Abstract
Background
Outcomes data among patients with heart failure (HF) with reduced ejection fraction treated with sacubitril/valsartan (SAC/VAL) are largely limited to clinical trial results. We compared hospitalization and healthcare costs among real‐world patients with HF with reduced ejection fraction treated with SAC/VAL versus angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker (ACEI/ARB).
Methods and Results
Using retrospective administrative claims data, stable patients with HF with reduced ejection fraction treated with SAC/VAL or ACEI/ARB from October 2015 to June 2016 were identified. Postindex hospitalization and healthcare costs were assessed in propensity‐matched cohorts using robust variance estimation. Time to first hospitalization was modeled using unadjusted Kaplan–Meier estimates and multivariable models. Postindex all‐cause healthcare costs were modeled using an adjusted multivariable model. Among 279 patients per matched cohort, postindex hospitalization risk was lower for SAC/VAL compared with ACEI/ARB using Kaplan–Meier estimation and unadjusted Cox models. For HF hospitalization, the hazard ratio (95% CI) was 0.56 (0.33–0.94; P=0.030). Adjusted results were similar to unadjusted. Mean (SD) monthly healthcare costs were lower for SAC/VAL versus ACEI/ARB for all categories except pharmacy, with hospital costs being particularly disparate between cohorts: for HF hospitalization, $248 ($1588) for SAC/VAL versus $1122 ($7290) for ACEI/ARB. The adjusted risk of incurring increased all‐cause postindex costs was lower for SAC/VAL versus ACEI/ARB (cost ratio [95% CI] 0.74 [0.59–0.94]; P=0.013).
Conclusions
In clinical practice, patients with HF with reduced ejection fraction treated with SAC/VAL were less likely to be hospitalized than matched patients treated with ACEI/ARB. Despite higher pharmacy costs, SAC/VAL–treated patients incurred lower monthly medical and total healthcare costs.
Keywords: healthcare costs, heart failure, hospitalization, retrospective studies, sacubitril/valsartan
Subject Categories: Heart Failure, ACE/Angiotension Receptors/Renin Angiotensin System, Quality and Outcomes, Cost-Effectiveness, Health Services
Clinical Perspective
What Is New?
In real‐world clinical practice settings, patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan were less likely to be hospitalized than patients treated with an angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker after controlling for patient demographics, comorbid conditions, and other factors.
Patients treated with sacubitril/valsartan also had lower hospitalization costs and total all‐cause healthcare costs than those treated with angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker.
What Are the Clinical Implications?
Cost is often implicated as a barrier to use of novel pharmacological therapy for heart failure with reduced ejection fraction; however, in our real‐world analysis, increased pharmacy costs for sacubitril/valsartan –treated patients were mitigated by lower medical costs.
Healthcare providers need to consider clinical benefit and total costs in addition to drug costs when making treatment decisions.
Introduction
Heart failure (HF) is a major cause of morbidity and mortality; as recently as 2014, ≈900 000 hospital discharges for HF occurred in the United States.1 The cost associated with HF—estimated at $31 billion in 2012—is forecasted to increase to $70 billion by 2030, driven by an aging population and epidemiological factors such as obesity, hypertension, diabetes mellitus, and coronary artery disease.2, 3
Although HF is poised to remain a heavy burden on the US healthcare system for years to come, in recent decades pharmacological therapy enhancements have reduced morbidity and mortality among patients with HF with reduced ejection fraction (HFrEF).4 Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin‐receptor blockers (ARBs) improve clinical outcomes in HFrEF4 and were the mainstays of HFrEF renin‐angiotensin system blockade therapy until 2015, when sacubitril/valsartan (SAC/VAL), a combination neprilysin inhibitor and ARB, was approved by the US Food and Drug Administration. SAC/VAL is used in patients with chronic HFrEF and New York Heart Association functional class II to IV symptoms. Approval of SAC/VAL was based largely on the strength of results from the PARADIGM‐HF (Prospective Comparison of Angiotensin Receptor‐Neprilysin Inhibitor with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, in which SAC/VAL reduced the risk of cardiovascular death and first HF hospitalization among patients with HFrEF by 20% compared with the ACEI, enalapril.5, 6 Reducing hospitalization risk is critical, given that HF hospitalization has been associated with higher 30‐ to 60‐day mortality and readmission rates7 and accounts for the majority of costs attributable to HF in the United States.8
Although PARADIGM‐HF illustrated superior efficacy of SAC/VAL compared with enalapril in a clinical trial setting,6 real‐world data regarding outcomes associated with SAC/VAL use—including potential economic benefit—are limited. This study was conducted to compare hospitalization and healthcare costs among stable patients with HFrEF treated with SAC/VAL versus an ACEI or ARB in clinical practice.
