Abstract
Background:
There are no agreed-upon variables for predicting progression from unimpaired cognition to amnestic mild cognitive impairment (aMCI), or from aMCI to AD.
Objective:
Use ADNI data to develop a ‘Framingham-like’ prediction model for a 4-year period.
Methods:
We developed models using the strongest baseline predictors from six domains (demographics, neuroimaging, CSF biomarkers, genetics, cognitive tests, and functional ability). We chose the best predictor from each domain, which was dichotomized into more vs. less harmful.
Results:
There were 224 unimpaired individuals and 424 aMCIs with baseline data on all predictors, of whom 37 (17%) and 150 (35%) converted to aMCI and AD, respectively, during 4 years of follow-up. For the unimpaired, CSF tau/AB ratio, hippocampal volume, and a memory score predicted progression. For those aMCI a baseline, the same predictors plus APOE4 status and functional ability predicted progression. Demographics and family history were not important predictors for progression for either group. The fit statistic was good for the unimpaired-aMCI model (C-statistic 0.80) and very good for the aMCI-AD model (C-statistic 0.91). Among the unimpaired, those with no harmful risk factors had a 4-year predicted 2% risk of progression, while those with the most harmful risk factors had a predicted 35% risk. The aMCIs with no harmful risk factors had a predicted 1% risk of progression those with all six harmful risk factors had a predicted 90% risk.
Conclusion:
Our parsimonious model accurately predicted progression from unimpaired to aMCI with three variables, and from aMCI to AD with five variables.
Keywords: biomarkers, imaging, cerebral spinal fluid, mild cognitive impairment, dementia, Alzheimer’s disease
Introduction
There is great heterogeneity in the clinical manifestation of Alzheimer’s disease (AD) and other neurodegenerative disorders. This complicates both the ability to predict an individual’s level of risk and to detect the disease early in its course. While there is accumulating evidence that a subset of risk factors may increase one’s level of risk for subsequent disease development, earlier and better prediction is needed. Below we briefly summarize existing meta-analyses of studies regarding risk factors for progression as well as the prior longitudinal studies using the ADNI dataset, before adding our contribution of a 4-year, multifactorial risk profile for those transitioning from unimpaired cognition to amnestic mild cognitive impairment (aMCI) and from aMCI to Alzheimer’s disease (AD) dementia, based on ADNI data.
There are numerous longitudinal studies in which the risk of conversion from mild cognitive impairment (MCI) to AD or to non-AD dementia is predicted over a specific time period based on different baseline variables. Six meta-analyses of these studies have been conducted considering either genetic (APOE4), positron emission tomography (PET), or cerebral spinal fluid (CSF) AD biomarkers [1–6]. Each of these risk factors had significant predictive ability. Schmand et al. [7] conducted a meta-analysis of progression, which included either conversion from cognitively unimpaired to MCI/AD or conversion from MCI to AD. These investigators found significant predictive effects of memory and CSF for progression to MCI, with less of an effect for brain imaging. A more recent publication by Chen et al. [8] studied the progression from unimpaired to MCI in 254 participants from the UC Davis Alzheimer’s Disease Center, and found age, low executive function, and worse functional ability as significant risk factors for progression. While neuroimaging was included in Chen et al. [8], CSF markers were not.
Few studies, however, report the risk of progression over a given time period while including a full combination of demographic, cognitive, functional, genetic, neuroimaging, and CSF biomarker information in their analyses. This may be because such comprehensive data sets are rare. An exception to this is the Alzheimer’s Disease Neuroimaging Initiative (ADNI). While ADNI remains one of the most useful datasets for examining combined risk factors, the number of individuals possessing data on all of these variables in ADNI still remains relatively sparse.
Multiple investigators have reported predictive models for progression using baseline or longitudinal ADNI data, but most of these have focused only on the conversion from MCI to AD, and each publication has emphasized a different combination of risk factors [9–16]. Among the various predictors, CSF is the least commonly available in the ADNI data, and is therefore often excluded from many predictive models. Cognitive performance variables, for which there are well-established relationships between performance and disease, consistently perform as robust predictors [10–14, 16]. Hohman and colleagues [9] examined combined risk using most of the available categories of data within ADNI, including demographic, genetic, imaging, cognitive, and CSF characteristics. Rather than predicting risk, however, they created a latent variable model of global resilience.
We have previously used ADNI data to document the predictive power among those unimpaired at baseline of demographics, APOE4, MRI imaging, and CSF for decline on the ADAS-Cog test [17]. Here we extend our earlier work by using ADNI data to predict progression from unimpaired cognition to MCI, and also from MCI to dementia. We considered possible predictors from six domains: demographics, neuroimaging, CSF biomarkers, genetic predisposition (e.g., APOE4, family history), cognitive tests, and functional ability, and we chose the best predictors from each of these domains. Our goal was to develop a risk prediction model based on values of specific variables from each of these domains, which we dichotomized into ‘more harmful’ or ‘less harmful’ for the purpose of risk prediction. We estimate the risk of progression to a worse diagnosis after 4-years from baseline data for 424 MCIs at baseline and for 224 cognitively unimpaired individuals at baseline. These data use a longer follow-up and a larger number of subjects than prior analyses of ADNI data, with a systematic evaluation of possible predictors within each of six domains, and the choice of a single predictor to represent each domain.
