Abstract
Aims
Myocardial ischemia/reperfusion (I/R) injury is a leading cause of cardiomyocyte loss and subsequent ventricular dysfunction after restoring the coronary blood flow and contributes to considerable increase in morbidity and mortality. Resveratrol has been declared to confer cardioprotection against in vivo and ex vivo myocardial I/R injury. Here, we have sought to investigate the effects of preconditioning with resveratrol on myocardial I/R damage across the small animal studies.
Methods and Results
The MEDLINE, Google Scholar, PubMed, and Cochrane databases were searched for preclinical studies investigating resveratrol vs. vehicle published from the inception to July 2018. Eventually, 10 in vivo and 7 ex vivo studies with 261 animals (130 for resveratrol; 131 for vehicle) were included for meta-analysis. Pooled estimates for primary outcomes demonstrated that pretreatment with resveratrol significantly reduced the infarct size after myocardial I/R injury irrespective of in vivo (weighted mean difference (WMD): -13.42, 95% CI: -16.63 to -10.21, P ≤ 0.001) or ex vivo (WMD: -15.05, 95% CI: -18.23 to -11.86, P ≤ 0.001) studies. Consistently, stratified analysis according to the reperfusion duration, route of administration, or timing regimen of pretreatment all showed the infarct-sparing benefit of resveratrol. Metaregression did not indicate any difference in infarct size based on species, sample size, state, route of administration, reperfusion duration, and timing regimen of pretreatment. Meanwhile, sensitivity analysis also identified the cardioprotection of resveratrol with robust results in spite of significant heterogeneity.
Conclusions
Preconditioning with resveratrol appears to prevent the heart from I/R injury in comparison with vehicle, as evidenced by limited infarct size in a preclinical setting. Studies with large animals or randomized controlled trials will add more evidence and provide the rationale for clinical use.
1. Introduction
Acute myocardial infarction is the leading cause of disability and mortality worldwide [1]. Although timely and effective revascularization (i.e., percutaneous coronary intervention, thrombolytic therapy, or coronary artery bypass graft) results in reduction in infarct size, the process of myocardial reperfusion is associated with a further death of cardiomyocytes, which contributes up to 50% of final myocardial damage [2, 3]. So far, the cellular and molecular mechanism underlying myocardial I/R injury remains unclear; experimental evidences show that oxidative stress, inflammation, apoptosis, or calcium overload is deeply involved [2–5]. For decades, novel strategies mitigating lethal reperfusion injury in addition to current reperfusion treatments have been intensively investigated.
Resveratrol is a natural polyphenolic compound, mainly found in edible plants such as peanut, grape, and berry [6]. Moreover, it is also abundant in red wine. Previous studies have demonstrated that resveratrol attenuates the pathological progression in a variety of disease models (i.e., diabetes mellitus, cancer, or neurodegenerative disease) [7–9]. Importantly, it has been currently reported to confer a promising cardioprotective effect against ischemic heart disease in vivo, especially myocardial I/R injury, by modulating angiogenesis, oxidative stress, inflammatory, cardiomyocyte apoptosis, and mitochondrial function, along with energy metabolism [10–16]. Furthermore, it also prevents the heart from fibrotic remodeling and hypertrophy. Well-designed experimental studies could provide a deep insight into the efficacy of resveratrol; nevertheless, there are still many discrepancies between the preclinical and the clinical studies due to the complexity of the clinical situation which therefore preclude further application.
Thus, we conduct a comprehensive systematic review and meta-analysis to assess the critical role of resveratrol on myocardial I/R injury across the in vivo and ex vivo small animal studies.
2. Methods
2.1. Search Strategy
We systematically searched the MEDLINE, Google Scholar, PubMed, and Cochrane databases for evidence of the cardioprotective effect of resveratrol in an animal model of myocardial I/R injury published from the inception to July 2018, without any language restriction. The following terms were used for the search: “ischemia/reperfusion injury” or “ischemia-reperfusion injury” or “I/R injury” AND “resveratrol”. In addition, we scrutinized the reference of review articles, meeting abstracts, and comments for additional citations.
2.2. Inclusion and Exclusion Criteria
Studies that met the following inclusion criteria were included for further meta-analysis: (1) reported the infarct size determined by a recognized method (i.e., Evans blue/TTC staining for in vivo studies or only TTC staining for ex vivo studies). After reperfusion, the coronary artery was reoccluded, and Evans blue was injected intravenously to identify the area at risk. The heart was then excised, sliced, and incubated in TTC to denote the infarct size for in vivo studies. And for ex vivo studies, only TTC staining was used for evaluating the infarct size after reperfusion; (2) resveratrol vs. vehicle treatment; (3) nonhuman setting; (4) all the procedures and animal care were confirmed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH publication No. 85-23, revised 1996), and the animals were anesthetized before sacrifice, and the heart was excised for further analysis; and (5) no additional anti-inflammatory drugs were used. The exclusion criteria are as follows: (1) studies including animals with cardiovascular comorbidity (i.e., diabetes or obesity), (2) animals treated with resveratrol analogues, and (3) in vitro studies.
