.36
Liu et al.33 discovered a non‐exportable mutant version (preMalE‐A14E) with an Ala‐to‐Glu mutation at position 14. Because preMalE‐A14E is not exported, it is readily expressed and purified for study as a stand‐in for cytoplasmic preMalE. Beena et al.34 characterized extensively the stability and folding kinetics of MalE and preMalE‐A14E. Equilibrium thermal denaturation studies of MalE and preMalE‐A14E using CD spectroscopy revealed that the mature form of MalE is more stable (denaturation temperature midpoint ≈63°C) than the immature form (midpoint ≈55°C). Beena et al. performed their experiments in buffered 150 mM sodium chloride. We carried out similar measurements using 50 mM KF and 200 mM KGlu (Fig. 4). We found the precursor to be less stable than the mature form in both KF (57.3 vs. 62.3°C) and KGlu (61.3 vs. 63.0°C). However, while KGlu had only a minor effect on the stability of the mature form, it stabilized the precursor by about 4°C. Thus, KGlu stabilizes at least one preprotein, whether it stabilizes others as well remains to be determined. An important question to be answered is how glutamate will affect the kinetics of folding of MalE and its interaction with SecB, which are believed to be of critical importance for successful secretion of preproteins.31, 32, 33, 34, 35, 36
Figure 4.
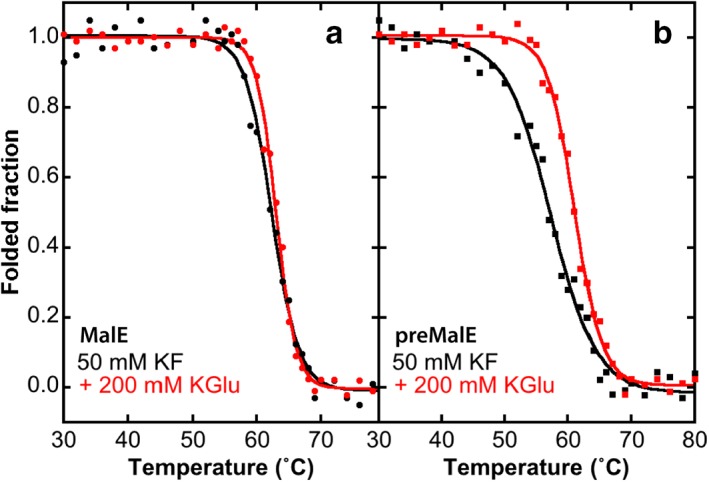
KGlu stabilizes a precursor form of MalE maltose‐binding protein. Stability was measured in KF by CD spectroscopy at a wavelength of 222 nm. KF was used to reduce spectral absorption in the near UV.37 (a) Stability of MalE. In potassium fluoride, the transition midpoint temperature is 62.3 ± 0.2°C while in KGlu it is 63.0 ± 0.1°C. (b) Stability of preMalE‐A14E. In potassium fluoride, the transition midpoint temperature is 57.3 ± 0.1°C while in KGlu it is 61.3 ± 0.1°C. The precursor is thus less stable than the mature form in both KF and KGlu. However, while KGlu has only a minor effect on the stability of the mature form, it stabilizes the precursor by 4°C.
Conflict of Interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix S1: Supplementary Information
Acknowledgments
This research was supported by Grant GM‐74637 from the National Institutes of Health. We gratefully acknowledge the technical support provided by Dr. Gargi Dasgupta. We thank Dima Fishman of the Laser Spectroscopy Labs at UC Irvine for the use of the CD spectrometer.
References
- 1. Blobel G, Dobberstein B (1975) Transfer of proteins across membranes. J Cell Biol 67:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliver DB, Beckwith J (1982) The identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli . J Bacteriol 150:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliver DB, Beckwith J (1982) Regulation of a membrane component required for protein secretion in Escherichia coli . Cell 30:311–319. [DOI] [PubMed] [Google Scholar]
- 4. Crane JM, Randall LL (2017) The Sec system: Protein export in Escherichia coli . EcoSalplus. 10.1128/ecosalplus.ESP-0002-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rapoport TA, Li L, Park E (2017) Structural and mechanistic insights into protein translocation. Annu Rev Cell Dev Biol 33:369–390. [DOI] [PubMed] [Google Scholar]
- 6. Hunt JF, Weinkauf S, Henry L, Fak JJ, McNicholas P, Oliver DB, Deisenhofer J (2002) Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 297:2018–2026. [DOI] [PubMed] [Google Scholar]
- 7. du Plessis DJF, Nouwen N, Driessen AJM (2011) The Sec translocase. Biochim Biophys Acta 1808:851–865. [DOI] [PubMed] [Google Scholar]
- 8. Papanikou E, Karamanou S, Economou A (2007) Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol 5:839–851. [DOI] [PubMed] [Google Scholar]
- 9. Ding H, Hunt JF, Mukerji I, Oliver D (2003) Bacillus subtilis SecA ATPase exists as an antiparallel dimer in solution. Biochemisty 42:8729–8738. [DOI] [PubMed] [Google Scholar]
- 10. Wowor AJ, Yu D, Kendall DA, Cole JL (2011) Energetics of SecA dimerization. J Mol Biol 408:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maki JL, Krishnan B, Gierasch LM (2012) Using a low denaturant model to explore the conformational features of translocation‐active SecA. Biochemistry 51:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wowor AJ, Yan Y, Auclair SM, Yu D, Zhang J, May ER, Gross ML, Kendell DA, Cole JL (2014) Analysis of SecA dimerization in solution. Biochemistry 53:3248–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song M, Kim H (1997) Stability and solvent accessibility of SecA protein from Escherichia coli . J Biochem 122:1010–1018. [DOI] [PubMed] [Google Scholar]
- 14. Ulbrandt ND, London E, Oliver DB (1992) Deep penetration of a portion of Escherichia coli SecA protein into model membranes is promoted by anionic phospholipids and by partial unfolding. J Biol Chem 267:15184–15192. [PubMed] [Google Scholar]
