Abstract
Background
Multiple lines of evidence point to the potential importance of early-life environmental factors in the rapid increase in the incidence of eosinophilic esophagitis (EoE), but potential exposures have not been extensively studied.
Objective
We sought to assess the association between prenatal, intrapartum, and postnatal factors and the development of pediatric EoE using a case-control study.
Methods
Patients with EoE were recruited from an existing registry at Cincinnati Children’s Hospital Medical Center (CCHMC). Population-based community control subjects were identified from a separate CCHMC registry. Mothers of study subjects were contacted and completed a Web-based questionnaire. Crude and adjusted models were used to estimate associations.
Results
Mothers of 127 cases and 121 control subjects were included. We observed a positive association between several early-life factors and EoE, including prenatal (maternal fever: adjusted odds ratio [aOR], 3.18; 95% CI, 1.27–7.98; preterm labor: aOR, 2.18; 95% CI, 1.06–4.48), intrapartum (cesarean delivery: aOR, 1.77; 95% CI, 1.01, 3.09), and infancy (antibiotic use: aOR, 2.30; 95% CI, 1.21–4.38; use of an acid suppressant: aOR, 6.05; 95% CI, 2.55–14.40) factors. We observed an inverse association between having a furry pet in infancy and EoE (aOR, 0.58; 95% CI, 0.34–0.97). No associations were observed for breast-feeding or maternal multivitamin or folic acid supplement use.
Conclusion
Early-life factors, including maternal fever, preterm labor, cesarean delivery, and antibiotic or acid suppressant use in infancy, were associated with risk of pediatric EoE; having a pet in the home was protective. These results add to growing evidence that implicate early-life exposures in EoE pathogenesis.
Keywords: Allergy, antibiotics, acid suppressant, breast-feeding, cesarean delivery, early-life factors, environment, eosinophilic esophagitis, furred pet, neonatal intensive care unit, acid-suppressive agents
Eosinophilic esophagitis (EoE) has progressed over the past 2 decades from a case-reportable disease (0.4 cases/100,000 person-years) to a major cause of upper gastrointestinal morbidity1,2 with an estimated incidence of 10 cases/100,000 person-years and a prevalence of 50 to 100 cases/100,000 persons.3,4 In the United States, where 150,000 to 400,000 persons are affected,5 the estimated annual health care costs associated with EoE approach $1 billion.6 Although increased disease recognition is likely a contributory factor to the increase in EoE incidence,7 environmental factors are also likely contributory,8,9 as evidenced from twin studies that have disentangled genetics from environment and demonstrated that environmental factors account for nearly 85% of the disease cause.
Patients with EoE often have atopic comorbidities, respond to elimination diets, relapse on food re-exposure, and have a TH2-like transcriptome response in the esophagus, suggesting that the disease is allergen mediated.10,11 Furthermore, experimental modeling in mice has shown that esophageal eosinophilia with pathologic features of EoE can be induced by exposing mice to allergens through respiratory, cutaneous, and intraesophageal routes.12
Recent research on other atopic diseases has shifted focus on the contribution of environmental risk factors to early-life perturbations believed to lead to dysbiosis in colonization of the gut and subsequent dysregulation in immunity. Independent of dysbiosis, these environmental factors can also lead to epigenetic changes that might lead to disease development. Epigenetic modifications in relation to environmental factors have not been explored in patients with EoE but have been suggested to contribute to the development of other atopic disease conditions.13 Identified environmental factors of interest for atopic diseases have included mode of delivery11,14,15; maternal, intrapartum, and infancy antibiotic use16,17; preterm delivery11,18,19; and neonatal hospitalization.20
To date, only 3 studies have investigated possible early-life determinants for EoE.9,21,22 All provided general support for the contribution of early-life factors to disease etiology, although different factors were associated, and there was a lack of consistency in results across studies. All 3 studies had small sample sizes and potential for selection bias.9,21,22 Because control subjects were hospital based, the heterogeneity in results could be attributable to differences in the underlying exposure distribution in the control subjects. If the exposure distribution in control subjects were different from the population from which cases arose, there could be bias.23 The goal of the present study was to assess the association between prenatal, intrapartum, and postnatal factors and the development of pediatric EoE by using a case-control study with population-based control subject. We aimed to examine a relatively large number of cases, expand the scope of factors assessed to include factors implicated in other atopic and immune-mediated diseases,24 and draw on population-based control subjects representative of the catchment population for the tertiary hospital from which cases were derived.
METHODS
We conducted a case-control study. Cases were recruited from children identified through the EoE database at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center (CCHMC). All cases were observed to have 15 or more eosinophils/high-power field (hpf) at the diagnostic endoscopy.25 We used a cumulative sampling approach for selection of control subjects, specifically sampling control subjects from the population of noncases at the time prevalent cases were assembled. Control subjects were recruited from the CCHMC Genomic Control Cohort, a population-based control cohort representative of the greater Cincinnati population.26,27 Cases and control subjects were identified through a study of genetic factors for EoE that was restricted to non-Hispanic white subjects. Cases and control subjects were less than 18 years of age at the time of enrollment. Mothers of children who were identified were contacted by telephone to confirm eligibility and to assess willingness to participate. After consenting, mothers of subjects were e-mailed the link to complete the survey to assess early-life exposures. All data were recorded electronically and were deidentified.