2.3. Data Extraction
Two reviewers (Zhi-Jie Mao and Hui Lin) extracted the data independently from included studies, and discrepancies were resolved by consensus. The following information of each study was extracted and summarized in Table 1: (1) studies' characteristics (i.e., author's name, state, year of publication, number of included animals, and duration of I/R injury), (2) animals' characteristics (i.e., species, sex, body weight/age, and anesthetics), (3) information on interventions (i.e., route of administration, dosage, type of vehicle, and time of treatment), and (4) data about the infarct size of both approaches (to minimize the publication bias, mean and standard deviation rather than standard error were used for further analysis).
Table 1.
Characteristics of the in vivo studies included in the meta-analysis.
| Author | Year | State | Species | Weight/year | Anesthetic | Animal numbers | I/R duration | Vehicle | Resveratrol treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Resveratrol | Dosage | Approach | Time | ||||||||
| Dong et al. [10] | 2015 | China | Rats, SD, M | 270-300 g | Pentobarbital | 10 | 10 | 30 min/2 h | DMSO | 10 mg/L | i.p. | 60 min before I/R |
| Cheng et al. [11] | 2015 | China | Rats, SD, M | 250-300 g | Sodium pentobarbital | 10 | 10 | 30 min/2 h | 0.9% NaCl | 100 μmol/L | i.v. | 5 min before I/R |
| Li et al. [12] | 2014 | China | Rats, SD, M | 250-300 g | Sodium pentobarbital | 10 | 10 | 30 min/2 h | 0.9% NaCl | 100 μmol/L | i.v. | 5 min before I/R |
| Cong et al. [13] | 2014 | China | Rats, SD, M | 250-300 g | Sodium pentobarbital | 10 | 10 | 30 min/2 h | 0.9% NaCl | 100 μmol/L | i.v. | 5 min before I/R |
| Du et al. [29] | 2014 | China | Mice, M | 3 months | 2% isoflurane | 8 | 8 | 30 min/24 h | Ethanol | 10 mg/L | i.p. | 60 min before I/R |
| Shalwala et al. [28] | 2014 | USA | Mice, ICR, M | 35.5 ± 5 g | Sodium pentobarbital | 6 | 6 | 30 min/24 h | DMSO | 5 mg/L | i.p. | 24 h before I/R |
| Naumenko et al. [30] | 2013 | Russia | Rats, Wistar, M | 339.5 ± 9.8 g | Urethane | 10 | 9 | 30 min/2 h | DMSO | 10 μmol/L | i.v. | 30 min before I/R |
| Shen et al. [31] | 2006 | China | Rats, SD, M | 275-300 g | Sodium pentobarbital | 8 | 8 | 30 min/2 h | DMSO | 10 μmol/L | i.v. | 15 min before I/R |
| Hung et al. [26] | 2004 | China | Rats, SD, M | 270-300 g | Urethane | 8 | 8 | 60 min/3 h | DMSO | 1 mg/L | i.p. | 60 min before I/R |
| Hale and Kloner [32] | 2001 | USA | Rabbits, NZW, M | 2.2-3.0 kg | Ketamine/xylazine | 8 | 8 | 30 min/3 h | Ethanol | 1.5 mg/L | i.v. | 15 min before I/R |
SD: Sprague-Dawley rats; ICR: Institute of Cancer Research mice; NZW: New Zealand White rabbit; M: male; DMSO: dimethyl sulfoxide; i.p.: intraperitoneal injection; i.v.: intravenous injection.
2.4. Quality Assessment
The quality of included studies was assessed and graded by two reviewers (Yi-He Chen and Hui Lin) based on published criteria for animal experiments [17]. Each of the following was scored as one point: peer-reviewed publication, random allocation to groups, blinded assessment of outcome, sample size calculation, compliance with animal welfare regulations, and a statement of a potential conflict of interest. Discrepancies were resolved by consensus or another reviewer (Jian-Wen Hou) when necessary.
2.5. Statistical Analysis
We performed two separate analyses for in vivo and ex vivo studies, respectively. Weighted mean difference (WMD) measured the difference of means for infarct size from each included studies and therefore reflects the efficacy of resveratrol treatment. The WMD and respective 95% CIs were measured for continuous variables by using DerSimonian and Laird random effects meta-analysis. The extent of heterogeneity among studies was assessed with Cochran's Q test and further quantified by I2 statistics, which determined the inconsistency across results and presented the proportion of total variation in study estimates that was due to heterogeneity rather than sampling error. Evidence for potential publication bias was evaluated by Begg's and Egger's test. Begg's test assessed if there was a significant correlation between the ranks of the effect estimates and the ranks of their variances. Egger's test used linear regression to assess the relation between the standardized effect estimates and the standard error. Thus, both tests indicated whether the pooled results were affected by publication bias. We stratified the meta-analysis of the primary results by the route of administration (i.p. or i.v.) and timing regimen of pretreatment (short-term or long-term). Sensitivity analysis was conducted by removing one study in turn to estimate the influence of each study. Metaregressions were conducted to explore the impact of potential effect modifiers (species, sample size, state, route of administration, reperfusion duration, and timing regimen of pretreatment) on outcomes and the possible sources of heterogeneity. Statistical analyses were performed with STATA version 12.0 (STATA Corporation, College Station, TX, USA), with P values ≤ 0.05 considered statistically significant.
