Abstract
Background
The influences of low‐carbohydrate diets in cardiovascular disease are controversial. Few studies have examined the relationship of carbohydrate intake and risk of incident atrial fibrillation (AF). We aimed to evaluate the association between carbohydrate intake and the risk of incident AF in the ARIC (Atherosclerosis Risk in Communities) Study.
Methods and Results
We included 13 385 participants (age, 54.2±5.8 years; 45.1% men and 74.7% white) who completed a dietary questionnaire at baseline (1987–1989) in the ARIC Study. The primary outcome was incident AF, which was identified by ECG performed during study examinations, hospital discharge codes, and death certificates. We used multivariable Cox hazard regression models to assess the association between carbohydrate intake and incident AF. We further explored the effects of specific food source (animal versus plant based) used to replace carbohydrate intake in the low‐carbohydrate intake setting. During a median follow‐up of 22.4 years, 1808 cases (13.5%) of AF occurred. The hazard ratio for incident AF associated with a 1‐SD (9.4%) increase in carbohydrate intake as a percentage of energy intake was 0.82 (95% CI, 0.72–0.94), after adjustment for traditional AF risk factors and other diets factors. Results were similar when individuals were categorized by carbohydrate intake quartiles (hazard ratio, 0.64; 95% CI, 0.49–0.84; comparing extreme quartiles). No association was found between the type of protein or fat used to replace the carbohydrate and risk of incident AF.
Conclusions
Low‐carbohydrate diets were associated with increased risk of incident AF, regardless of the type of protein or fat used to replace the carbohydrate.
Keywords: atrial fibrillation, diet, epidemiology, risk factor
Subject Categories: Atrial Fibrillation, Cardiovascular Disease, Diet and Nutrition, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
In this large, prospective, community‐based cohort study with a long‐term follow‐up, we were the first to evaluate the association of carbohydrate intake with incident atrial fibrillation and found that people with a low‐carbohydrate diet may have had a higher risk of incident atrial fibrillation, regardless of the type of protein or fat used to replace the carbohydrate.
What Are the Clinical Implications?
Low‐carbohydrate diets are associated with increased risk of incident atrial fibrillation, indicating that this popular weight control method, by restricting carbohydrate intake, should be recommended cautiously and more studies should be conducted to evaluate the effect.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, with an estimated lifetime risk of 25%.1, 2 As AF is related to substantial increased morbidity, mortality, and economic costs,3 it is important to recognize modifiable risk factors, such as dietary factors, as a step to provide preventive strategies for this disease.
Low‐carbohydrate diets, which restrict carbohydrate intake, in favor of increased protein or fat intake, have gained substantial popularity because of their ability to induce short‐term weight loss.4, 5 Nevertheless, the long‐term effect of carbohydrate restriction is still controversial, especially in the influence on cardiovascular disease.6, 7, 8, 9 Recently, the 2017 PURE (Prospective Urban‐Rural Epidemiology) Study, of 135 335 participants from 18 countries across 5 continents, reported that higher carbohydrate intake was associated with an increased risk of total mortality but not with the risk of cardiovascular disease (myocardial infarction, stroke, and heart failure) or cardiovascular disease mortality.10 Another recent study of a large cohort, the ARIC (Atherosclerosis Risk in Communities) Study, reported a U‐shaped association between carbohydrate intake and total mortality, whereas no association was found with cardiovascular mortality.5 However, to the best of our knowledge, no study has examined the relationship of carbohydrate intake and risk of incident AF. As a result, we analyzed the ARIC Study data set to assess the association of carbohydrate intake and incident AF.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of human subjects’ restrictions. However, interested investigators can contact the ARIC Study Coordinating Center at the University of North Carolina–Chapel Hill to request overall access to ARIC Study data.11
Study Populations
The ARIC Study is a population‐based, prospective, cohort study of cardiovascular risk factors in 4 US communities (Forsyth County, North Carolina; Jackson, MD; suburbs of Minneapolis, MN; and Washington County, Maryland), initially consisting of 15 792 participants, aged 45 to 64 years, recruited between 1987 and 1989 (visit 1). Four subsequent study visits were conducted: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), and visit 5 (2011–2013). Participants are being followed up by annual or semiannual telephone interviews and active surveillance of ARIC Study community hospitals. Further details about the study design have been previously described.12 The ARIC Study has been approved by institutional review boards at all participating institutions, and all participants provided written informed consent. In the present analysis, of 15 792 participants at baseline (visit 1), we excluded participants who had race other than white or black (n=103), participants with prevalent AF or missing data of AF (n=243), participants with missing dietary information or with extreme caloric intake (defined as <600 or >4200 kcal/d for men and <500 or >3600 kcal/d for women) (n=327), and participants missing other covariates (n=1734). The final sample size was 13 385 (Figure 1).
Figure 1.
