Abstract
Jen-Chieh Shiao, Chien-Yu Chen, Jie Zhang, and Yoshiyuki Iizuka (2016) Salangid icefish (Salangidae) are commercially important species and are widely distributed in lakes, rivers, estuaries, and coastal area of Asia. This study examined their habitat use and migratory patterns by analyzing otolith microstructure and Sr/ Ca ratios. Neosalanx tangkahkeii and Protosalanx chinesis collected in the isolated freshwater Taihu Lake in China showed consistently low otolith Sr/Ca ratios (< 5.0 × 10-3, mean + 2 SD), which were used to represent the freshwater residence. Another batch of P. chinesis collected from the Yangtze River estuary of China also showed low otolith Sr/Ca ratios (< 5.5 × 10-3, mean + 2 SD) throughout the life history, suggesting that these fish only use freshwater environments. A group of N. tangkahkeii collected in the Pearl River estuary of China showed otolith Sr/Ca ratios between 10.0 × 10-3 and 30.0 × 10-3, indicating habitat shifts between brackish and marine environments. Salanx ariakenesis collected in the Yangtze River estuary showed variable and higher otolith Sr/Ca ratios between 1.6 × 10-3 and 36.5 × 10-3, exhibiting the diverse migratory patterns between the river and the sea with the habitat shifts occurring at the juvenile, young, and adult stages. Neosalanx anderssoni collected in the Bohai Sea, China only used marine habitats based on their consistently high otolith Sr/Ca ratios with the mean values of each fish varying between 20.7 × 10-3 and 24.6 × 10-3. The habitat use by the icefish may differ within and among species. Different migratory patterns can coexist in the same species e.g., S. ariakenesis. The euryhaline icefish, even those living in the estuary or coastal water, do not necessarily migrate between the sea and rivers, suggesting their high plasticity of habitat use and facultative anadromous behaviors
Keywords: Otolith, Sr/Ca ratios, Icefish, Life history, Migration
BACKGROUND
Icefish belong to the family Salangidae and comprise five genera and 18 species (Zhang and Qiao 1994; Zhang et al. 2007). Salangid icefish are believed to have originated from the inshore areas of the East China Sea and have adapted to very different environments through multiple unrelated founding events (Zhao et al. 2011). The icefish are endemic to eastern Asia and are widely distributed in lakes, rivers, estuaries and coastal areas of the northwest Pacific (Sokolovskaya et al. 1998; Nakabo 2002). The icefish feed mainly on zooplankton and have a life span of about one year (Liu 2001). All the icefish are small-sized, transparent and high valued fish. The biomass of some icefish species has seriously declined in recent years due to the destruction of spawning grounds, over-fishing, water pollution, and habitat fragmentation (Wang et al. 2005; Kang et al. 2013).
Studying the habitat use and migratory life history of the fishes can provide useful knowledge for the effective management and conservation of these important fishery species. However, it is not possible to trace the migration of the icefish by the conventional tagging due to their fragile and small body, usually less than 10 cm. Therefore, reports for freshwater residence or anadromous migration of the icefish are usually based on field observation (Chen 1956; Sun 1990). Alternatively, Xie et al. (2001) reported the migration of juvenile Ariake icefish (Salanx ariakensis) from Yalujiang estuary, Liaoning Province to the Yellow Sea for overwintering based on few samples analyzed for the otolith Sr/Ca ratio. Yamaguchi et al. (2004) applied the same approach of otolith chemical analysis on a large sample size of Salangichthys microdon and further revealed wide habitat use and diverse migratory patterns of this species in Japan.
Otoliths are biomineral structures inside the inner ear of teleost fish, and are part of the balance and auditory systems. The periodic increments in the otolith provide a means of determining age, and incremental width changes can be associated with important life history events such as hatching in different seasons (Wu et al. 2011) and metamorphosis (e.g., Campana 2005). Otolith elemental compositions such as Sr/Ca ratio naturally mark periods of freshwater (i.e., low Sr/Ca ratio) and seawater (i.e., high Sr/Ca ratio) residence (Gillanders 2005; Elsdon et al. 2008) and have been used to reveal the occupancy pattern of many diadromous species including the catadromous eel (Shiao et al. 2006), anadromous grenadier anchovy (Coilia mystus) in the Yangtze River estuary (Yang et al. 2006) and the grey mullet (Chang and Iizuka 2012).