3. Results
Of 201 records identified in the initial search, 147 were removed after title and abstract screening, and the remaining 54 records were retrieved for more detailed evaluation. As a result, 17 literatures (including 10 in vivo and 7 ex vivo studies) met our selection criteria (Figure 1). Baseline characteristics of each study were summarized in Table 1 and Table 2. A total of 261 animals were enrolled for comparing resveratrol (n = 130) vs. vehicle (n = 131) in the setting of myocardial I/R injury. All the eligible studies except one used rodents; however, all the animals included were male. Myocardial I/R injury was achieved by establishing the in vivo left coronary artery occlusion-reperfusion model or ex vivo Langendorff-perfused heart model. For in vivo studies, animals were preconditioned with resveratrol by either intravenous or intraperitoneal injection. Accordingly, Evans blue/TTC staining was used for assessing the infarct size post-myocardial I/R injury in vivo; nonetheless, only TTC staining was performed in ex vivo studies, while for ex vivo studies, resveratrol was orally administrated or perfused before the assault. The dosing and time regimen of resveratrol treatment varied substantially among the studies. In addition, the majority of these studies were conducted in China (n = 9), with the remaining in the USA (n = 6), Africa (n = 1), or Russia (n = 1). The score of included studies ranged from 2 to 4, with a median of 3 out of 6, which may possibly suggest a low risk of bias (Table 3). Furthermore, Table 4 and Table 5 listed the potential molecular and cellular mechanisms of resveratrol in protecting the heart from I/R damage in in vivo and ex vivo studies.
Figure 1.
Flow diagram of the study selection process.
Table 2.
Characteristics of the ex vivo studies included in the meta-analysis.
| Author | Year | State | Species | Weight/year | Anesthetic | Animal numbers | I/R duration | Vehicle | Resveratrol treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Resveratrol | Dosage | Approach | Time | ||||||||
| Yang et al. [14] | 2016 | China | Rats, SD, M | 10 weeks | Chloral hydrate | 10 | 10 | 30 min/1 h | K-H solution | 10 μmol/L | Perfusion | 15 min before I/R |
| Liao et al. [15] | 2014 | China | Mice, KM, M | NA | Sodium pentobarbital | 6 | 6 | 15 min/30 min | Ethanol | 2.0 mg/kg.d | Gavage | 6 weeks before I/R |
| Adam et al. [16] | 2012 | Africa | Rats, LE, M | 9 weeks | Sodium pentobarbital | 6 | 6 | 30 min/1 h | K-H solution | 10 mmol/L | Perfusion | 25 min before I/R |
| Mukhopadhyay et al. [33] | 2010 | USA | Rats, SD, M | 250-300 g | Sodium pentobarbital | 6 | 6 | 30 min/2 h | NA | 5 mg/kg/d | Gavage | 21 days before I/R |
| Lekli et al. [34] | 2009 | USA | Rats, SD, M | 250-300 g | Sodium pentobarbital | 3 | 3 | 30 min/2 h | NA | 2.5 mg/kg/d | Gavage | 15 days before I/R |
| Das et al. [35] | 2006 | USA | Rats, SD, M | 250-300 g | Sodium pentobarbital | 6 | 6 | 30 min/2 h | K-H solution | 10 μmol/L | Perfusion | 20 min before I/R |
| Ray et al. [36] | 1999 | USA | Rats, SD, M | 275–300 g | Sodium pentobarbital | 3 | 3 | 30 min/2 h | K-H solution | 10 μmol/L | Perfusion | 15 min before I/R |
SD: Sprague-Dawley rats; KM: Kun-Ming; M: male; LE: Long-Evans; K-H solution: Krebs-Henseleit solution; DMSO: dimethyl sulfoxide.
Table 3.
The research quality of included studies.
| Authors | A | B | C | D | E | F | Score |
|---|---|---|---|---|---|---|---|
| In vivo studies | |||||||
| Dong et al. | Y | Y | N | N | Y | Y | 4 |
| Cheng et al. | Y | Y | N | N | Y | Y | 4 |
| Li et al. | Y | Y | N | N | Y | N | 3 |
| Cong et al. | Y | Y | N | N | Y | N | 3 |
| Du et al. | Y | N | N | N | Y | Y | 3 |
| Shalwala et al. | Y | N | Y | N | Y | N | 3 |
| Naumenko et al. | Y | Y | N | N | Y | Y | 4 |
| Shen et al. | Y | Y | N | N | N | N | 2 |
| Hung et al. | Y | N | N | N | Y | N | 2 |
| Hale and Kloner | Y | Y | N | N | Y | N | 3 |
|
| |||||||
| Ex vivo studies | |||||||
| Yang et al. | Y | Y | N | N | Y | N | 3 |
| Liao et al. | Y | Y | N | N | Y | Y | 4 |
| Adam et al. | Y | N | N | N | Y | Y | 3 |
| Mukhopadhyay et al. | Y | N | N | N | Y | Y | 3 |
| Lekli et al. | Y | Y | N | N | Y | N | 3 |
| Das et al. | Y | Y | N | N | Y | N | 3 |
| Ray et al. | Y | Y | N | N | Y | N | 3 |
A: peer-reviewed publication; B: random allocation to groups; C: blinded assessment of outcomes; D: sample size calculation; E: compliance with animal welfare regulations; F: a statement of a potential conflict of interest. Y: yes; N: no.
Table 4.
