Abstract
The serine/threonine protein kinase TBK1 (Tank-binding Kinase-1) is a noncanonical member of the IkB kinase (IKK) family. This kinase regulates signaling pathways in innate immunity, oncogenesis, energy homeostasis, autophagy, and neuroinflammation. Herein, we report the discovery and characterization of a novel potent and highly selective TBK1 inhibitor, GSK8612. In cellular assays, this small molecule inhibited toll-like receptor (TLR)3-induced interferon regulatory factor (IRF)3 phosphorylation in Ramos cells and type I interferon (IFN) secretion in primary human mononuclear cells. In THP1 cells, GSK8612 was able to inhibit secretion of interferon beta (IFNβ) in response to dsDNA and cGAMP, the natural ligand for STING. GSK8612 is a TBK1 small molecule inhibitor displaying an excellent selectivity profile and therefore represents an ideal probe to further dissect the biology of TBK1 in models of immunity, neuroinflammation, obesity, or cancer.
Keywords: TBK1, kinase inhibitor, interferon, inflammation
TBK1 is a member of the noncanonical IKK family of serine/threonine kinases and a central player in innate immunity.1,2 Following ligation of TLR3 or TLR4, TBK1 is activated via the adaptor protein TRIF, resulting in the phosphorylation of its target protein IRF3. In a variety of cells including conventional dendritic cells (DC) and macrophages, IRF3 activation subsequently triggers the secretion of IFNβ, an immunomodulatory chemokine.2−7 Through an autocrine loop via type I IFN receptor (IFNAR) and induction of IRFs and interferon-stimulated genes (ISGs), IFNβ typically primes stimulated cells to produce high levels of IFNα.8,9
TLR3 is naturally stimulated by viral double stranded (ds)RNA or by its synthetic mimetic polyinosine–polycytidylic acid (poly(I:C)), a suitable tool to trigger a type I IFN response in vitro.8 Further, TBK1 is activated following dsDNA sensor ligation via the signaling intermediate STING, likewise leading to IRF activation and type I IFN expression.3,10 In turn, poly(I:C) and IFNα promote STING expression in macrophages and DCs, forming another mechanism of amplifying the IFN-mediated antiviral immune response.10
Misregulation of TBK1 activity is associated with autoimmune disorders and has therefore been considered a target for the treatment of (auto)inflammatory diseases.11−13 This concept is, however, challenged by the observation that TBK1 inhibition may have proinflammatory effects due to its regulatory function in the noncanonical NF-κB pathway.14−16 More recently, roles for TBK1 in energy homeostasis and tumorigenesis have been revealed, identifying TBK1 as potential target for obesity and cancer.16,17 Key roles in autophagy and neuroinflammation have also been reported for TBK1.18 The discovery of TBK1 inhibitors has therefore gained attention from both industrial and academic groups.19 In this context, the selective degradation of TBK1 using proteolysis targeting chimeras (PROTACs) has been recently explored.20 Further validation of the effects of TBK1 inhibition in disease-relevant models is required to improve the understanding of the biological function of this key signaling mediator. To this end, the availability of potent and selective chemical probes would greatly facilitate such target validation efforts.21 We report here the discovery of GSK8612, a highly potent and selective inhibitor for TBK1 and its activity in cellular models.
The application of affinity enrichment based chemoproteomics to the discovery of selective probes for PI3Kγ,22 mTOR,23 and TNKS24 was previously described.25 Using these methods, the kinase selectivity of two small molecules used to explore TBK1 biology, BX795(26,27) and MRT67307,28,29 was established (Table S1 and Figure S1). BX795 and MRT67307 were determined to have an average pKd of 7.7 and 7.1, respectively, for TBK1 when profiled with kinobeads30 in a mixture of cell lines and tissue extracts (optimized for a broad protein kinase coverage). However, both molecules show affinity for more than 20 other kinases within a 10-fold window of their TBK1 binding affinity (see Table S1 for full list of kinases). The inhibitor of nuclear factor kappa-B kinase subunit ε (IKKε), a close homologue of TBK1 sharing high sequence identity in the kinase domain, is among these 20 kinases. Of note, both molecules demonstrated highest affinity for AP2-associated protein kinase 1 (AAK1), a protein involved in neuropathic pain.31 Kinase inhibitors can be classified according to their binding site (catalytic site or allosteric site) and the form of the kinase bound (active or nonactive and Asp-Phe-Gly (DFG) loop in or out).32 The kinobeads are generated by immobilization of kinase inhibitors that ligate the ATP binding site of kinases. Therefore, molecules that demonstrate competitive binding behavior with the kinobeads are concluded to bind into the ATP site of kinases. In order to investigate to which kinase state these TBK1 inhibitors bind, profiling experiments with the kinobeads were performed using cell extracts with and without enrichment of phosphorylated kinases. The enrichment was achieved by treating Ramos cells with the phosphatase inhibitor Calyculin A before cell lysis. In the Calyculin A extracts, BX795 and MRT67307 were determined to have an average pKd of 7.4 and 7.5, respectively, compared to 6.8 and 6.6 in the control extracts (Table S1 and Figure S2). This shows that BX795 and MRT67307 may preferentially bind to the phosphorylated form of TBK1 and the pKds determined against this form are within the expected range considering the reported TBK1 inhibition potencies in activity assays.26,28 Interestingly, in the two different cell lysates, no significant pKd value changes were observed for other target kinases, such as BMP-2-inducible protein kinase (BMP2K) and serine/threonine kinase 17B (STK17B), which cannot be readily explained (note, AAK1 protein kinase was not detected in Ramos cells).