For the icefish living in the coastal areas and estuaries, their habitat use and migratory patterns remain unclear due to difficulties in species identification and limitations with current methods of field observation and catch analysis. Furthermore, sympatry is very common in salangid icefish belonging to different genera (Chen 1956; Takita 1995). Whether the sympatric species have different microhabitat preferences (e.g., salinity tolerance) or temporal shifts when using coastal areas and estuaries, remain unknown. The wide distributions of the icefish across diverse environments suggest phenotypic plasticity in habitat use of the same species. Therefore, this study aims to clarify the habitat use and migratory life history of icefish belonging to three genera and four species, including Neosalanx anderssoni, N. tangkahkeii, Salanx ariakensis and Protosalanx chinensis. The results of this study may reveal the ambiguous parts of icefish autecology and will also provide further insight into the habitat preferences of closely related icefish species in the estuary and costal waters.
MATERIALS AND METHODS
Fish collection and sampling areas
Although icefish resource has been heavily exploited, the four species in the present study are not on any IUCN Red List or Chinese Red List. These species are high valued fish, being harvested only by fyke nets with small mesh sizes, and such commercial catching of icefish has a long history in China. Icefish are small-sized with fragile skin and very vulnerable to low oxygen. Thus, it is very difficult to bring individuals alive to the lab after being collected. All the samples were purchased from the local fishermen. Neosalanx anderssoni were collected in the Bohai Sea near Qinhuangdao Port, Hebei Province (Fig. 1), where seawater salinity fluctuates between 30.1 to 31.5 (mean = 31.0). Salanx ariakensis and Protosalanx chinensis were collected in the Yangtze River estuary (Shanghai City) from freshwater to brackish environments (salinity: 0.01- 9.7). Neosalanx tangkahkeii were collected in the Pearl River estuary (Guangdong Province), which is a brackish to marine environment (salinity: 13.2- 25.6, mean = 19.4) (Wong et al. 1995). Additional batches of N. tangkahkeii and P. chinensis were collected in the freshwater Taihu Lake (Jiangsu Province, salinity: < 1, Ni and Zhu 2005). Since Taihu Lake is isolated from both the Yangtze River and the East China Sea, the icefish collected from this lake only record the freshwater signature in the otolith Sr/Ca ratios. Sampling information is summarized in Table 1. Species were identified following Zhang and Qiao (1994) and Zhang et al. (2007). In order to ensure accurate species identification for the neotenic family, morphology- based species identification was further confirmed by DNA barcodes (Zhang et al. 2007).
Fig. 1.
Fig. 1. Approximate sampling locations (indicated by the arrows) of the icefish in the Bohai Sea, the Yangtze River estuary, Taihu Lake and the Pearl River estuary.
Table 1. Biological and sampling information of the icefishes used for otolith Sr/Ca ratio analysis.
| Species (code) | Sampling site | Date | Standard length (cm) | Mean (range) otolith Sr/Ca ratios |
| Neosalanx anderssoni (NA1) | Bohai Sea | Spring 2004 | 8.6 ± 0.6 | 22.6 ± 3.9 × 10-3 |
| (12-32 × 10-3, N = 9) | ||||
| Neosalanx tangkahkeii (NT5) | Taihu Lake | 15 October, 2009 | 5.7 ± 0.6 | 3.0 ± 1.0 × 10-3 |
| (0-6 × 10-3, N = 8) | ||||
| Neosalanx tangkahkeii (NT3) | Pearl River estuary | 1 July, 2004 | 4.4 ± 0.2 | 19.5 ± 1.2 × 10-3 |
| (10-29 × 10-3, N = 10) | ||||
| Protosalanx chinensis (PC1) | Taihu Lake | 31 March, 2005 | 9.9 ± 0.4 | 3.4 ± 0.2 × 10-3 |
| (0-7 × 10-3, N = 8) | ||||
| Protosalanx chinensis (PC2) | Yangtze River estuary | Autumn, 2006 | 10.0 ± 0.4 | 3.3 ± 1.1 × 10-3 |
| (0-6 ×10-3, N = 8) | ||||
| Salanx ariakensis (SA1) | Yangtze River estuary | 9 August, 2006 | 12.3 ± 0.7 | 19.9 ± 5.5 × 10-3 |
| (7-32 ×10-3, N = 10) | ||||
| Salanx ariakensis (SA2) | Yangtze River estuary | 28 September, 2006 | 12.8 ± 1.9 | 19.7 ± 6.0× 10-3 |
| (2-32 ×10-3, N = 8) |
Otolith preparation and Sr/Ca analysis
Sagittal otoliths were extracted from the fish under a stereo microscope, cleaned, dried and embedded in Epofix resin (Struers, Demark) after measuring the total length and weight of the fish. The embedded otoliths were then mounted on a slide and polished along the sagittal plane by a Buehler Metaserv grinder-polisher (Buehler, USA) at a speed of 300 rpm with wet-polishing paper of 2,400-grit. The polished otoliths were periodically checked under a compound light microscope (BX- 51 Olympus, Japan). When the core was revealed on the surface, final polishing was done with a wet-polishing cloth and 0.05 μm alumina powder (Buehler, USA) to smooth the otolith surface. The otoliths were coated with a layer of carbon (Q150T E, Quorum Technologies Ltd., UK) to increase the electron conductance when analyzed by the electron probe microanalyzer (EPMA, JEOL JXA- 8900R, JEOL, Japan).
