Abstract
Hiroshi Yamasaki (2016) No kinorhynchs have previously been reported at the species level from Vietnamese waters. Two new species in the Echinoderes coulli-group, Echinoderes regina sp. nov. and Echinoderes serratulus sp. nov., are described from Nha Trang, Vietnam, Southeast Asia. Echinoderes regina sp. nov. is characterized by the large trunk (451-503 μm), a middorsal spine on segment 4, sublateral spines on segments 6 and 7, lateroventral tubules on segment 5, sublateral tubules on segment 8, laterodorsal tubules on segment 10, large sieve plates on segment 9, a primary pectinate fringe with fringe tips wider middorsally to ventrolaterally and thinner ventromedially on segments 2-9, and lateral terminal spines (15-20% of trunk length). Echinoderes serratulus sp. nov. is characterized by a middorsal spine on segment 4, sublateral spines on segments 6 and 7, lateroventral tubules on segments 5 and 8, midlateral tubules on segment 9, laterodorsal tubules on segment 10, large sieve plates on segment 9, primary pectinate fringes with long fringe tips ventrally on segments 1-9, with the ventromedial tips obliquely orientated, pointing towards the midventral line on segments 2-5, and short, plump lateral terminal spines (12-15% of trunk length).
Keywords: Echinoderes coulli-group, Meiofauna, Southeast Asia, Taxonomy, Distribution
BACKGROUND
Echinoderes is the most species-rich genus in the marine meiobenthic phylum Kinorhyncha. To date, 87 Echinoderes species have been reported worldwide, from the intertidal zone to abyssal depths and from polar to tropical regions (Neuhaus 2013; Sørensen 2013, 2014; Herranz et al. 2014; Sørensen and Landers 2014; Yamasaki and Fujimoto 2014; Sørensen et al. 2016).
So far, no kinorhynchs identified to the species level have been recorded from Vietnamese waters. While two ecological studies reported kinorhynchs from Vietnam, both studies identified them only to the phylum level, but did not provide any information for identification to species (Mokievsky et al. 2011; Ngo et al. 2013). This study describes two new species in the Echinoderes coulli-group, representing the first species-level records of kinorhynchs from Vietnam.
MATERIALS AND METHODS
Muddy sediment samples were collected by scuba diving at 10 m depth near Hon Mieu Island, Nha Trang, Vietnam (12°11.60'N, 109°13.96'E) on 25 November 2014 (Fig. 1). The station is ca. 2.6 km far from the nearest river mouth, but Hon Mieu Island would reduce the influence of freshwater income from the river. Although the fluctuation of the salinity at the station has not been measured, the salinity at a few meters depth nearby the station was 33 PSU at low tide on the sampling day. Kinorhynchs were subsequently extracted from the samples by using the bubbling and blot method (Higgins 1988; Sørensen and Pardos 2008). Extracted animals including kinorhynchs were washed with fresh water and preserved in 99% EtOH. In the laboratory, kinorhynch specimens were sorted under a stereomicroscope, and observed by light microscopy (LM) or scanning electron microscopy (SEM).
Fig. 1.
Fig. 1. Map of Vietnam, with enlargements indicating the sampling sites. A, enlarged map of Nha Trang.
Specimens for LM were transferred into glycerin to replace the ethanol with glycerin and were then mounted in Fluoromount G® between two cover slips attached to a plastic H-S slide. They were observed, sketched, and photographed with an Olympus BX51 microscope equipped with a Nikon DS-Fi1c camera and a drawing tube. Line arts were drawn in Adobe Illustrator CS5, based on scanned camera lucida drawings of mounted specimens. Measurements were made with a Nikon DS-L3 camera control unit.
Specimens for SEM were immersed in 100% butanol for several minutes, freeze dried, mounted on aluminum stubs, sputter-coated with gold-palladium, and observed with a JEOL JSM- 6060LV scanning electron microscope at 15 kV accelerating voltage.
The terminology follows Neuhaus and Higgins (2002), Sørensen and Pardos (2008), and Neuhaus (2013). Type material has been deposited in the invertebrate collection of the Hokkaido University Museum (formerly the Zoological Institute), Hokkaido University (ZIHU), Sapporo, Japan.
RESULTS
SYSTEMATICS
Class Cyclorhagida [Zelinka (1896)] Sørensen et al. (2015)
Order Echinorhagata Sørensen et al. (2015)
Family Echinoderidae Zelinka (1894)
Genus Echinoderes Claparède (1863)
Echinoderes regina sp. nov. Yamasaki
urn:lsid:zoobank.org:act: 0776C0CE-4E5E-458A-A350- 3EDC96B979FF
[New Japanese name: Jouou togekawa]
Material examined: All specimens collected 25 November 2014 at 10 m depth near Hon Mieu Island, Nha Trang, Vietnam (12°11.60'N, 109°13.96'E). Holotype: adult female (ZIHU- 5045), mounted in Fluoromount G®. Allotype: adult male (ZIHU-5046); mounted in Fluoromount G®. Paratypes: four adult females and two adult males (ZIHU-5047-5052), mounted in Fluoromount G®. Additional material: six specimens for SEM (two adult female, two adult males, and two adults gender undetermined), mounted on aluminum stubs.
Etymology: The species name regina is from Latin, meaning ‘queen’, referring to the species’ large trunk length, similar to that of Echinoderes rex Lundbye et al. (2011)(rex, ‘king’ in Latin). Diagnosis: Echinoderes with large trunk (451-503 m); with short acicular spine middorsally on segment 4 and sublaterally on segments 6 and 7; short tubules lateroventrally on segment 5 and sublaterally on segment 8; laterodorsal tubules on segment 10; large sieve plates on segment 9; primary pectinate fringe of segments 2-9 with conspicuously wider tips in middorsal to ventrolateral areas and thinner tips in ventromedial area; lateral terminal spines length 15-20% of trunk length.