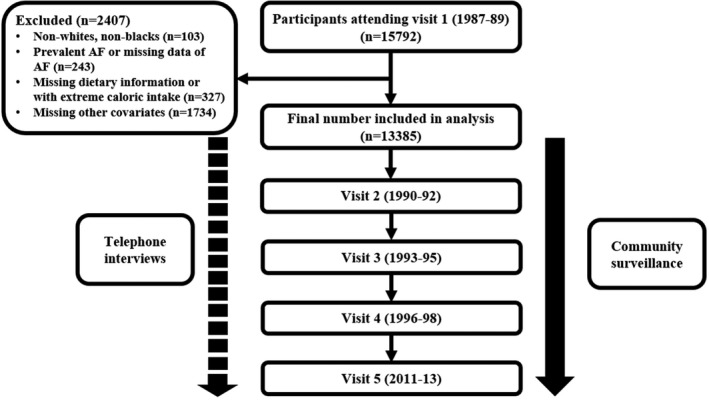
Flow diagram of participants in the ARIC (Atherosclerosis Risk in Communities) Study. AF indicates atrial fibrillation.
Dietary Assessment
At visits 1 and 3, participants completed an interview that included a 66‐item semiquantitative food frequency questionnaire, a modified version of the 61‐item instrument developed by Willett et al.13 Participants reported the frequency of particular foods and beverages consumed on a 9‐category scale, ranging from never or <1 time per month to ≥6 times per day. Standard portion sizes were provided as a reference based on picture and food models. Foods were grouped into dairy foods, fruits, vegetables, meats, sweets, baked goods, cereals, miscellaneous, beverages, and other dietary items. In addition, detailed information about alcohol intake was ascertained on a separate interview form. Nutrient intakes were derived from the food frequency questionnaire responses using the Harvard Nutrient Database. The macronutrients (carbohydrate, fat, and protein) were expressed as percentage of energy, calculated as the daily calories of the macronutrient divided by the total number of calories for the day.13
AF Ascertainment
Detailed ascertainment of AF, including both AF proper and atrial flutter, has been described previously.14 Incident AF was identified by ECGs performed during study examinations, hospital discharge codes, and death certificates. At each study visit, a 12‐lead ECG was performed with the participant lying in a supine position. Electrocardiographic data were transmitted electronically to a reading center (EpiCare, Wake Forest University, Winston‐Salem, NC), reviewed, and analyzed using the GE Marquette 12‐SL program (GE Marquette, Milwaukee, WI). ECGs automatically coded as AF or atrial flutter were visually checked and confirmed by a cardiologist.
Hospitalizations during follow‐up were identified through annual telephone calls and surveillance of local hospitals. Trained abstractors collected information from all participants’ hospitalizations, including all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), codes for diagnoses. AF was considered to be present if the ICD‐9‐CM code 427.31 (AF) or 427.32 (atrial flutter) was present in any hospitalization. AF events associated with open cardiac surgery were excluded. Besides, AF was also defined if ICD‐9‐CM code 427.31 or 427.32 was listed as a cause of death.
Measurement of Other Covariates
All covariates were assessed at visit 1, except for body mass index, which was also a measure at visits 2 and 3. Race, age, sex, education level, smoking, and alcohol consumption status were self‐reported. Height and weight were measured with the participant wearing light clothes, and body mass index was calculated as weight (in kilograms) divided by squared height (in meters). Body surface area was calculated according to the Mosteller formula as the square root of [height (cm)×weight (kg)/3600]. Sport and physical activity during leisure time was accessed using the validated Baecke questionnaire. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg, or blood pressure medicine use in the past 2 weeks. Diabetes mellitus was defined if the participants had fasting blood glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, use of antidiabetic medicines, or self‐reported physician diagnosis of diabetes mellitus. Stroke was identified by 6 associated symptoms (speech, vision, double vision, numbness, paralysis, and dizziness) corresponding to the specific artery. Prevalent coronary heart disease and heart failure were defined as previously described.15, 16 Total cholesterol, high‐density lipoprotein cholesterol, and triglycerides were measured using standardized enzymatic assays, and low‐density lipoprotein cholesterols were than calculated on the basis of the Friedewald formula.17 Creatinine was measured using a modified kinetic Jaffe method, and uric acid was measured by the method of Haeckel.12
Statistical Analysis
We primarily modeled carbohydrate intake as a continuous variable, and we rescaled the data by dividing by the SD (1 SD=9.4%). Then, we categorized carbohydrate intake into quartiles based on the sample distribution. Baseline characteristics of participants were compared between groups using the 1‐way ANOVA test, the χ2 test, and the Kruskal‐Wallis test, as appropriate. We used multivariable Cox hazard regression models to assess the association between baseline carbohydrate intake and incident AF. Time of follow‐up was defined as time from visit 1 (baseline) to incident of AF, loss to follow‐up, death, or December 31, 2012, whichever occurred first. We also used a restricted cubic spline with 4 knots to express the dose‐response association between total energy from carbohydrate intake and incident AF. The initial model adjusted for age, sex, and race. A second model additionally adjusted for total energy intake, total fat intake as a percentage of energy, animal fat intake as a percentage of energy, total protein intake as a percentage of energy, animal protein intake as a percentage of energy, dietary fiber intake, glycemic index, and glycemic load. In a final model, we further adjusted for body mass index, body surface area, smoking, drinking, education level, sport, physical activity, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, creatinine, uric acid, hypertension, stroke, diabetes mellitus, prevalent coronary artery disease, and prevalent heart failure. We did a time‐varying sensitivity analysis for participants who were identified with incident AF, loss to follow‐up, or death before visit 3; carbohydrate intake was calculated on the basis of responses from the baseline (visit 1) food frequency questionnaire. From visit 3 onwards, the carbohydrate intake was calculated on the basis of the mean of visit 1 and visit 3 food frequency questionnaire responses. Carbohydrate exposures were not updated for participants who developed heart disease, diabetes mellitus, or stroke before visit 3 for reducing potential confounding from changes in diet that could arise from the diagnosis. For the missing of dietary information at visit 3 in 2743 participants, we did a further sensitivity analysis: we excluded the missing data in the first analysis, and in the second we used dietary information in visit 1 to replace missing data. In addition, we performed prespecified subgroup analysis by age, sex, and race and tested for potential interactions of these covariates with carbohydrate intake separately. To minimize the potential of reverse causation, we did a sensitivity analysis whereby individuals with prevalent coronary artery disease, heart failure, stroke, or diabetes mellitus at baseline were excluded from the analysis. Because of the high rate of hypertension at the baseline, we also did a subgroup analysis of participants with or without hypertension and tested for interaction of hypertension and carbohydrate intake.