Exposures were assessed by using an expanded version of the questionnaire developed in our initial study of early-life exposures, which was generated through an interactive process that included cognitive interviewing and testing. 21 Survey domains included prenatal, intrapartum, and postnatal exposures, including those previously demonstrated to be associated with EoE,9,21,22,28,29 but also assessment of associations with maternal infections, use of acid suppressants in infancy, and exposure to pets in in the home (see Appendix E1 in this article’s Online Repository at www.jacionline.org).
Statistical analyses
First, we examined the extent of missing data and distribution of responses to each of the early-life factors of interest. We then examined the demographic distribution of cases and control subjects and described the presence of atopic comorbidities in cases and control subjects.
Next, we used generalized linear models (logit link and binomial distribution) to examine the odds of disease in those exposed relative to those unexposed. We conducted crude and adjusted analyses. For adjustment, we considered maternal factors that could act as potential confounders of the association between early-life exposures in relation to disease development. Specifically, we adjusted for maternal education as a proxy for socioeconomic status because this could act as both an antecedent factor in the exposures assessed and for the diagnosis of EoE.30 Crude and adjusted odds ratios (aORs) with 95% CIs were reported.
Specific exposures were addressed as follows. Maternal infection was denoted by the presence of a temperature of at least 100.4°F during pregnancy, and the trimester during which the fever occurred was also recorded. We characterized maternal smoking as any smoking during pregnancy. Folic acid use was characterized as use of any dose of folic acid supplement alone or in addition to a prenatal multivitamin during pregnancy. Prenatal vitamin use was characterized as regular ingestion of any prenatal multivitamin during pregnancy. Although all prenatal supplements likely contain folic acid, the amount of folic acid included varies (typically 400–800 μg). Furthermore, some women might choose to take a folic acid supplement either because they have been instructed to take a higher therapeutic dose (4 mg for women whose offspring are at increased risk of experiencing a birth defect, such as women with type 1 diabetes) or because they cannot tolerate multivitamins. Folic acid used independently of prenatal vitamins is typically a higher dose than that prescribed through use of a multivitamin. We sought to assess whether these higher doses of folic acid could be associated with development of EoE, thus contributing to the mixed evidence to suggest that folic acid supplement use can be associated with atopic disease and expanding this to include assessment of EoE.31
Secondarily, we combined prenatal and folic acid supplementation to reflect varying levels of folic acid intake, specifically characterizing whether neither a folic acid supplement nor a multivitamin was taken, whether just a prenatal multivitamin was taken, or whether a folic acid supplement was taken (58 of 60 respondents who reported taking a folic acid supplement reported also taking a multivitamin). Acid suppressant use was characterized as any use of a proton pump inhibitor (PPI), H2 antagonist, or other acid suppressant in the first year of life. Preterm labor and birth were defined as labor or delivery before 37 weeks of completed gestation. Although we assessed pregnancy complications independently, there were too few responses in any one category, with the exception of preterm labor, to examine these associations independently. Thus we created a single combined category of indication of having experienced a pregnancy complication, which included gestational diabetes; anemia; pre-eclampsia or pregnancy-induced hypertension; eclampsia; chorioamnionitis; hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; hyperemesis; preterm labor; premature rupture of the membranes; or other complication. Admission to the neonatal intensive care unit (NICU) was characterized as any length of stay in the NICU. For examining the association between use of acid suppressants during the first year of life and EoE and to address concerns of possible protopathic bias or bias resulting from the potential that PPIs were used to address symptoms associated with undiagnosed EoE, we restricted our case sample to those children with symptoms of EoE beginning at age 3 or older. Antibiotic use was restricted to reported administration during the first 12 months of life. Breast-feeding was characterized as any breast-feeding. Pets were restricted to having a pet with fur (ie, dogs, cats, and rabbits) in the home during infancy.
Because we noted that cases arose from a region broader than the greater Cincinnati area, we performed a secondary analysis using the χ2 test to assess for differences in the exposure distribution between those who resided within or outside of the greater Cincinnati area. Where there were sparse data (count of ≤5), we used the Fisher exact test.
Secondary analysis
Exposures examined could be associated with one another (eg, preterm birth and NICU admission), and the direct effect of each exposure might not be fully estimable. We conducted secondary analyses, adjusting for those related factors that might act as mediators as a means of estimating the direct effect and under the assumption that no factors were confounding the association between the exposure and the mediator.32 We specifically focused on maternal fever (mediated by preterm delivery and delivery route) and NICU admission (mediated by antibiotic use and an acid suppressant in infancy). The study was approved by the CCHMC Institutional Review Board.