Methods
ADNI Data source
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org.
Participants
Participants were enrolled in ADNI (adni.loni.usc.edu), a multi-center longitudinal investigation to identify structural and functional brain changes and other biological markers to predict the progression to MCI and AD. As an AD-focused study, all the MCI participants were required to have an amnestic subtype, as classified by the presence of a memory complaint or a memory problem that was noted by their partner, a specified education adjusted cutoff score on Logical Memory, a MMSE score between 24–30, and a CDR score (including the Memory Box score) of 0.5. The initial goal of ADNI was to recruit 800 subjects, but ADNI has been followed up by ADNI-GO and ADNI-2. To date, these three protocols have recruited over 1500 adults, ages 55 to 90, who had either unimpaired cognition, MCI, or early AD at baseline. The follow up duration of each group is specified in the protocols. We included persons who were involved in any of the three rounds of ADNI data collection, provided they had complete baseline data on all our predictors of interest. Most data were taken from the data set ADNI-MERGE, which combines the three ADNIs.
Using a dataset downloaded on 05/25/2017, we found individuals with unimpaired cognition or amnestic MCI (aMCI) at baseline. We restricted the sample to those who had at least four visits (including the baseline visit). There were various batches of CSF analysis, and the median of the measures across batches at baseline was used in the present analysis. Diagnostic and etiologic data came from the following two files compiled by ADNI, “DXSUM_PDXCONV_ADNIALL” and “ADNIMERGE”. Baseline imaging data was sometime missing from “ADNI-MERGE”, but was found in the“MRIMETA/MRI3META” dataset.
We created separate predictive models for the conversion from unimpaired to aMCI and from aMCI to AD. We excluded those who transitioned to aMCI and reverted back to unimpaired cognition (1 person fell into this category), as well as those who transitioned to AD and reverted back to MCI or unimpaired cognition (37 persons fell into this category), and any who were missing a diagnosis. Our final data set consisted of 224 unimpaired participants at baseline and 424 aMCIs at baseline.
Subjects with complete data included in our analyses numbered 224/417 (54%) unimpaired at baseline participants and 356/872 (48%) aMCIs at baseline. For both groups, the main variable for which subjects were missing data was the CSF (65% of MCIs, 48% of the unimpaired), followed by hippocampal volume, and having less than 4 visits. However, for both the unimpaired and aMCI groups, there were no significant (at the 0.05 level) differences between the included and excluded participants on values of mean age, percent male, percent white, percent APOE4 positive, MMSE, FAQ, memory summary, executive function summary, brain volumes (whole, hippocampal, and middle temporal), or CSF variables (AB, p-tau, total tau, and tau/AB ratio).
Predictors
Demographics, neuroimaging, CSF biomarkers, genetic predisposition (e.g., APOE4, family history), cognitive tests, and functional ability were the domains of interest. We considered depression and PET imaging, but a large number of participants were missing data on these risk factors and therefore we did not include them. Diagnostic co-morbidities were initially considered, but were sparse and did not perform well.
For demographics, we considered age, gender, education, and race. Age was considered as both a continuous and categorical (quartile) variable, while education was categorized into four groups: less than high school, high school, some college, and college or more. Race was categorized as white and non-white.
For imaging, we considered whole brain volume, medial temporal lobe (MTL) volume, and hippocampal volume. All neuroimaging data in ADNI1 was acquired using a field strength of 1.5 or 3 Tesla, and post-processed cross-sectionally using FreeSurfer Version 4.3 at the University of California San Francisco prior to being made available through ADNI (http://adni.loni.ucs.edu/). Cortical reconstruction and volumetric segmentation is performed with the FreeSurfer image analysis suite. FreeSurfer analysis was completed using Version 4.3 for ADNI1 cross-sectional data [UCSFFSX], and Version 5.1 for ADNI GO and 2 data [UCSFFSX51]. Further details can be found in Jack et al. [18]
Data on follow-up time proved to be a highly significant predictor of conversion rates, and was included in both models (unimpaired to aMCI, aMCI to AD). Data on field strength proved to be a predictor of both brain volumes and conversion rates, the latter association due partly to the correlation between follow-up time and conversion rates (those with more follow-up time primarily were from the early ADNI data and had imaging done with 1.5 Tesla field strength, and those with greater follow-up time were more likely to convert). Inclusion of follow-up time in the model for unimpaired to aMCI eliminated the importance of field strength, but field strength proved an important predictor in the aMCI to AD conversion, and was retained in that model.
For CSF we considered AB, p-tau, total tau, and tau/AB ratio. All ADNI CSF samples are collected according to a standard protocol (http://www.adni-info.org/Scientists/doc/CSF_Biomarker_Test_Instr.pdf) and are then sent to a central location for analysis (ADNI Biomarker Core, University of Pennsylvania Medical School), using methods described in the literature [19]. Test-retest R-squares have been high at the ADNI Biomarker Core, typically on the order of 90% or higher, depending on the marker (http://www.alz.org/research/funding/partnerships/2013_meeting/5-biomarkers-core-adni.pdf.)