To further explore the effects of specific food sources (animal versus plant based) that are used to replace carbohydrate intake in the low‐carbohydrate intake setting, we created animal‐ and plant‐based low‐carbohydrate diet scores, as previously described.8 Either animal‐ or plant‐based fat and protein and carbohydrate intake as a percentage of energy were divided into deciles. For carbohydrate, participants got 10 points in the lowest decile and 0 points in the highest decile. The order was reversed for animal‐ or plant‐based fat and protein. Animal‐based low‐carbohydrate diet scores were calculated by summing up the points for carbohydrate, animal‐based fat, and animal‐based protein. Plant‐based low‐carbohydrate diet scores were calculated by summing up the points for carbohydrate, plant‐based fat, and plant‐based protein (Table S1). As a result, the highest score represented low‐carbohydrate and high animal‐ or plant‐based fat and protein intake. We used the Cox hazard regression model to determine the association of incident AF with animal‐ or plant‐based low‐carbohydrate scores.
Results
Baseline characteristics are shown in Table 1. The mean value of carbohydrate intake as a percentage of energy for 13 384 participants was 44.8±9.4%. The average age was 54.2±5.8 years, and 45.1% of participants were men. Participants with a relatively low percentage of energy from carbohydrate were more likely to be young, men, white, smokers, and ever drinkers; and to have diabetes mellitus, a high education level, a high high‐density lipoprotein cholesterol level, a high uric acid level, high total fat intake, high animal fat intake, high total protein intake, high animal protein intake, low plant protein intake, low dietary fiber intake, a low glycemic index, and a low glycemic load. Total energy intake or plant fat intake had reverse U‐shaped relationship across carbohydrate intake quartiles: participants in both the first and fourth quartiles had lower total energy intake and plant fat intake than those in the intermediate quartiles. There was no significant difference in change in body mass index at the time points of 3 and 6 years from baseline across carbohydrate quartiles. The prevalence of hypertension, stroke, coronary artery disease, and heart failure was similar across carbohydrate quartiles (Table 1).
Table 1.
Baseline Characteristics of Study Participants by Quartiles of Carbohydrate Intake as a Percentage of Energy
| Characteristic | Total (n=13 384) | Quartile 1 (n=3344) | Quartile 2 (n=3345) | Quartile 3 (n=3349) | Quartile 4 (n=3347) | P Value |
|---|---|---|---|---|---|---|
| Carbohydrate, % of energy | 48.8±9.4 | 37.2±4.7 | 45.8±1.7 | 51.5±1.8 | 60.8±5.3 | <0.001 |
| Age, y | 54.2±5.8 | 53.9±5.7 | 54.2±5.8 | 54.3±5.8 | 54.3±5.8 | 0.018 |
| Sex | <0.001 | |||||
| Men | 6036 (45.1) | 1772 (53.0) | 1547 (46.2) | 1477 (44.1) | 1240 (37.0) | |
| Women | 7349 (54.9) | 1572 (47.0) | 1798 (53.8) | 1872 (55.9) | 2107 (63.0) | |
| Race | <0.001 | |||||
| Black | 3393 (25.3) | 730 (21.8) | 814 (24.3) | 879 (26.2) | 970 (29.0) | |
| White | 9992 (74.7) | 2614 (78.2) | 2531 (75.7) | 2470 (73.8) | 2377 (71.0) | |
| BMI, kg/m2 | 27.6±5.3 | 27.8±5.1 | 27.8±5.3 | 27.5±5.3 | 27.3±5.5 | <0.001 |
| Change in BMI, kg/m2 | ||||||
| 3‐y Change | 0.36±1.7 | 0.35±1.7 | 0.34±1.7 | 0.33±1.7 | 0.42±1.7 | 0.127 |
| 6‐y Change | 0.93±2.1 | 0.91±2.3 | 0.92±2.2 | 0.92±2.1 | 0.96±2.1 | 0.812 |
| BSA, m2 | 1.91±0.2 | 1.94±0.2 | 1.92±0.2 | 1.90±0.2 | 1.87±0.2 | <0.001 |
| Hypertension | 4750 (34.1) | 1116 (33.4) | 1118 (33.4) | 1136 (33.9) | 1200 (35.9) | 0.108 |
| Stroke | 625 (4.7) | 160 (4.8) | 151 (4.5) | 159 (4.7) | 155 (4.6) | 0.953 |
| Diabetes mellitus | 1239 (9.3) | 356 (10.6) | 334 (10.0) | 278 (8.3) | 271 (8.1) | <0.001 |
| Coronary artery disease | 643 (4.8) | 151 (4.5) | 155 (4.6) | 151 (4.5) | 186 (5.6) | 0.132 |
| Heart failure | 607 (4.5) | 144 (4.3) | 137 (4.1) | 147 (4.4) | 179 (5.3) | 0.067 |
| Smoking | <0.001 | |||||
| Current smoker | 3504 (26.2) | 1056 (31.6) | 897 (26.8) | 785 (23.4) | 766 (22.9) | |
| Former smoker | 4377 (32.7) | 1191 (35.6) | 1100 (32.9) | 1105 (33.0) | 981 (29.3) | |