In an effort to discover new inhibitors of TBK1, the kinase selectivity data of proprietary compounds was interrogated. That revealed a series of 2,4-diaminopyrimidines with good affinity for TBK1, and optimization of this series culminated in the discovery of GSK8612 (Figure 1). This molecule can be readily synthesized by two sequential nucleophilic aromatic substitution reactions (Scheme S1; full synthetic details are given in the Supporting Information).
Figure 1.
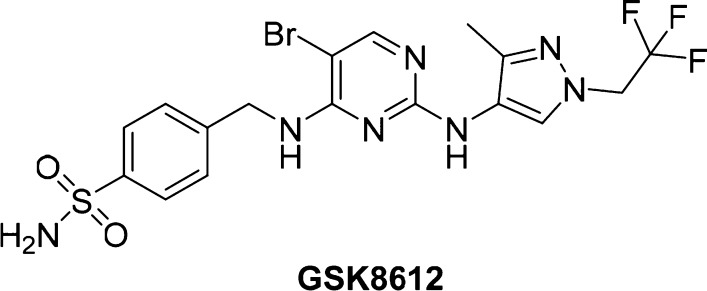
Structure of TBK1 inhibitor GSK8612.
Determination of the physical chemical properties of GSK8612 (Table 1) revealed that the low log D value translates into aqueous solubility greatly exceeding the determined affinity for TBK1 without being detrimental to its cellular permeability. The low lipophilicity may also contribute to GSK8612’s low microsomal clearance in human and rat, and low to medium clearance in mouse. Nonetheless, despite the low log D, GSK8612 is highly protein bound in mouse, rat, and human blood.
Table 1. Properties of TBK1 Inhibitor GSK8612.
| assay | value |
|---|---|
| solubility (CLND) | 119 μM |
| CHROM LogD (pH 7.4) | 3.6 |
| artificial membrane permeability | 2.1 × 10–5 cm/s |
| fraction bound in blood (mouse, rat, human) | 99.5%, 99.6%, 99.3% |
| microsomal clearance (mouse, rat, human) | 8.5, 2.1, 1.1 mL/min/g tissue |
| microsomal half-life (mouse, rat, human) | 8.5, 34.2, 67.4 min |
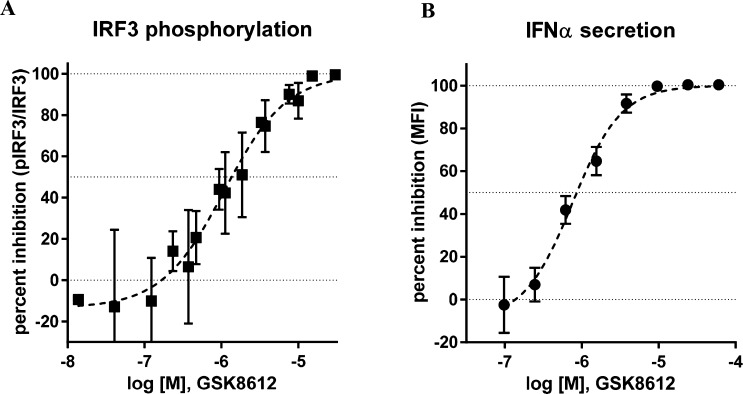
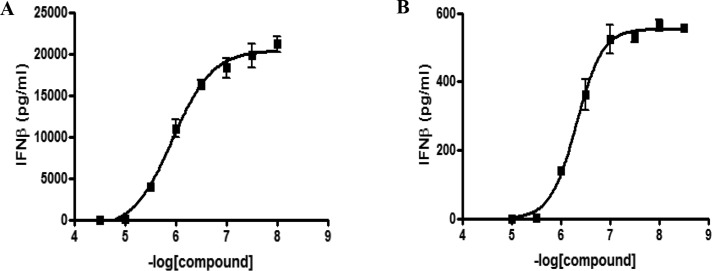
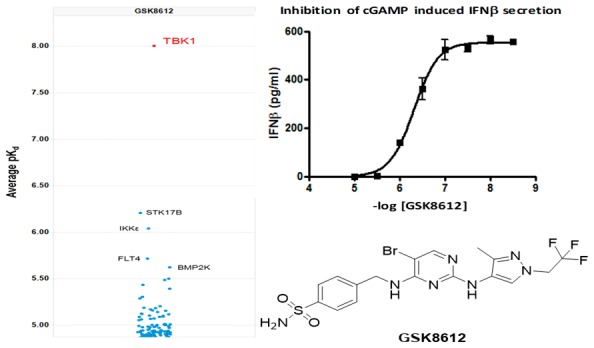
Kinases are attractive and extensively explored drug targets,33,34 and the 2,4-diaminopyrimidine core is a common structural motif found in many kinase inhibitors; indeed, both BX795 and MRT67307 possess this structural feature. The majority of kinase inhibitors that bind in the ATP binding pocket make two conserved hydrogen bonding interactions with the hinge region of the kinase backbone. GSK8612 was docked into a crystal structure of TBK1 bound to MRT67307 (PDB 41WQ),35 and the predicted binding mode is shown in Figure S3. The model predicts that the N1 of the pyrimidine nitrogen and the NH linking the pyrimidine and pyrazole make the hinge region interactions with the backbone NH and carbonyl of Cys89, respectively. It is further predicted that the bromine sits toward a lipophilic pocket near Met86 and that the pyrazole may bind in two orientations projected toward the solvent interface. The sulfonamide NH2 is predicted to make two hydrogen bonds, one with the side chain of Asn140 and the second with the side chain of Asp157, helping to explain the high affinity imparted by this moiety. The combination of the substituted pyrazole moiety on the N2-amine and the benzyl-sulfonamide moiety on the N4-amine of the pyrimidine conferred GSK8612 with high TBK1 affinity and good kinase selectivity. This was revealed in the kinobead selectivity profile of GSK8612 in extracts from a mixture of cell lines and tissue (Figure 2 and Table S1 for a full list of kinases). An average pKd of 8.0 was determined for GSK8612 against TBK1. Within 10-fold affinity with respect to TBK1, no off-targets of GSK8612 could be identified. The highest affinity protein in our study was STK17B