Methods
Study Design and Data Sources
Research materials, data, and analytical methods will not be made available to other researchers for purposes of replicating analysis procedures or reproducing study results.
This retrospective study was conducted using administrative claims data from October 1, 2014 through September 30, 2016 from the Optum Research Database (ORD) with merged mortality data from the US Social Security Administration public death master file. The ORD is a large, population‐representative database containing de‐identified medical and pharmacy claims data and linked enrollment information for individuals enrolled in US commercial and Medicare Advantage health plans. Medical claims included diagnosis and procedure codes from the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD‐9‐CM and ICD‐10‐CM); Current Procedural Terminology or Healthcare Common Procedure Coding System codes; site of service codes; paid amounts; and other information. Pharmacy claims included drug name, dosage form, drug strength, fill date, number of days’ supply, and financial information for health plan–provided outpatient pharmacy services. Because no identifiable protected health information was accessed during this study, institutional review board approval or waiver of authorization was not required.
Patient Identification and Cohort Assignment
The study included patients with at least 1 pharmacy claim for SAC/VAL, ACEI, or ARB from October 1, 2015 through June 30, 2016 (identification period). Adults with a claim for SAC/VAL during the identification period and no previous claims for SAC/VAL were assigned to the SAC/VAL cohort, and those with a claim for ACEI or ARB during the identification period and no claims for SAC/VAL from July 7, 2015 (market approval) through September 30, 2016 (end of the study period) were assigned to the ACEI/ARB cohort. The date of the first claim for the index therapy was defined as the index date. Continuous enrollment in the health plan with both medical and pharmacy coverage was required during the 12 months preceding the index date (preindex period) and for a minimum 3‐month postindex period starting on the index date and ending on the earliest of the following: end of the study period, health plan disenrollment, or death.
Included patients were required to have claims evidence of HFrEF and a stable clinical status. Criteria for claims evidence of HFrEF and stable status are given in Figure 1.9 Patients were also required to be aged ≥18 years (and <65 years if they had a commercial health plan) and to have no missing demographic data. Finally, in order to control for possible early differences in days’ supply of the index therapy (eg, 30‐ versus 90‐day supply) between cohorts, only patients with proportion of days covered ≥0.80 in the first 3 months of the postindex period were retained in the study sample.
Figure 1.
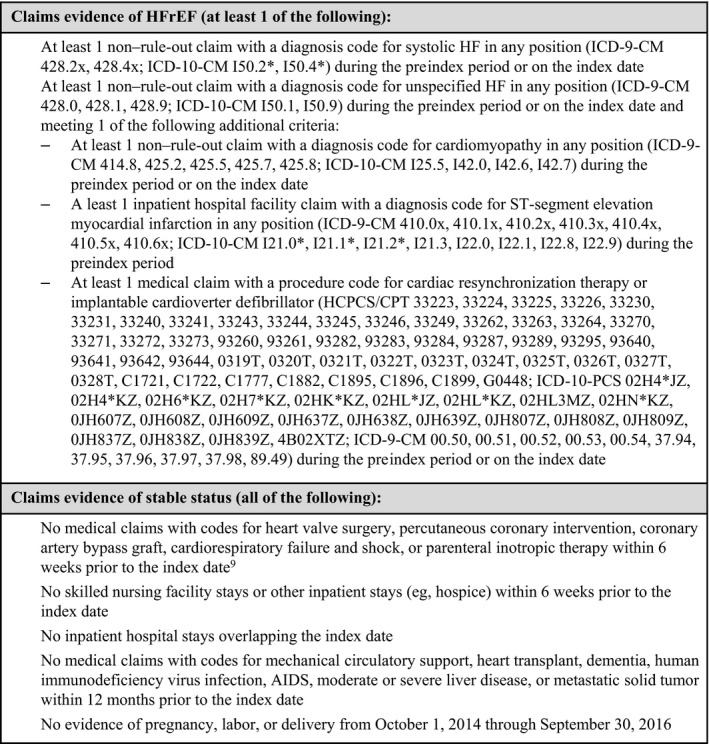
Criteria for claims evidence of HFrEF and stable status. Rule‐out claims (diagnostic services claims) included medical claims with a diagnosis code and diagnostic service procedure code(s) without any other services. Non–rule‐out claims represented medical claims other than rule‐out claims. AIDS indicates acquired immunodeficiency syndrome; CPT, current procedural terminology; HCPCS, Healthcare Common Procedure Coding System; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD‐9‐CM, International Classification of Diseases, Ninth Edition, Clinical Modification; ICD‐10‐CM, International Classification of Diseases, Tenth Edition, Clinical Modification.