| Never smoker | 5504 (41.1) | 1097 (32.8) | 1348 (40.3) | 1459 (43.6) | 1600 (47.8) | |
| Drinking | <0.001 | |||||
| Current drinker | 7650 (57.2) | 2379 (71.1) | 2034 (60.8) | 1801 (53.8) | 1436 (42.9) | |
| Former drinker | 2488 (18.6) | 480 (14.4) | 575 (17.2) | 625 (18.7) | 808 (24.1) | |
| Never drinker | 3247 (24.3) | 485 (14.5) | 736 (22.0) | 923 (27.6) | 1103 (33.0) | |
| Education level | <0.001 | |||||
| Basic or 0 y | 3048 (22.8) | 662 (19.8) | 730 (21.8) | 780 (23.3) | 876 (26.2) | |
| Intermediate | 5492 (41.0) | 1319 (39.4) | 1319 (39.4) | 1369 (40.9) | 1404 (41.9) | |
| Advanced | 4845 (36.2) | 1363 (40.8) | 1246 (37.2) | 1165 (34.8) | 1071 (32.0) | |
| Sport | 2.3 (1.8–3.0) | 2.3 (1.8–3.0) | 2.3 (1.8–3.0) | 2.3 (1.8–3.0) | 2.3 (1.8–3.0) | 0.003 |
| Physical activity | 2.3 (2.0–2.8) | 2.3 (2.0–2.8) | 2.3 (2.0–2.8) | 2.3 (2.0–2.8) | 2.3 (2.0–2.8) | <0.001 |
| Total cholesterol, mmol/L | 5.5±1.1 | 5.5±1.1 | 5.5±1.1 | 5.5±1.1 | 5.6±1.1 | 0.266 |
| HDL‐C, mmol/L | 1.3±0.4 | 1.4±0.5 | 1.3±0.4 | 1.3±0.4 | 1.3±0.4 | <0.001 |
| LDL‐C, mmol/L | 3.6±1.0 | 3.5±1.0 | 3.6±1.0 | 3.5±1.0 | 3.6±1.0 | 0.042 |
| Lg triglycerides, lg(mmol/L) | 0.099±0.21 | 0.097±0.21 | 0.096±0.21 | 0.099±0.21 | 0.104±0.21 | 0.345 |
| Creatinine, mg/dL | 1.1±0.4 | 1.1±0.4 | 1.1±0.5 | 1.1±0.3 | 1.1±0.3 | 0.007 |
| Uric acid, mg/dL | 6.0±1.6 | 6.2±1.6 | 6.0±1.6 | 6.0±1.5 | 6.0±1.6 | <0.001 |
| Total energy intake, kcal | 1623.4±609.0 | 1592.9±604.2 | 1660.0±601.5 | 1656.3±596.3 | 1584.6±629.9 | <0.001 |
| Total fat, % of energy | 32.9±6.8 | 38.4±6.3 | 35.1±4.5 | 31.9±4.0 | 26.1±4.8 | <0.001 |
| Animal fat, % of energy | 19.9±6.2 | 25.6±5.9 | 21.4±4.4 | 18.5±3.9 | 14.3±4.0 | <0.001 |
| Plant fat, % of energy | 13.0±5.1 | 12.9±5.4 | 13.8±5.1 | 13.4±4.8 | 11.8±4.6 | <0.001 |
| Total protein, % of energy | 17.9±4.2 | 20.3±4.3 | 18.6±3.5 | 17.5±3.4 | 15.3±3.6 | <0.001 |
| Animal protein, % of energy | 13.5±4.3 | 16.4±4.5 | 14.2±3.6 | 12.9±3.4 | 10.6±3.3 | <0.001 |
| Plant protein, % of energy | 4.4±1.2 | 4.0±1.1 | 4.4±1.1 | 4.6±1.2 | 4.7±1.5 | <0.001 |
| Dietary fiber, g | 17.2±8.2 | 14.0±6.7 | 17.0±7.2 | 18.4±7.7 | 19.6±9.8 | <0.001 |
| Glycemic index | 588.6±263.3 | 447.7±196.6 | 574.8±226.6 | 641.8±259.4 | 689.9±294.7 | <0.001 |
| Glycemic load | 10 439±4673 | 7532±3189 | 9928±3661 | 11 295±4168 | 12 995±5516 | <0.001 |
Data are median (interquartile range), mean±SD, or number (percentage), unless otherwise indicated. Baseline characteristics are from the study population (n=13 384) at baseline visit 1, according to quartiles of carbohydrate intake as a percentage of energy intake. BMI indicates body mass index; BSA, body surface area; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
During a median follow‐up of 22.4 years, 1808 cases (13.5%) of AF occurred. In the model that measured carbohydrate intake as a continuous variable, an increase of 9.4% in carbohydrate intake (corresponding to 1 SD) was associated with an 18% lower rate of incident AF (hazard ratio, 0.82; 95% CI, 0.72–0.94), after adjusting for all covariates (Table 2). Results were similar when we categorized individuals by carbohydrate intake quartiles: the highest risk of incident AF was observed in the lowest carbohydrate intake subgroup, in both unadjusted and adjusted models (P<0.001, Table 2). In the final model, the hazard ratios for incident AF comparing the second, third, and fourth quartiles of carbohydrate intake as a percentage of energy with the first quartile were 0.79 (95% CI, 0.68–0.92), 0.77 (95% CI, 0.64–0.93), and 0.64 (95% CI, 0.49–0.84) separately (Table 2, Figure 2). Figure 3 shows the restricted cubic splines of the risk of incident AF across levels of carbohydrate intake as a percentage of energy intake. Consistent with the analysis using quartiles of sample distribution, the risk of incident AF increased in participants with a lower carbohydrate intake. However, there was no significant difference for risk of incident AF in participants with carbohydrate intake as a percentage of energy >62%, compared with the reference level of 50% (Figure 3).