The proposed molecular and cellular mechanism of the cardioprotective effect of resveratrol in in vivo studies.
| Studies | Year | Dosage | Proposed mechanism |
|---|---|---|---|
| Dong et al. [10] | 2015 | 10 mg/L | Downregulation of inflammatory response (NALP3 inflammasome, IL-1β, IL-18) and caspase-1 expression |
| Cheng et al. [11] | 2015 | 100 μmol/L | Attenuate inflammation (MPO), oxidative stress (SOD, MDA, GSH-PX) possibly via Nrf2/ARE pathway |
| Li et al. [12] | 2014 | 100 μmol/L | Deactivation of TLR4/NF-κB signaling, anti-inflammation (MPO, TNF-α, NO) |
| Cong et al. [13] | 2014 | 100 μmol/L | Activation of cGMP/NO signaling, anti-inflammation (MPO, TNF-α) |
| Du et al. [29] | 2014 | 10 mg/L | Activation of AMPK/Kir6.2-containing K-ATP channel signaling |
| Shalwala et al. [28] | 2014 | 5 mg/L | Activation of SIRT1 signaling |
| Naumenko et al. [30] | 2013 | 10 μmol/L | NA |
| Shen et al. [31] | 2006 | 10 μmol/L | Attenuate oxidative stress (MDA, NO) |
| Hung et al. [26] | 2004 | 1 mg/L | NO-independent |
| Hale and Kloner [32] | 2001 | 1.5 mg/L | NA |
Table 5.
The proposed molecular and cellular mechanism of cardioprotective effect of resveratrol in ex vivo studies.
| Studies | Year | Dosage | Proposed mechanism |
|---|---|---|---|
| Yang et al. [14] | 2016 | 10 μmol/L | Upregulation of VEGF-B signaling, attenuate oxidative stress (ROS, MnSOD) |
| Liao et al. [15] | 2014 | 2.0 mg/kg d | Upregulation of VDAC1 signaling, inhibit mitochondria-mediated apoptosis (mPTP, caspase-3, cytochrome c) |
| Adam et al. [16] | 2012 | 10 mmol/L | SIRT1-independent |
| Mukhopadhyay et al. [33] | 2010 | 5 mg/kg/d | Regulation of miRNA expression (miR-21) |
| Lekli et al. [34] | 2009 | 2.5 mg/kg/d | Regulation of autophagy |
| Das et al. [35] | 2006 | 10 μmol/L | Activation of MAPK (ERK 1/2, p38 MAPK)/MSK1/CREB signaling, antiapoptosis |
| Ray et al. [36] | 1999 | 10 μmol/L | Attenuate oxidative stress (MDA) |
3.1. In Vivo Studies
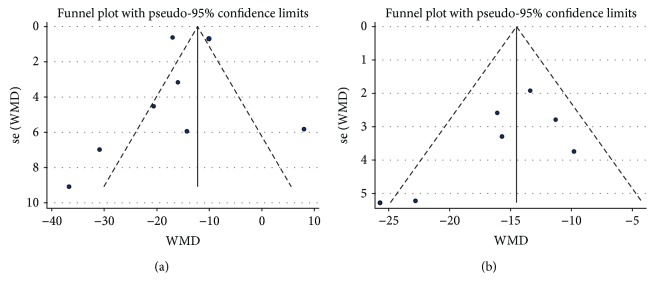
In the pooled analysis using a random effects model, preconditioning with resveratrol in vivo markedly diminished the infarct size when compared with vehicle treatment (WMD: -13.42, 95% CI: -16.63 to -10.21, P ≤ 0.001) (Figure 2). There were evidences of high heterogeneity among the studies (I2 = 92.7%, P ≤ 0.001). Absence of publication bias was identified by Begg's (P = 0.210) and Egger's test (P = 0.673) in spite of a minimal asymmetrical funnel plot (Figure 3(a)). In addition, by systematically excluding each study, the infarct size was still significantly reduced with resveratrol over vehicle treatment in the myocardial I/R injury setting. Notably, stratified analysis suggested that the pooled estimates for improvement of infarct size did not depend on the reperfusion duration, route of administration, or timing regimen of pretreatment (Table 6). Metaregression did not unmask a significant impact of covariates (i.e., species, sample size, state, route of administration, reperfusion duration, and timing regimen of pretreatment) on the beneficial effect of resveratrol (Table 7).
Figure 2.
Pooled estimates of infarct size for resveratrol vs. vehicle in vivo. Treatment with resveratrol was associated with a smaller infarct size in in vivo studies (WMD: -13.42, 95% CI: -16.63 to -10.21, P ≤ 0.001). Gray squares represent WMDs in studies. The 95% CIs for each studies are denoted by lines and those for the pooled WMDs by open diamonds. Meta-analysis is performed by random effects model.
Figure 3.
Funnel plot for assessment of publication bias for the infarct size in vivo (a) and ex vivo (b). Funnel plots were scatter plots (blue points) of the effect sizes of the included studies versus a measure of their precision usually standard error. The funnel plot appeared to have minimal asymmetry in (a); however, it was symmetrical in (b).
Table 6.