with an average pKd of 6.2, and 100-fold selectivity was determined over the close family member IKKε (average pKd = 6.0). Binding of GSK8612 to AAK1, the highest affinity target for both BX795 and MRT67307, showed a pKd of 5.1, 1000-fold lower than for TBK1. Profiling of GSK8612 using the lipid kinase affinity matrix in mixed cell extracts revealed that GSK8612 bound to one lipid kinase, phosphatidylinositol 4-kinase beta (PI4Kβ), though with only an average pKd of 5.3 (Table S1). The preference of GSK8612 to bind to activated or nonactivated TBK1 was investigated using extracts from Ramos cells with and without Calyculin A treatment (Table S1 and Figure S2). This revealed that GSK8612 has lower affinity for phosphorylated TBK1. An average pKd for TBK1 of 6.8 was determined in the extracts from Calyculin A treated cells compared to 7.7 in the extracts without Calyculin A treatment. In line with the affinity determined in extracts from Calyculin A-activated cells, GSK8612 inhibited recombinant TBK1 with an average pIC50 of 6.8 in a biochemical functional assay.36 Interestingly, the pKd of the closest off-targets of GSK8612, such as IKKε, did not show any significant change between Calyculin A treated and control Ramos cells. Therefore, the actual selectivity of GSK8612 for TBK1 in the cells will depend on the activation state of TBK1. The effects of TBK1 inhibition with GSK8612 in live cells were then investigated. First, Ramos cells were stimulated with the TLR3 ligand poly(I:C), and the phosphorylation of IRF3 was measured by Western blot. GSK8612 was able to inhibit phospho-IRF3 with an average pIC50 of 6.0 (Figure 3A), confirming effective inhibition of TBK1 kinase activity in live cells. Next, the ability of GSK8612 to block TBK1-dependent functional responses was evaluated also in primary cells. Type I IFN secretion was measured in poly(I:C)-stimulated human peripheral blood mononuclear cells (PBMCs).37 Since IFNβ was below the limit of detection of the assay (MSD readout, data not shown) and IFNα could be robustly detected by flow cytometry (CBA assay), the latter was chosen as readout. GSK8612 inhibited the release of IFNα with a pIC50 of 6.1, demonstrating submicromolar potency in primary immune cells (Figure 3B). Of note, IFNα secretion from TLR3-stimulated PBMC is believed to result from a priming effect by IFNβ, a well characterized IFN positive feedback response.7,9,10 Accordingly, a blocking antibody against IFNAR ablated poly(I:C)-triggered IFNα release in PBMC, confirming IFNα secretion to be a secondary, though biologically relevant downstream effect of TBK1 inhibition (Figure S4). Together, TBK1 inhibition interferes with the activation of the TLR3-IFN axis and disrupts the IFN positive feedback response, resulting in the abrogation of IFNα secretion. It is also well-known that TBK1 propagates biological signaling of IFNβ downstream of DNA sensing by cGAS and STING.38 To test whether GSK8612 inhibits this pathway, THP-1 cells were stimulated with dsDNA containing virus (bacmam) or the natural STING ligand cGAMP. GSK8612 was able to completely inhibit secretion of IFNβ with a measured pIC50 of 5.9 and 6.3 for the dsDNA virus and cGAMP stimulated cells, respectively (Figure 4).