Table 2.
Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy
| Carbohydrate Intake (% of Energy) | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Quartiles | ||||||
| 1 (≤42.70) | 1.00 (Reference) | ··· | 1.00 (Reference) | ··· | 1.00 (Reference) | ··· |
| 2 (42.71–48.55) | 0.84 (0.74–0.95) | 0.007 | 0.77 (0.67–0.90) | 0.001 | 0.79 (0.68–0.92) | 0.002 |
| 3 (48.56–54.74) | 0.84 (0.74–0.96) | 0.008 | 0.73 (0.61–0.88) | 0.001 | 0.77 (0.64–0.93) | 0.007 |
| 4 (≥54.75) | 0.79 (0.69–0.90) | <0.001 | 0.62 (0.48–0.81) | <0.001 | 0.64 (0.49–0.84) | 0.001 |
| Per 1 SD (9.4%) | 0.93 (0.89–0.98) | 0.003 | 0.79 (0.70–0.91) | 0.001 | 0.82 (0.72–0.94) | 0.005 |
AF indicates atrial fibrillation; HR, hazard ratio.
Adjusted for age, sex, and race.
Further adjusted for total energy intake, total fat intake as a percentage of energy, animal fat intake as a percentage of energy, total protein intake as a percentage of energy, animal protein intake as a percentage of energy, dietary fiber intake, glycemic index, and glycemic load.
Further adjusted for body mass index, body surface area, smoking, drinking, education level, sport, physical activity, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, creatinine, uric acid, hypertension, stroke, diabetes mellitus, coronary artery disease, and heart failure.
Figure 2.
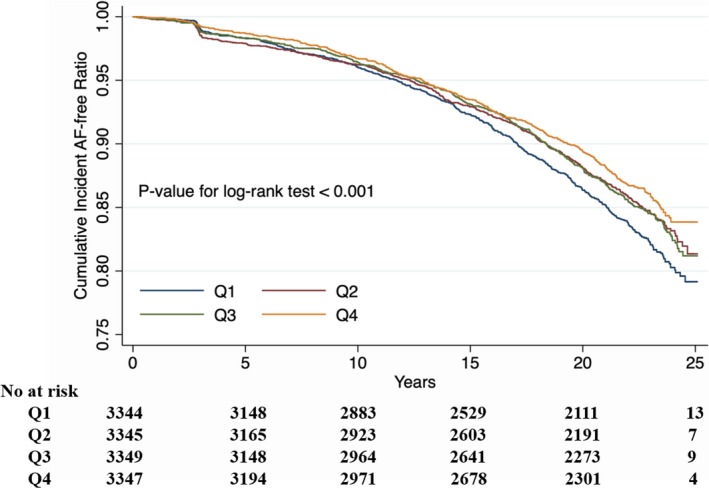
Kaplan‐Meier curve of incident atrial fibrillation (AF) by quartiles of carbohydrate intake as a percentage of energy.
Figure 3.