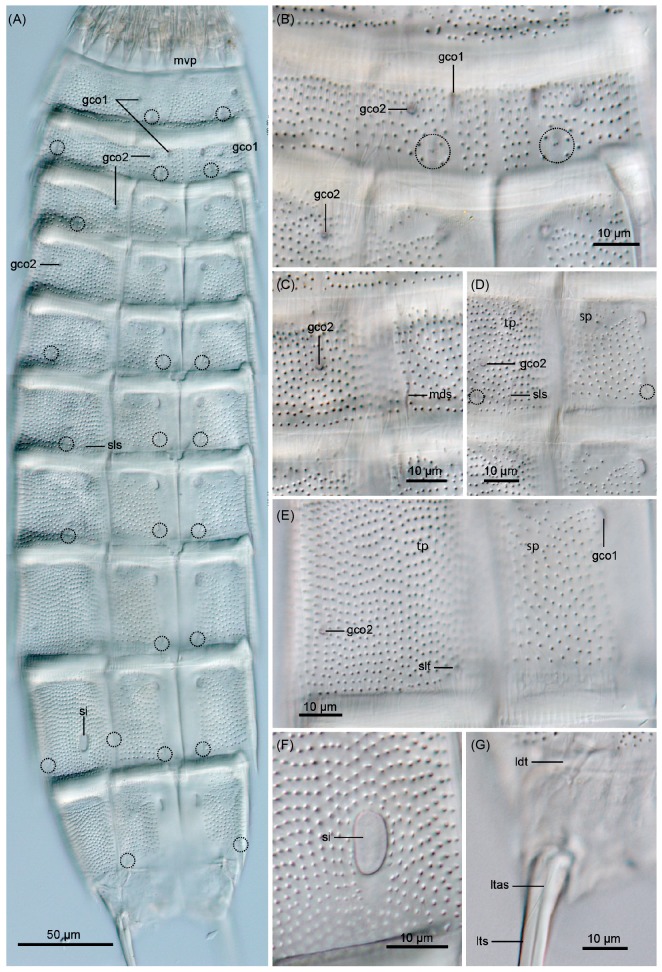
Description: Adult with head, neck, and eleven trunk segments (Figs. 2A, B, 3A, 4A). See table 1 for measurements. Table 2 indicates the positions of cuticular structures (sensory spots, glandular cell outlets, spines, tubules, and sieve plates).
Fig. 2.
Fig. 2. Echinoderes regina sp. nov., camera lucida drawings. A, B, Holotype, female (ZIHU-5045), entire animal, dorsal and ventral views, respectively; C, D, allotype, male (ZIHU-5046), segments 9-11, dorsal and ventral views, respectively. White, grey, and black circles or ovals indicate sensory spots, type-1 glandular cell outlets, and type-2 glandular cell outlets, respectively. Abbreviations: gco1, type-1 glandular cell outlet; gco2, type-2 glandular cell outlet; ldt, laterodorsal tubule; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvt, lateroventral tubule; mds, middorsal acicular spine; ps, penile spine; si, sieve plate; sls, sublateral acicular spine; slt, sublateral tubule; ss, sensory spot.
Fig. 3.
Fig. 3. Echinoderes regina sp. nov., Nomarski photomicrographs of holotype female (ZIHU-5045). A, entire animal, ventral view; B, segments 2 and 3, ventral view; C, segment 4, dorsal view; D, segments 6 and 7, lateroventral view; E, segment 8, lateroventral view; F, segment 9, sublateral view; G, segments 10 and 11, dorsal view. Dashed circles indicate sensory spots. Abbreviations: gco1, type-1 glandular cell outlet; gco2, type-2 glandular cell outlet; ldt, laterodorsal tubule; ltas, lateral terminal accessory spine; lts, lateral terminal spine; mds, middorsal acicular spine; mvp, midventral placid; si, sieve plate; sls, sublateral acicular spine; slt, sublateral tubule; sp, sternal plate; tp, tergal plate.
Fig. 4.
Fig. 4. Echinoderes regina sp. nov., scanning electron micrographs. A, entire animal, lateral view; B, head, lateral view; C, mouth cone, lateral view; D, introvert, lateral view; E, introvert, laterodorsal view. Abbreviations: int, introvert; lts, lateral terminal spine; mc, mouth cone; oos, outer oral style; psc, primary spinoscalid; sec, sector; seg, segment.
Table 1. Measurements for adult Echinoderes regina sp. nov. (in micrometers). Columns N and SD are sample size and standard deviation, respectively.
| Character | N | Range | Mean | SD |
| TL | 8 | 451-503 | 481 | 18.92 |
| MSW-8 | 8 | 63-75 | 69 | 4.69 |
| MSW-8/TL | 8 | 13.3-16.3% | 14.4% | 1.09% |
| SW-10 | 8 | 56-70 | 64 | 4.90 |
| SW-10/TL | 8 | 12.0-15.4% | 13.4% | 1.15% |
| S1 | 8 | 44-49 | 46 | 1.70 |
| S2 | 8 | 38-42 | 40 | 1.43 |
| S3 | 8 | 35-44 | 40 | 2.57 |
| S4 | 8 | 42-45 | 44 | 0.97 |
| S5 | 8 | 45-50 | 46 | 1.77 |
| S6 | 8 | 47-54 | 51 | 2.13 |
| S7 | 8 | 53-60 | 56 | 2.70 |
| S8 | 8 | 57-64 | 62 | 2.44 |
| S9 | 8 | 63-69 | 67 | 1.92 |
| S10 | 8 | 57-62 | 59 | 2.08 |
| S11 | 8 | 34-41 | 37 | 2.25 |
| MD4 (ac) | 7 | 7-11 | 10 | 1.47 |
| LV5 (tu) | 8 | 6-11 | 8 | 1.84 |
| SL6 (ac) | 8 | 7-10 | 8 | 1.47 |
| SL7 (ac) | 8 | 7-11 | 9 | 1.49 |
| SL8 (tu) | 8 | 7-10 | 8 | 1.46 |
| LD10 (tu) (m) | 3 | 35-41 | 38 | 2.92 |
| LD10 (tu) (f) | 5 | 22-26 | 23 | 1.73 |
| LTS | 8 | 76-85 | 79 | 3.11 |
| LTAS | 5 | 13-17 | 15 | 1.98 |
| LTS/TL | 8 | 15.2-18.9% | 16.6% | 1.21% |
| LTAS/TL | 5 | 2.8-3.4% | 3.1% | 0.30% |
Abbreviations: (ac), acicular spine; (f), female; LD, length of laterodorsal tubule; LTAS, length of lateral terminal accessory spine; LTS, length of lateral terminal spine; LV, length of lateroventral tubule; (m), male; MD: length of middorsal acicular spine; MSW, maximum sternal width; S, segment length; SL, length of sublateral spine/tubule; SW, standard width; TL, trunk length; (tu), tubule.
Table 2. Summary of location of cuticular structures, tubules, and spines in Echinoderes regina sp. nov.
| Position / segment | MD | PD | SD | LD | ML | SL | LV | VL | VM |
| 1 | gco1 | ss | ss | gco1 | ss | ||||
| 2 | gco1, ss | gco2 | ss, gco2, ss | gco2, ss, gco1 | |||||
| 3 | gco1 | ss | ss | gco2 | gco1 | ||||
| 4 | ac | gco1 | gco2 | gco2 | gco1 | ||||
| 5 | gco1 | ss | gco2, tu | gco1, ss | |||||
| 6 | gco1 | ss | gco2, ss | ac | gco1, ss | ||||
| 7 | gco1 | ss | gco2, ss | ac | gco1, ss | ||||
| 8 | gco1 | ss | gco2 | tu | gco1, ss | ||||
| 9 | gco1 | ss, ss | ss | si | ss | gco1, ss | |||
| 10 | gco1, gco1 | ss | tu | ss | gco1 | ||||
| 11 | ss | ltas (f), ps (m) | lts | ss (f) |
Abbreviations: ac, acicular spine; (f), female condition of sexually dimorphic character; gco1, type-1 glandular cell outlet; gco2, type-2 glandular cell outlet; LD, laterodorsal; ltas, lateral terminal accessory spine; lts, lateral terminal spine; LV, lateroventral; (m), male condition of sexually dimorphic character; MD, middorsal; ML, midlateral; PD, paradorsal; ps, penile spine; SD, subdorsal; si, sieve plate; SL, sublateral; ss, sensory spot; tu, tubule; VL, ventrolateral; VM, ventromedial.