RESULTS
Demographics
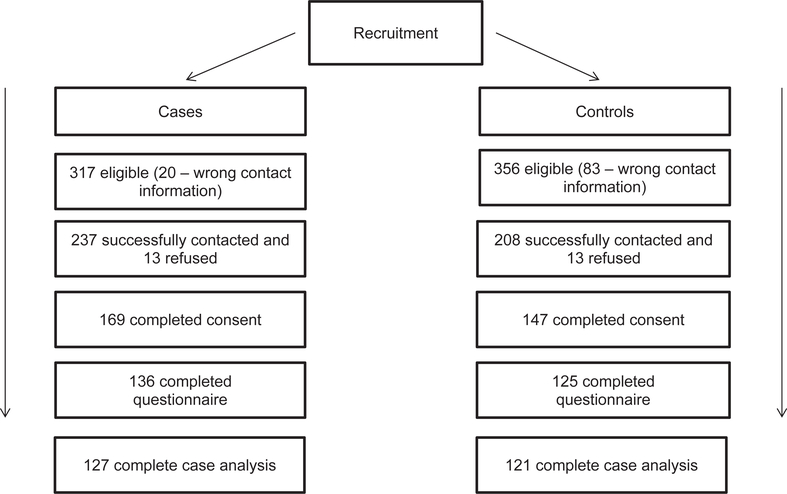
Of the 237 cases contacted successfully, 169 (71%) consented to participation. Of these subject-mother pairs, 136 (80%) mothers completed the questionnaire. Similarly, for control subjects, of the 208 potential subjects successfully contacted, 147 (71%) consented to participation, and of these subject-mother pairs, 125 (85%) mothers completed the questionnaire (Fig 1). An examination of the mean age among those who did and did not consent (of all those that were eligible) among both cases and control subjects suggested that unselected cases and control subjects were comparable with those who responded. The mean age of those EoE cases who were not enrolled was 11.1 (SD, 3.4) (compared with 10.6 [SD, 3.8] enrolled), and the mean age of control subjects not enrolled was 12.9 (SD, 2.8; compared with 13.8 [SD, 2.8] enrolled). Our complete case analytic sample included 127 cases and 121 control subjects (Fig 1). The demographic features of the cases and control subjects were similar, with the exception of atopic disease comorbidities and sex (Table I). Consistent with the descriptive epidemiologic literature on EoE,5,11 cases had a higher prevalence of atopic disease than control subjects, particularly food allergies (83.3% of cases vs 12.4% of control subjects, respectively), and were more likely to be male (80.3% of cases vs 52.1% of control subjects).
FIG 1.
Recruitment of cases and control subjects and final study sample.
TABLE I.
Characteristics of the study population
| Characteristic | Control subjects, n = 125, (% or mean [SD]) | Cases, n = 136 (% or mean [SD]) |
|---|---|---|
| Age at enrollment (y), mean (SD) | 13.8 (2.8) | 10.6 (3.8) |
| Sex (% male) | 52.1 | 80.3 |
| Atopic illnesses (%) | ||
| Food allergies | 12.4 | 83.3 |
| Environmental allergies | 42.2 | 69.3 |
| Antibiotic allergies | 14.5 | 25.8 |
| Eczema | 21.5 | 59.1 |
| Asthma | 18.2 | 44.9 |
| Maternal marital status (%) | ||
| Married or civil union | 93.6 | 91.9 |
| Single | 6.4 | 5.9 |
| Missing | 0.0 | 2.2 |
| Maternal education (%) | ||
| <HS | 0.0 | 2.2 |
| HS or GED | 17.6 | 11.0 |
| Technical or Associate’s degree | 20.8 | 18.4 |
| Bachelor’s degree | 39.2 | 35.3 |
| Graduate or professional | 20.0 | 27.9 |
| Missing | 2.4 | 5.2 |
| Maternal smoking (%) | ||
| Yes | 9.6 | 5.9 |
| No | 90.4 | 94.1 |
| Missing | 0.0 | 0.0 |
| Folic acid supplements (%) | ||
| Yes | 20.0 | 27.9 |
| No | 80.0 | 71.3 |
| Missing | 0.0 | 0.7 |
| Prenatal vitamin supplements (%) | ||
| Yes | 94.4 | 94.1 |
| No | 5.6 | 5.9 |
| Missing | 0.0 | 0.0 |
| Maternal fever (%) | ||
| Yes | 6.4 | 14.7 |
| No | 78.4 | 60.3 |
| Missing | 15.2 | 25.0 |
| Preterm labor (%) | ||
| Yes | 11.2 | 20.6 |
| No | 88.8 | 79.4 |
| Missing | 0.0 | 0.0 |
| Preterm delivery (%) | ||
| Yes | 14.4 | 19.1 |
| No | 84.8 | 80.2 |
| Missing | 0.8 | 0.7 |
| Pregnancy complications (%)* | ||
| Yes | 40.0 | 54.4 |
| No | 59.2 | 44.9 |
| Missing | 0.8 | 0.7 |
| Cesarean delivery (%) | ||
| Yes | 22.4 | 36.0 |
| No | 77.6 | 64.0 |
| Missing | 0.0 | 0.0 |
| NICU admission (%) | ||
| Yes | 11.2 | 20.6 |
| No | 88.8 | 79.4 |
| Missing | 0.0 | 0.0 |
| Breast-feeding, any (%) | ||
| Yes | 76.0 | 80.2 |
| No | 24.0 | 19.9 |
| Missing | 0.0 | 0.0 |
| Antibiotic use in infancy (%) | ||
| Yes | 57.6 | 66.9 |
| No | 28.8 | 15.4 |
| Missing | 13.6 | 17.7 |
| Acid suppressant use in infancy (%) | ||
| Yes | 14.4 | 55.9 |
| No | 82.4 | 42.7 |
| Missing | 3.2 | 1.5 |
| Furry pet in infancy (%) | ||
| Yes | 54.4 | 45.6 |
| No | 45.6 | 54.4 |
| Missing | 0.0 | 0.0 |
GED, General Education Development test certification; HS, high school.
Includes gestational diabetes, anemia, pre-eclampsia or pregnancy-induced hypertension, eclampsia, chorioamnionitis, HELLP syndrome, hyperemesis, preterm labor, premature rupture of the membranes, or other complication.