Secondary and backscattered electron images were used to guide the analysis on target positions located along the longest axis from the primordium to the edge at 10 μm intervals. Beam conditions were 15 kV for the acceleration voltage and 3 nA for the current, with a 5 × 4 μm rectangular scanning beam size. The wavelength dispersive spectrum at the Sr Lα peak position was measured for 80 s and each of the upper and lower baselines for 20 s. The peak concentration of Ca Kα was measured for 20 s and each of the upper and lower baselines for 10 s. Synthesized aragonite (CaCO3) and strontianite [(Sr0.95Ca0.05) CO3; NMNH R10065] were used as standards to calibrate the concentration of Ca and Sr in the icefish otoliths, respectively. The Sr/Ca ratio was calculated after a correction using the PRZ (phi- rho-z) method (Goldstein et al. 1992; Reed 1997). Analytical errors were smaller than 0.05 wt% in Sr (Iizuka 2012).
Age determination
After microchemical analysis, the otoliths were polished to remove the carbon coating and etched with 0.1 M HCl for 15 seconds to reveal the growth increment (Fig. 2) for age determination under a compound light microscope equipped with a digital camera (DP-71, Olympus, Japan). A daily growth increment was validated for the icefish P. hyalocranius (Fu et al. 1997) and examined for N. taihuensis (Wu et al. 2011). The otolith growth increment was assumed deposited daily for the icefish examined in this study.
Fig. 2.

Fig. 2. Otolith of the icefish (Neosalanx anderssoni) collected in Qinhuangdao (Bohai Sea) showing the daily growth increments and electron microprobe transect from the core to the edge for measuring Sr/Ca ratios. Arrows point out the rectangular beam marks after the analysis by the eletron microprobe.
RESULTS
Neosalanx tangkahkeii (NT5) and Protosalanx chinensis (PC1) collected from the freshwater Taihu Lake showed consistently low Sr/Ca ratios from the otolith core to the edge (Figs. 3a, b), with the mean values of each fish varying from 2.8 × 10-3 (NT5-8) to 3.6 × 10-3 (PC1-3). The mean values (± 1 standard deviation) for all NT5 and PC1 icefish were 3.0 ± 1.0 × 10-3 (NT5-8) and 3.3 ± 1.1 × 10-3, respectively. The otolith Sr/Ca profiles of these icefish from the Taihu Lake were regarded as typical freshwater residence.
Fig. 3.
Fig. 3. Consistently low otolith Sr/Ca ratios of the icefish, Neosalanx tangkahkeii (a) and, Protosalanx chinensis (b) collected from Taihu Lake and Protosalanx chinensis (c) collected from the Yangtze River estuary.
Another group of P. chinensis (PC2) collected from the Yangtze River estuary also showed low otolith Sr/Ca ratios throughout their life, with the mean values for each individual varying from 3.1 × 10-3 (PC2-4) to 3.5 × 10-3 (PC2-9) (Fig. 3c). These profiles were consistent with icefish collected from the Taihu Lake, suggesting that these fish resided all their life in the freshwater environment of the Yangtze River.