Head consists of retractable mouth cone and introvert (Figs. 4A-E, 5). Mouth cone with inner oral styles and nine outer oral styles. Exact number and arrangement of inner oral styles not examined. Each outer oral style consists of rectangular basal part and triangular distal part (Fig. 4C). Basal parts of outer oral styles alternating in size: five large in odd sectors of the introvert, and four small in even sectors (Figs. 4B, C, 5). Three rows of filose structures present on posterior to basal parts of outer oral styles (Fig. 4C). Anteriormost row consists of three to five longer spinose processes for each outer oral style. Middle row consists of multiple, much shorter and thinner processes, seamlessly covering mouth cone. Posteriormost row consists of one to five cracked-tip spinose processes for each outer oral style. Introvert composed of seven rings of spinoscalids and one ring of trichoscalids (Figs. 4B, D, E, 5). Ring 01 includes ten primary spinoscalids, each with basal sheath and smooth long end-piece (Figs. 4D, E). Each basal sheath with three overlapping fringes. Proximal fringe extends into three flat projections, like a trident, covering next fringe. Middle fringe with two lateral projections overlapping end-piece. Distal fringe with five threads projecting between two projections of middle fringe. End-piece of primary spinoscalids is longest unit. Rings 02 and 04 with 10 spinoscalids; rings 03 and 05 with 20 spinoscalids. Spinoscalids of rings 02-05 similar in length. Rings 06 and 07 not examined in detail, but ring 06 with at least five, and ring 07 with at least three relatively short spinoscalids (Fig. 5). Six trichoscalids attached with trichoscalid plate in sectors 2, 4, 5, 7, 8, and 10.
Fig. 5.
Fig. 5. Diagram of mouth cone, introvert, and placids in Echinoderes regina sp. nov. Grey area and heavy line arcs show mouth cone and placids, respectively. The table lists the scalid arrangement by sector.
Neck with 16 placids (Figs. 2A, B, 3A, 5). Midventral placid broadest (ca. 18 m wide at base, ca. 12 m wide at tip). Remaining placids similar in size (ca. 11 m in basal width and ca. 5 m in tip width).
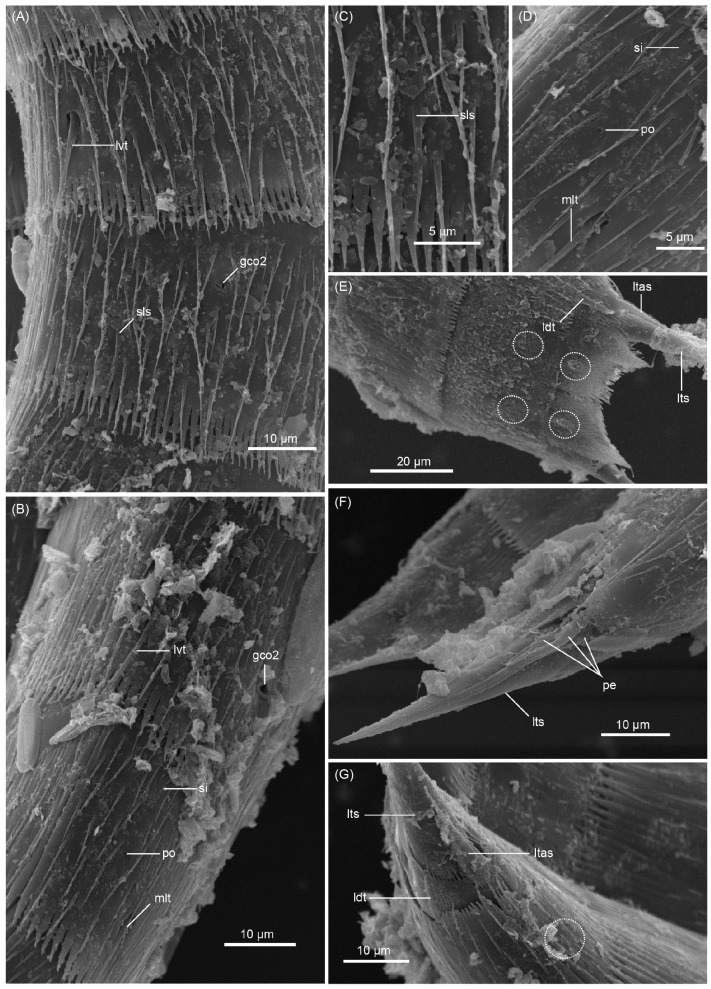
Segment 1 consists of complete cuticular ring. This and following nine segments with thick pachycycli at anterior margin (Figs. 2A-D, 3A). Non-bracteate cuticular hairs densely cover entire segment (Fig. 6A), but perforation sites are not visible in posterior quarter of the segment (Figs. 2A, B, 3A, B). Rounded subdorsal and laterodorsal sensory spots located close to anterior margin of the segment (Figs. 2A, 6A, D). Rounded ventrolateral sensory spots located centrally between anterior and posterior segment margins (Figs. 2B, 3A, 6B). Type-1 glandular cell outlets situated anteriorly in middorsal and lateroventral positions (Figs. 2A, B, 3A). Posterior part of segment with primary pectinate fringe, with all fringe tips similar in length and width (Figs. 2A, B, 6A, B).
Fig. 6.
Fig. 6. Echinoderes regina sp. nov., scanning electron micrographs. A, segments 1-3, laterodorsal view; B, introvert and segments 1-3, ventral view; C, segment 2, ventrolateral view; D, laterodorsal sensory spot on segment 1; E, segment 2, laterodorsal view; F, segment 4, dorsal view. Dashed circles indicate sensory spots. Abbreviations: gco2, type-2 glandular cell outlet; mds, middorsal acicular spine; ppf, primary pectinate fringe.