Prenatal factors
We observed a strong association between maternally reported fever during pregnancy and risk of EoE (aOR, 3.18; 95% CI, 1.27–7.98; Table II). Although data were too sparse for estimating trimester-specific associations, the distribution of fevers was clustered in the second trimester for cases, with mothers reporting a fever in the second trimester for 11 (9%) cases versus 1 (1%) control subject. We observed no association of maternal smoking or prenatal vitamin use with risk of EoE. We observed a nonsignificant positive association between folic acid use and EoE (aOR, 1.56; 95% CI, 0.86–2.83). Although the odds of EoE among those consuming both a multivitamin and folic acid was higher than the odds of EoE for those using only one or the other, the associations were not significant (data not shown). Analysis of possible trend for association across possible increase in dose was also not significant (P for trend = .10). Preterm labor was positively associated with EoE (aOR, 2.18; 95% CI, 1.06–4.48; Table II), as were pregnancy complications (aOR, 1.87; 95% CI, 1.12–3.12; Table II). Without preterm labor included, the association was attenuated (aOR, 1.29; 95% CI, 0.99–1.69).
TABLE II.
Association between prenatal factors and EoE
| Prenatal factor | Control subjects (n = 121) | Cases (n = 127) | OR | aOR* |
|---|---|---|---|---|
| Maternal fever† | ||||
| No | 95 | 79 | Referent | Referent |
| Yes | 7 | 19 | 3.26 (1.31–8.16) | 3.18 (1.27–7.98) |
| Maternal smoking | ||||
| No | 110 | 119 | Referent | Referent |
| Yes | 11 | 8 | 0.67 (0.26–1.73) | 0.70 (0.27–1.80) |
| Prenatal vitamin use | ||||
| No | 7 | 8 | Referent | Referent |
| Yes | 114 | 119 | 0.91 (0.32–2.60) | 0.85 (0.30–2.45) |
| Folic acid supplement use‡ | ||||
| No | 97 | 91 | Referent | Referent |
| Yes | 24 | 36 | 1.60 (0.89–2.89) | 1.56 (0.86–2.83) |
| Pregnancy complications§ | ||||
| No | 72 | 58 | Referent | Referent |
| Yes | 46 | 68 | 1.84 (1.10–3.05) | 1.87 (1.12–3.12) |
| Preterm labor | ||||
| No | 108 | 101 | Referent | Referent |
| Yes | 13 | 26 | 2.13 (1.04–4.39) | 2.18 (1.06–4.48) |
Includes adjustment for maternal education.
The sample was reduced to 98 cases and 102 control subjects because of missing data. Fever was defined as having a temperature of >100.4°F.
Characterized as use of a folic acid supplement independent of any use of a prenatal vitamin.
Includes gestational diabetes, anemia, pre-eclampsia or pregnancy-induced hypertension, eclampsia, chorioamnionitis, HELLP syndrome, hyperemesis, preterm labor, premature rupture of the membranes, or other complication.
Intrapartum factors
Cesarean delivery, compared with vaginal delivery, conferred a 77% increase in risk of EoE (aOR, 1.77; 95% CI, 1.01–3.09). A nonsignificant positive association was observed between preterm birth and EoE (aOR, 1.39; 95% CI, 0.71–2.72; Table III).
TABLE III.
Association between intrapartum factors and EoE
| Intrapartum factor | Control subjects (n = 121) | Cases (n = 127) | OR | aOR* |
|---|---|---|---|---|
| Mode of delivery | ||||
| Vaginal | 93 | 83 | Referent | Referent |
| Cesarean | 28 | 44 | 1.76 (1.01–3.08) | 1.77 (1.01–3.09) |
| Preterm birth | ||||
| No | 103 | 103 | Referent | Referent |
| Yes | 18 | 24 | 1.33 (0.68–2.60) | 1.39 (0.71–2.72) |
Includes adjustment for maternal education.
Infancy factors
We observed a suggestive association between NICU admission and EoE, although this did not reach significance (aOR, 1.92; 95% CI, 0.95–3.89). Use of antibiotics in infancy was positively associated with EoE (aOR, 2.30; 95% CI, 1.21–4.38). Having a dog or cat in the home during infancy was inversely associated with EoE (aOR, 0.58; 95% CI, 0.34–0.97); no subjects reported having pet rabbits. We observed no association between breastfeeding and EoE. Report of acid suppressant use in infancy was strongly associated with EoE (aOR, 7.41; 95% CI, 4.00–13.74). Restricting the case sample to those reporting symptoms at age 3 years or older had minimal effect on the association observed (aOR, 6.05; 95% CI, 2.55–14.40). Nearly half of the cases reporting development of EoE symptoms at age 3 years or older reported receiving an acid suppressant during infancy (Table IV).
TABLE IV.
Association between Infancy factors and EoE
| Infancy factor | Control subjects (n = 121) | Cases (n = 127) | OR | aOR* |
|---|---|---|---|---|
| NICU admission | ||||
| No | 107 | 101 | Referent | Referent |
| Yes | 14 | 26 | 1.97 (0.97–3.98) | 1.92 (0.95–3.89) |
| Breast-feeding, any | ||||
| No | 28 | 26 | Referent | Referent |
| Yes | 93 | 101 | 1.17 (0.64–2.14) | 1.11 (0.60–2.05) |
| Antibiotic use (infancy)† | ||||
| No | 35 | 19 | Referent | Referent |
| Yes | 70 | 88 | 2.32 (1.22–4.40) | 2.30 (1.21–4.38) |
| Acid suppressant use (infancy)‡ | ||||
| No | 99 | 19 | Referent | Referent |
| Yes | 18 | 18 | 5.21 (2.30–11.80) | 6.05 (2.55–14.40) |
| Cats or dogs in home (infancy) | ||||
| No | 40 | 58 | Referent | Referent |
| Yes | 81 | 69 | 0.59 (0.35–0.98) | 0.58 (0.34–0.97) |
Includes adjustment for maternal education.