Figure 2.
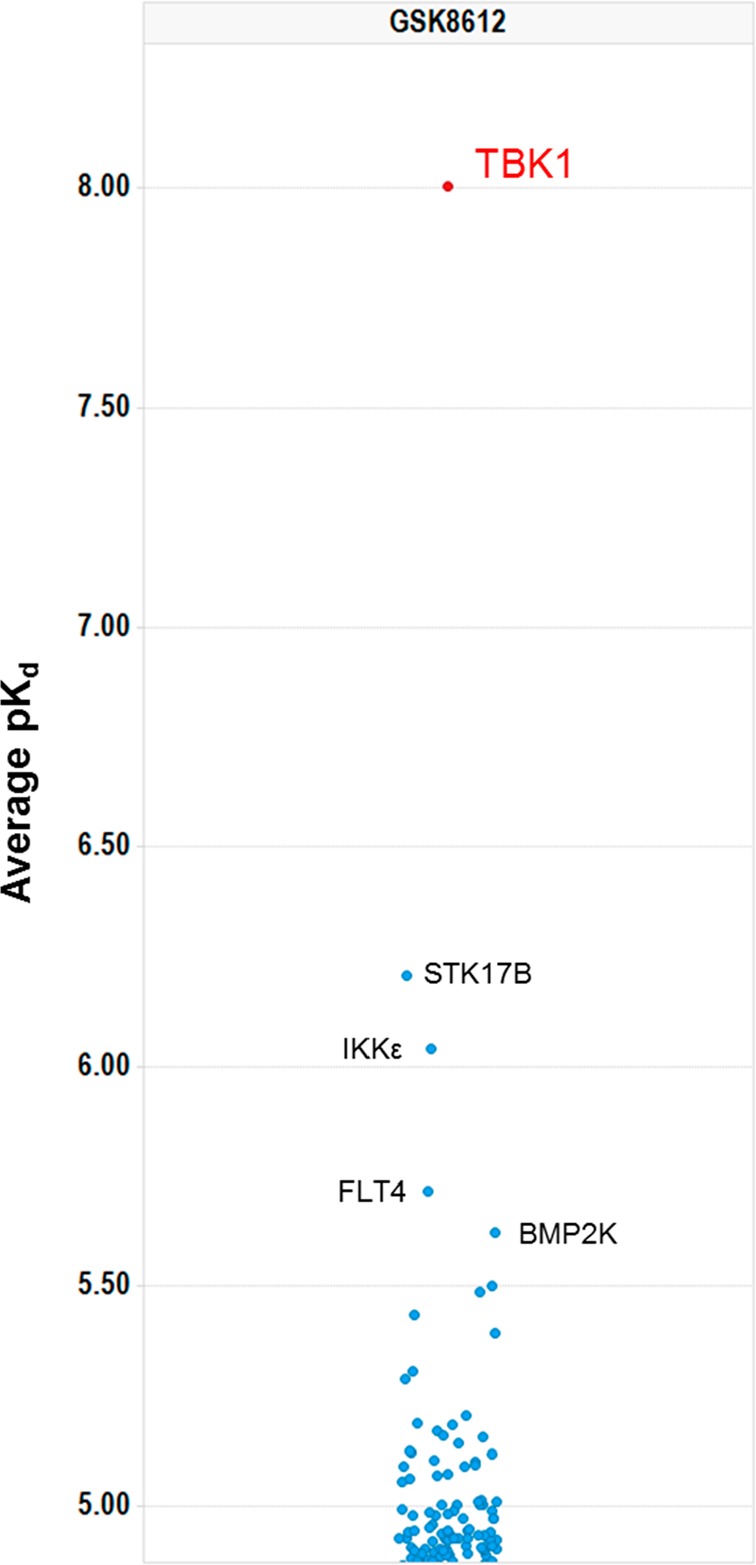
Chemoproteomics based kinase selectivity of GSK8612 determined using kinobeads in mixed HEK293, K-562, HepG2, and placenta cell extracts and lipid kinobeads in mixed HeLa, Jurkat, and K-562 cell extracts. pKd values (shown on the y-axis) were determined for 285 kinases.
