Abstract
Medicinal chemists have increasing opportunities to transition from the pharmaceutical industry to academic medical centers interested in translational research. This Viewpoint highlights some of the differences between these two cultures and strategies to succeed in academic drug discovery.
Keywords: Medicinal chemistry, academic drug discovery, translational science, innovation
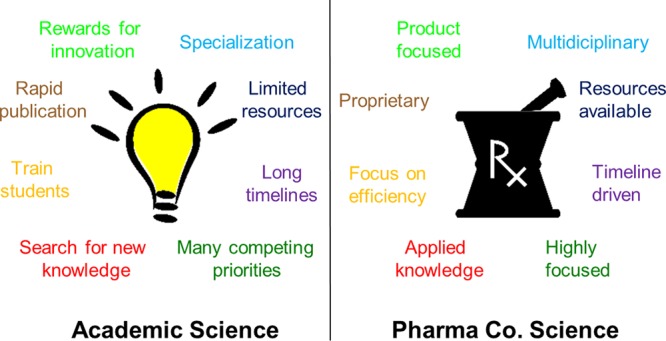
The glory days of pharmaceutical research and development laboratories peaked in the early 2000s, as optimism of ever-increasing research and development spending leading to new drug approvals gave way to the harsh reality of declining return on R&D investment.1 Over the last 15 years, mergers, acquisitions, and the resulting organizational churn prompted a number of experienced medicinal chemists to look for other opportunities to do drug discovery. This time also corresponded to an increased call from government and private funding agencies for more translational research, and academic drug discovery centers began to appear or grow across many campuses.2 University technology transfer offices also sought to replicate windfall licensing deals from a few select academic laboratories,3 and companies began to realize the benefits of facilitating early stage research at universities.4 As a result, many medicinal chemists with significant experience in both large and small companies have made, and continue to make, the switch back to academic environments. Their return provides valuable insight on the drug discovery process to an audience of eager students as well as faculty looking to commercialize the fruits of their work. While each academic institution has their own model for how drug discovery is organized, there are significant differences in culture between most academic institutions and a traditional pharmaceutical company. Academic drug discovery laboratories are challenged with bridging these two cultures if they are to build a sustainable pipeline of novel therapeutics. Rather than a review of academic drug discovery,5,6 this Viewpoint is intended to highlight opportunities and common pitfalls for industrially experienced medicinal chemists making the transition to a university setting.
The mission of most research universities is driven by the quest for new knowledge. In principle, academic scientists have the freedom to pursue any novel idea for the sole purpose of gaining insight on a previously unstudied area of science. In reality, most academic laboratories in the health sciences have practical limitations based on what they can get funded, but our collective knowledge of biology is so incomplete that it leaves a wide swath of interesting science to pursue as long as a funding agency agrees. Drug discovery takes that new knowledge and turns it into a treatment for patients, usually by invention or application of a variety of technologies in a coordinated cycle of design, synthesis, and testing.7 This work is by no means less innovative and insightful than purely hypothesis-driven studies, as many challenges are faced in turning basic research observations into robust and predictive assays that lead to therapeutic agents. This type of science is less amenable to the standard grant application format of 3–4 independent aims that can be completed by a couple of diligent students and postdoctoral fellows, but rather takes a multidisciplinary team with expertise in a variety of areas such as in vitro and in vivo pharmacology, medicinal chemistry, drug metabolism, and safety/toxicology. This proficiency is often available at a large academic medical center, but it needs to be effectively coordinated. Scientists returning from industry, especially medicinal chemists, often play this role because they provide a perspective on chemistry and drug development that is different from a traditional chemistry department. At smaller universities, collaborations across institutions are likely needed to amass all the requisite expertise. It is important for new medicinal chemist faculty to make themselves visible across the institution and actively build relationships with potential collaborators, most of which will be very excited to have chemistry collaborators for projects.
In the process of doing knowledge-enhancing curiosity-driven research, academic laboratories have another key mission of training the next generation of scientists. Laboratories have a range of participants, from “green” undergraduates getting their first taste of research to postdoctoral fellows honing their skills in preparation for independent academic careers or joining teams in industry. The training aspect can often slow down the pace of discovery as senior members of the lab take time to educate younger lab members, or instruments go down for repairs from someone “learning the hard way”. Further, trainees need to rapidly publish their work, which can sometimes be at odds with the desire to keep drug discovery insights proprietary until effective patent protection can be achieved. Many laboratories have balanced these needs by having dedicated professional staff complement the trainees in the lab. Students can develop chemical probes8 that may not have all the properties of a drug candidate but can be used to explore the basic biology of the system, while the staff members focus on developing the most drug-like compounds and performing routine assays. While getting this balance correct can be challenging, it is very rewarding to see young scientists learn and ultimately flourish with new ideas and enthusiasm for drug discovery. A well-characterized chemical probe is often the basis for the creation of a new biotech company, which is another means to separate academic and commercial interests.
The resources available for drug discovery in academic laboratories are more limited than found in pharma. This often limits the pace and throughput through the traditional drug discovery cycle, thereby necessitating careful selection of assays for maximum impact on the project. Laboratories can mitigate this disparity by use of core facilities, judicious outsourcing, and collaborations that often bring unique capabilities and data sets to a project. While collaborations can greatly enhance the resources available to a project, care must be taken to set expectations about how many compounds can be tested by a collaborator and what the turn-around time will be. Nothing is more frustrating for a medicinal chemist than completing the synthesis of a tough analog that will set the direction of future chemistry efforts, only to have to wait months for data. The most optimal scenario is to have the primary, structure–activity relationship-driving in vitro assay closely coupled to the chemistry lab, which may necessitate having dedicated staff in a chemistry lab for doing assays. Despite the plethora of assay “kits” from vendors, it takes a lot of careful optimization to make sure implementation is robust, and this work can be taken for granted by industry chemists who are often spared the details of assay optimization. While not as generously funded, academic laboratories usually have the luxury of time to work through difficult challenges that may discourage a profit-driven organization to terminate a project that is not showing fast enough progress.