Another 10 fish of N. tangkahkeii (NT3) collected from the Pearl River estuary demon- strated high otolith Sr/Ca ratios with data varying between 10.0 × 10-3 to 30.0 × 10-3 (Fig. 4). The otolith Sr/Ca ratios before 60 μm from the core differed among the fish with large variations from 10.0 × 10-3 to 25.0 × 10-3. After that, the values gradually increased to the highest values near or at the otolith edge for all individuals. The mean otolith Sr/Ca ratios of each individual varied from 17.6 × 10-3 to 21.2 × 10-3.
Fig. 4.

Fig. 4. Icefish (Neosalanx tangkahkeii) collected from the Pearl River estuary display consistently high otolith Sr/Ca ratios, indicating brackish and marine residence for these 10 fish.
N. anderssoni (NA1) collected from Bohai Sea (Fig. 5) also showed high Sr/Ca ratios from the core to the edge of the otolith with mean values of each fish varying from 20.7 × 10-3 to 24.6 × 10-3. Before 80 μm from the core, the values varied from 10 × 10-3 to 27 × 10-3 among the nine fish, without a consensus pattern. The values then increased and reached a plateau between 150-200 μm for all individuals, followed by a decreasing trend to the otolith edge.
Fig. 5.

Fig. 5. Icefish (Neosalanx anderssoni) collected from Qinhuangdao (Bohai Sea) display variably high otolith Sr/Ca ratios, indicating marine residence for the fish.
S. ariakensis (SA1 and SA2) collected from the Yangtze River estuary showed different ontogenetic patterns of otolith Sr/Ca ratios among the 18 fish (Fig. 6), with a minimal value of 1.6 × 10-3 (SA2-4) to a maximal value of 36.5 × 10-3 (SA1-7). SA2-4 had the lowest otolith Sr/ Ca ratios (< 3 × 10-3) before 30 μm from the core suggesting that the fish hatched and resided in freshwater during its early life stage. The values then dramatically increased to a peak of 33.6 × 10-3 at 120 μm, followed by a decrease to a level between 15 × 10-3 to 26 × 10-3, suggesting migration to seawater and remaining in the high saline environments for the remainder of its life. SA1-1, SA1-5 and SA2-3 also had relative low otolith Sr/Ca ratios from approximately 8.0 × 10-3 to 10.5 × 10-3 near the core, with increasing values to > 30.0 × 10-3 around 90-120 μm (Fig. 6a). These Sr/Ca ratio profiles suggested that SA2-4, SA1- 1, SA1-5 and SA2-3 were hatched and resided in the freshwater or brackish water during the first five to ten days post hatching before these fish were transported to seawater environments for the remaining life. Another 5 fish (SA1-2, SA1- 7, SA2-6, SA2-7, SA2-10) showed migration from the sea to the river during the adult stage based on a declining Sr/Ca ratios from > 20.0 × 10-3 to around 6.0 × 10-3 to 9.0 × 10-3 near the otolith edge (Fig. 6b). The remaining individuals have otolith Sr/Ca ratios > 12 × 10-3 from the core to the edge suggesting whole-life residence in the ocean (Fig. 6c).
Fig. 6.
Fig. 6. Icefish (Salanx ariakensis) collected from the Yangtze River estuary show diverse otolith Sr/Ca profiles that represent whole-life residence in the sea (a), the movement from the river to the sea at during juvenile stage (b) and the movement from the sea to the river at adult stage (c).
DISCUSSION
Reliability of otolith Sr/Ca ratios in interpreting habitat use of icefish
Otolith Sr/Ca ratio is positively correlated with Sr concentration in the water and salinity (Campana 1999; Bath et al. 2000) and more than 80% of the otolith Sr content is derived from the surrounding water for both freshwater and marine species (Farrell and Campana 1996; Walther and Thorrold 2006). Fish living in Sr-poor fresh water usually show consistent low levels of otolith Sr/Ca ratios (e.g., Shiao et al. 2006; Lamson et al. 2006; Thibault et al. 2007, 2010) while fish living in Sr- rich fresh water may have similar or even higher values than the otolith Sr/Ca ratios of marine fishes (Kraus and Secor 2004). In this study, we used the habitat discrimination critical value method (e.g., Jessop et al. 2012) to determine a threshold value between freshwater and brackish/seawater residences of the fish although other method e.g., linear discriminant analysis can also be used (Jessop et al. 2013).