For cognitive tests, we considered the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog), and the Rey Auditory Verbal Learning Test (RAVLT), as well as summary measures of executive function and memory that were developed by a team of researchers utilizing latent variable modeling within the ADNI cognitive battery. The summary measure for memory is a factor score made up of items from the ADAS-Cog, RAVLT, Logical Memory, and MMSE, while the factor score for the composite measure of executive function is derived from items on Category Fluency, Trails A and B, Digit Span backwards, WAIS-R Digit Symbol Test, and the Clock Drawing Test [20,21]. For functional ability, we used the Functional Activities Questionnaire (FAQ)[22].
For our genetic predictors, we focused on family history (first degree relatives) and APOE4 genotype given its strong relationship to increased risk by allele status. APOE4 genotyping was performed at the time of participant enrollment and included in the ADNI database [19].
Data Analysis
In the ADNI study, participants undergo annual evaluations. As such, it is not possible to provide an exact date for diagnosis or diagnostic conversion. Conversion to a worse diagnosis is instead measured through intervals between study visits. Furthermore, for those participants who were MCI at baseline, we did not know the true incidence date of MCI, which may have preceded the baseline presentation. These factors hinder the use of a time to conversion analysis. Given this type of interval censoring, a typical survival analysis (with exact survival times) via Cox regression is not appropriate. We analyzed our data two different ways, via a Cox proportional hazard models (SAS ICPHREG, https://support.sas.com/documentation/onlinedoc/stat/132/icphreg.pdf) for interval censoring and via logistic regression with follow-up time in the model. These two methods provided similar results. We present the results from the logistic regression because this analysis fit the data slightly better, when comparing observed to predicted conversions.
We selected specific variables to be included in the final model based on comparative goodness of fit values. We did not use a formal step-wise procedure, but began with variables from all six domains and then identified the most predictive variables for each domain by comparing Akaike’s Information Criterion (AIC) when there were multiple measures within a single domain. Our goal was to develop a parsimonious model for relatively simple risk prediction based on a small number of key variables dichotomized into ‘more harmful’ vs. ‘less harmful’, along the lines of the Framingham algorithm to predict heart disease risk (the Framingham risk prediction score is based on 10, 5, 2, 4, and 4 categories for the following variable domains: age, cholesterol, smoking, high-density cholesterol, and systolic blood pressure, respectively.(https://en.wikipedia.org/wiki/Framingham_Risk_Score
We started developing models using continuous variables when available, and then proceeded to analyze the data by quartiles of continuous measures, to assess whether trends identified with continuous variables were consistent (monotonic). Goodness of fit for final models was determined using the C-statistic, which analyzes concordance between observed and predicted conversions and ranges from 0 to 1, with 1 being perfect concordance.
We included in final dichotomous models all variables with p<0.10 as a continuous variable, and which also showed reasonably consistent increasing trends in quartile analysis, as the risk factor profie worsened. In the case of the unimpaired model, not all domains were represented. One variable, hippocampal volume, was found to be am important predictor in continuous and quartile models, and was retained in the final dichotomous variable even though when dichotomized it was no longer significant at the 0.05 level.
Once a final best model had been identified using continuous and categorical variables in quartiles, we dichotomized all variables in order to develop our 4-year risk predictions, in keeping with our goal to create a final simple model along the lines of Framingham. To derive cut-points for optimal dichotomization, we considered either Receiver Operator Curves (ROC), or the CART algorithm (Classification and Regression Trees)[23]. For the sparser data for the unimpaired, CART was unable to provide a simple tree for dichotomizing the three predictor variables, and we used the ROCs. For the progression from aMCI to AD, CART provided a tree with dichotomous cut-points for all five variables in the model. However, one variable (hippocampal volume) was not a statistically significant predictor using the CART cut-point, and we used the ROC cut-point for that variable because it improved prediction and resulted in a better fit to the data.
Finally we used the final dichotomous model results to develop general predictions for conversion based on the presence of ‘worse’ levels of the predictors from the model. We first calculated a risk for the referent group (for either the unimpaired or the aMCIs), and then the baseline risk was then multiplied by the odds ratio for each of the dichotomous risk factors, or for a combination of risk factors.
Results
Table 1 provides a description of cognitively unimpaired and aMCI subjects included in our study. Those who were diagnosed as MCI at baseline had worse values of CSF markers, lower cognitive scores, and lower hippocampal volume than did those with unimpaired cognition at baseline. However, these two groups did not significantly differ in gender, race, education, or whole brain or middle-temporal lobe volume. Mean age was higher in the unimpaired group but categorical analyses of age showed no significant differences It should be noted that there were few non-whites in either group (6% in aMCIs, 8% in unimpaired).
Table 1.