- 15. den Blaauwen T, Van der Wolk JPW, van der Does C, van Wely KHM, Driessen AJM (1999) Thermodynamaics of nucleotide binding to NBS‐1 of the Bacillus subtilis preprotein translocase subunit SecA. FEBS Lett 458:145–150. [DOI] [PubMed] [Google Scholar]
- 16. Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz J (2009) Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli . Nat Chem Biol 5:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dinnbier U, Limpinsel E, Schmid R, Bakker EP (1988) Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K‐12 to elevated sodium chloride concentrations. Arch Microbiol 150:348–357. [DOI] [PubMed] [Google Scholar]
- 18. Cayley DS, Guttman HJ, Jr Record MT (2000) Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Biophys J 78:1748–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sengupta R, Pantel A, Cheng X, Shkel I, Peran I, Stenzoski N, Raleigh DP, Jr Record MT (2016) Positioning the intracellular salt potassium glutamate in the Hofmeister series by chemical unfolding studies of NTL9. Biochemistry 55:2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng X, Guinn EJ, Buechel E, Wong R, Sengupta R, Shkel IA, Record MT Jr (2016) The basis of protein stabilization by K glutamate: Unfavorable interactions with carbon, oxygen groups. Biophys J 111:1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anumalla B, Prabhu NP (2018) Glutamate induced thermal equilibirium intermediate and counteracting effect on chemical denaturation of proteins. J Phys Chem B 122:1132–1144. [DOI] [PubMed] [Google Scholar]
- 22. Karamanou S, Vrontou E, Sianidis G, Baud C, Roos T, Kuhn A, Politou AS, Economou A (1999) A molecular switch in SecA protein couples ATP hydrolysis to protein translocation. Mol Microbiol 34:1133–1145. [DOI] [PubMed] [Google Scholar]
- 23. Kim J‐S, Ahn T, Ko J, Park C, Kim H (2001) Effect of divalent cations on the ATPase activity of Escherichia coli SecA. FEBS Lett 493:12–16. [DOI] [PubMed] [Google Scholar]
- 24. Natale P, Swaving J, van der Does C, de Keyzer J, Driessen AJM (2004) Binding of SecA to the SecYEG complex accelerates the rate of nucleotide exchange on SecA. J Biol Chem 279:13769–13777. [DOI] [PubMed] [Google Scholar]
- 25. Ding H, Mukerji I, Oliver D (2001) Lipid and signal peptide‐induced conformational changes within the C‐domain of Escherichia coli SecA protein. Biochemistry 40:1835–1843. [DOI] [PubMed] [Google Scholar]
- 26. Natale P, den Blaauwen T, van der Does C, Driessen AJM (2005) Conformational state of the SecYEG‐bound SecA probed by single tryptophan fluorescence spectroscopy. Biochemistry 44(17):6424–6432. [DOI] [PubMed] [Google Scholar]
- 27. Zimmer J, Nam Y, Rapoport TA (2008) Structure of a complex of the ATPase SecA and the protein‐translocation channel. Nature 455:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roe AJ, McLaggan D, Davidson I, O'Bryrne C, Booth IR (1998) Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol 180:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenthal AZ, Kim Y, Gralla JD (2008) Regulation of transcription by acetate in Escherichia coli: in vivo and in vitro comparisons. Mol Microbiol 68:907–917. [DOI] [PubMed] [Google Scholar]
- 30. Woodbury RL, Hardy SJS, Randall LL (2002) Complex behavior in solution of homodimeric SecA. Protein Sci 11:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park S, Liu G, Topping TB, Cover WH, Randall LL (1988) Modulation of folding pathways of exported proteins by the leader sequence. Science 239:1033–1035. [DOI] [PubMed] [Google Scholar]
- 32. Chun S‐Y, Strobel S, Bassford P, Randall LL (1993) Folding of maltose‐binding protein. J Biol Chem 268:20855–20862. [PubMed] [Google Scholar]
- 33. Liu G, Topping TB, Cover WH, Randall LL (1988) Retardation of folding as a possible means of suppression of a mutation in the leader sequence of an exported protein. J Biol Chem 263:14790–14793. [PubMed] [Google Scholar]
- 34. Beena K, Udgaonkar JB, Varadarajan R (2004) Effect of signal peptide on the stability and folding kinetics of maltose binding protein. Biochemistry 43:3608–3619. [DOI] [PubMed] [Google Scholar]
- 35. Kulothungan SR, Das MK, Johnson M, Ganesh C, Varadarajan K (2009) Effects of crowding agents, signalpeptide, and chaperone SecB on the folding and aggregation of E. coli maltose binding protein. Langmuir 25:6637–6648. [DOI] [PubMed] [Google Scholar]
- 36. Hardy SJS, Randall LL (1991) A kinetic partitioning model of selective binding of nonnative proteins by the bacterial chaperone SecB. Science 251:439–443. [DOI] [PubMed] [Google Scholar]
- 37. Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim Biophys Acta 1751:119–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