To account for potential selection bias, cohorts were 1:1 propensity‐score matched on selected demographics and preindex patient characteristics (see Table 1), including exact matching on health plan type (commercial or Medicare Advantage). For each patient in the SAC/VAL cohort, a patient in the ACEI/ARB cohort with the closest propensity score within a caliper of 0.20 of the SD of the estimated logit was selected. Unmatched patients were excluded from the analysis.
Table 1.
Patient Characteristics
| Characteristic | SAC/VAL Cohort (n=279) | ACEI/ARB Cohort (n=279) | P Value | Used for Matcha | Prematch SMD (%) | Postmatch SMD (%) |
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 68.2 (12.4) | 67.6 (12.9) | 0.400 | ✓b | −29.78 | 5.51 |
| Male, n (%) | 192 (68.8) | 188 (67.4) | 0.720 | ✓ | 14.94 | 3.02 |
| Medicare Advantage health plan, n (%) | 205 (73.5) | 205 (73.5) | ··· | ✓ | −25.68 | −0.00 |
| Geographical region, n (%) | 0.786 | ✓ | 5.20 | −5.31 | ||
| Northeast | 54 (19.4) | 45 (16.1) | 0.313 | ✓ | 6.92 | 8.36 |
| Midwest | 77 (27.6) | 82 (29.4) | 0.630 | ✓ | −23.93 | −3.85 |
| South | 129 (46.2) | 133 (47.7) | 0.726 | Ref. | 19.77 | −2.92 |
| West | 19 (6.8) | 19 (6.8) | 1.000 | ✓ | −4.46 | 0.00 |
| Duration of postindex period, days, mean (SD) | 186.9 (69.2) | 183.0 (71.2) | 0.352 | ✓b | −177.2 | 5.82 |
| Selected preindex comorbid conditions, n (%)c | ||||||
| Hypertension | 256 (91.8) | 251 (90.0) | 0.457 | 0.51 | 6.33 | |
| Dyslipidemia (including hypercholesterolemia) | 226 (81.0) | 221 (79.2) | 0.584 | ✓ | 1.29 | 4.56 |
| Ischemic heart disease (including MI) | 215 (77.1) | 200 (71.7) | 0.137 | 11.77 | 12.34 | |
| Diabetes mellitus (including complications) | 156 (55.9) | 157 (56.3) | 0.932 | ✓ | 12.54 | −0.72 |
| Atrial fibrillation | 125 (44.8) | 132 (47.3) | 0.545 | 4.56 | −5.05 | |
| Renal disease | 89 (31.9) | 93 (33.3) | 0.703 | 1.06 | −3.08 | |
| Chronic obstructive pulmonary disease | 76 (27.2) | 82 (29.4) | 0.581 | −4.08 | −4.76 | |
| Sleep apnea | 74 (26.5) | 70 (25.1) | 0.708 | ✓ | 16.22 | 3.39 |
| Anemia (including iron deficiency) | 37 (13.3) | 42 (15.1) | 0.530 | 1.44 | −5.27 | |
| Selected preindex signs and symptoms, n (%)c | ||||||
| Shortness of breath (not including sleep apnea) | 228 (81.7) | 230 (82.4) | 0.823 | ✓ | 31.47 | −1.67 |
| Altered consciousness | 103 (36.9) | 118 (42.3) | 0.188 | −0.68 | −11.10 | |
| Tachycardia | 88 (31.5) | 93 (33.3) | 0.653 | ✓ | 17.04 | −3.98 |
| Edema and fluid overload | 60 (21.5) | 72 (25.8) | 0.232 | ✓ | −5.20 | −10.32 |
| Pulmonary edema | 38 (13.6) | 36 (12.9) | 0.803 | ✓ | 11.29 | 2.23 |
| Number of preindex guideline‐recommended therapies,d n (%) | ||||||
| 0 | 7 (2.5) | 2 (0.7) | 0.097 | −8.83 | 10.16 | |
| 1 | 23 (8.2) | 28 (10.0) | 0.447 | −41.19 | −5.08 | |
| 2 | 92 (33.0) | 98 (35.1) | 0.591 | −28.14 | −4.44 | |
| 3 | 109 (39.1) | 105 (37.6) | 0.730 | 35.95 | 3.15 | |
| 4 | 45 (16.1) | 42 (15.1) | 0.722 | 39.81 | 3.54 | |
| 5 | 3 (1.1) | 4 (1.4) | 0.706 | 14.42 | −4.12 | |
| Preindex CRT/ICD, n (%) | 172 (61.6) | 157 (56.3) | 0.163 | ✓ | 55.33 | 11.15 |
| Preindex HF hospitalization, n (%) | 66 (23.7) | 64 (22.9) | 0.835 | ✓b | 27.10 | 1.85 |