Stratified analysis of pooled estimates of infarct size in vivo and ex vivo.
| Pooled estimates | No. of studies | WMD (95% CI) | P value |
|---|---|---|---|
| In vivo studies | |||
| Reperfusion duration | |||
| <24 h | 8 | -12.26 (-15.67, -8.84) | ≤0.001 |
| ≥24 h | 2 | -22.15 (-36.52, -7.77) | 0.003 |
| Route of administration | |||
| i.p. | 4 | -20.57 (-26.81, -14.33) | ≤0.001 |
| i.v. | 6 | -10.14 (-11.98, -8.29) | ≤0.001 |
| Timing regimen of pretreatment | |||
| ≥60 min | 4 | -20.57 (-26.81, -14.33) | ≤0.001 |
| <60 min | 6 | -10.14 (-11.98, -8.29) | ≤0.001 |
| Overall | 10 | -13.42 (-16.63, -10.21) | ≤0.001 |
|
| |||
| Ex vivo studies | |||
| Reperfusion duration | |||
| ≤1 h | 3 | -15.38 (-21.93, -8.82) | ≤0.001 |
| >1 h | 4 | -15.06 (-19.15, -10.96) | ≤0.001 |
| Route of administration | |||
| Perfusion | 4 | -17.75 (-23.11, -12.40) | ≤0.001 |
| Gavage | 3 | -12.92 (-16.70, -9.14) | ≤0.001 |
| Timing regimen of pretreatment | |||
| ≥60 min | 3 | -12.92 (-16.70, -9.14) | ≤0.001 |
| <60 min | 4 | -17.75 (-23.11, -12.40) | ≤0.001 |
| Overall | 7 | -15.05 (-18.23, -11.86) | ≤0.001 |
Stratified analysis investigated whether particular categorical covariates explain any of the heterogeneity of treatments between studies.
Table 7.
Metaregression analysis in vivo and ex vivo.
| Covariates | Infarct size (in vivo studies) | Infarct size (ex vivo studies) | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Species | 6.122819 | -6.043731; 18.28937 | 0.279 | 5.763822 | -8.045439; 19.57308 | 0.332 |
| Sample size | 1.662638 | -1.379227; 4.704503 | 0.243 | 0.2914345 | -0.869869; 1.452738 | 0.547 |
| State | 1.953709 | -11.39187; 15.29928 | 0.744 | -0.774244 | -6.697155; 5.148667 | 0.750 |
| Route of administration | 4.398078 | -12.15415; 20.9503 | 0.557 | 4.566912 | -4.397479; 13.5313 | 0.247 |
| Reperfusion duration | -9.196691 | -29.21135; 10.81797 | 0.320 | 1.047275 | -5.011723; 7.106273 | 0.675 |
| Timing regimen of pretreatment | 2.226924 | -3.267802; 7.721649 | 0.377 | 0.7420125 | -1.891014; 3.375039 | 0.501 |
Metaregression provided valuable information regarding the interaction between the continuous covariates and treatment effect of resveratrol in reducing the infarct size and may explore the source of heterogeneity. Consistent P value showed that none of the covariates below had an impact on the cardioprotection of resveratrol in myocardial I/R injury.
3.2. Ex Vivo Studies
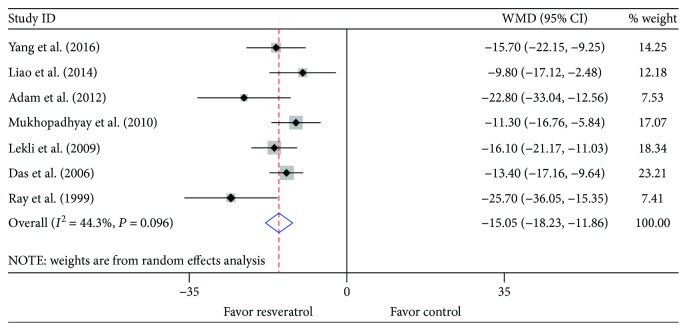
In accordance with the data from in vivo studies, administration of resveratrol ex vivo was also associated with a significant limitation in infarct size when compared with vehicle treatment (WMD: -15.05, 95% CI: -18.23 to -11.86, P ≤ 0.001) (Figure 4). There was evidence of moderate heterogeneity among the studies (I2 = 44.3%, P = 0.096). No publication bias was detected both visually (Figure 3(b)) or mathematically (Begg's test: P = 0.368; Egger's test: P = 0.155). Unsurprisingly, sensitivity analysis by systematically removing each study provided a consistent estimation of the benefit of resveratrol treatment in reducing the infarct size. Stratified analysis by reperfusion duration, route of administration, or timing regimen of pretreatment had no impact on the effect size and the P value (Table 6). Similarly, there was no relationship between the prespecified covariates and pooled estimates by metaregression (Table 7).
Figure 4.
Pooled estimates of infarct size for resveratrol vs. vehicle ex vivo. Resveratrol treatment reduced the infarct size in ex vivo studies (WMD: -15.05, 95% CI: -18.23 to -11.86, P ≤ 0.001). Gray squares represent WMDs in studies. The 95% CIs for each studies are denoted by lines and those for the pooled WMDs by open diamonds. Meta-analysis is performed by the random effects model.
4. Discussion
To our knowledge, this is the first preclinical meta-analysis to investigate the cardioprotective effect of resveratrol in animals subjected to myocardial I/R injury. Our findings indicate that as compared with vehicle, resveratrol is associated with a significantly improved infarct size of hearts post-I/R injury in both in vivo and ex vivo small animal studies. The marked benefits of resveratrol are not affected by either the duration of reperfusion or route and timing regimen of administration.