Segment 2 with complete cuticular ring, like segment 1 (Figs. 2A, B). Bracteate cuticular hairs densely covering whole area. One droplet- shaped sensory spot in middorsal position, two pairs in laterodorsal position, and one pair in ventromedial position (Figs. 2A, B, 3A, B, 6A-C, E). All sensory spots central in position. Type-1 glandular cell outlets of segment 2 and following eight segments situated in anterior part of segment in ventromedial position (Figs. 2B, 3A, B). Type-1 glandular cell outlet situated anteriorly in middorsal position (Fig. 2A). Paired type-2 glandular cell outlets in paradorsal, laterodorsal, and ventromedial positions (Figs. 2A, B, 3A, B, 6A- C, E). All type-2 glandular cell outlets of segment 2 and following six segments situated centrally on segment. Posterior margin of segment ends as primary pectinate fringe showing tips similar to those of the preceding segment in ventrolateral to middorsal position, and smaller and thinner tips in ventromedial position (Figs. 2A, B, 6A, B).
Segment 3 and following eight segments consist of one tergal and two sternal plates (Figs 2A, B). This and following seven segments entirely covered with bracteate cuticular hairs except for anterior area. Paired sensory spots in subdorsal and midlateral positions (Figs. 2A, B, 3A). Type-1 glandular cell outlet situated anteriorly in middorsal position (Fig. 2A). Pair of type-2 glandular cell outlets in lateroventral position (Figs. 2B, 3A, B). Pectinate fringe on segment 3 and following six segments as on segment 2 (Figs. 2A, B).
Segment 4 with short middorsal acicular spine (Figs. 2A, 3C, 6F), and without sensory spots. Pair of type-1 glandular cell outlets on segment 4 and following five segments, situated in anterior part of segment in paradorsal position (Fig. 2A). Type-2 glandular cell outlets in subdorsal and laterodorsal positions (Figs. 2A, B, 3A, C, 6F, 7A).
Fig. 7.
Fig. 7. Echinoderes regina sp. nov., scanning electron micrographs. A, segments 4-6, laterodorsal view; B, segments 5-7, ventrolateral view; C, segments 8-10, ventral view; D, sublateral acicular spine on segment 6; E, segments 10 and 11 in female, dorsal view; F, segments 10 and 11 in male, laterodorsal view. Dashed circles indicate sensory spots. Abbreviations: gco2, type-2 glandular cell outlet; ldt, laterodorsal tubule; ltas, lateral terminal acicular spine; lts lateral terminal spine; lvt, lateroventral tubule; ps, penile spine; sls, sublateral acicular spine; slt, sublateral tubule.
Segment 5 with lateroventral tubules (Figs. 2B, 7B). Sensory spots in laterodorsal and ventromedial positions (Figs. 2A, B, 3A, 7A, B). Paired type-2 glandular cell outlets anteriorly in lateroventral position (Fig. 2B).
Segment 6 with short sublateral acicular spine (Figs. 2A, B, 3D, 7B, D), and subdorsal, midlateral, and ventromedial paired sensory spots (Figs. 2A, B, 3A, D, 7B). Paired type-2 glandular cell outlets in midlateral position (Figs. 2A, B, 3A, D, 7B).
Segment 7 similar to segment 6 (Figs. 2A, B, 3A, 7B).
Segment 8 with sublateral tubules (Figs. 2A, B, 3E, 7C). Paired sensory spots in subdorsal and ventromedial positions (Figs. 2A, B, 3A, 7C). Paired type-2 glandular cell outlets in laterodorsal position (Figs. 2A, B, 3E).
Segment 9 with two pairs of subdorsal sensory spots and one pair each of laterodorsal, ventrolateral, and ventromedial sensory spots (Figs. 2A-D, 3A, 7C). Pair of sieve plates with oval-shaped sieve area and single posterior pore situated in midlateral position (Figs. 2A-D, 3A, F).
Segment 10 with laterodorsal tubules (Figs. 2A, C, 3G, 7E, F). Laterodorsal tubules in males about twice as long as in females (Table 1). Paired subdorsal and ventrolateral sensory spots present (Figs. 2B, D, 3A, 7E, F). Two type-1 glandular cell outlets present in middorsal position. Posterior margin ends as primary pectinate fringe with short, thin tips.
Segment 11 with lateral terminal spines (Figs. 2A-D, 3A, G, 4A, 7E, F). Short, thin lateral terminal accessory spines present only in females (Figs. 2A, B, 3G, 7E), and three pairs of penile spines only in males (Figs. 2C, D, 7F). Cuticular hairs absent. Paired sensory spots present in subdorsal position (Figs. 2A, C, 7E, F). Additional paired sensory spots present in ventrolateral position in female. Tergal plate projects laterally and ends in short, pointed tergal extensions (Figs. 2A, C, 3G, 7E, F).
Remarks: The combination of the arrange- ment of spines and tubules and the presence of large sieve plates assigns Echinoderes regina sp. nov to be a member of the Echinoderes coulli- group (see also DISCUSSION). Within the group, E. regina sp. nov. is most similar to Echinoderes annae Sørensen et al. (2016), Echinoderes maxwelli [Omer-Cooper (1957)], Echinoderes ohtsukai Yamasaki and Kajihara (2012), E. rex, Echinoderes teretis Brown, 1999 in Adrianov and Malakhov (1999), and Echinoderes serratulus sp. nov. (next description) in having a middorsal spine on segment 4 (Omer-Cooper 1957; Brown 1985; Adrianov and Malakhov 1999; Lundbye et al. 2011; Yamasaki and Kajihara 2012; Sørensen 2014; Yamasaki and Fujimoto 2014; Herranz and Leander 2016; Sørensen et al. 2016).
Echinoderes regina sp. nov. differs from E. annae in having an acicular spine laterally on segments 6 and 7 (E. regina sp. nov. has a short acicular spine sublaterally on segments 6 and 7, whereas E. annae lacks those) (Sørensen et al. 2016). Echinoderes regina sp. nov. also differs from E. annae in lacking midlateral tubules on segment 9 (Sørensen et al. 2016).
Echinoderes regina sp. nov. differs from E. maxwelli in the shape of the posterior edge on segment 10 (the edge on segment 10 is not projecting posteriorly in E. regina, whereas that is projecting over segment 11 in E. maxwelli) (Sørensen 2014).
Echinoderes regina sp. nov. shares with E. ohtsukai, E. rex, E. teretis, and E. serratulus sp. nov. acicular spines laterally on segments 6 and 7.
Echinoderes regina sp. nov. differs from E. ohtsukai in the shorter length of lateral terminal spines (76-85 m in E. regina sp. nov., 163-190 m in E. teretis), having conspicuous wide tips in the primary pectinate fringes in the middorsal to ventrolateral area on segments 2-9, and lacking any fringed tube (Yamasaki and Kajihara 2012; Herranz and Leander 2016).
Echinoderes regina sp. nov. is similar to E. rex in trunk length (451-503 m in E. regina sp. nov., 482-528 m in E. rex), but differs conspicuously in having a longer lateral terminal spine (15.2-18.9% of trunk length in E. regina sp. nov., 3.8-4.8% of trunk length in E. rex) (Lundbye et al. 2011).