N. tangkahkeii and P. chinensis living in the freshwater Taihu Lake only record freshwater signature in the otolith Sr/Ca ratios. The mean Sr/Ca ratios of N. tangkahkeii and P. chinensis plus 2 standard deviations were 5.0 × 10-3 and 5.5 × 10-3, respectively and these values were used as a threshold between freshwater and brackish waters in this study. This result agrees with previous studies and supports the hypothesis proposed by Chen (1956) that N. tangkahkeii living in the Taihu Lake only use freshwater habitats and is thus a landlocked population. Geological studies suggest that the Taihu Lake was a bay of the East China Sea during the Holocene and was gradually sealed into a freshwater lake by the growing deltas of the Yangtze and Qiantang rivers (Wu et al. 2012). Xie et al. (2001) did not report the criteria used to define the freshwater and brackish/seawater residence of S. ariakensis. However, the comparable values of otolith Sr/Ca ratios between 6.8 × 10-3 and 7.7 × 10-3 (Arai et al. 2003) or 4.7 × 10-3 (Yamaguchi et al. 2004) were used to discriminate freshwater from brackish/ seawater residence of the icefish Salangichthys microdon. The asymptotic relationship between otolith Sr/Ca ratios and salinity makes it difficult to define the boundary between brackish and seawater residence of the icefish by otolith Sr/Ca ratios as found in other species (e.g., Tabouret et al. 2010). It has also been reported that variations of otolith Sr/Ca ratios of marine fish cannot be fully explained by Sr concentration in the sea water (Elsdon et al. 2008; Brown and Severin 2009). Fish living in a brackish estuary might have similar or higher otolith Sr/Ca ratios than the values in the marine fish otoliths (Tabouret et al. 2010). Simultaneous use of Sr/Ca and Ba/Ca ratios may be better at distinguishing brackish and marine residence of the fish (Tabouret et al. 2010; Feutry et al. 2011). Nevertheless, otolith Sr/Ca ratios by themselves remain useful for the detection of habitat shifts between freshwater and brackish/sea waters of the icefish.
Anomaly of high Sr/Ca ratios in the otoliths of icefish
It is worth noting that the highest values of icefish otolith Sr/Ca ratios are larger than the values reported for most other species. Otolith Sr/Ca ratios can reach more than 20.0 × 10-3 in P. chinensis and 30.0 × 10-3 to 37.0 × 10-3 in N. anderssoni, S. ariakensis and N. tangkahkeii. Otolith Sr/Ca ratios as high as 25.0 × 10-3 to 30.0 × 10-3 have been reported in the icefish Salangichthys microdon (Arai et al. 2003; Yamaguchi et al. 2004). Such high Sr/Ca ratios are only found in stout eelblenny (Anisarchus medius, Sr/Ca ratio = 30.0 × 10-3) and anadromous whitefish (Coregonus nasus, Sr/Ca ratio = 48.0 × 10-3) (Brown and Severin 2009). Low growth rates caused by low temperature might enhance Sr incorporation into otoliths (Sadovy and Severin 1994) and could explain the high otolith Sr/Ca ratios of A. medius and C. nasus, which distributed in the Arctic zones. Anguilliformes leptocephali also had otolith Sr/Ca ratios as high as 20.0 × 10-3, which corresponded to the lowest growth rate of the otoliths (Shiao et al. 2002). However, the highest Sr/Ca ratios did not correspond to the lowest growth rate of the icefish otolith. Therefore, other factors that might influence Sr incorporation into the otoliths of the icefish shall be considered. Icefish have transparent bodies and lack any scales. Their skeletons are not fully ossified and consist largely of cartilage which is believed to be neotenic i.e., retaining some larval features during the adult stage. Possibly, the body surface of icefish has a weaker regulatory ability for some elements e.g., Sr in ambient water, and is more permeable for Sr entering the fish’s tissues, which is consequently deposited in the otolith with high concentrations. The underlying biological mechanism for the high otolith Sr/Ca ratios in the icefish desires further investigation and is beyond the scope of this study.