Reperfusion injury is a devastating consequences for reestablishment of blood flow to the ischemic myocardium, which induced additional damage inflicted on the heart [2]. It is first reported by Jennings and Reimer that reperfusion exacerbated the cell necrosis of irreversible injured cardiomyocytes [18]. Subsequently, experimental and clinical studies also confirm the paradoxical phenomenon and further investigate the cellular and molecular mechanism underlying the pathophysiological progress [1–3]. Although not fully elucidated, accumulating evidence has demonstrated a causality between the myocardial I/R injury and intracellular calcium overload, inflammation, and oxidative stress [4, 5, 19]. Mitochondrial dysfunction is recognized as the main source of reactive oxygen species in the pathogenesis of reperfusion injury and also promotes inflammatory response and endothelial damage [20]. Meanwhile, mPTP opening and subsequent cytochrome c released from impaired mitochondria trigger the intrinsic apoptotic process by activation of caspase-9/3 signaling pathway. Cardiomyocyte apoptosis along with necrosis collectively contributes to an extended infarct size post-I/R injury. Autophagy has also played an important role in the development of reperfusion damage; previous researches show that activation or inhibition of autophagy could exert either a beneficial or detrimental effect in the context of myocardial I/R injury [21]. In addition, platelet aggregation induced by I/R injury contributed to microvascular obstruction, characterized by microcirculatory spasm, intraluminal thrombosis, and notably swollen and dysfunctional endothelial cells, and finally caused slow or no-reflow [22]. Based on the aforementioned results, it provides the rationale for therapeutic strategies targeted against these adverse pathways.
Resveratrol is a unique plant-derived polyphenol and has been demonstrated to exhibit impressively beneficial effects in attenuating the progression of various illness, including ageing, obesity, cancer, inflammatory bowel disease, depressant, and diabetes mellitus, along with cardiovascular disease [6, 23]. The biological and pharmacological properties of resveratrol have been well established, i.e., antioxidant, anti-inflammation, antimitochondrial dysfunction, and antiapoptotic potency. Moreover, metabolic modulation and angiogenesis are also identified as the therapeutic actions of resveratrol [24]. It is worth noticing that resveratrol exerted cardioprotection on ischemic heart disease, especially the myocardial I/R injury. After reperfusion injury, inflammatory response and oxidative stress mainly contribute to the substantial loss of myocytes and consequent enlarged infarct area. An experimental study by Dong et al. shows that resveratrol protects the myocardium against I/R damage through deactivation of NALP3 inflammasome and suppression of IL-1β- and IL-18-mediated inflammatory cascade [10]. Neutrophils are also deeply implicated in the inflammatory response, and robust accumulation of neutrophils in reperfused areas results in negative repercussions for cardiomyocyte survival [25]. In addition, impaired mitochondria and infiltrated immune cells cause substantial ROS generation and consequent excessive oxidative stress [20]. Evidence from previous studies have identified resveratrol as an antioxidant that regulates the multistep process of redox system [19]. Recently, pretreatment with resveratrol decreases the ROS level by DCFH-DA staining, inhibits MDA formation, and is inversely correlated with increased expression of antioxidant enzymes, i.e., MnSOD and catalase in both in vivo and ex vivo myocardial I/R models [11, 14]. Meanwhile, inflammation and/or oxidative stress evidently trigger apoptosis cascades via either intrinsic or extrinsic apoptotic signaling pathway leading to cardiomyocyte loss and adverse ventricular remodeling, further deteriorating contractile function post-myocardial I/R injury. In accordance with the favorable effects of resveratrol in scavenging ROS production and alleviating inflammatory response, apoptosis assessed by TUNEL staining is also markedly diminished by resveratrol administration in the heart of I/R damage [12]. Similarly, resveratrol prevents mPTP opening, cytochrome c release from mitochondria, and subsequent caspase-3 activation during I/R injury and thus protects against mitochondrial dysfunction-induced cell death [15]. Noteworthy, contemporary studies find that resveratrol conferred vasoprotection through attenuating endothelial dysfunction and prompt angiogenesis in the reperfused myocardium, evidenced by restored expression of eNOS, nNOS, and VEGF-B [14, 26]. Intriguingly, pretreatment with a NOS inhibitor (L-NAME) or cGMP inhibitor (MB) significantly abolished the cardioprotection of resveratrol, which demonstrated a critical role of proangiogenic effect underlying the impressively beneficial effects of resveratrol [12]. Importantly, it has been reported that TLR4/NF-κB signaling pathway is involved in the biological effects of resveratrol [12]. TLR4 is rapidly upregulated to mediate a multitude of proinflammatory cytokines in response to I/R injury, which conspire to induce myocardial damage [27]. Subsequently, it predominantly activates the NF-κB family, the key transcription factors in modulating the inflammatory response, and cell death genes linked to the pathogenesis of cardiovascular disease. Surprisingly, resveratrol treatment decreases the expression of TLR4 and NF-κB in reperfused myocardium, accompanied with lower levels of TNF-α and reduced infarct size. However, whether there is a causal relationship between resveratrol and TLR4/NF-κB signaling needed further investigation. Other studies suggest Nrf2, SIRT1, and AKT/GSK3β as potential targets of resveratrol [11, 14, 16, 28]. Nevertheless, of course, detailed molecular and cellular mechanisms of resveratrol are far more sophisticated than we have got from the experimental results (Table 4 and Table 5). Unexpectedly, there is very little success in transforming resveratrol into clinical practice with a desired efficacy in relevant patients in spite of promising infarct-limiting effects from animal studies. Theoretically, animal models help to explore the probable mechanism; however, there is still a huge anatomic and/or physiological gap between the different species which may possibly be responsible for the inconsistency between preclinical studies and clinical studies. Since the overall conclusion of this work mainly depends on evidence from animal studies, large animal studies and/or well-designed RCTs are of pressing need for evaluating the expected cardioprotection of resveratrol.