Descriptive data for unimpaired cognition or aMCI at Baseline
| MCI (n=424) | Unimpaired (n=224) |
p value | |
|---|---|---|---|
| Baseline age, years Baseline age category <=74 75–79 >=80 |
72.6 ± 7.12 258 (60.8) 103 (24.3) 63 (14.9) |
74.4 ± 5.77 125 (55.8) 62 (27.7) 37 (16.5) |
0.0008 0.46 |
| Male | 243 (57.3) | 115 (51.3) | 0.15 |
| White | 401 (94.6) | 204 (91.1) | 0.09 |
| Baseline education, years | 16.0 ± 2.79 | 16.3 ± 2.68 | 0.18 |
| APOE4 positive | 208 (49.1) | 52 (23.2) | <0.0001 |
| MMSE | 27.7 ± 1.79 | 29.0 ± 1.13 | <0.0001 |
| FAQ total | 3.16 ± 4.19 | 0.16 ± 0.68 | <0.0001 |
| Memory summary | 0.19 ± 0.66 | 1.04 ± 0.57 | <0.0001 |
| Executive function | 0.23 ± 0.79 | 0.81 ± 0.71 | <0.0001 |
| Hippocampus (mm3) | 6800 ± 1133 | 7420 ± 850 | <0.0001 |
| Whole Brain (mm3) | 1044600 ± 108159 | 1035437 ± 103695 | 0.23 |
| Middle Temporal (mm3) | 19816 ± 2869 | 20386 ± 2510 | 0.011 |
| TAU (pg/ml) | 92.4 ± 52.7 | 67.7 ± 31.5 | <0.0001 |
| PTAU (pg/ml) | 40.0 ± 21.3 | 30.4 ± 17.5 | <0.0001 |
| ABETA (pg/ml) | 169 ± 51.3 | 202 ± 51.7 | <0.0001 |
| Ratio of total TAU to Aβ | 0.65 ± 0.51 | 0.38 ± 0.25 | <0.0001 |
| Follow-up years, mean ± std Follow-up years, median (p25 – p75) |
4.0 ± 1.9 4.0 (3.0 – 5.0) |
4.6 ± 2.6 4.0 (3.0 – 5.0) |
0.001 0.09 |
Those with unimpaired cognition at baseline (n=224) were followed for a median of 4-years (interquartile range: 3.0–5.0, mean: 4.6, std: 2.6); 37 converted to amnestic MCI during follow-up (17%). Those with amnestic MCI at baseline (n=424) were followed for a median of 4-years (interquartile range 3.0–5.0, mean: 4.0, std: 1.9); 150 converted to AD during follow-up (35%).
Variables retained in final models for unimpaired individuals included only hippocampal volume, tau/AB ratio, and a summary measure of memory. Variables included in the final model for those with aMCI at baseline included APOE4 status, a summary measure of memory, hippocampal volume, FAQ, and tau/AB ratio. Both models included a variable for MRI field strength (either 1.5 or 3 Tesla).
Surprisingly, neither demographic variables nor family history proved to be significant predictors when the above variables were in their respective models. This may be simply a function of limited sample size. Increased age showed greater risk for both the progression of the unimpaired to aMCI, and of the aMCI subjects to AD, but these risks were not statistically significant and not consistently linear via quartile analysis. For family history in a first degree relative, there was no consistent pattern of increased risk in either model.
Tables 2a and 2b give the odds ratios from the final models using quartiles of predictors, as well as the p-value for the trend, as determined using the continuous variable for each predictor. Trends in hazard ratios by quartile were generally but not always monotonically increasing, as values worsened for the predictor variables, and all trends were statistically significant at the p<0.05 level, except for hippocampal volume in the aMCI to AD model, for which the p-value was 0.06. Tables 3a and 3b give the final model after dichotomizing all variables to use for our risk estimation, based on ROC curves or CART (cut-points listed in Table).
Table 2a.
Quartile model for progression for unimpaired at baseline
| Effect | Odds ratio | p value | p-value for trend* |
|---|---|---|---|
| Memory quartile 1 <=0.61 | 12.6 (2.56 – 62.0) | 0.0018 | 0.0002 |
| 0.6 < Memory quartile 2 <=0.96 | 8.65 (1.79 – 41.9) | 0.0073 | |
| 0.96< Memory quartile 3 <=1.43 | 3.37 (0.64 – 17.8) | 0.15 | |
| Memory quartile 4 (referent) (>1.43) | |||
| Hippocampus quartile 1 <=6898.5 | 2.47 (0.74 – 8.20) | 0.14 | 0.007 |
| 6898.5< Hippocampus quartile 2 <=7464 | 1.62 (0.47 – 5.51) | 0.44 | |
| 7464< Hippocampus quartile <=7903 | 0.61 (0.14 – 2.62) | 0.50 | |
| Hippocampus quartile 4 (referent) (>7903) | |||
| tau/Aβ ratio quartile 4 >0.451 | 4.16 (1.19 – 14.5) | 0.0252 | 0.01 |
| 0.280< au/Aβ ratio quartile 3 <=0.451 | 3.34 (0.90 – 12.4) | 0.07 | |
| 0.208<tau/Aβ ratio quartile 2 <=0.280 | 0.37 (0.06 – 2.22) | 0.28 | |
| tau/Aβ ratio quartile 1 (<=0.208) | |||
| Follow-up years | 1.54 (1.31 – 1.82) | <0.0001 |
C statistic 0.87, trend tests for quartiles based on p-value for coefficient of continuous variable
Table 2b.