| Preindex all‐cause hospitalization, n (%) | 124 (44.4) | 136 (48.7) | 0.312 | 0.24 | −8.65 | |
ACEI/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker; CRT/ICD, cardiac resynchronization therapy or implantable cardioverter‐defibrillator; HF, heart failure; ref., reference; MI, myocardial infarction; SAC/VAL, sacubitril/valsartan; SMD, standardized mean difference.
In addition to the patient characteristics indicated, SAC/VAL and ACEI/ARB cohorts were matched for preindex Quan–Charlson comorbidity score (category), selected preindex comorbid conditions (ischemic heart disease [other than MI], pulmonary vascular disease, peripheral artery disease, liver disease, anxiety [including adjustment disorders with anxiety], and substance abuse/dependence [including drugs and alcohol]), preindex HF‐related outpatient pharmacotherapy (ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor agonist, loop diuretic, thiazide diuretic, and digoxin), selected preindex other outpatient pharmacotherapy (anticoagulant, antiplatelet agent, nondihydropyridine calcium‐channel blocker, dihydropyridine calcium‐channel blocker, lipid‐altering medication, vasodilator, insulin, and noninsulin hypoglycemic agent), preindex revascularization, preindex all‐cause medical and pharmacy costs (health plan–dependent quintiles), and encounter with cardiologist in the month preindex. Each preindex outpatient pharmacotherapy was calculated as total days’ supply.
Match performed using categorical version.
Identified using ICD‐9‐CM and ICD‐10‐CM diagnosis codes.
Guideline‐recommended medical therapies included ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor agonist, isosorbide dinitrate+hydralazine, digoxin, and ivabradine.
Patient Characteristics and Outcomes
Patient characteristics included demographic information (age, sex, health plan type, and geographical region); duration of the postindex period; preindex Quan–Charlson comorbidity score;10 selected preindex comorbid conditions, signs and symptoms (based on ICD‐9‐CM or ICD‐10‐CM diagnosis codes), preindex HF‐related outpatient pharmacotherapy (ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor agonist, loop diuretic, thiazide diuretic, and digoxin); selected preindex other outpatient pharmacotherapy (anticoagulant, antiplatelet agent, nondihydropyridine calcium‐channel blocker, dihydropyridine calcium‐channel blocker, lipid‐altering medication, vasodilator, insulin, or noninsulin hypoglycemic agent); number of preindex HF‐related guideline therapies (ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor agonist, isosorbide dinitrate+hydralazine, digoxin, and ivabradine); preindex cardiac resynchronization therapy or implantable cardioverter defibrillator; preindex revascularization; preindex hospitalization (all‐cause and HF); preindex all‐cause medical costs; preindex all‐cause outpatient pharmacy costs; and encounter with cardiologist in the month preindex. HF hospitalization was identified on the basis of facility claims for hospitalizations with an HF diagnosis code (ICD‐9‐CM 402.x1, 404.x1, 404.x3, 428.xx; ICD‐10‐CM I11.0, I13.0, I13.2, I50.1, I50.2*, I50.3*, I50.4*, I50.9, I97.13*) in the primary position.