Habitat use and migratory life history of icefish
Icefish are found in the freshwater and coastal waters with a few species showing a diadromous life pattern (Chen 1956; Sun 1990). According to the otolith Sr/Ca ratio profiles, P. chinensis collected from the Taihu Lake and the Yangtze River estuary only live in freshwater environments. Diadromous migration and habitat shift were found in other species. N. tangkahkeii can complete the life cycle in freshwater environments such as the Taihu Lake or shift habitats across different salinity ranges in the Pearl River estuary. These results suggest that N. tangkahkeii have high plasticity and flexibility for adapting to different saline environments. Owing to this capability, N. tangkahkeii from the Taihu Lake have been transplanted into reservoirs and lakes in most areas of China since 1979 to enhance fish production (Kang et al. 2013).
S. ariakensis is traditionally considered to be an anadromous species (Sun 1990; Xie et al. 2001). We only found one S. ariakensis (SA2-4) that was hatched in the freshwater environment in the Yangtze River estuary then the fish was transported passively by the downstream current or along shore current in the estuary to the sea for feeding and growth. Although the migratory pattern of SA2-4 could be considered as anadromous behavior, SA2-4 only stayed very short time (< 10 days) in the fresh water during the larval to early juvenile stages. This result suggested that anadromous S. ariakensis spawned near the upper estuary, which can facilitate the young fish quickly dispersing into the sea. Otolith Sr/Ca ratios suggest that most S. ariakensis live in sea waters from birth until capture at the adult stage and some of them e.g., SA2-3, SA2-6, SA2-7 and SA2- 10 may return to the estuary during their young to adult stages. Therefore, this study suggests that anadromous life history is not obligatory but facultative for S. ariakensis. Different migratory life histories of S. ariakensis were consistent with their wide distribution in the Yangtze River estuary of Shanghai City and neighboring marine waters with salinities between 0.01-20 (Sun 1990).
N. anderssoni is widely distributed in coastal waters from Yalujiang River to the Yangtze River estuary (Zhang 1987). Otolith Sr/Ca ratios analysis indicate no freshwater or diadromous migrations for N. anderssoni although there are several rivers connecting to the coastal waters around Qinhuangdao Port. Therefore, N. anderssoni, unlike other icefish species, may be restricted to seawater environments and have not evolved freshwater and diadromous contingents.
CONCLUSIONS
Our results highlight the difference in habitat use among four icefish species. We also confirmed the hypothesis of phenotypic plasticity in the habitat use of the icefish. The habitat use and different migratory patterns of the icefish species are depicted in figure 7. The euryhaline icefish living in the estuary or coastal water do not necessarily undergo migratory life history between the rivers and the ocean. In addition, icefish of different migratory histories coexist in the estuary or coastal waters. These phenomena indicate that the habitat selection of icefish during the growth phase may be opportunistic but not obligatory since they can survive in different salinity environments.
Fig. 7.
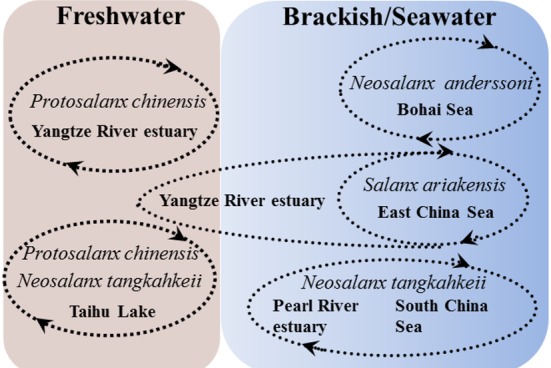
Fig. 7. A diagram shows the habitat use and migratory life history of the icefish species reconstructed from their otolith Sr/ Ca profiles.
Acknowledgments
Acknowledgments: We are grateful to XL Chen, SG Fan and JH Gu for their kind help in sample collections and, to BM Jessop for useful comments. This study was financially supported by the Chinese Academy of Sciences (KSCX2-EW-J-2) and the Ministry of Science and Technology of Taiwan (MOST 103-2611-M-002 -001). JZ collected the fish samples. CY extracted the otoliths from the fish and prepared the otoliths for Sr/Ca ratio analysis. YY carried out the analysis of otolith Sr/Ca ratio. JC drafted the manuscript. All authors read and approved the final manuscript.
Abbreviations
- IUCN
International Union for Conservation of Nature and Natural Resources
- Sr/Ca
Strontium/ Calcium
References
- Arai T, Hayano H, Asami H, Miyazaki N. 2003. Coexistence of anadromous and lacustrine life histories of the shirauo, Salangichthys microdon. Fish Oceanogr 12(2):134-139.