Echinoderes regina sp. nov. differs from E. teretis in the much longer trunk length (451-503 μm in E. regina sp. nov., 207-264 μm in E. teretis) and overall trunk shape (slender, with all trunk segments similar in segment circumference in E. regina sp. nov.; plump, with segments 4-6 broader circumferences than other segments) (Brown 1985).
Echinoderes regina sp. nov. differs from E. serratulus sp. nov. in lacking midlateral tubules on segment 9, in having slender lateral terminal spines, and in the shape of the primary pectinate fringes, especially on segments 2-9 (width of tips of primary pectinate fringe different between middorsal to ventrolateral area and ventromedial area in E. regina sp. nov., similar in E. serratulus sp. nov.).
Echinoderes serratulus sp. nov. Yamasaki
urn:lsid:zoobank.org:act: 68D196A3-A3D0-48AD-92FC- 9406562D8CD0
[New Japanese name: Nokogiri togekawa]
Material examined: All specimens collected 25 November 2014 at 10 m depth near Hon Mieu Island, Nha Trang, Vietnam (12°11.60'N, 109°13.96'E). Holotype: adult female (ZIHU- 5053), mounted in Fluoromount G®. Allotype: adult male (ZIHU-5054), mounted in Fluoromount G®. Paratypes: one adult female and two adult males (ZIHU-5055-5057), mounted in Fluoromount G®. Additional material: four specimens for SEM (three adult females and one adult male), mounted on aluminum stubs.
Etymology: The species name serratulus is from Latin, meaning ‘serration’, referring to the species’ conspicuous primary pectinate fringe.
Diagnosis: Echinoderes with short acicular spine middorsally on segment 4 and sublaterally on segments 6 and 7; lateroventral tubules on segments 5 and 8, midlateral tubules on segment 9, and laterodorsal tubules on segment 10; large sieve plates on segment 9; primary pectinate fringes ventrally on segments 1-9 conspicuous, with longer fringe tips; tips of ventromedial primary pectinate fringes on segments 2-5 obliquely orientated, pointing towards midventral line; lateral terminal spines short and plump, length 12-15% of trunk length.
Description: Adult with head, neck and eleven trunk segments (Figs. 8A, B, 9A, 10A). See table 3 for measurements, and table 4 for positions of cuticular structures (sensory spots, glandular cell outlets, acicular spines, tubules and sieve plates).
Fig. 8.
Fig. 8. Echinoderes serratulus sp. nov., camera lucida drawings. A, B, Holotype, female (ZIHU-5053), entire animal, dorsal and ventral views, respectively; C, D, allotype, male (ZIHU-5054), segments 9-11, dorsal and ventral views, respectively. Double, gray, and black circles indicate sensory spots, type-1 glandular cell outlets, and type-2 glandular cell outlets, respectively. Abbreviations: gco1, type-1 glandular cell outlet; gco2, type-2 glandular cell outlet; ldt, laterodorsal tubule; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvt, lateroventral tubule; mds, middorsal acicular spine; mlt, midlateral tubule; ps, penile spine; si, sieve plate; sls, sublateral acicular spine; ss, sensory spot.
Fig. 9.
Fig. 9. Echinoderes serratulus sp. nov., Nomarski photomicrographs of holotype female (ZIHU-5053). A, entire animal, ventral view; B, segments 2 and 3, ventral view; C, segments 1 and 2, dorsal view; D, segments 5 and 6, lateroventral view; E, segments 8 and 9, lateroventral view; F, segments 9-11, laterodorsal view; G, segments 10 and 11, ventrolateral view. Dashed circles indicate sensory spots. Abbreviations: gco1, type-1 glandular cell outlet; gco2, type-2 glandular cell outlet; ldt, laterodorsal tubule; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvt, lateroventral tubule; mlt, midlateral tubule; mvp, midventral placid; ppf, primary pectinate fringe; si, sieve plate; sls, sublateral acicular spine; sp, sternal plate.
Fig. 10.
Fig. 10. Echinoderes serratulus sp. nov., scanning electron micrographs. A, entire animal, lateroventral view; B, mouth cone, lateroventral view; C, introvert, ventrolateral view; D, segments 1-7, ventral view; E, segment 5, ventromedial view; F, segments 3-6, ventrolateral view; G, segments 3-5, dorsal view. Dashed circles indicate sensory spots. Abbreviations: gco2, type-2 glandular cell outlet; ha, hair; int, introvert; lts, lateral terminal spine; lvt, lateroventral tubule; mds, middorsal acicular spine; oos, outer oral style; ppf, primary pectinate fringe; psc, primary spinoscalid; sec, sector; seg, segment; spf, secondary pectinate fringe.
Table 3. Measurements for adult Echinoderes serratulus sp. nov. (in micrometers). Columns N and SD are sample size and standard deviation, respectively.
| Character | N | Range | Mean | SD |
| TL | 5 | 321-359 | 348 | 15.32 |
| MSW-6 | 5 | 62-69 | 66 | 3.09 |
| MSW-6/TL | 5 | 17.9-19.9% | 18.9% | 1.00% |
| SW-10 | 5 | 54-59 | 56 | 1.76 |
| SW-10/TL | 5 | 15.1-17.0% | 16.1% | 0.81% |
| S1 | 5 | 36-39 | 37 | 1.25 |
| S2 | 5 | 33-37 | 35 | 1.82 |
| S3 | 5 | 28-31 | 30 | 1.21 |
| S4 | 5 | 31-34 | 33 | 1.28 |
| S5 | 5 | 34-38 | 37 | 1.68 |
| S6 | 5 | 38-42 | 40 | 1.51 |
| S7 | 5 | 40-48 | 45 | 2.86 |
| S8 | 5 | 46-53 | 50 | 2.67 |
| S9 | 5 | 47-53 | 50 | 2.37 |
| S10 | 5 | 44-46 | 45 | 0.66 |
| S11 | 5 | 44-48 | 46 | 1.75 |
| MD4 (ac) | 5 | 10-14 | 12 | 1.68 |
| LV5 (tu) | 5 | 11-13 | 12 | 1.14 |
| SL6 (ac) | 5 | 9-12 | 10 | 1.53 |
| SL7 (ac) | 5 | 9-13 | 11 | 1.71 |
| LV8 (tu) | 5 | 9-13 | 12 | 1.59 |
| ML9 (tu) | 5 | 16-21 | 19 | 1.96 |
| LD10 (tu) (m) | 3 | 39-41 | 40 | 1.11 |
| LD10 (tu) (f) | 2 | 16-17 | 17 | 0.96 |
| LTS | 5 | 44-49 | 46 | 1.65 |
| LTAS | 2 | 20-21 | 20 | 0.66 |
| LTS/TL | 5 | 12.4-14.7% | 13.4% | 0.86% |
| LTAS/TL | 2 | 5.6-5.8% | 5.7% | 0.11% |
Abbreviations: (ac), acicular spine; (f), female; LD, length of laterodorsal tubule; LTAS, length of lateral terminal accessory spine; LTS, length of lateral terminal spine; LV, length of lateroventral tubule; (m), male; MD: length of middorsal acicular spine; ML, length of midlateral tubule; MSW, maximum sternal width; S, segment length; SL, length of sublateral acicular spine; SW, standard width; TL, trunk length; (tu), tubule.