Study outcomes included postindex hospitalization (HF and all‐cause; measured as per‐patient‐per‐month [PPPM] count, indicator variable, and time to first event) and healthcare costs (HF hospital and all‐cause; measured as PPPM costs). HF hospital costs were defined as costs associated with HF hospitalization. Healthcare costs were calculated as combined health plan plus patient‐paid amounts, adjusted to 2016 US dollars using the annual medical care component of the Consumer Price Index.11 Patient‐paid amounts were also calculated separately. All‐cause healthcare costs were reported as total costs and for subcategories of medical, hospital, and outpatient pharmacy costs.
Statistical Analysis
The pre‐ and postmatch balances of patient characteristics between cohorts were evaluated using standardized mean differences (SMDs; by convention, values ≥10% indicated meaningful imbalance).12 Postindex hospitalization (PPPM counts) and healthcare costs between matched cohorts were compared using robust variance estimation. Time to first postindex hospitalization (HF and all‐cause) was modeled using Kaplan–Meier failure probability estimates and multivariable Cox proportional hazards models. Postindex all‐cause healthcare costs were modeled using a multivariable generalized linear model with gamma distribution and log link. In addition to cohort, adjustment variables for multivariable modeling included age and health plan type interaction, sex, selected (a priori) preindex chronic comorbid conditions, selected (a priori) preindex signs and symptoms, number of preindex guideline therapies, preindex cardiac resynchronization therapy/implantable cardioverter defibrillator, preindex revascularization, preindex HF hospitalization, preindex all‐cause medical costs, and preindex all‐cause outpatient pharmacy costs. Because the precision of signs/symptoms identified from ICD‐9/10‐CM codes may vary, sensitivity analyses in which preindex signs and symptoms were excluded from the multivariable models were also performed. Statistical analysis was performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC). P<0.05 was considered statistically significant.
Results
Study Sample
Among patients who initiated 1 of the index therapies during the identification period, the remaining selection criteria were satisfied by 287 in the SAC/VAL cohort and 22 469 in the ACEI/ARB cohort (Figure 2). Before matching, several patient characteristics differed between cohorts. Compared with ACEI/ARB–treated patients, SAC/VAL–treated patients were younger (mean age, 68.0 versus 71.6 years; SMD=−29.8); had a higher percentage of commercial enrollees (26.8% versus 16.3%; SMD=25.7); had a lower mean duration of the postindex period (185 versus 303 days; SMD=−177.2); had higher percentages of selected preindex comorbid conditions and symptoms, including pulmonary vascular disease (15.3% versus 7.6%; SMD=24.4) and shortness of breath (81.5% versus 68.0%; SMD=31.5); and had a higher percentage with preindex HF hospitalization (24.0% versus 13.5%; SMD=27.10; Table 1).
Figure 2.
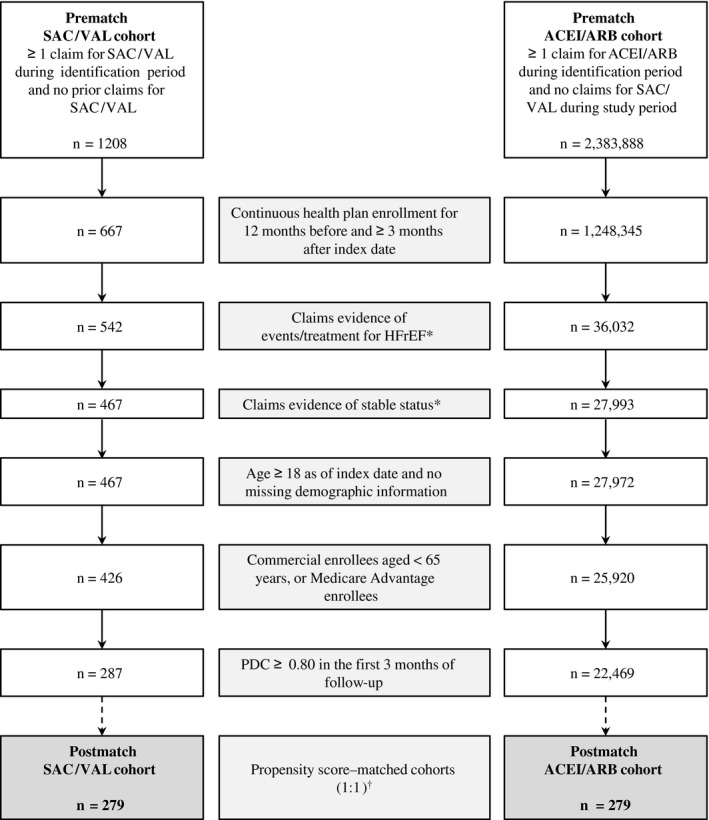
Patient selection and attrition. Identification period: October 1, 2015 through June 30, 2016. Index date: date of first pharmacy claim for SAC/VAL or ACEI/ARB. ACEI/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker; CRT/ICD, cardiac resynchronization therapy/implantable cardioverter‐defibrillator; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction; PDC, proportion of days covered; SAC/VAL, sacubitril/valsartan. *Criteria for claims evidence of HF and stable status are provided in Figure 1. †Variables used in propensity score matching are provided in Table 1.