5. Limitation
Several limitations should be considered: First, we performed meta-analysis at an aggregate study level (based on mean value and standard deviation) due to unavailability of individual animal-level data. We could not conduct stratified analyses to evaluate the impact of treatment in relation to other relevant variables (i.e., body weight, age, or level of left ventricular dysfunction). Second, our results can only be generalized to the overall animals subjected to myocardial I/R injury without any cardiovascular comorbidities (i.e., diabetes mellitus, obesity, hypertension, hyperlipidemia, or even renal dysfunction). Third, it was worthwhile to note that the absence of data on large animals in our statistical analysis, which shared more pathophysiological characteristics with human, may also possibly limit the interpretation and extension of our results and thus warranted large animal studies to further confirm the favorable effects of resveratrol on the heart with I/R damage. Fourth, variation in route of pretreatment, dosing, and timing regimens may have a possible impact on the cardioprotection of resveratrol. Finally, we found statistical heterogeneity among the studies; however, the robustness of the data across sensitivity analysis and stratified analysis can help minimize the potential effect of heterogeneity on the reliability of our conclusions. Furthermore, metaregression also did not reveal any possible source which may be responsible for the high heterogeneity due to the consistent effect of resveratrol irrespective of study level covariates.
6. Conclusion
From the available data of small animal studies, preconditioning with resveratrol presented a favorable infarct-limiting effect against myocardial I/R damage. Nonetheless, large animal studies and well-designed randomized controlled trials were needed in order to further confirm the cardioprotection of resveratrol before clinical application.
Acknowledgments
We thank Professor Yi-He Chen and Zhou-Qing Huang for assisting and guiding in the revision of the article. We thank Professor Zhao-Yang Lu for correcting the mistakes in spelling and grammatical errors. We also thank all the study participants. This work was supported by a Natural Science Foundation of China (NSFC) Grant (No. 81270258) to Y.-G. L.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Zhi-Jie Mao and Hui Lin contributed equally to this work.
References
- 1.Santos-Gallego C. G., Picatoste B., Badimón J. J. Pathophysiology of acute coronary syndrome. Current Atherosclerosis Reports. 2014;16(4):p. 401. doi: 10.1007/s11883-014-0401-9. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy D. J., Yellon D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. The Journal of Clinical Investigation. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yellon D. M., Hausenloy D. J. Myocardial reperfusion injury. The New England Journal of Medicine. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 4.Turer A. T., Hill J. A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. The American Journal of Cardiology. 2010;106(3):360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltzschig H. K., Eckle T. Ischemia and reperfusion: from mechanism to translation. Nature Medicine. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauf A., Imran M., Suleria H. A. R., Ahmad B., Peters D. G., Mubarak M. S. A comprehensive review of the health perspectives of resveratrol. Food & Function. 2017;8(12):4284–4305. doi: 10.1039/C7FO01300K. [DOI] [PubMed] [Google Scholar]
- 7.Gao F., Deng G., Liu W., Zhou K., Li M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncology Reports. 2017;37(2):1203–1211. doi: 10.3892/or.2017.5347. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y. J., Dong S. Y., Cui X. X., et al. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Molecular Nutrition & Food Research. 2016;60(10):2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Z., Wang J., Xu M., Wang Y., Zhang M., Zhou Y. Resveratrol improves cognitive impairment by regulating apoptosis and synaptic plasticity in streptozotocin-induced diabetic rats. Cellular Physiology and Biochemistry. 2016;40(6):1670–1677. doi: 10.1159/000453216. [DOI] [PubMed] [Google Scholar]
- 10.Dong W., Yang R., Yang J., et al. Resveratrol pretreatment protects rat hearts from ischemia/reperfusion injury partly via a NALP3 inflammasome pathway. International Journal of Clinical and Experimental Pathology. 2015;8(8):8731–8741. [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L., Jin Z., Zhao R., Ren K., Deng C., Yu S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. International Journal of Clinical and Experimental Medicine. 2015;8(7):10420–10428. [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Xie C., Zhuang J., et al. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-κB signaling pathway. Molecular Medicine Reports. 2015;11(2):1120–1126. doi: 10.3892/mmr.2014.2955. [DOI] [PubMed] [Google Scholar]
- 13.Cong X., Li Y., Lu N., et al. Resveratrol attenuates the inflammatory reaction induced by ischemia/reperfusion in the rat heart. Molecular Medicine Reports. 2014;9(6):2528–2532. doi: 10.3892/mmr.2014.2090. [DOI] [PubMed] [Google Scholar]