Sample was reduced to 105 cases and 107 control subjects because of missing data.
Restricting analysis to those reporting symptoms at age 3 years or later for cases (n = 37); there were missing data on 4 control subjects.
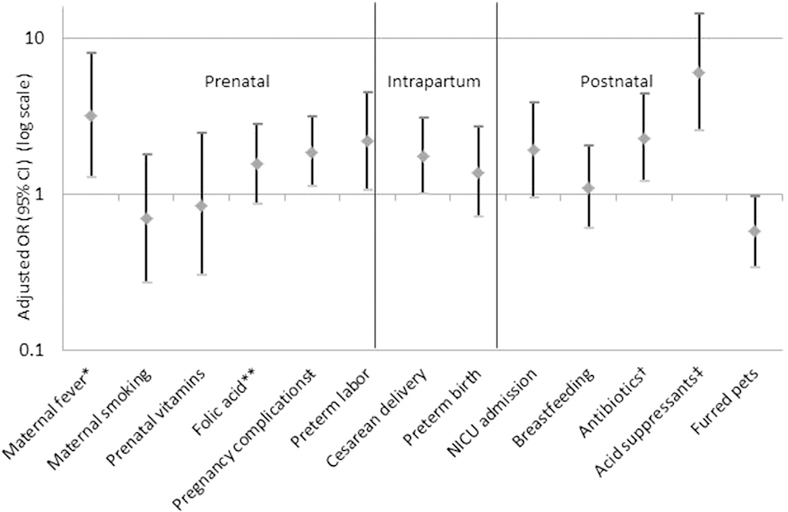
None of the control subjects were from outside of the greater Cincinnati area. For cases, examining the distribution of exposures in those residing within and outside of the greater Cincinnati area identified no significant differences in exposure distribution for all of the early-life factors examined, with the exception of maternal smoking; there was a significantly higher proportion of smokers among cases from within the Cincinnati area (see Table E1 in this article’s Online Repository at www.jacionline.org). As an additional analysis, to account for this difference in exposure distribution, we examined the association between maternal smoking and EoE, restricting the case sample to those within greater Cincinnati only. Within this subsample, the association between maternal smoking and EoE still did not reach significance (aOR, 2.06; 95% CI, 0.65–6.48). The results of all the exposures examined are summarized in Fig 2.
FIG 2.
Association between prenatal, intrapartum, and postnatal factors and EoE. *Sample was reduced to 98 cases and 102 control subjects because of missing data; fever was defined as having a temperature of >100.4°F. **Characterized as use of a folic acid supplement independent of any use of a prenatal vitamin. ŧIncludes gestational diabetes, anemia, pre-eclampsia or pregnancy-induced hypertension, eclampsia, chorioamnionitis, HELLP syndrome, hyperemesis, preterm labor, premature rupture of the membranes, or other complication. †Sample was reduced to 105 cases and 107 control subjects because of missing data. ‡Restricting analysis to those reporting symptoms at age 3 years or later for cases (n = 37); there were missing data on 4 control subjects.
Secondary analysis
We attempted to disentangle the effects of related mediating factors by adjusting for these factors. For the association between maternal fever and EoE, further adjustment for preterm birth and delivery route attenuated the observed association slightly (aOR, 2.94; 95% CI, 1.16–7.44). For NICU admission, adjustment for antibiotic use and use of an acid suppressant in infancy attenuated the association observed (aOR, 1.12; 95% CI, 0.33–3.77).
DISCUSSION
The present study is the largest to date examining the associations between early-life factors, including those in the prenatal, intrapartum, and postpartum period, and pediatric EoE and selecting population-based control subjects designed to be representative of the catchment area from which cases arose. Here we report that EoE risk is (1) positively associated with several prenatal exposures, including maternal fever (aOR, 3.18) and preterm labor (aOR, 2.18); (2) positively associated with cesarean delivery (aOR, 1.77); (3) positively associated with antibiotic use during infancy (aOR, 2.30); (4) dramatically and positively associated with use of acid suppression therapy during infancy (aOR, 6.05); (5) negatively associated with having a furred pet in the home during the postnatal period (aOR, 0.58); and (6) not apparently associated with breast-feeding, maternal multivitamin, or folic acid supplement use. Accounting for possible mediating factors in the association between maternal fever and EoE had no substantive effect on the estimates observed, whereas adjustment for antibiotic and acid suppressant use suggested no independent effect of NICU admission on the risk of EoE. This analysis assumes that the direct effect can be estimated, which might not be a reasonable assumption in the present study if there are confounding factors associated with the exposure and mediating factor or factors.32
A strength of the current study is the use of population-based control subjects from the catchment area of the hospital from which cases were identified, although we note that cases arose from a larger catchment area, possibly because of the relative rarity of EoE. As with 2 of the 3 previous studies conducted on early-life factors and EoE,9,21 we observed positive associations between both cesarean delivery and antibiotic use in infancy with EoE. No association was observed with folic acid supplement use or use of a prenatal multivitamin; however, the lack of available data on folic acid dose might make these results less interpretable. Although a suggestive inverse association between breast-feeding and EoE had been noted previously,21 this association was observed in neither the present study nor the other 2 studies conducted to date.9,22 The inverse association previously noted for smoking22 was also not observed. However, this inverse association had been observed for postnatal environmental tobacco smoke exposure only, whereas in the present study we examined prenatal exposure only.