- Bath GE, Thorrold SR, Jones CM, Campana SE, Mclaren JW, Lam JWH. 2000. Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim Cosmochim Ac 64:1705-1714.
- Brown RJ, Severin KP. 2009. Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Can J Fish Aquat Sci 66:1790-1808.
- Campana SE. 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263-297.
- Campana SE. 2005. Otolith science entering the 21st century. Mar Freshw Res 56:485-495.
- Chang CW, Iizuka Y. 2012. Estuarine use and movement patterns of seven sympatric Mugilidae fishes: The Tatu Creek estuary, central western Taiwan. Estuar Coast Shelf S 106:121-126.
- Chen NS. 1956. On the salangid fishes of Lake Taihu. Acta Hydrobiol Sin 2:324-335.
- Elsdon TS, Wells BK, Campana SE, Gillander BM, Jones CM, Limburg KE, Secor DH, Thorrold SR, Walther BD. 2008. Otolith chemistry to describe movements and life- history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanogr Mar Biol 46:297- 330.
- Farrell J, Campana SE. 1996. Regulation of calcium and strontium deposition on the otoliths of juvenile tilapia, Oreochromis niloticus. Comp Biochem Physiol A 115:103- 109.
- Feutry P, Keith P, Pecheyran C, Claverie F, Robinet T. 2011. Evidence of diadromy in the French Polynesian Kuhlia malo (Teleostei: Percoidei) inferred from otolith microchemistry analysis. Ecol Freshw Fish 20:636-645.
- Fu LJ, Xie YH, Li B, Zhao HC. 1997. Study on daily-growth increment of otolith and the growth of larvae icefish. J Fish Sci China 4:21-27.
- Gillanders BM. 2005. Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuar Coast Shelf S 64:47-57.
- Goldstein JI, Newbury DE, Echlin P, Joy DC, Fiori C. 1992. Scanning Electron Microscopy and X-ray Microanalysis-A Text for Biologists, Materials Scientists, and Geologists. New York, Plenum Press.
- Iizuka Y. 2012. Electron microprobe study of otolith: migratory behavior and habitat of three major temperate species of eels. JEOL News 47(1):33-50.
- Jessop BM, Shiao JC, Iizuka Y. 2013. Methods for estimating a critical value for determining the freshwater/estuarine habitat residence of American eels from otolith Sr:Ca data. Estuar Coast Shelf S 133:293-303.
- Jessop BM, Wang CH, Tzeng WN, You CF, Shiao JC, Lin SH. 2012. Otolith Sr:Ca and Ba:Ca may give inconsistent indications of estuarine habitat use for American eels (Anguilla rostrata). Environ Biol Fish 93:193-207.
- Kang B, Deng JM, Wang ZM, Zhang J. 2013. Transplantation of icefish (salangidae) in China: glory or disaster? Rev Aquac 5:1-15.
- Kraus RT, Secor DH. 2004. Incorporation of strontium into otoliths of an estuarine fish. J Exp Mar Biol Ecol 302:85- 106.
- Lamson HM, Shiao JC, Iizuka Y, Tzeng WN, Cairns DK. 2006. Movement patterns of American eels (Anguilla rostrata) between salt and fresh water in a small coastal watershed, based on otolith microchemistry. Mar Biol 149:1567-1576.
- Liu ZW. 2001. Diet of the zooplanktivorous icefish Neosalanx pseudotaihuensis Zhang. Hydrobiologia 459:51-56.
- Nakabo T. 2002. Fishes of Japan with pictorial keys to the species. Tokyo, Tokai University Press.
- Ni Y, Zhu C. 2005. Fishes of the Taihu Lake. Shanghai, Shanghai Scientific and Technical Publishers.
- Reed SJB. 1997. Electron microprobe analysis. Cambridge, Cambridge University Press.
- Shiao JC, Ložys L, Iizuka Y, Tzeng WN. 2006. Migratory patterns and contribution of stocking to the population of European eels Anguilla anguilla by otolith Sr:Ca ratio analysis. J Fish Biol 69:749-769.
- Shiao JC, Tzeng WN, Collins A, Iizuka Y. 2002. Role of marine larval duration and growth rate of glass eels in determining the distribution of Anguilla reinhardtii and A. australis on Australian eastern coasts. Mar Freshwater Res 53:687-695.