Quartile model for progression from aMCI to AD
| Effect | Odds ratio | p value | p-value for trend |
|---|---|---|---|
| APOE4 positive | 1.85 (0.97 – 3.53) | 0.06 | |
| FAQ quartile 4 >=5 | 23.3 (9.33 – 58.4) | <0.0001 | <0.0001 |
| 2<=FAQ quartile 3 <=4 | 11.9 (4.87 – 29.2) | <0.0001 | |
| FAQ quartile 2 =1 | 11.4 (4.24 – 30.6) | <0.0001 | |
| FAQ quartile 1 (referent, FAQ=0) | |||
| Memory quartile 1 <= −0.27 | 7.66 (2.72 – 21.5) | 0.0001 | <0.0001 |
| −0.27< Memory quartile 2 <=0.12 | 6.10 (2.20 – 17.0) | 0.0005 | |
| 0.12< Memory quartile 3 <=0.63 | 3.04 (1.08 – 8.55) | 0.04 | |
| Memory quartile 4 (referent) (>0.63) | |||
| Hippocampus quartile 1 <=5988 | 2.56 (1.04 – 6.28) | 0.041 | 0.06 |
| 5988< Hippocampus quartile 2 <=6802.0 | 1.78 (0.71 – 4.41) | 0.22 | |
| 6802< Hippocampus quartile 3 <=7620 | 1.09 (0.44 – 2.69) | 0.85 | |
| Hippocampus quartile 4 (referent) (>7620) | |||
| 1.5 Tesla vs 3 Tesla MRI | 3.22 (1.67 – 6.21) | 0.0005 | |
| tau/Aβ ratio quartile 4 >0.87 | 5.01 (1.79 – 14.0) | 0.002 | 0.0001 |
| 0.51< tau/Aβ ratio quartile 3 <=0.87 | 2.48 (0.93 – 6.61) | 0.07 | |
| 0.27< tau/Aβ ratio quartile 2 <=0.51 | 1.34 (0.51 – 3.55) | 0.55 | |
| tau/Aβ ratio quartile 1 (<=0.272) | |||
| Follow-up years | 1.26 (1.07 – 1.48) | 0.005 |
C-statistic 0.91, trend tests for quartiles based on p-value for coefficient of continuous variable, not applicable (n.a.) for other variables in model (APOE4, Tesla strength, follow-up years)
Table 3a.
Dichotomous model for unimpaired transition to aMCI (n=224)
| Effect | Odds ratio | p value |
|---|---|---|
| Memory summary score<0.899 | 2.72 (1.17 – 6.34) | 0.0204 |
| Hippocampus<7310 | 2.08 (0.92 – 4.73) | 0.08 |
| Tau/Aβ ratio>=0.341 | 3.74 (1.62 – 8.65) | 0.0020 |
| Follow-up years | 1.45 (1.26 – 1.68) | <0.0001 |
C statistic from logistic regression: 0.80, cutpoints determined from ROC curves
Table 3b.
Dichotomous model for MCI transition to AD (n=424)
| Effect | Odds ratio | p value |
|---|---|---|
| APOE4 positive | 1.83 (0.99 – 3.36) | 0.05 |
| FAQ>=0.5 | 19.0 (8.33 – 43.1) | <0.0001 |
| Memory summary score<0.26 | 7.37 (3.81 – 14.3) | <0.0001 |
| Hippocampus<6696 | 1.46 (0.80 – 2.65) | 0.21 |
| 1.5 Tesla vs 3 Tesla MRI | 3.32 (1.72 – 6.42) | 0.0004 |
| Tau/Aβ ratio>=0.43 | 4.91 (2.43 – 9.89) | <0.0001 |
| Follow-up years | 1.30 (1.10 – 1.53) | 0.0021 |
C statistic: 0.91. Cutpoints chosen by CART except for hippocampus, chosen via ROC
The C-statistic showed good fit for the final dichotomized model (C-statistic 0.80 for the unimpaired, and very good fit for the aMCIs progressing to AD (C-statistic, 0.91). Figures 1 and 2 show that the correspondence between observed and predicted risk among our study subjects was good both for the unimpaired progressing to aMCI and for the aMCIs at baseline progressing to AD.
Figure 1.