Antibiotics and cesarean delivery have been demonstrated to alter colonization of the gut in early life.33 Although some studies suggest that these changes can be transient, early infancy is a period of unique susceptibility for immune development. Colonization of the gut microbiota in early life is essential to establishing the mucosal barrier, developing tolerance, and promoting immune maturation.34,35 It is notable that the 2 main EoE susceptibility genes, TSLP and CAPN14, are genes encoded by the mucosal epithelium, which would presumably be the tissue to directly encounter microbial content. In addition, CAPN14 has been shown to regulate esophageal barrier function, which presumably affects the extent of exposure to the environmental factors identified.36
We also observed several novel positive associations of preterm labor, maternal fever, and pregnancy complications with EoE. Maternal fever has been implicated as a possible contributing factor in patients with other atopic illnesses,37 and fetal exposure to proinflammatory cytokines, as a result of maternal infection, might interfere with the development of the fetal immune system.38 Pregnancy complications, including pre-eclampsia, have been associated with atopic illnesses.37,39 Pre-eclampsia is associated with increased maternal proinflammatory cytokines, which might contribute to an altered immunologic development in the fetus.40 The inverse association with pet exposures has been observed in studies of other atopic illnesses,41 although the evidence is mixed, with some studies suggesting a protective association for cats, dogs, or both and other studies suggesting no association.42
Interestingly, the strongest new association we observed was for infant use of an acid suppressant. Although this association might simply reflect treatment of early EoE symptoms,43,44 there is some evidence in support of a causal association, especially when given in early life.45–48 Animal models, clinical studies, and observational studies suggest that antisecretory agents might act to incite an inappropriate immune response through direct alteration of the gut mucosal permeability or through interference with normal digestion of dietary proteins, thus preserving antigens that induce a TH2 response.48 In both animal49,50 and human51 studies, antisecretory agents have promoted IgE formation toward dietary antigens. Conversely, some studies have suggested that an overabundance of acid might impair esophageal barrier function52 and that PPIs can, in some instances, restore esophageal barrier function.53
Although the number of cases included in this study was the largest assembled to date, an even larger sample size would facilitate characterizing the environmental factors with a higher degree of detail. Moreover, exposures were characterized based on maternal recall. Given that these exposures occurred roughly a decade earlier, there is the potential for recall bias. However, some factors, such as cesarean delivery, breast-feeding, preterm birth, NICU admission, and maternal smoking, are unlikely to be hampered by recall.
Although there were minimal missing data across most exposures (Table I), responses for antibiotic use and fever during pregnancy had more than 10% missing. For antibiotic use, missingness was roughly comparable between cases and control subjects (17% vs 14% missing, respectively). If those missing a response were all assumed to be unexposed, the estimate would have attenuated to an odds ratio of 1.66 (95% CI, 0.99–2.79). For maternal fever, mothers of cases were less able to recall exposure to fever during pregnancy (25% cases vs 15% control subjects missing). Assuming all of these were indicative of no exposure to maternal fever, the estimate would have attenuated to an odds ratio of 2.46 (95% CI, 1.03–5.85). Although this provides some reassurance that the qualitative positive association observed for antibiotic use and maternal fever is not likely an artifact of selection bias, the potential remains that the inability to recall is associated with both exposure status and case status and could reflect a biased estimate. For antibiotic use, there is also the potential that the association observed could be attributed to confounding by indication, whereby some unknown factor is contributing to the use of antibiotics and is also associated with development of EoE. For acid suppressant use, there remains the potential for protopathic bias. The mean age of diagnosis among our cases was 4.9 (SD, 3.5) years. We restricted this analysis to cases in which symptoms were reported at age 3 years or older. However, it remains possible that these children received an acid-suppressive agent as a result of very early and unrecognized manifestations of disease.
Roughly 30% of those eligible for contact were successfully consented for participation, and of these, 80% to 85% completed the questionnaire. Although the success rate for enrolling subjects was comparable across cases and control subjects, there is the potential that those participating represent a selected population no longer representative of the population eligible for participation. Furthermore, the sample of cases and control subjects was restricted to non-Hispanic white subjects. Although EoE is more commonly diagnosed in white subjects,54 this might limit the generalizability of the study findings to other racial and ethnic groups.
In our study cases had a median of 50 eosinophils/hpf (interquartile range, 37.5–96.0 eosinophils/hpf). However, we do not have any other histologic or clinical data by which disease severity could be characterized.55 Peak eosinophil count per hpf has been demonstrated to correlate poorly with disease symptoms,55,56 and thus we were not able to characterize exposures in relation to disease severity. Additionally, we did not restrict our cases to those who underwent a PPI trial before diagnostic endoscopy. PPI use before diagnosis was not a discriminating factor in our 2 prior genome-wide association studies,27,57 and the mechanistic factors for EoE and PPI-responsive esophageal eosinophilia are believed to overlap.58 Finally, clinical and histologic data suggest no differences between EoE and PPI-responsive esophageal eosinophilia.59
In conclusion, the present study is the largest to date examining the associations between early-life factors, including those in the prenatal, intrapartum, and postpartum periods, and pediatric EoE. We report that EoE risk is (1) positively associated with several prenatal exposures, including maternal fever (aOR, 3.18) and preterm labor (aOR, 2.18); (2) positively associated with cesarean delivery (aOR, 1.77); (3) positively associated with antibiotic use during infancy (aOR, 2.30); (4) dramatically and positively associated with use of acid suppressant therapy during infancy (aOR, 6.05); (5) negatively associated with having a furred pet in the home during infancy (aOR, 0.58); and (6) not apparently associated with breast-feeding or maternal multivitamin or folic acid supplement use. Because many of the factors identified in this study are potentially modifiable, our findings have implications for prevention. On a clinical level, patients exposed to the identified environmental factors might receive differential evaluations for EoE based on the particular risk factor’s association. Further studies focused on gene-environment interactions might improve the predictive value of these exposures; specifically, patients who harbor certain genetic variants in EoE susceptibility genes can have differential responses to each environmental factor.