Figure 3.
Cell-based activity of GSK8612. (A) GSK8612 inhibits IRF3 phosphorylation in Ramos cells. Western blot analysis reveals inhibition of IRF3 phosphorylation (Ser396) by GSK8612 in poly(I:C)-stimulated Ramos cells with an average pIC50 = 6.0 (n = 5). Western blot densitometry data for pIRF3 normalized to total IRF3 are displayed as percentage of maximal inhibition. (B) GSK8612 inhibits IFNα secretion in human PBMC with an average pIC50 = 6.1 (n = 3). IFNα was measured in supernatants of poly(I:C)-stimulated human PBMCs at 16 h by FACS-based Cytometric Bead Array (CBA) assay. Percent of maximal inhibition was derived from normalizing mean fluorescence intensity (MFI) data to vehicle-treated, poly(I:C)-stimulated samples (0% inhibition).
Figure 4.
GSK8612 inhibits secretion of IFNβ from THP-1 cells. GSK8612 inhibits secretion of IFNβ (pg/mL) in THP-1 cells stimulated with (A) dsDNA-containing virus (Baculovirus) with a pIC50 = 5.9 (n = 3) or (B) 60 μg/mL cGAMP with a pIC50 = 6.3 (n = 3).
In summary, the biological activity of GSK8612 was demonstrated, resulting in inhibition of IRF3 phosphorylation in Ramos cells, IFNα secretion from human PBMCs, and IFNβ secretion from THP-1 cells with low micromolar potency. GSK8612 is a highly selective TBK1 inhibitor, thus representing an ideal tool to further dissect the physiological roles of TBK1 in biological models of immunity, neuroinflammation, obesity, and cancer.
Acknowledgments
We thank Cellzome mass spectrometry department for help with mass spectrometry sample preparation and mass spectrometry instruments; also, the cell and tissue culture group for preparation of cell extracts. We would like to thank colleagues within GSK who performed the in vitro and in vivo DMPK experiments and determined the compounds’ physicochemical properties.
Glossary
ABBREVIATIONS
- IKK
inhibitor of nuclear factor kappa-B kinase
- IFNα
interferon-alpha
- IFNβ
interferon-beta
- IFNAR
interferon-α/β receptor
- IL-12
interleukin 12
- IRF3
interferon regulatory factor 3
- ISG
interferon stimulated gene
- poly(I:C)
polyinosinic–polycytidylic acid
- TBK1
TANK-binding kinase 1
- TLR
toll-like receptor
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00027.
Synthesis and characterization of GSK8612, protocols for selectivity profiling, cellular assays and in vitro physchem properties determination (PDF) Figures on kinase selectivity of BX795 and MRT67307, docking studies and inhibition of IFNα secretion by IFNAR-blocking antibody (PDF)
List of proteins identified and pKd determined in kinase-binding profiling (XLSX)
Author Contributions
⊥ These authors contributed equally to manuscript. K.S., J.P., D.P., V.K., and G.S.P. contributed to the design, execution, and analysis of the cellular experiments; N.Z., C.R., and B.D. contributed to the design, execution, and analysis of the proteomic experiments; A.P.G. executed and analyzed computation experiments; D.W.T. and A.J.W. contributed to the chemical design and synthesis; M.M. led the chemistry efforts; J.M.R. and M.B. contributed to data interpretation; D.W.T., M.M., D.P., N.Z., A.J.W., G.S.P., and G.B. wrote the manuscript; G.B. designed and supervised the study. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): The authors are employees or former employees of Cellzome GmbH and GlaxoSmithKline, and the company funded the work.