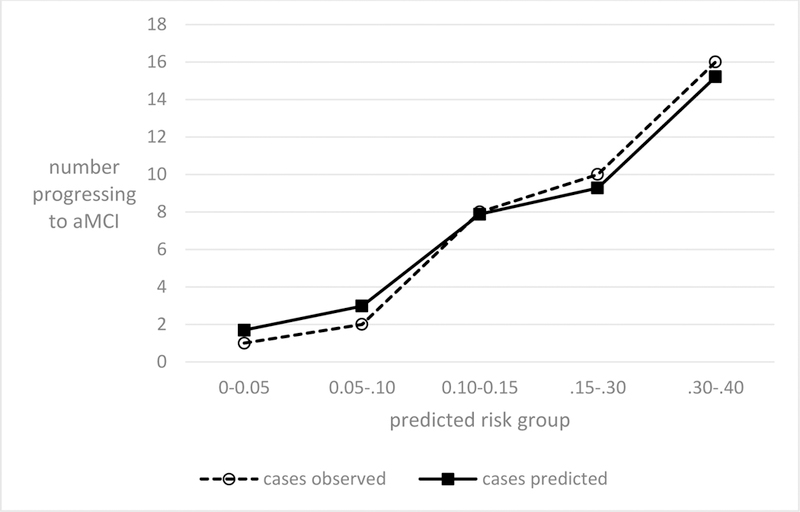
Observed and predicted MCIs among the unimpaired, by predicted risk groups
Figure 2.

Observed and predicted dementia among the MCIs, within predicted risk groups
Table 4a gives the 4-year predicted risk of progression to a worse diagnosis for those with unimpaired cognition at baseline, for each possible combination of risk factors, in order of increasing risk. Table 4b gives the predicted risk of progression for broad categories for the aMCIs at baseline, according to the number of ‘more harmful’ risk factors present. In these calculations the variables for field strength and follow-up time were not used to create predictions, except insomuch as their average values for each group (unimpaired, mean follow-up 4 years, aMCIs, mean follow-up 4 years, 30% Tesla 1.5) were entered into the estimation of the risk for the referent group (eg, for the aMCIs, referent risk was p=(exp(b0 + b1*average follow-up + b3*percent Tesla1.5)/ (1+ p=(exp(b0 + b1*average follow-up + b3*percent Tesla1.5))).
Table 4a.
4-year predicted risk of progression from unimpaired to aMCI, by number of dichotomous risk factors
| Group | Number in group |
4-year predicted risk |
|---|---|---|
| All ADNI unimpaired combined (mixture of risk factors) | 224 | 0.17* |
| No risk factor | 44 | 0.02 |
| Low hippocampal volume | 31 | 0.05 |
| Low memory score | 39 | 0.06 |
| High tau/AB | 24 | 0.09 |
| Low memory and low hippocampal volume | 21 | 0.12 |
| Low hippocampal volume and high tau/AB | 20 | 0.16 |
| Low memory and high tau/AB | 24 | 0.20 |
| All three risk factors | 21 | 0.35 |
Observed risk. All other risks are predicted from model with dichotomized risk factors (Table 3a), assuming an average 4 years of follow-up
Table 4b.
4-year predicted risk of progression from aMCI to AD, by number of dichotomous risk factors with worse values
| Group | Number in group |
4-year risk |
|---|---|---|
| All ADNI MCIs combined (mixture of risk factors) | 424 | 0.37* |
| no risk factor | 33 | 0.01 |
| 1 risk factor | 71 | 0.05 |
| 2 risk factors | 91 | 0.18 |
| 3 risk factors | 85 | 0.43 |
| 4 risk factors | 75 | 0.72 |
| 5 risk factors | 69 | 0.90 |
Observed risk. All other risks are predicted from model with dichotomized risk factors (Table 3b), assuming (based on the observed data) an average 4 years of follow-up and that 30% of aMCI subjects had imaging at 1.5 Tesla at baseline. Subjects are depending on the number of ‘worse’ risk factors, eg, someone with low hippocampal volume and low memory score but low FAQ, no APOE4 variant, and low tau/AB would be in the group with 2 risk factors.
Discussion
Our data provide an estimate of 4-year risk of progression based on risk factors from six domains: demographics, neuroimaging, CSF biomarkers, genetic predisposition (APOE4, family history), cognitive tests, and functional ability. Only three predictors (hippocampal volume, tau/AB ratio, and summary memory score) were significant for predicting progression to aMCI for those unimpaired at baseline. The fit of this model to the observed data was reasonably good, with a C-statistic of 0.80 (see Figure 1). It should be noted that we could not evaluate the role of recent depression due to lack of data in ADNI; we and others have found recent depression to be a strong risk factor for the unimpaired progressing to MCI [24,25].
In contrast, five predictors from six domains (APOE4, tau-AB ratio, hippocampal volume, FAQ, and a summary score for memory) were significant or borderline-significant in the model for aMCIs at baseline progressing to AD. The predictive ability of the aMCI-to-AD model was quite good (C statistic 0.91 and Figure 2), suggesting that most major predictors for this progression had been identified.
As ADNI participants accumulate more follow-up time, and as new participants are recruited, these predictive models can be further improved. One might expect, for example, that age, race, and education will prove important predictors with increased sample size.
Barnes et al. [10] conducted a similar exercise to predict the progression of MCI to AD. Using ADNI data they developed a point-based score. However, their follow-up time was shorter than ours (3 years vs. 4 year). Like us, they found that MRI imaging, cognitive tests, and the FAQ were significant predictors, with a good fit to the data (C-statistic 0.78), but they did not find APOE4 to be a significant predictor and they did not use CSF data, which was a strong predictor in our models.