After propensity‐score matching, there were 279 patients in each cohort. Preindex characteristics were similar for SAC/VAL versus ACEI/ARB and SMDs were ≤10% for most variables (range, 0.0–12.5%), although differences remained for ischemic heart disease (77.1% versus 71.7%; SMD=12.3), altered consciousness (36.9% versus 42.3%; SMD=−11.1), and edema and fluid overload (21.5% versus 25.8%; SMD=−10.3). Other preindex comorbid conditions that were highly prevalent though similar between cohorts included hypertension (91.8% SAC/VAL, 90.0% ACEI/ARB), dyslipidemia (81.0% SAC/VAL, 79.2% ACEI/ARB), diabetes mellitus (55.9% SAC/VAL, 56.3% ACEI/ARB), and atrial fibrillation (44.8% SAC/VAL, 47.3% ACEI/ARB).
Postindex Hospitalization
Mean (SD) number of postindex PPPM hospitalizations was lower in the SAC/VAL versus the ACEI/ARB cohort for HF hospitalizations (0.01 [0.06] versus 0.03 [0.10]; P=0.003) and all‐cause hospitalizations (0.05 [0.11] versus 0.11 [0.20]; P<0.001). Kaplan–Meier estimation revealed a lower probability of first postindex HF hospitalization and all‐cause hospitalization with SAC/VAL versus ACEI/ARB at 30, 91, and 183 days (Figure 3). Risk‐based postindex hospitalization in unadjusted Cox proportional hazards models was also lower among SAC/VAL–treated versus ACEI/ARB–treated patients (HF hospitalization hazard ratio [HR], 0.56; 95% CI, 0.33–0.94; P=0.030 and all‐cause hospitalization HR, 0.57; 95% CI, 0.42–0.77; P<0.001). Multivariable analysis results were similar after adjustment for preindex demographics and characteristics (HF hospitalization HR, 0.42; 95% CI, 0.23–0.79; P=0.007 and all‐cause hospitalization HR, 0.51; 95% CI, 0.36–0.71; P<0.001; Tables 2 and 3, respectively). In sensitivity analyses that excluded signs and symptoms from the multivariable models, adjusted hospitalization risk remained lower for patients treated with SAC/VAL compared with ACEI/ARB (HF hospitalization HR, 0.43; 95% CI, 0.24–0.80; P=0.007 and all‐cause hospitalization HR, 0.50; 95% CI, 0.36–0.69; P<0.001; full results not shown).
Figure 3.
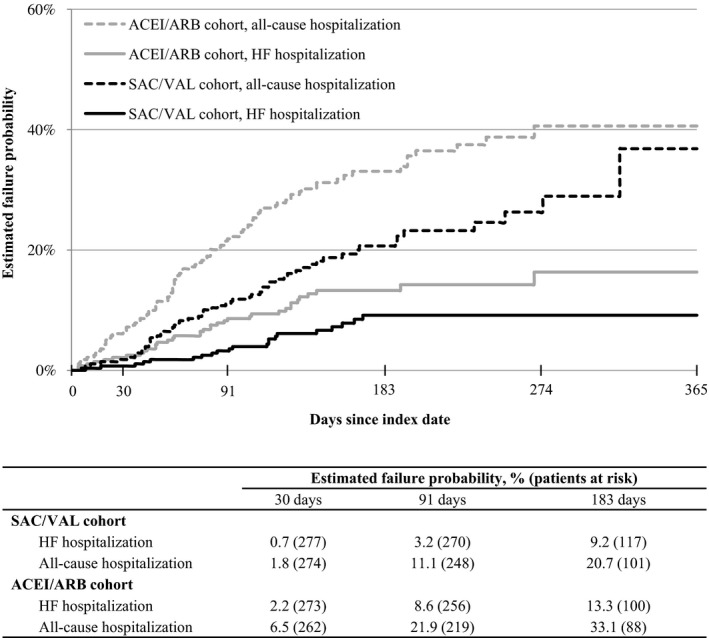
Kaplan–Meier plots of first postindex hospitalization. Patient data are observed until each event of interest (HF hospitalization or all‐cause hospitalization) or patient postindex is censored (earliest of end of study period, health plan disenrollment, or death). Log‐rank tests: P=0.031 for HF hospitalization; P<0.001 for all‐cause hospitalization (SAC/VAL cohort vs ACEI/ARB cohort). ACEI/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker; HF, heart failure; SAC/VAL, sacubitril/valsartan.