Supplementary Material
References
- Zhan Z.; Cao H.; Xie X.; Yang L.; Zhang P.; Chen Y.; Fan H.; Liu Z.; Liu X. Phosphatase PP4 Negatively Regulates Type I IFN Production and Antiviral Innate Immunity by Dephosphorylating and Deactivating TBK1. J. Immunol. 2015, 195 (8), 3849–3857. 10.4049/jimmunol.1403083. [DOI] [PubMed] [Google Scholar]
- Tanaka Y.; Chen Z. J. STING Specifies IRF3 Phosphorylation by TBK1 in the Cytosolic DNA Signaling Pathway. Sci. Signaling 2012, 5 (214), ra20. 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C.; Burns C.; Wicks I. TANK-Binding Kinase 1-Dependent Responses in Health and Autoimmunity. Front. Immunol. 2018, 10.3389/fimmu.2018.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S. M.; Chiang S.-H.; Decker S. J.; Chang L.; Uhm M.; Larsen M. J.; Rubin J. R.; Mowers J.; White N. M.; Hochberg I.; Downes M.; Yu R. T.; Liddle C.; Evans R. M.; Oh D.; Li P.; Olefsky J. M.; Saltiel A. R. An Inhibitor of the Protein Kinases TBK1 and IKK-ε Improves Obesity-Related Metabolic Dysfunctions in Mice. Nat. Med. 2013, 19 (3), 313–321. 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M. O.; Sweet M. J.; Mansell A.; Kellie S.; Kobe B. TRIF-Dependent TLR Signaling, Its Functions in Host Defense and Inflammation, and Its Potential as a Therapeutic Target. J. Leukocyte Biol. 2016, 100 (1), 27–45. 10.1189/jlb.2RI1115-531R. [DOI] [PubMed] [Google Scholar]
- Longhi M. P.; Trumpfheller C.; Idoyaga J.; Caskey M.; Matos I.; Kluger C.; Salazar A. M.; Colonna M.; Steinman R. M. Dendritic Cells Require a Systemic Type I Interferon Response to Mature and Induce CD4+ Th1 Immunity with Poly IC as Adjuvant. J. Exp. Med. 2009, 206 (7), 1589–1602. 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskardin T. L. W.; Niewold T. B. Type I Interferon in Rheumatic Diseases. Nat. Rev. Rheumatol. 2018, 14 (4), 214–228. 10.1038/nrrheum.2018.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T.; Akira S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11 (5), 373–384. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Richez C.; Yasuda K.; Watkins A. A.; Akira S.; Lafyatis R.; van Seventer J. M.; Rifkin I. R. TLR4 Ligands Induce IFN-alpha Production by Mouse Conventional Dendritic Cells and Human Monocytes after IFN-beta Priming. J. Immunol. 2009, 182 (2), 820–828. 10.4049/jimmunol.182.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F.; Li B.; Yu Y.; Iyer S. S.; Sun M.; Cheng G. Positive Feedback Regulation of Type I Interferon by the Interferon-Stimulated Gene STING. EMBO Rep. 2015, 16 (2), 202–212. 10.15252/embr.201439366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M.; Yan N. Therapeutic Potential of Targeting TBK1 in Autoimmune Diseases and Interferonopathies. Pharmacol. Res. 2016, 111, 336–342. 10.1016/j.phrs.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K. A.; McWhirter S. M.; Faia K. L.; Rowe D. C.; Latz E.; Golenbock D. T.; Coyle A. J.; Liao S.-M.; Maniatis T. IKKε and TBK1 Are Essential Components of the IRF3 Signaling Pathway. Nat. Immunol. 2003, 4 (5), 491–496. 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Yu T.; Yi Y.-S.; Yang Y.; Oh J.; Jeong D.; Cho J. Y. The Pivotal Role of TBK1 in Inflammatory Responses Mediated by Macrophages. Mediators Inflammation 2012, 2012, 1–8. 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.; Xiao Y.; Chang J.-H.; Yu J.; Hu H.; Starr R.; Brittain G. C.; Chang M.; Cheng X.; Sun S.-C. The Kinase TBK1 Controls IgA Class Switching by Negatively Regulating Noncanonical NF-ΚB Signaling. Nat. Immunol. 2012, 13 (11), 1101–1109. 10.1038/ni.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlik E.; Thakker P.; Carlson T.; Jiang Z.; Ryan M.; Marusic S.; Goutagny N.; Kuang W.; Askew G. R.; Roberts V.; Benoit S.; Zhuo T.; Ling V.; Pfeifer R.; Stedman N.; Fitzgerald K. A.; Lin L.-L.; Hall J. P. Mice Lacking Tbk1 Activity Exhibit Immune Cell Infiltrates in Multiple Tissues and Increased Susceptibility to LPS-Induced Lethality. J. Leukocyte Biol. 2010, 88 (6), 1171–1180. 