Table 4. Summary of location of cuticular structures, tubules, and spines in Echinoderes serratulus sp. nov.
| Position / segment | MD | PD | SD | LD | ML | SL | LV | VL | VM |
| 1 | gco1 | ss | ss | gco1 | ss | ||||
| 2 | ss, gco1 | gco2 | ss, gco2 | ss | gco2 | ss, gco1 | |||
| 3 | gco1, gco1 | ss | ss | ss | gco1 | ||||
| 4 | ac | gco1 | gco2 | ss | gco1 | ||||
| 5 | gco1 | ss | ss, gco2 | tu | ss, gco1 | ||||
| 6 | gco1 | ss | ss, gco2 | ac | ss, gco1 | ||||
| 7 | gco1 | ss | ss, gco2 | ac | ss, gco1 | ||||
| 8 | gco1 | ss | ss | gco2 | tu | gco1 | |||
| 9 | gco1 | ss | ss | tu | si | ss | gco1 | ||
| 10 | gco1 | ss | tu | ss | gco1 | ||||
| 11 | ss | ltas (f), ps (m) | lts |
Abbreviations: ac, acicular spine; (f), female condition of sexually dimorphic character; gco1, type-1 glandular cell outlet; gco2, type-2 glandular cell outlet; LD, laterodorsal; ltas, lateral terminal accessory spine; lts, lateral terminal spine; LV, lateroventral; (m), male condition of sexually dimorphic character; MD, middorsal; ML, midlateral; PD, paradorsal; ps, penile spine; SD, subdorsal; si, sieve plate; SL, sublateral; ss, sensory spot; tu, tubule; VL, ventrolateral; VM, ventromedial.
Head consists of retractable mouth cone and introvert (Figs. 10A-C). Mouth cone with inner oral styles and nine outer oral styles. Exact number and arrangement of inner oral styles not observed. Each outer oral style composed of rectangular basal part and triangular distal part (Fig. 10B). Basal parts of outer oral styles alternate in size: five large anterior to odd sectors of introvert, and four slightly smaller ones in even sectors (Figs. 10B, 11). Introvert composed of seven rings of spinoscalids and one ring of trichoscalids (Figs. 10C, 11). Ring 01 includes ten primary spinoscalids, each with basal sheath and smooth long end-piece (Fig. 10C). Each basal sheath with three overlapping fringes. Proximal fringe extends into three flat projections, like a trident, covering next fringe. Middle fringe with two lateral projections overlapping end-piece. Distal fringe with five threads projecting between two projections of middle fringe. End-piece of primary spinoscalids is longest unit. Rings 02 and 04 with 10 spinoscalids; rings 03 and 05 with 20 spinoscalids. Spinoscalids in rings 02-05 similar in length. Rings 06 and 07 not examined with detail, but ring 06 with at least seven, and ring 07 with at least ten relatively short spinoscalids (Fig. 11). Six trichoscalids attached with trichoscalid plate in sectors 2, 4, 5, 7, 8, and 10.
Fig. 11.
Fig. 11. Diagram of mouth cone, introvert, and placids in Echinoderes serratulus sp. nov. Grey area and heavy line arcs show mouth cone and placids, respectively. The table lists the scalid arrangement by sector.
Neck with 16 placids (Figs. 8A, B, 9A, 11). Midventral placid broadest (ca. 17 μm wide at base, ca. 12 μm wide at tip); remaining placids with similar size (ca. 10 μm wide at base, ca. 4 μm wide at tip).
Segment 1 consists of complete cuticular ring (Figs. 8A, B, 9A). Non-bracteate cuticular hairs densely cover entire segment. Paired, rounded subdorsal and laterodorsal sensory spots located close to anterior margin of segment (Figs. 8A, 9C). Rounded ventromedial sensory spots centered between anterior and posterior margins (Figs. 8B, 10D). Type-1 glandular cell outlets situated anteriorly in middorsal and lateroventral positions (Figs. 8A, B, 9A, C). Primary pectinate fringe conspicuous, with longer fringe tips, especially on ventral side (Figs. 8A, B, 9A-C).
Segment 2 also with complete cuticular ring (Figs. 8A, B), with thick pachycyclus at anterior margin (Figs. 8A, B, 9A-C). Entire cuticular surface, except anterior area, covered with bracteate cuticular hairs on this and following eight segments. Oval sensory spots in middorsal, laterodorsal, midlateral, and ventromedial positions (Figs. 8A, B, 9A-C, 10D). Unpaired type- 1 glandular cell outlet present in middorsal position (Fig. 8A). Paired type-1 glandular cell outlets close to anterior margin in ventromedial position on this and following eight segments (Figs. 8B, D, 9A, B). Type-2 glandular cell outlets in subdorsal, laterodorsal, and ventrolateral positions (Figs. 8A, B, 9A-C). Type-2 glandular cell outlets show single large pore in LM and SEM (Figs. 9B, C; see also figures 12A, B for type-2 glandular cell outlet on segments 6 and 8). Posterior margin of segment with primary pectinate fringe having long tips, with ventromedial tips obliquely orientated, pointing towards the midventral line (Figs. 8A, B, 9A-C).
Segment 3 and following eight segments consist of one tergal and two sternal plates (Figs. 8A, B, 9A). Each plate with thicker pachycycli in anterior area (Figs. 8A, B, 9A, B). Sensory spots in subdorsal, midlateral, and sublateral positions (Figs. 8A, 9A). Two unpaired type-1 glandular cell outlets present middorsally (Fig. 8A). Primary pectinate fringes on this and following two segments as on segment 2. Secondary pectinate fringes, consisting of single belt of minute teeth, visible on anterior dorsal plate in SEM observation, at least on this and following five segments (Fig. 10G).
Segment 4 with short middorsal acicular spine (Figs. 8A, 10G). Paired laterodorsal sensory spots and paired subdorsal type-2 glandular cell outlets present (Figs. 8A, 10G). Paired type-1 glandular cell outlets present paradorsally on this and following six segments. Fringe tips on dorsal, lateral, and ventral sides similar in length and width.
Segment 5 with lateroventral tubules (Figs. 8B, 9D, 10D, F, 12A). Paired sensory spots in subdorsal, midlateral, and ventromedial positions (Figs. 8A, B, 9A, 10D-F). Pair of type-2 glandular cell outlets in midlateral position (Figs. 8A, B).