- 14.Yang L., Zhang Y., Zhu M., et al. Resveratrol attenuates myocardial ischemia/reperfusion injury through upregulation of vascular endothelial growth factor B. Free Radical Biology & Medicine. 2016;101:1–9. doi: 10.1016/j.freeradbiomed.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Liao Z., Liu D., Tang L., et al. Long-term oral resveratrol intake provides nutritional preconditioning against myocardial ischemia/reperfusion injury: involvement of VDAC1 down-regulation. Molecular Nutrition & Food Research. 2015;59(3):454–464. doi: 10.1002/mnfr.201400730. [DOI] [PubMed] [Google Scholar]
- 16.Adam T., Sharp S., Opie L. H., Lecour S. Loss of cardioprotection with ischemic preconditioning in aging hearts: role of sirtuin 1? Journal of Cardiovascular Pharmacology and Therapeutics. 2013;18(1):46–53. doi: 10.1177/1074248412458723. [DOI] [PubMed] [Google Scholar]
- 17.Macleod M. R., O’Collins T., Howells D. W., Donnan G. A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 18.Jennings R. B., Reimer K. A. Factors involved in salvaging ischemic myocardium: effect of reperfusion of arterial blood. Circulation. 1983;68(2) Part 2:I25–I36. [PubMed] [Google Scholar]
- 19.Madamanchi N. R., Runge M. S. Redox signaling in cardiovascular health and disease. Free Radical Biology & Medicine. 2013;61:473–501. doi: 10.1016/j.freeradbiomed.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagano G., Aiello Talamanca A., Castello G., et al. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward mitochondria-targeted clinical strategies. Oxidative Medicine and Cellular Longevity. 2014;2014:27. doi: 10.1155/2014/541230.541230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T., Jiao Y. R., Wang L. H., Zhou Y. H., Yao H. C. Autophagy in myocardial ischemia reperfusion injury: friend or foe? International Journal of Cardiology. 2017;239:p. 10. doi: 10.1016/j.ijcard.2017.01.083. [DOI] [PubMed] [Google Scholar]
- 22.Takaya N., Katoh Y., Iwabuchi K., et al. Platelets activated by collagen through the immunoreceptor tyrosine-based activation motif in the Fc receptor γ-chain play a pivotal role in the development of myocardial ischemia-reperfusion injury. Journal of Molecular and Cellular Cardiology. 2005;39(6):856–864. doi: 10.1016/j.yjmcc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y., Zhou J., Jiang B., Miao M. Resveratrol and inflammatory bowel disease. Annals of the New York Academy of Sciences. 2017;1403(1):38–47. doi: 10.1111/nyas.13426. [DOI] [PubMed] [Google Scholar]
- 24.Cho S., Namkoong K., Shin M., et al. Cardiovascular protective effects and clinical applications of resveratrol. Journal of Medicinal Food. 2017;20(4):323–334. doi: 10.1089/jmf.2016.3856. [DOI] [PubMed] [Google Scholar]
- 25.Dobaczewski M., Gonzalez-Quesada C., Frangogiannis N. G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. Journal of Molecular and Cellular Cardiology. 2010;48(3):504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung L. M., Su M. J., Chen J. K. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radical Biology & Medicine. 2004;36(6):774–781. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Qian Y., Fang Q., et al. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nature Communications. 2017;8:p. 13997. doi: 10.1038/ncomms13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shalwala M., Zhu S. G., Das A., Salloum F. N., Xi L., Kukreja R. C. Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS One. 2014;9(1, article e86977) doi: 10.1371/journal.pone.0086977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du R. H., Dai T., Cao W. J., Lu M., Ding J. H., Hu G. Kir6.2-containing ATP-sensitive K+ channel is required for cardioprotection of resveratrol in mice. Cardiovascular Diabetology. 2014;13(1):p. 35. doi: 10.1186/1475-2840-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naumenko S. E., Latysheva T. V., Gilinsky M. A., et al. Cardioprotective effect of resveratrol and resveratroloside. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2013;11(3):207–210. doi: 10.2174/187152571103140120103302. [DOI] [PubMed] [Google Scholar]
- 31.Shen M., Jia G. L., Wang Y. M., Ma H. Cardioprotective effect of resvaratrol pretreatment on myocardial ischemia–reperfusion induced injury in rats. Vascular Pharmacology. 2006;45(2):122–126. doi: 10.1016/j.vph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Hale S. L., Kloner R. A. Effects of resveratrol, a flavinoid found in red wine, on infarct size in an experimental model of ischemia/reperfusion. Journal of Studies on Alcohol. 2001;62(6):730–735. doi: 10.15288/jsa.2001.62.730. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay P., Mukherjee S., Ahsan K., Bagchi A., Pacher P., Das D. K. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS One. 2010;5(12, article e15705) doi: 10.1371/journal.pone.0015705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lekli I., Ray D., Mukherjee S., et al. Co-ordinated autophagy with resveratrol and γ-tocotrienol confers synergetic cardioprotection. Journal of Cellular and Molecular Medicine. 2010;14(10):2506–2518. doi: 10.1111/j.1582-4934.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das S., Tosaki A., Bagchi D., Maulik N., Das D. K. Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. The Journal of Pharmacology and Experimental Therapeutics. 2006;317(3):980–988. doi: 10.1124/jpet.105.095133. [DOI] [PubMed] [Google Scholar]
- 36.Ray P. S., Maulik G., Cordis G. A., Bertelli A. A. E., Bertelli A., Das D. K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radical Biology and Medicine. 1999;27(1-2):160–169. doi: 10.1016/S0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.