Supplementary Material
Key messages.
Heritability estimates for EoE suggest that both genetics and early-life environmental factors are implicated in disease pathogenesis.
Prenatal (maternal fever, preterm labor), intrapartum (cesarean delivery), and postnatal (antibiotic use and use of acid-suppressive agents) factors are associated with increased risk of EoE (aOR, 1.8–6.1). In contrast, having a furred pet in infancy was protective against EoE (aOR, 0.6).
Identifying modifiable factors for disease risk not only offers the potential opportunity to develop a further understanding of the pathogenesis of disease but also opportunities for modifying disease development.
Acknowledgments
Supported by the American College of Gastroenterology–Clinical Research Award (to E.S.D. and E.T.J.) and R01 DK101856 (to E.S.D.).
Disclosure of potential conflict of interest: E. T. Jensen receives grant support from the American College of Gastroenterology and Nutricia. L. J. Martin receives grant support from the National Institutes of Health (NIH). M. E. Rothenberg receives grant support from the NIH and US-Israel Binational grant PCORI; serves as a consultant for Receptos, Immune Pharmaceuticals, NKT Therapeutics, and PulmOne Therapeutics; received payment for lectures from Merck; holds patents with Miraca Life Science; receives royalties from Teva Pharmaceuticals and Miraca Life Sciences; and holds stock options from PulmOne, NKT Therapeutics, and Immune Pharmaceuticals. E. S. Dellon receives grant support from the American College of Gastroenterology Clinical Research Grant, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; serves as a consultant for Adare, Alivio, Banner, Celgene/Receptos, GlaxoSmithKline, Regeneron, and Shire; and receives an educational grant from Banner. J. T. Kuhl declares that he has no relevant conflicts of interest.
Abbreviations used
- aOR
Adjusted odds ratio
- CCHMC
Cincinnati Children’s Hospital Medical Center
- EoE
Eosinophilic esophagitis
- HELLP
Hemolysis, elevated liver enzymes, and low platelet count
- hpf
High-power field
- NICU
Neonatal intensive care unit
- PPI
Proton pump inhibitor
REFERENCES
- 1.Veerappan GR, Perry JL, Duncan TJ, Baker TP, Maydonovitch C, Lake JM, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009;7: 420–6. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–92. [DOI] [PubMed] [Google Scholar]
- 3.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am 2014;43:201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a nationwide cohort. Gastroenterology 2012; 142(suppl 1):S1138. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014; 12:589–96.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015;110:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa’ad AH, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol 2010;126:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hruz P, Bussmann C, Heer P, Simon HU, Zwahlen M, Beglinger C, et al. Escalating epidemiology of eosinophilic esophagitis: 21 years of prospective population-based documentation in Olten County. Gastroenterology 2011; 140(suppl 1):S238–9. [Google Scholar]
- 9.Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract 2014;2:475–7.e1. [DOI] [PubMed] [Google Scholar]
- 10.Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr 2016;62:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009;48:30–6. [DOI] [PubMed] [Google Scholar]
- 12.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001;107: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North ML, Ellis AK. The role of epigenetics in the developmental origins of allergic disease. Ann Allergy Asthma Immunol 2011;106:355–62. [DOI] [PubMed] [Google Scholar]
- 14.Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol 2008;122:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo JH, Kim HY, Jung YH, Lee E, Yang SI, Yu HS, et al. Interactions between innate immunity genes and early-life risk factors in allergic rhinitis. Allergy Asthma Immunol Res 2015;7:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee E, Kwon JW, Kim HB, Yu HS, Kang MJ, Hong K, et al. Association between antibiotic exposure, bronchiolitis, and TLR4 (rs1927911) polymorphisms in childhood asthma. Allergy Asthma Immunol Res 2015;7:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo EX, Shek LP, Goh A, Teoh OH, Chan YH, Soh SE, et al. Atopic dermatitis in early life: evidence for at least three phenotypes? Results from the GUSTO study. Int Arch Allergy Immunol 2015;166:273–9. [DOI] [PubMed] [Google Scholar]
- 18.Edwards MO, Kotecha SJ, Lowe J, Richards L, Watkins WJ, Kotecha S. Early-term birth is a risk factor for wheezing in childhood: a cross-sectional population study. J Allergy Clin Immunol 2015;136:581–7.e2. [DOI] [PubMed] [Google Scholar]
- 19.Rosas-Salazar C, Ramratnam SK, Brehm JM, Han YY, Boutaoui N, Forno E, et al. Prematurity, atopy, and childhood asthma in Puerto Ricans. J Allergy Clin Immunol 2014;133:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel AL, Mutlu EA, Sun Y, Koenig L, Green S, Jakubowicz A, et al. Longitudinal survey of microbiota in hospitalized preterm very low birth weight infants. J Pediatr Gastroenterol Nutr 2016;62:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2013;57:67–71. [DOI] [PubMed] [Google Scholar]
- 22.Slae M, Persad R, Leung A-T, Gabr R, Brocks D, Huynh H. Role of environmental factors in the development of pediatric eosinophilic esophagitis. Dig Dis Sci 2015;60:3364–72. [DOI] [PubMed] [Google Scholar]
- 23.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol 1992;135:1029–41. [DOI] [PubMed] [Google Scholar]