Fig. 12.
Fig. 12. Echinoderes serratulus sp. nov., scanning electron micrographs. A, segments 5 and 6, lateral view; B, segments 8 and 9, lateroventral view; C, segment 6, sublateral view; D, segment 9, sublateral view; E, segments 9-11 in female, dorsal view; F, segments 10 and 11 in male, lateral view; G, segments 10 and 11 in female, lateral view. Dashed circles indicate sensory spots. Abbreviations: gco2, type-2 glandular cell outlet; ldt, laterodorsal tubule; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvt, lateroventral tubule; mlt, midlateral tubule; pe, penile spine; po, pore; si, sieve area; sls, sublateral acicular spine.
Segment 6 similar to segment 5 except for absence of lateroventral tubules and presence of short sublateral acicular spines (Figs. 8A, B, 9A, D, 10D, F, 12A, C). Primary pectinate fringes of this and following three segments with long, conspicuous fringe tips.
Segment 7 similar to segment 6.
Segment 8 with lateroventral tubules (Figs. 8B, 9A, E, 12B). Paired sensory spots in subdorsal and laterodorsal positions (Fig. 7A). Paired type- 2 glandular cell outlets in midlateral position (Figs. 8A, B, 12B).
Segment 9 with midlateral tubules (Figs. 8A, C, D, 9F, 12B, D). Paired subdorsal, laterodorsal, and ventrolateral sensory spots present (Figs. 8A- D, 9A, E). Sieve plates with oval sieve area and single posterior pore in sublateral position (Figs. 8B-D, 9E, 12B, D).
Segment 10 with long laterodorsal tubules in males, short laterodorsal tubules in females (Figs. 8A, C, 9F, 12E, G; Table 1). Paired subdorsal and ventrolateral sensory spots present (Figs. 8A-D, 12E, G). Primary pectinate fringe with shorter and thinner tips than preceding segments in middorsal to ventrolateral areas, and with tips similar in length to those of preceding segments in ventromedial area.
Segment 11 with short, plump lateral terminal spines (Figs. 8A-D, 9A, F, G, 10A, 12E-G). Pair of short lateral terminal accessory spines present in females (Figs. 8B, 9G, 12E, G), and three pairs of penile spines present in males (Figs. 8D, 12F). Cuticular hairs absent. Paired sensory spots in subdorsal position (Figs. 8A, C, 12E). Tergal plate partially divided at middorsal line from central to posterior part. Posterior edge of tergal plate projects laterally and ends in pointed tergal extensions (Figs. 8A-D, 12E).
Remarks: The combination of the arrange- ment of spines and tubules and the presence of large sieve plates assigns Echinoderes serratulus sp. nov to be a member of the Echinoderes coulli-group (see also DISCUSSION). Within the group, Echinoderes serratulus sp. nov. is similar to E. annae and Echinoderes hwiizaa Yamasaki and Fujimoto (2014) in having paired tubules on segment 9, and plump lateral terminal spines on segment 11 (Yamasaki and Fujimoto 2014; Sørensen et al. 2016). It is also similar to Echinoderes aspinosus Sørensen et al. (2012) in having paired large sieve plates on segment 9, and the tips of the ventral primary pectinate fringes pointing towards the midventral line on segments 2-5 (Sørensen et al. 2012).
Echinoderes serratulus sp. nov. differs from E. annae in having minute sublateral acicular spines on segments 6 and 7 (Sørensen et al. 2016).
Echinoderes serratulus sp. nov. differs from E. hwiizaa in having a middorsal acicular spine on segment 4 and a conspicuous ventral primary pectinate fringe on segments 1 and 2, and in lacking midlateral tubules on segment 8 (Yamasaki and Fujimoto 2014).
Echinoderes serratulus sp. nov. easily differs from E. aspinosus in having acicular spines and tubules on segments 4-10 (E. aspinosus lacks any acicular spines and tubules on segments 4-10) (Sørensen et al. 2012).
DISCUSSION
The morphological features of both Echinoderes regina sp. nov. and E. serratulus sp. nov. match the following diagnosis of the E. coulli-group proposed by Yamasaki and Fujimoto (2014): Echinoderes without acicular middorsal spines, or with a very short spine only on segment 4; lateral spines very short on segments 6 and 7, or absent; midlateral, sublateral, lateral accessory, or lateroventral tubules on segments 5 and 8; sieve plates relatively large, consisting of oval or inverted-triangular sieve area and single posterior pore; and lateral terminal accessory spines poorly developed or completely absent in females. Thus the affiliation of the two new species to the E. coulli-group is undoubtable.
To date, 12 species of E. coulli-group including two new species have been reported worldwide: E. annae from Singapore; Echinoderes applicitus Ostmann et al. (2012) from Java Island, Indonesia; Echinoderes coulli Higgins (1977) from South Carolina and Louisiana, United States; E. hwiizaa from Ryukyu Islands, Japan; Echinoderes komatsui Yamasaki and Fujimoto (2014) from Ryukyu Islands, Japan; Echinoderes marthae Sørensen (2014) from Araçá, São Sebastião, Brazil; E. maxwelli from the estuary of the Kleinemonde River, South Africa; E. ohtsukai from Seto Inland Sea, Japan and from Boundary Bay, southeast from Vancouver, Canada; E. regina sp. nov. from Nha Trang, Vietnam; E. rex from the southern coast of South Korea; E. serratulus sp. nov. from Nha Trang, Vietnam; E. teretis from the south eastern coast of Australia (Omer-Cooper 1957; Higgins 1977; Brown 1985; Lundbye et al. 2011; Ostmann et al. 2012; Sørensen et al. 2012; Yamasaki and Kajihara 2012; Sørensen 2014; Yamasaki and Fujimoto 2014; Fleeger et al. 2015; Herranz and Leander 2016; Sørensen et al. 2016; this study) (Fig. 13). Most of them have been reported from estuarine environments, whereas three species, E. regina sp. nov., E. rex, and E. serratulus sp. nov. inhabit subtidal marine waters. Although it remains to be open to question whether adaptation to an estuarine environment occurred once or several times within the group, the discovering of E. regina sp. nov. and E. serratulus sp. nov. suggests inhabiting not estuarine but subtidal marine waters is not anomaly in the E. coulli-group.
Fig. 13.
Fig. 13. World map showing the locations of records of species in the Echinoderes coulli-group.
CONCLUSIONS
Two new species of Echinoderes are described from Vietnam, Southeast Asia. These are the first named kinorhynchs reported from Vietnamese waters. The two species are the eleventh and twelfth species described in the Echinoderes coulli-group.