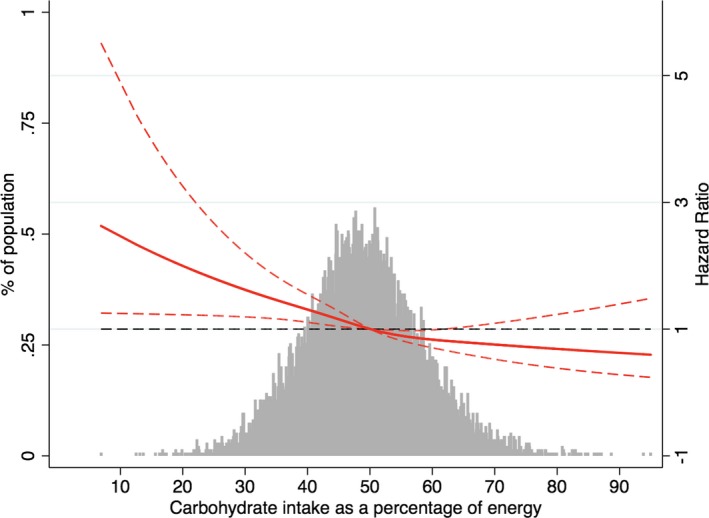
Adjusted hazard ratios of atrial fibrillation by baseline carbohydrate intake as a percentage of energy. Each hazard ratio was computed with a carbohydrate intake level of 50% as the reference. The hazard ratio was adjusted for age, race, total energy intake, total fat intake as a percentage of energy, animal fat intake as a percentage of energy, total protein intake as a percentage of energy, animal protein intake as a percentage of energy, dietary fiber intake, glycemic index, glycemic load, body mass index, smoking, drinking, education level, sport, physical activity, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, creatine, uric acid, hypertension, stroke, diabetes mellitus, coronary artery disease, and heart failure. Red solid line represents the hazard ratio of carbohydrate intake across the whole range. Red dotted lines represent the 95% CI. Black dotted lines is the reference line as hazard ratio =1. Histograms represent the frequency distribution of carbohydrate intake as a percentage of energy at baseline.
There were similar results in the time‐varying sensitivity analysis (Tables S2 and S3). When stratified by age, sex, race, and presence of hypertension, the associations between carbohydrate intake and incident AF were stronger in white participants, women, older participants, and participants with hypertension; however, all interactions were not statistically significant (P>0.05 for all interactions, Tables S4 through S7). In sensitivity analysis, the association between carbohydrate intake and incident AF persisted after excluding participants with prevalent coronary artery disease, prevalent heart failure, prevalent stroke, or diabetes mellitus at baseline (Table S8).
To further explore the effects of source of fat and protein alternatives to low‐carbohydrate intake, we analyzed the association of animal‐ or plant‐based low‐carbohydrate diet scores with incident AF using Cox hazard regression analysis. However, no significant relationship could be found (Tables S9 and S10).
Discussion
In this large, prospective, cohort study with a long‐term follow‐up of >20 years, we found that low‐carbohydrate intake as a percentage of energy was associated with a higher risk of incident AF, which was independent of other well‐known risk factors for incident AF. The association was consistently observed in several sensitivity analyses (Tables S2 through S8). No relationship was found in the further exploration of the effects of source of fat and protein alternatives to low‐carbohydrate intake. To the best of our knowledge, it is the first large prospective cohort study to assess the relationship of carbohydrate intake with risk of incident AF.
Previous assessments of dietary exposures in relation to AF mostly focused on the effect of omega‐3 fatty acid from fish, although with controversial conclusions.18, 19, 20, 21, 22, 23, 24 In the PREDIMED (Prevención con Dieta Mediterránea) trial,25 Martinez‐Gonzalez et al found that extravirgin olive oil in the context of a Mediterranean dietary pattern may reduce the risk of AF. Other studies26, 27, 28 also found inconsistent associations between chocolate, coffee, and AF. Similarly, our study also evaluated the relationship between diet factors and incident AF. The adverse association of carbohydrate intake with incident AF found in our study supplemented the relationship of macronutrient and risk of AF.
A low‐carbohydrate diet, with the reduction of carbohydrate intake and thereby encouragement of high protein or fat intake, is now widely recommended for weight control, for the effect of significant weight loss in the short‐term without feeling hungery.29 However, the long‐term effectiveness and safety of low‐carbohydrate diets remain controversial. Several studies reported that the weight loss effect of a low‐carbohydrate diet was observed for 6 months only and was no longer significant after 12 months, compared with the energy‐restricted low‐fat diet.30, 31, 32 The effect of weight loss might be attributable to excretion of bound water; decreased energy intake, by appetite suppression or satiation; and increased energy expenditure.29, 33 For the safety of a low‐carbohydrate diet, previous studies reported inconsistent conclusions. In the study by Lagiou et al,9 low‐carbohydrate–high‐protein diets were associated with increased risk of cardiovascular disease. On the contrary, Bazzano et al6 reported the reduction of cardiovascular risk factors in a low‐carbohydrate diet; and in the study by Halton et al,8 a lower‐carbohydrate diet was not associated with increased risk of coronary artery disease. Two recent studies for 2 large prospective cohorts (the PURE and ARIC Studies), combined with a meta‐analysis study of several previous studies, reported a U‐shaped association of carbohydrate intake and total mortality, whereas no association was found between carbohydrate intake and risk of cardiovascular disease (myocardial infarction, stroke, and heart failure) or cardiovascular mortality.5, 10 However, none of these studies assessed the potential relationship between carbohydrate intake and incident AF, which is also a common cardiovascular disease in clinical practice, with high mortality. Interestingly, our study, for the first time, discovered the adverse effect of a low‐carbohydrate diet to AF, which provided a novel potential risk factor for the primary prevention of AF. In view of the different effect of food source used to replace carbohydrate to the risk of total mortality, as the previous study described,5 we further explored the association of animal‐ or plant‐based low‐carbohydrate diet scores with incident AF; no association could be found, which suggests that the increased risk of AF caused by a low‐carbohydrate intake was not related to the source of food used to replace carbohydrate.