- 24.Jensen ET, Bertelsen RJ. Assessing early life factors for eosinophilic esophagitis: lessons from other allergic diseases. Curr Treat Options Gastroenterol 2016;14: 39–50. [DOI] [PubMed] [Google Scholar]
- 25.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20.e6. [DOI] [PubMed] [Google Scholar]
- 26.Kovacic MB, Myers JM, Wang N, Martin LJ, Lindsey M, Ericksen MB, et al. Identification of KIF3A as a novel candidate gene for childhood asthma using RNA expression and population allelic frequencies differences. PLoS One 2011;6:e23714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet 2014;46:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lexmond WS, Neves JF, Nurko S, Olszak T, Exley MA, Blumberg RS, et al. Involvement of the iNKT cell pathway is associated with early-onset eosinophilic esophagitis and response to allergen avoidance therapy. Am J Gastroenterol 2014; 109:646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen ET, Kuhl JT, Martin L, Rothenberg ME, Dellon E. 664 Prenatal, antenatal, and early life factors are associated with risk of eosinophilic esophagitis. Gastroenterology 2016;150:S135–6. [Google Scholar]
- 30.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 31.Brown SB, Reeves KW, Bertone-Johnson ER. Maternal folate exposure in pregnancy and childhood asthma and allergy: a systematic review. Nutr Rev 2014;72: 55–64. [DOI] [PubMed] [Google Scholar]
- 32.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol 2013;42:1511–9. [DOI] [PubMed] [Google Scholar]
- 33.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 2016;123:983–93. [DOI] [PubMed] [Google Scholar]
- 34.Mulder IE, Schmidt B, Lewis M, Delday M, Stokes CR, Bailey M, et al. Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity. PLoS One 2011;6: e28279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schokker D, Zhang J, Zhang LL, Vastenhouw SA, Heilig HG, Smidt H, et al. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS One 2014;9:e100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight 2016;1:e86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pesce G, Marcon A, Marchetti P, Girardi P, de Marco R. Febrile and gynecological infections during pregnancy are associated with a greater risk of childhood eczema. Pediatr Allergy Immunol 2014;25:159–65. [DOI] [PubMed] [Google Scholar]
- 38.Sandberg M, Frykman A, Ernerudh J, Berg G, Matthiesen L, Ekerfelt C, et al. Cord blood cytokines and chemokines and development of allergic disease. Pediatr Allergy Immunol 2009;20:519–27. [DOI] [PubMed] [Google Scholar]
- 39.Byberg KK, Ogland B, Eide GE, Oymar K. Birth after preeclamptic pregnancies: association with allergic sensitization and allergic rhinoconjunctivitis in late childhood; a historically matched cohort study. BMC Pediatr 2014;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herberth G, Hinz D, Roder S, Schlink U, Sack U, Diez U, et al. Maternal immune status in pregnancy is related to offspring’s immune responses and atopy risk. Allergy 2011;66:1065–74. [DOI] [PubMed] [Google Scholar]
- 41.Lodge CJ, Allen KJ, Lowe AJ, Hill DJ, Hosking CS, Abramson MJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies. Clin Dev Immunol 2012; 2012:176484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodrup Carlsen KC, Roll S, Carlsen KH, Mowinckel P, Wijga AH, Brunekreef B, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One 2012;7:e43214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol 2013;108:1854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011;9:110–7. [DOI] [PubMed] [Google Scholar]
- 45.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009;54:2312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merwat SN, Spechler SJ. Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol 2009; 104:1897–902. [DOI] [PubMed] [Google Scholar]
- 47.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 2007;102:1301–6. [DOI] [PubMed] [Google Scholar]
- 48.Jensen ET, Dellon ES. Environmental and infectious factors in eosinophilic esophagitis. Best Pract Res Clin Gastroenterol 2015;29:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol 2003;112:616–23. [DOI] [PubMed] [Google Scholar]
- 50.Pali-Scholl I, Yildirim AO, Ackermann U, Knauer T, Becker C, Garn H, et al. Anti-acids lead to immunological and morphological changes in the intestine of BALB/c mice similar to human food allergy. Exp Toxicol Pathol 2008;60:337–45. [DOI] [PubMed] [Google Scholar]
- 51.Untersmayr E, Bakos N, Scholl I, Kundi M, Roth-Walter F, Szalai K, et al. Antiulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J 2005;19:656–8. [DOI] [PubMed] [Google Scholar]
- 52.Farre R, De Vos R, Geboes K, Verbecke K, Vanden Berghe P, Depoortere I, et al. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut 2007;56:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenacchi G, Scialpi C, et al. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol 2005;100:537–42. [DOI] [PubMed] [Google Scholar]
- 54.Moawad FJ, Dellon ES, Achem SR, Ljuldjuraj T, Green DJ, Maydonovitch CL, et al. Effects of race and sex on features of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2016;14:23–30. [DOI] [PubMed] [Google Scholar]
- 55.Martin LJ, Franciosi JP, Collins MH, Abonia JP, Lee JJ, Hommel KA, et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol 2015;135:1519–28.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherrill JD, Rothenberg ME. Genetic dissection of eosinophilic esophagitis provides insight into disease pathogenesis and treatment strategies. J Allergy Clin Immunol 2011;128:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2015;135:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2016;65:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.