The same three specific risk factors that predicted the progression of those without impairment to MCI (hippocampal volume, tau/AB ratio, and summary memory score) also predicted the progression of MCI to dementia. These values are hallmarks of the clinical presentation commonly seen in sporadic AD cases in older aged individuals. They are also highly related to one another [26]. Given the priority for amnestic presentations within the ADNI sample recruitment, the summary memory measure is an expected cognitive predictor for both transitions. Two other risk factors important for the MCI to AD progression were not important in predicting the progression from unimpaired to MCI: APOE4 and FAQ. It is not surprising that the FAQ predicts the MCI to AD progression, given that functional impairments are one of the diagnostic criteria for the diagnosis of dementia. While it is possible for executive functioning to decline as a result of AD brain pathology, the initial clinical presentation reflects memory problems [27]. Regarding APOE4, MCI subjects at baseline had twice the frequency of APOE4 variants than did the unimpaired at baseline, yet APOE4 was not a significant predictor of MCI among the unimpaired. We note that Chen et al. [8] also did not find APOE4 to be an important predictor of the transition from unimpaired to MCI.
The greatest limitation of our data is the small sample size in ADNI. There were only 37 of 224 unimpaired individuals who progressed to MCI, and 150 of 424 MCI individuals who progressed to dementia. Hence, we are less confident of our predictions for them. Our limited sample size also precluded splitting our data into a training and validation set, typically used for prediction models – this would have provided some internal validation.
It is also difficult to know the generalizability of our findings. While our group of important risk factors may differ from those found in other studies, we note again that to our knowledge few other studies analyzed the whole gamut of risk factors considered here. In particular, most studies did not include CSF biomarkers, which are strong predictors for progression (unimpaired to MCI, MCI to dementia). Inclusion of CSF biomarkers may result in other factors being less important.
Our observed four-year risks were generally in line with the well-characterized Mayo clinic population, which reflects rates in the community in Rochester, Minnesota. In our data, the four-year risk of aMCI among the unimpaired was 17%. This is slightly higher than the rate in the Mayo clinic data, where the incidence is 12% [28]. Similarly, our four-year risk of AD among those with aMCI was 35%, while in the Mayo clinic population the four-year risk was approximately 30% [29], for a mixture of those with prevalent or incidence aMCI at baseline. Similar to the Mayo population, our population was a mix of prevalent and incidence cases at baseline. While these similarities in 4-year risk are reassuring, ultimately even if they were more markedly different – with lower or higher rates of progression - there is no a priori reason to believe that the etiologic effect of the risk factors would be different in other populations.
Future work should focus on the transition from unimpaired to MCI, given the somewhat weaker prediction for that group. Indeed, the field is transitioning to emphasize the preclinical manifestations of AD [30,31]. Efforts to recruit and follow younger samples of participants, beginning as early as the 20s and focusing on midlife, are underway. Other hopeful targets of future research prioritize the detection of cognitive change over time, even within those of normal cognitive status [17,32] as well as improved understanding of patients’ self-report of subjective declines [33].
Finally, we note that current brain imaging and CSF-based tests, among the most predictive variables we examined, have significant limitations for widespread use in clinical practice for predicting disease progression, including expense and/or invasiveness. Less expensive and minimally invasive biomarkers (e.g., surrogates for imaging and CSF) are urgently needed.
Acknowledgment:
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are Rev November 7, 2012 facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern.
This work was also supported by an NIH Grant for the Emory Alzheimer’s Disease Research Center (P50 AG025688). Alvaro Alonso is supported by grant U01HL096902.