- Sadovy Y, Severin KP. 1994. Elemental patterns in red hind (Epinephelus guttatus) otoliths from Bermuda and Puerto Rico reflect growth rate, not temperature. Can J Fish Aquat Sci 51:133-141.
- Sokolovskaya TG, Sokolovsky AS, Sobolevsky EI. 1998. A list of fishes of the Peter the Great Bay (the Sea of Japan). J Ichthyol 38:5-15.
- Sun G. 1990. Salanx ariakensis in Yangtze River estuary and the neighbouring marine water. Trans Oceanol Limnol 1:41-46.
- Tabouret H, Bareille G, Claverie F, Pecheyran C, Prouzet P, Donard OFX. 2010. Simultaneous use of strontium: calcium and barium:calcium ratios in otoliths as markers of habitat: application to the European eel (Anguilla anguilla) in the Adour basin, South West France. Mar Environ Res 70:35-45. [DOI] [PubMed]
- Takita T. 1995. Fishes of Ariake Sound. Mar Sci 12:105-115.
- Thibault I, Dodson JJ, Caron F, Tzeng WN, Iizuka Y, Shiao JC. 2007. Facultative catadromy in American eels: testing the conditional strategy hypothesis. Mar Ecol Prog Ser 344:219-229.
- Thibault I, Dodson JJ, Hedger RD, Shiao JC, Iizuka Y, Tzeng WN. 2010. Anadromy and the dispersal of an invasive fish species (Oncorhynchus mykiss) in Eastern Quebec, as revealed by otolith microchemistry. Ecol Freshw Fish 19:348-360.
- Walther BD, Thorrold SR. 2006. Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Mar Ecol Prog Ser 311:125-130.
- Wang ZS, Lu C, Hu HJ, Zhou Y, Xu CR, Lei GC. 2005. Freshwater icefishes (Salangidae) in the Yangtze River basin of China: Spatial distribution patterns and environmental determinants. Environ Biol Fish 73:253- 262.
- Wong CK, Chu KH, Chen QC, Ma XL. 1995. Environmental Research in Pearl River and Coastal Areas. Guangdong, Guangdong Higher Education Press.
- Wu L, Li F, Zhu C, Li L, Li B. 2012. Holocene environmental change and archaeology, Yangtze River Valley, China: review and prospects. Geosci Front 3(6):875-892.
- Wu L, Liu JS, Wang XL, Zhang G, Zhang ZY, Murphy BR, Xie SG. 2011. Identification of individuals born in different spawning seasons using otolith microstructure to reveal life history of Neosalanx taihuensis. Fish Sci 77:321-327.
- Xie YH, Tang ZP, Xie H, Li B, Zhang SD, Yu F. 2001. Microstructure and microchemistry in otolith of ariake icefish (Salanx ariakensis). Acta Zool Sin 47(2):215-220.
- Yamaguchi M, Katayama S, Omori M. 2004. Migration pattern of shirauo Salangichthys microdon Bleeker, in the Ishikari River system and adjacent nearshore sea area, Japan, as estimated by otolith microchemistry analysis. Fish Sci 70:546-552.
- Yang J, Arai T, Liu H, Miyazaki N. 2006. Environmental signature in the otolith elemental fingerprint of the tapertail anchovy, Coilia mystus, from the Changjiang estuary, China. J Appl Ichthyol 22:459-462.
- Zhang J, Li M, Xu MQ, Takita T, Wei FW. 2007. Molecular phylogeny of icefish Salangidae based on complete mtDNA cytochrome b sequences, with comments on estuarine fish evolution. Biol J Linn Soc 91:325-340.
- Zhang YL. 1987. A taxonomic study on the Chinese icefishes of the genus Neosalanx (Pisces: Salangidae), with description of a new species from the lake Taihu. Zool Res 8(3):277-286.
- Zhang YL, Qiao XG. 1994. Study on phylogeny and zoogeography of fishes of the family Salangidae. Acta Zool Taiwan 5:95-115.
- Zhao L, Zhang J, Liu Z, Li M. 2011. Multiple unrelated founding events for the long-distance Pleistocene dispersal of the Salangid, Neosalanx taihuensis: A general demographic model for inshore-orientated freshwater fish. Mol Phylogenet Evol 58:142-147. [DOI] [PubMed]