10.1189/jlb.0210071. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Wong K. I.; Sun X.; Reilly S. M.; Uhm M.; Liao Z.; Skorobogatko Y.; Saltiel A. R. TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell 2018, 172 (4), 731–743. 10.1016/j.cell.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz V. H.; Brekken R. A. Assessment of TANK-Binding Kinase 1 as a Therapeutic Target in Cancer. J. Cell Commun. Signal. 2018, 12 (1), 83–90. 10.1007/s12079-017-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad L.; Zhang S.-Y.; Casanova J.-L.; Sancho-Shimizu V. Human TBK1: A Gatekeeper of Neuroinflammation. Trends Mol. Med. 2016, 22 (6), 511–527. 10.1016/j.molmed.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.; Yang Y.; Yin D. Q.; Hong S.; Son Y.-J.; Kim J.-H.; Cho J. Y. TBK1 Inhibitors: A Review of Patent Literature (2011 – 2014). Expert Opin. Ther. Pat. 2015, 25 (12), 1385–1396. 10.1517/13543776.2015.1081168. [DOI] [PubMed] [Google Scholar]
- Crew A. P.; Raina K.; Dong H.; Qian Y.; Wang J.; Vigil D.; Serebrenik Y. V.; Hamman B. D.; Morgan A.; Ferraro C.; Siu K.; Neklesa T. K.; Winkler J. D.; Coleman K. G.; Crews C. M. Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. J. Med. Chem. 2018, 61 (2), 583–598. 10.1021/acs.jmedchem.7b00635. [DOI] [PubMed] [Google Scholar]
- Arrowsmith C. H.; Audia J. E.; Austin C.; Baell J.; Bennett J.; Blagg J.; Bountra C.; Brennan P. E.; Brown P. J.; Bunnage M. E.; Buser-Doepner C.; Campbell R. M.; Carter A. J.; Cohen P.; Copeland R. A.; Cravatt B.; Dahlin J. L.; Dhanak D.; Edwards A. M.; Frederiksen M.; Frye S. V.; Gray N.; Grimshaw C. E.; Hepworth D.; Howe T.; Huber K. V. M.; Jin J.; Knapp S.; Kotz J. D.; Kruger R. G.; Lowe D.; Mader M. M.; Marsden B.; Mueller-Fahrnow A.; Müller S.; O’Hagan R. C.; Overington J. P.; Owen D. R.; Rosenberg S. H.; Ross R.; Roth B.; Schapira M.; Schreiber S. L.; Shoichet B.; Sundström M.; Superti-Furga G.; Taunton J.; Toledo-Sherman L.; Walpole C.; Walters M. A.; Willson T. M.; Workman P.; Young R. N.; Zuercher W. J. The Promise and Peril of Chemical Probes. Nat. Chem. Biol. 2015, 11 (8), 536–541. 10.1038/nchembio.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini G.; Bell K.; Shimamura S.; Werner T.; Cansfield A.; Müller K.; Perrin J.; Rau C.; Ellard K.; Hopf C.; Doce C.; Leggate D.; Mangano R.; Mathieson T.; O’Mahony A.; Plavec I.; Rharbaoui F.; Reinhard F.; Savitski M. M.; Ramsden N.; Hirsch E.; Drewes G.; Rausch O.; Bantscheff M.; Neubauer G. A Selective Inhibitor Reveals PI3Kγ Dependence of TH17 Cell Differentiation. Nat. Chem. Biol. 2012, 8 (6), 576–582. 10.1038/nchembio.957. [DOI] [PubMed] [Google Scholar]
- Cansfield A. D.; Ladduwahetty T.; Sunose M.; Ellard K.; Lynch R.; Newton A. L.; Lewis A.; Bennett G.; Zinn N.; Thomson D. W.; Rüger A. J.; Feutrill J. T.; Rausch O.; Watt A. P.; Bergamini G. CZ415, a Highly Selective MTOR Inhibitor Showing in Vivo Efficacy in a Collagen Induced Arthritis Model. ACS Med. Chem. Lett. 2016, 7 (8), 768–773. 10.1021/acsmedchemlett.6b00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. W.; Wagner A. J.; Bantscheff M.; Benson R. E.; Dittus L.; Duempelfeld B.; Drewes G.; Krause J.; Moore J. T.; Mueller K.; Poeckel D.; Rau C.; Salzer E.; Shewchuk L.; Hopf C.; Emery J. G.; Muelbaier M. Discovery of a Highly Selective Tankyrase Inhibitor Displaying Growth Inhibition Effects against a Diverse Range of Tumor Derived Cell Lines. J. Med. Chem. 2017, 60 (13), 5455–5471. 10.1021/acs.jmedchem.7b00137. [DOI] [PubMed] [Google Scholar]