References
- 1.Mitchell AJ, Shiri-Feshki M (2009) Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119(4):252–65. [DOI] [PubMed] [Google Scholar]
- 2.Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, Verhey FR, Visser PJ (2011) Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: a meta-analysis. J Neurol Neurosurg Psychiatry. 82(10):1149–56 [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Zhang S, Li J, Zheng DM, Guo Y, Feng J, Ren WD (2014) Predictive accuracy of amyloid imaging for progression from mild cognitive impairment to Alzheimer disease with different lengths of follow-up: a meta-analysis. Medicine (Baltimore) 93(27):e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira D, Rivero-Santana A, Perestelo-Pérez L, Westman E, Wahlund LO, Sarría A, Serrano-Aguilar P (2014) Improving CSF Biomarkers’ Performance for Predicting Progression from Mild Cognitive Impairment to Alzheimer’s Disease by Considering Different Confounding Factors: A Meta-Analysis. Front Aging Neurosci 16;6:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fei M, Jianhua W (2013) Apolipoprotein ε4-allele as a significant risk factor for conversion from mild cognitive impairment to Alzheimer’s disease: a meta-analysis of prospective studies. J Mol Neurosci 50(2):257–63. [DOI] [PubMed] [Google Scholar]
- 6.Diniz BS, Pinto Júnior JA, Forlenza OV (2008) Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 9(3):172–82. [DOI] [PubMed] [Google Scholar]
- 7.Schmand B, Huizenga HM, van Gool WA (2010) Meta-analysis of CSF and MRI biomarkers for detecting preclinical Alzheimer’s disease. Psychol Med 40(1):135–45. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Denny KG, Harvey D, Farias ST, Mungas D, DeCarli C, Beckett L (2017) Progression from normal cognition to mild cognitive impairment in a diverse clinic-based and community-based elderly cohort. Alzheimers Dement 13(4):399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL, Weiner MW, Petersen R, Aisen P, Jack C, Jagust W (2016) Asymptomatic Alzheimer’s disease: Defining resilience. Neurology 87(23):2443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes DE, Cenzer IS, Yaffe K, Ritchie CS, Lee SJ, Alzheimer’s Disease Neuroimaging Initiative (2014) A point-based tool to predict conversion from mild cognitive impairment to probable Alzheimer’s disease. Alzheimers Dement 10(6):646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr, Feldman HH, Bokde AL, Alexander GE, Scheltens P, Vellas B, Dubois B, Weiner M, Hampel H, Alzheimer’s Disease Neuroimaging Initiative (2012) Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 33(7):1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dukart J, Sambataro F, Bertolino A. Accurate Prediction of Conversion to Alzheimer’s Disease using Imaging, Genetic, and Neuropsychological Biomarkers (2016) J Alzheimers Dis 49(4):1143–59. [DOI] [PubMed] [Google Scholar]
- 13.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE, Alzheimer’s Disease Neuroimaging Initiative (2011) Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Arch Gen Psychiatry. 68(9):961–9. [DOI] [PubMed] [Google Scholar]
- 14.Schmand B, Eikelenboom P, van Gool WA, Alzheimer’s Disease Neuroimaging Initiative (2012). Value of diagnostic tests to predict conversion to Alzheimer’s disease in young and old patients with amnestic mild cognitive impairment. J Alzheimers Dis 29(3):641–8 [DOI] [PubMed] [Google Scholar]
- 15.Spampinato MV, Langdon BR, Patrick KE, Parker RO, Collins H, Pravata E, Alzheimer’s Disease Neuroimaging Initiative (2016) Gender, apolipoprotein E genotype, and mesial temporal atrophy: 2-year follow-up in patients with stable mild cognitive impairment and with progression from mild cognitive impairment to Alzheimer’s disease. Neuroradiology 58(11):1143–1151 [DOI] [PubMed] [Google Scholar]
- 16.Li K, Chan W, Doody RS, Quinn J, Luo S, Alzheimer’s Disease Neuroimaging Initiative (2017) Prediction of Conversion to Alzheimer’s Disease with Longitudinal Measures and Time-To-Event Data. J Alzheimers Dis 58(2):361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenland K, Zhao L, Goldstein F, Cellar J, Lah J, Alzheimer’s Disease Neuroimaging Initiative (2014) Biomarkers for predicting cognitive decline in those with normal cognition. J Alzheimers Dis 40(3):587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L Whitwell J, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW, The Alzheimer’s Disease Neuroimaging Initiative (2008) MRI methods. J Magn Reson Imaging 27(4):685–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter WZ, Weiner MW, Jack CR Jr, Jagust W, Toga AW, Lee VM, Shaw LM, Alzheimer’s Disease Neuroimaging Initiative (2010) Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement 6:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crane PK, Carle A, Gibbons LE Crane PK, Insel P, Mackin RS, Gross A, Jones RN,Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D; Alzheimer’s Disease Neuroimaging Initiative (2012) Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6(4):502–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM,Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging Initiative (2012) A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6(4):517–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeffer RI, Kurosaki TT, Harrah CH Jr Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community. J Gerontol 37:323–329 [DOI] [PubMed] [Google Scholar]
- 23.Brieman L, Freidman J, Stone C, and Olshen R (1984). Classification and Regression Trees, Taylor & Francis, Boca Raton, Florida [Google Scholar]
- 24.Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A (2012). Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis 31(2):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM (2011) Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc 59(1):57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, & Stadlan EM (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDAWork Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7), 939–944. [DOI] [PubMed] [Google Scholar]
- 28.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Tangalos EG,Ivnik RJ, Rocca WA, Petersen RC (2012) The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 78(5):342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJ, Geda YE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA, Petersen RC (2014). Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 82(4):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR Jr (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12(3):292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlawish J, Jack CR, Rocca WA, Snyder HM, & Carrillo MC (2017). Alzheimer’s disease: The next frontier—Special Report 2017. Alzheimer’s and Dementia 13(4), 374–380. [DOI] [PubMed] [Google Scholar]
- 32.Duke Han S, Nguyen CP, Stricker NH, Nation DA (2017) Detectable Neuropsychological Differences in Early Preclinical Alzheimer’s Disease: A Meta-Analysis.Neuropsychol Rev 27(4):305–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, Ewers M, Hampel H, Klöppel S, Rami L, Reisberg B, Saykin AJ, Sikkes S, Smart CM, Snitz BE, Sperling R, van der Flier WM, Wagner M, Jessen F (2017) Subjective Cognitive Decline Initiative (SCD-I) Working Group. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement 13(3):296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]