Acknowledgments
Acknowledgments: This work and the new subspecies name have been registered with ZooBank under urn:lsid:zoobank.org:pub: 6DA5A911-09FD-4376-A09C-68DF8F71A669. I thank Dr. Le Doan Dung and the Research Institute for Marine Fisheries (RIMF) for permitting and assistance in collecting kinorhynchs in Vietnam; Dr. Akira Tsukagoshi (Shizuoka Univ.), Dr. Hiroshi Kajihara (Hokkaido Univ.), Dr. Toshihiko Fujita (National Museum of Nature and Science), Dr. Yuriko Nakao (Nihon Univ.), and Dr. Hayato Tanaka for helping collect kinorhynchs; Dr. Matthew H. Dick (Hokkaido Univ.) for editing my English; and three anonymous reviewers for peer reviews. This study was supported by a KAKENHI Grant (No. 26304011) from the Japan Society for the Promotion of Science.
References
- Adrianov AV, Malakhov VV. 1999. Cephalorhyncha of the World Ocean. KMK, Moscow.
- Brown R. 1985. Developmental and taxonomic studies of Sydney Harbor Kinorhyncha. PhD dissertation, Macquarie University.
- Claparède E. 1863. Beobachtungen über Anatomie und Entwicklungsgeschichte wirbelloser Tiere an der Küste der Normandie angestellt. Wilhelm Englemann, Leipzig.
- Fleeger JW, Carman KR, Riggio MR, Mendelsshohn IA, Lin QX, Hou A, Deis DR, Zengel S. 2015. Recovery of salt marsh benthic microalgae and meiofauna following the Deepwater Horizon oil spill linked to recovery of Spartina alterniflora. Mar Ecol Prog Ser 536:39-54. doi:10.3354/ meps11451.
- Herranz M, Leander BS. 2016. Redescription of Echinoderes ohtsukai Yamasaki and Kajihara, 2012 and E. kozloffi Higgins, 1977 from the northeastern Pacific coast, including the first report of a potential invasive species of kinorhynch. Zool Anz (in press). doi:10.1016/ j.jcz.2016.02.004.
- Herranz M, Sánchez N, Pardos F, Higgins RP. 2014. New Kinorhyncha from Florida coastal waters. Helgol Mar Res 68:59-87. doi:10.1007/s10152-013-0369-9.
- Higgins RP. 1977. Two new species of Echinoderes (Kinorhyncha) from South Carolina. Transact Am Microsc Soc 96:340-354. doi:10.2307/3225864.
- Higgins RP. 1988. Kinorhyncha. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington.
- Lundbye H, Rho HS, Sørensen MV. 2011. Echinoderes rex n. sp. (Kinorhyncha: Cyclorhagida), the largest Echinoderes species found so far. Sci Mar 75:41-51. doi:10.3989/ scimar.2011.75n1041.
- Mokievsky VO, Tchesunov AV, Udalov AA, Toan ND. 2011. Quantitative distribution of meiobenthos and the structure of the free-living nematode community of the mangrove intertidal zone in Nha Trang Bay (Vietnam) in the South China Sea. Russ J Mar Biol 37:272-283. doi:10.1134/ S1063074011040109.
- Neuhaus B. 2013. Kinorhyncha (= Echinodera). In: Schmidt- Rhaesa A (ed) Handbook of Zoology. Gastrotricha, Cycloneuralia and Gnathifera. Volume 1: Nematomorpha, Priapulida, Kinorhyncha, Loricifera. De Gruyter, Berlin.
- Neuhaus B, Higgins RP. 2002. Ultrastructure, biology and phylogenetic relationships of Kinorhyncha. Integr Comp Biol 42:619-632. doi:10.1093/icb/42.3.619. [DOI] [PubMed]
- Ngo XQ, Smol N, Vanreusel A. 2013. The meiofauna distribution in correlation with environmental characteristics in 5 Mekong estuaries, Vietnam. Cah Biol Mar 54:71-83.
- Omer-Cooper J. 1957. Deux nouvelle especes de Kinorhyncha en provenance de L’Afrique du Sud. Bulletin Mensuel de la Société Linnéenne de Lyon 26:213-216.
- Ostmann A, Nordhaus I, Sørensen MV. 2012. First recording of kinorhynchs from Java, with the description of a new brackish water species from a mangrove-fringed lagoon. Mar Biodiv 42:79-91. doi:10.1007/s12526-011-0094-z.
- Sørensen MV. 2013. Phylum Kinorhyncha. Zootaxa 3703:63- 66. doi:10.11646/zootaxa.3703.1.13.
- Sørensen MV. 2014. First account of echinoderid kinorhynchs from Brazil, with the description of three new species. Mar Biodiv 44:251-274. doi:10.1007/s12526-013-0181-4.
- Sørensen MV, Dal-Zotto M, Rho HS, Herranz M, Sánchez N, Pardos F, Yamasaki H. 2015. Towards a phylogeny of Kinorhyncha, based on morphology and two molecular loci. PLOS ONE 10:e0133440. doi:10.1371/journal. pone.0133440. [DOI] [PMC free article] [PubMed]
- Sørensen MV, Gąsiorowski L, Randsø PV, Sánchez N, Neves RC. 2016. First report of kinorhynchs from Singapore, with the description of three new species. Raffles Bull Zool 64:3-27.
- Sørensen MV, Landers SC. 2014. Two new species of Echinoderes (Kinorhyncha: Cyclorhagida) from the Gulf of Mexico. Front Mar Sci 1:8. doi:10.3389/fmars.2014.00008.
- Sørensen MV, Pardos F. 2008. Kinorhynch systematics and biology - an introduction to the study of kinorhynchs, inclusive identification keys to the genera. Meiofauna Marina 16:21-73.
- Sørensen MV, Rho HS, Min W, Kim D, Chang CY. 2012. An exploration of Echinoderes (Kinorhyncha: Cyclorhagida) in Korean and neighboring waters, with the description of four new species and a redescription of E. tchefouensis Lou, 1934. Zootaxa 3368:161-196.
- Yamasaki H, Fujimoto S. 2014. Two new species in the Echinoderes coulli group (Echinoderidae, Cyclorhagida, Kinorhyncha) from the Ryukyu Islands, Japan. ZooKeys 382:27-52. doi:10.3897/zookeys.382.6761. [DOI] [PMC free article] [PubMed]
- Yamasaki H, Kajihara H. 2012. A new brackish-water species of Echinoderes (Kinorhyncha: Cyclorhagida) from the Seto Inland Sea, Japan. Species Diversity 17:109-118. doi:10.12782/sd.17.1.109.
- Zelinka C. 1894. Über die Organisation von Echinoderes. Verhandlungen der Deutschen Zoologischen Gesellschaft. 4:46-49.
- Zelinka C. 1896. Demonstration von Tafeln der Echinoderes- Monographie. Ver Dtsc Gesellsch 6:197-199.