Several potential mechanisms may explain the observed inverse association. First, a low‐carbohydrate diet may lead to lower intake of vegetables, fruits, and grain, as well as the vitamins they contain, which may reduce their anti‐inflammatory effects34 and stimulate inflammatory pathways. As the association between a proinflammatory state and incidence of AF has been extensively demonstrated,25, 35 reducing intake of these anti‐inflammatory foods may be one of the important mechanisms for the risk of incident AF. Second, a low‐carbohydrate diet with increased protein and fat consumption may stimulate oxidative stress,36 which was also demonstrated to be associated with incident AF.37 Finally, the effect could result from the increased risk of other cardiovascular disease during the follow‐up, which is a known risk factor for AF.38 Nevertheless, the effect of a low‐carbohydrate intake on other cardiovascular disease is still controversial, as mentioned above.
Strengths and Limitations
Our analysis has important strengths. We used a large community‐based biracial cohort with a long follow‐up duration and adequate AF events to test our hypotheses. The ARIC Study's design, with the extensive and rigorous measurement of covariates, allows us to perform comprehensive statistical adjustment and reduce confounding as much as possible. AF incidence in the ARIC Study is consistent with other population‐based studies, and the use of hospital discharge records for the AF ascertainment has been previously validated in the ARIC Study.14, 39, 40, 41 There are also some limitations to consider. First, in the method of AF ascertainment, most AF events were found through hospital discharge codes. As a result, individuals with asymptomatic AF or those managed in an outpatient setting, not requiring hospital admission, were unable to be identified. Second, we are unable to classify AF type (paroxysmal, persistent, or permanent AF) accurately in the ARIC Study, so the relationship between carbohydrate intake and incident AF we found was not detailed enough. Third, our study was based on the diet information at the baseline, and dietary patterns could change during >20 years of follow‐up. We conducted a time‐varying sensitivity analysis spanning 6 years to minimize the bias as possible, and the result was similar, although the change after 6 years could not be assessed because of the unavailable data in the ARIC Study. Fourth, some degree of measurement error is unavoidable for the dietary assessment methods. As a result, the interpretation of absolute intakes should be cautious. Last, as it is an observational study, we could not exclude residual confounding, despite the fact that we adjusted for potential covariates as much as possible. More randomized controlled trials, with rigorously controlled food types and alternative energy sources, are needed to confirm this hypothesis, although it is difficult because of the long duration of study required.
In conclusion, we found that a low‐carbohydrate intake was associated with increased risk of incident AF, regardless of the type of protein and fat used to replace the carbohydrate. A low‐carbohydrate diet, a way to control weight, should be cautiously recommended, especially considering the potential influence on arrhythmia.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) Study is performed as a collaborative trial supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Liao is also supported by the National Natural Science Foundation of China (81600206) and the Natural Science Foundation of Guangdong Province (2016A030310140/20160903).
Disclosures
None.
Supporting information
Table S1. Criteria for Determining the Low‐Carbohydrate‐Diet Scores
Table S2. Time Varying Sensitivity Analysis for Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy*
Table S3. Time Varying Sensitivity Analysis for Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy*
Table S4. Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy, Stratified by Race
Table S5. Risk of Incident AF for Coronary Intake as a Percentage of Energy, Stratified by Sex
Table S6. Risk of Incident AF for Coronary Intake as a Percentage of Energy, Stratified by Age
Table S7. Risk of Incident AF for Coronary Intake as a Percentage of Energy, Stratified by Hypertension
Table S8. Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy, Excluded of Patients With Cardiovascular Disease or Diabetes
Table S9. Risk of Incident AF for Animal‐Based Low‐Carbohydrate Diet Scores
Table S10. Risk of Incident AF for Plant‐Based Low‐Carbohydrate Diet Scores
Acknowledgments
We thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) Study for their important contributions.
(J Am Heart Assoc. 2019;8:e011955 DOI: 10.1161/JAHA.119.011955.)
Contributor Information
Zhimin Du, Email: dujiaoshou7890@126.com.
Xinxue Liao, Email: liaoxinx@mail.sysu.edu.cn.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JJ, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd‐Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WJ, Brehm BJ, Bucher HC. Effects of low‐carbohydrate vs low‐fat diets on weight loss and cardiovascular risk factors: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. [DOI] [PubMed] [Google Scholar]
- 5. Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, Rimm EB, Willett WC, Solomon SD. Dietary carbohydrate intake and mortality: a prospective cohort study and meta‐analysis. Lancet Public Health. 2018;3:e419–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, Chen C, Klag MJ, Whelton PK, He J. Effects of low‐carbohydrate and low‐fat diets. Ann Intern Med. 2014;161:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low‐carbohydrate diets and all‐cause and cause‐specific mortality: two cohort studies. Ann Intern Med. 2010;153:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low‐carbohydrate‐diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. [DOI] [PubMed] [Google Scholar]
- 9. Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate‐high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel‐Viljoen E, Rosengren A, Amma LI, Avezum A, Chifamba J, Diaz R, Khatib R, Lear S, Lopez‐Jaramillo P, Liu X, Gupta R, Mohammadifard N, Gao N, Oguz A, Ramli AS, Seron P, Sun Y, Szuba A, Tsolekile L, Wielgosz A, Yusuf R, Hussein Yusufali A, Teo KK, Rangarajan S, Dagenais G, Bangdiwala SI, Islam S, Anand SS, Yusuf S; Prospective Urban Rural Epidemiology (PURE) study investigators . Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390:2050–2062. [DOI] [PubMed] [Google Scholar]
- 11. Collaborative Studies Coordinating Center. Atherosclerosis Risk in Communities Study. University of North Carolina at Chapel Hill . Available at: https://www2.cscc.unc.edu/aric/desc. Accessed February 16, 2019.