- McClure R. A.; Williams J. D. Impact of Mass Spectrometry-Based Technologies and Strategies on Chemoproteomics as a Tool for Drug Discovery. ACS Med. Chem. Lett. 2018, 9 (8), 785–791. 10.1021/acsmedchemlett.8b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.; Plater L.; Peggie M.; Cohen P. Use of the Pharmacological Inhibitor BX795 to Study the Regulation and Physiological Roles of TBK1 and IκB Kinase ϵ: A DISTINCT UPSTREAM KINASE MEDIATES SER-172 PHOSPHORYLATION AND ACTIVATION. J. Biol. Chem. 2009, 284 (21), 14136–14146. 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J.; Plater L.; Elliott M.; Shpiro N.; Hastie C. J.; Mclauchlan H.; Klevernic I.; Arthur J. S. C.; Alessi D. R.; Cohen P. The Selectivity of Protein Kinase Inhibitors: A Further Update. Biochem. J. 2007, 408 (3), 297–315. 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.; Peggie M.; Plater L.; Sorcek R. J.; Young E. R. R.; Madwed J. B.; Hough J.; McIver E. G.; Cohen P. Novel Cross-Talk within the IKK Family Controls Innate Immunity. Biochem. J. 2011, 434 (1), 93–104. 10.1042/BJ20101701. [DOI] [PubMed] [Google Scholar]
- Clark K.; Takeuchi O.; Akira S.; Cohen P. The TRAF-Associated Protein TANK Facilitates Cross-Talk within the I B Kinase Family during Toll-like Receptor Signaling. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (41), 17093–17098. 10.1073/pnas.1114194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantscheff M.; Eberhard D.; Abraham Y.; Bastuck S.; Boesche M.; Hobson S.; Mathieson T.; Perrin J.; Raida M.; Rau C.; Reader V.; Sweetman G.; Bauer A.; Bouwmeester T.; Hopf C.; Kruse U.; Neubauer G.; Ramsden N.; Rick J.; Kuster B.; Drewes G. Quantitative Chemical Proteomics Reveals Mechanisms of Action of Clinical ABL Kinase Inhibitors. Nat. Biotechnol. 2007, 25 (9), 1035–1044. 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- Kostich W.; Hamman B. D.; Li Y.-W.; Naidu S.; Dandapani K.; Feng J.; Easton A.; Bourin C.; Baker K.; Allen J.; Savelieva K.; Louis J. V.; Dokania M.; Elavazhagan S.; Vattikundala P.; Sharma V.; Lal Das M.; Shankar G.; Kumar A.; Holenarsipur V. K.; Gulianello M.; Molski T.; Brown J. M.; Lewis M.; Huang Y.; Lu Y.; Pieschl R.; O’Malley K.; Lippy J.; Nouraldeen A.; Lanthorn T. H.; Ye G.; Wilson A.; Balakrishnan A.; Denton R.; Grace J. E.; Lentz K. A.; Santone K. S.; Bi Y.; Main A.; Swaffield J.; Carson K.; Mandlekar S.; Vikramadithyan R. K.; Nara S. J.; Dzierba C.; Bronson J.; Macor J. E.; Zaczek R.; Westphal R.; Kiss L.; Bristow L.; Conway C. M.; Zambrowicz B.; Albright C. F. Inhibition of AAK1 Kinase as a Novel Therapeutic Approach to Treat Neuropathic Pain. J. Pharmacol. Exp. Ther. 2016, 358 (3), 371–386. 10.1124/jpet.116.235333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R. Classification of Small Molecule Protein Kinase Inhibitors Based upon the Structures of Their Drug-Enzyme Complexes. Pharmacol. Res. 2016, 103, 26–48. 10.1016/j.phrs.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Cohen P.; Alessi D. R. Kinase Drug Discovery – What’s Next in the Field?. ACS Chem. Biol. 2013, 8 (1), 96–104. 10.1021/cb300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson F. M.; Gray N. S. Kinase Inhibitors: The Road Ahead. Nat. Rev. Drug Discovery 2018, 17 (5), 353–377. 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- Larabi A.; Devos J. M.; Ng S.-L.; Nanao M. H.; Round A.; Maniatis T.; Panne D. Crystal Structure and Mechanism of Activation of TANK-Binding Kinase 1. Cell Rep. 2013, 3 (3), 734–746. 10.1016/j.celrep.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Anastassiadis T.; Deacon S. W.; Devarajan K.; Ma H.; Peterson J. R. Comprehensive Assay of Kinase Catalytic Activity Reveals Feactures of Kinase Inhibitor Selectivity. Nat. Biotechnol. 2011, 29, 1039–1045. 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan C.; Krutzik S.; Schenk M.; Scumpia P. O.; Lu J.; Pang Y. L. J.; Russell B. S.; Lim K. S.; Shell S.; Prestwich E.; Su D.; Elashoff D.; Hershberg R. M.; Bloom B. R.; Belisle J. T.; Fortune S.; Dedon P. C.; Pellegrini M.; Modlin R. L. Mycobacterium Tuberculosis Transfer RNA Induces IL-12p70 via Synergistic Activation of Pattern Recognition Receptors within a Cell Network. J. Immunol. 2018, 200 (9), 3244–3258. 10.4049/jimmunol.1701733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Sun L.; Chen Z. J. Regulation and Function of the CGAS–STING Pathway of Cytosolic DNA Sensing. Nat. Immunol. 2016, 17 (10), 1142–1149. 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.