Academic laboratories are generally rewarded for innovative research published in prestigious journals by additional grant funding and prominent lectures. This lessens the motivation to rigorously test key assays for reproducibility, especially critical in vivo experiments. In an academic drug discovery setting, these in vivo experiments are often done by collaborators who need extra reminding about rigorous protocols including randomization, blinding, and appropriate controls. Chemists need to play a close role in planning these experiments, especially making sure appropriate formulation of the drug for dosing is done. Obtaining plasma samples for analysis of drug levels, either in the actual test subjects or in a satellite group, is probably the most important aspect of interpreting resulting in vivo data. Academic laboratories are used to “dose–response” but need to be reminded of the importance of getting drug “exposure–response” to allow differentiation of test compounds and establishing pharmacokinetic–pharmacodynamic relationships. These considerations often lead to large experiments with multiple arms, which on its face may appear to be a significant drag on time and add additional cost. However, having to repeat in vivo experiments is often even more time-consuming. Further, the execution of well-designed studies is more likely to result in additional grant funding or partnerships with pharmaceutical companies.
Academic scientists who have spent years, sometimes decades, studying a particular disease process are often reluctant to do the “killer” experiment. Theoretical flaws are present for any novel therapeutic approach, and designing pivotal experiments (and living with the results) to understand possible risks is an important part of deciding whether to spend additional time and money on a project. Industrially experienced scientists often provide this critical eye, and each potential collaboration should be judged on the feasibility of getting to these key experiments. The most critical answers usually come from in vivo studies: “did the drug reach appropriate concentration in the tissue of interest to engage the target?” While the term “appropriate concentration” will need to be determined empirically, starting in the vicinity of the EC50 or IC50 of a compound (determined in a cell based functional assay) is usually a good initial target, and chemists need to make sure they can deliver a compound with the appropriate properties to test the mechanism.
The academic world is full of competing priorities, from things like grant writing, teaching commitments, academic seminars, peer review, and multiple collaborations. At many medical schools, grant writing is critical since the majority of salary support comes from grants, so extensive time needs to be dedicated to this activity. This makes it difficult to focus on a single project, which is often how industrial medicinal chemists operate. That focus is required to push a project forward quickly to meet management or investor expectations, and projects are often resourced with many dedicated chemists (either internal or outsourced) to ensure rapid development of structure–activity relationships. On the academic side, often a single chemist will be supporting multiple projects, although some of these will be early stage without high-throughput assays in place. In industry laboratories, interesting findings are often set aside so that resources are focused on getting the drug candidate delivered on time. On the academic side, opportunities for side projects around unexpected results are always lurking. While follow-up can be distracting from the goal of identifying drug candidates, there are often eager students that can flesh out details, which hopefully leads to new insight. Managing all these competing priorities may be the biggest initial challenge for industrial chemists returning to a university environment, especially because of the talent and perspective industrial chemists bring is in high demand. Success will be measured by the ability to maintain focus and deliver on core projects while also being opportunistic when compelling new science offers an avenue to have impact.
Overall, the recent emphasis on translational research from a variety of funding sources provides ample opportunities for industrial medicinal chemists to return to academia and continue a rewarding career in drug discovery. Adaptation to this new environment is challenging due to different priorities of academic research compared to the pharmaceutical industry, but medicinal chemists with an industrial background can have significant impact in translational science.
Acknowledgments
The author gratefully acknowledges Takashi Tsukamoto and Allen Reitz for constructive feedback.
The author declares no competing financial interest.
Notes
Views expressed in this Viewpoint are those of the author and not necessarily the views of the ACS.
References
- Scannell J. W.; Blanckley A.; Boldon H.; Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discovery 2012, 11 (3), 191–200. 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- Huryn D. M.; Resnick L. O.; Wipf P. Contributions of Academic Laboratories to the Discovery and Development of Chemical Biology Tools: Miniperspective. J. Med. Chem. 2013, 56 (18), 7161–7176. 10.1021/jm400132d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia W. D.University start-ups: Critical for improving technology transfer. In Center for Technology Innovation at Brookings, 2013; https://www.brookings.edu/wp-content/uploads/2016/06/Valdivia_Tech-Transfer_v29_No-Embargo.pdf.
- Hunter J.; Stephens S. Is open innovation the way forward for big pharma?. Nat. Rev. Drug Discovery 2010, 9 (2), 87–88. 10.1038/nrd3099. [DOI] [Google Scholar]
- Huryn D. M. Drug discovery in an academic setting: playing to the strengths. ACS Med. Chem. Lett. 2013, 4 (3), 313–5. 10.1021/ml400012g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J. R. Academic drug discovery: current status and prospects. Expert Opin. Drug Discovery 2015, 10 (9), 937–944. 10.1517/17460441.2015.1059816. [DOI] [PubMed] [Google Scholar]
- Dahlin J. L.; Inglese J.; Walters M. A. Mitigating risk in academic preclinical drug discovery. Nat. Rev. Drug Discovery 2015, 14 (4), 279. 10.1038/nrd4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith C. H.; Audia J. E.; Austin C.; Baell J.; Bennett J.; Blagg J.; Bountra C.; Brennan P. E.; Brown P. J.; Bunnage M. E. The promise and peril of chemical probes. Nat. Chem. Biol. 2015, 11 (8), 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]