- 12. Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 14. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 16. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18. Shen J, Johnson VM, Sullivan LM, Jacques PF, Magnani JW, Lubitz SA, Pandey S, Levy D, Vasan RS, Quatromoni PA, Junyent M, Ordovas JM, Benjamin EJ. Dietary factors and incident atrial fibrillation: the Framingham Heart Study. Am J Clin Nutr. 2011;93:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gronroos NN, Alonso A. Diet and risk of atrial fibrillation—epidemiologic and clinical evidence. Circ J. 2010;74:2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mozaffarian D, Wu JHY, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Silletta MG, Tavazzi L, Marchioli R. Fish oil and post‐operative atrial fibrillation. J Am Coll Cardiol. 2013;61:2194–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu JHY, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, Heckbert SR, Siscovick DS, Mozaffarian D. Association of plasma phospholipid long‐chain omega‐3 fatty acids with incident atrial fibrillation in older adults. Circulation. 2012;125:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virtanen JK, Mursu J, Voutilainen S, Tuomainen T. Serum long‐chain n‐3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;120:2315–2321. [DOI] [PubMed] [Google Scholar]
- 23. Gronroos N, Chamberlain A, Folsom A, Soliman E, Agarwal S, Nettleton J, Alonso A. Fish, fish‐derived n‐3 fatty acids, and risk of incident atrial fibrillation in the Atherosclerosis Risk in Communities (ARIC) Study. PLoS One. 2012;7:e36686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JCM. Intake of very long‐chain n‐3 fatty acids from fish and incidence of atrial fibrillation: the Rotterdam Study. Am Heart J. 2006;151:857–862. [DOI] [PubMed] [Google Scholar]
- 25. Martinez‐Gonzalez MA, Toledo E, Aros F, Fiol M, Corella D, Salas‐Salvado J, Ros E, Covas MI, Fernandez‐Crehuet J, Lapetra J, Munoz MA, Fito M, Serra‐Majem L, Pinto X, Lamuela‐Raventos RM, Sorli JV, Babio N, Buil‐Cosiales P, Ruiz‐Gutierrez V, Estruch R, Alonso A. Extravirgin olive oil consumption reduces risk of atrial fibrillation: the PREDIMED (Prevencion con Dieta Mediterranea) trial. Circulation. 2014;130:18–26. [DOI] [PubMed] [Google Scholar]
- 26. Mostofsky E, Berg Johansen M, Tjønneland A, Chahal HS, Mittleman MA, Overvad K. Chocolate intake and risk of clinically apparent atrial fibrillation: the Danish Diet, Cancer, and Health Study. Heart. 2017;103:1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsson SC, Drca N, Jensen‐Urstad M, Wolk A. Chocolate consumption and risk of atrial fibrillation: two cohort studies and a meta‐analysis. Am Heart J. 2018;195:86–90. [DOI] [PubMed] [Google Scholar]
- 28. Thelle DS. Coffee, caffeine and atrial fibrillation. Eur J Prev Cardiol. 2018;25:1053–1054. [DOI] [PubMed] [Google Scholar]
- 29. Astrup A, Meinert LT, Harper A. Atkins and other low‐carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. [DOI] [PubMed] [Google Scholar]
- 30. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Williams T, Gracely EJ, Stern L. A low‐carbohydrate as compared with a low‐fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. [DOI] [PubMed] [Google Scholar]
- 31. Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie‐restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. [DOI] [PubMed] [Google Scholar]
- 32. Foster GD, Wyatt HR, Hill JO. A randomized trial of a low‐carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. [DOI] [PubMed] [Google Scholar]
- 33. Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation. J Am Coll Cardiol. 2006;48:677–685. [DOI] [PubMed] [Google Scholar]
- 35. Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. [DOI] [PubMed] [Google Scholar]
- 36. Jiao L, Kramer JR, Chen L, Rugge M, Parente P, Verstovsek G, Alsarraj A, El‐Serag HB. Dietary consumption of meat, fat, animal products and advanced glycation end‐products and the risk of Barrett's oesophagus. Aliment Pharmacol Ther. 2013;38:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Youn J, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN, Cai H. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e102 DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 40. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 41. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Criteria for Determining the Low‐Carbohydrate‐Diet Scores
Table S2. Time Varying Sensitivity Analysis for Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy*
Table S3. Time Varying Sensitivity Analysis for Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy*
Table S4. Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy, Stratified by Race
Table S5. Risk of Incident AF for Coronary Intake as a Percentage of Energy, Stratified by Sex
Table S6. Risk of Incident AF for Coronary Intake as a Percentage of Energy, Stratified by Age
Table S7. Risk of Incident AF for Coronary Intake as a Percentage of Energy, Stratified by Hypertension
Table S8. Risk of Incident AF for Carbohydrate Intake as a Percentage of Energy, Excluded of Patients With Cardiovascular Disease or Diabetes
Table S9. Risk of Incident AF for Animal‐Based Low‐Carbohydrate Diet Scores
Table S10. Risk of Incident AF for Plant‐Based Low‐Carbohydrate Diet Scores