Table 2.
Multivariable Proportional Hazards Model of Follow‐up HF Hospitalization
| Independent Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| SAC/VAL cohort (ref.: ACEI/ARB cohort)a | 0.42 (0.23–0.79) | 0.007 |
| Health plan type, patient age (ref.: commercial, 18–64 y) | ||
| Medicare Advantage, 18 to 64 y | 2.48 (0.79–7.78) | 0.119 |
| Medicare Advantage, ≥65 y | 2.50 (0.89–7.08) | 0.084 |
| Male (ref.: female) | 0.65 (0.33–1.25) | 0.196 |
| Baseline comorbid conditions | ||
| Dyslipidemia | 0.63 (0.29–1.35) | 0.231 |
| Ischemic heart disease other than MI | 2.93 (1.13–7.61) | 0.027 |
| Diabetes mellitus (including complications) | 0.73 (0.37–1.46) | 0.377 |
| Atrial fibrillation | 1.67 (0.87–3.20) | 0.122 |
| Renal disease | 1.57 (0.76–3.23) | 0.224 |
| Chronic obstructive pulmonary disease | 0.68 (0.36–1.32) | 0.257 |
| Peripheral artery disease | 0.96 (0.43–2.17) | 0.924 |
| Cerebrovascular disease | 0.73 (0.27–1.95) | 0.529 |
| Pulmonary edema | 0.45 (0.17–1.17) | 0.103 |
| Asthma | 0.95 (0.41–2.19) | 0.906 |
| Pulmonary vascular disease | 0.85 (0.35–2.07) | 0.712 |
| Primary malignancy | 1.74 (0.74–4.12) | 0.205 |
| Liver disease | 1.38 (0.41–4.64) | 0.600 |
| Baseline symptoms | ||
| Shortness of breath | 2.27 (0.76–6.73) | 0.141 |
| Altered consciousness | 0.71 (0.33–1.54) | 0.383 |
| Tachycardia | 1.58 (0.85–2.94) | 0.145 |
| Edema and fluid overload | 1.68 (0.81–3.51) | 0.167 |
| Palpitations | 1.35 (0.62–2.95) | 0.454 |
| Baseline number of HF guideline‐recommended therapiesb (ref.: 0–1) | ||
| 2 | 5.77 (1.08–30.96) | 0.041 |
| 3 | 3.82 (0.75–19.56) | 0.108 |
| ≥4 | 6.27 (1.16–33.92) | 0.033 |
| Baseline CRT/ICD | 2.33 (1.18–4.57) | 0.014 |
| Baseline revascularization | 0.32 (0.07–1.38) | 0.125 |
| Baseline HF hospitalizations (ref.: 0) | ||
| 1 | 3.09 (1.24–7.74) | 0.016 |
| ≥2 | 11.22 (3.50–35.95) | <0.001 |
| Baseline all‐cause medical costsc (ref.: quintile 1) | ||
| Quintile 2 | 2.07 (0.78–5.50) | 0.147 |
| Quintile 3 | 1.14 (0.36–3.54) | 0.827 |
| Quintile 4 | 1.19 (0.41–3.42) | 0.751 |
| Quintile 5 | 0.68 (0.21–2.22) | 0.527 |
| Baseline all‐cause outpatient pharmacy costsc (ref.: quintile 1) | ||
| Quintile 2 | 0.61 (0.19–1.95) | 0.406 |
| Quintile 3 | 0.87 (0.30–2.51) | 0.789 |
| Quintile 4 | 1.77 (0.66–4.74) | 0.256 |
| Quintile 5 | 1.05 (0.36–3.01) | 0.931 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CRT/ICD, cardiac resynchronization therapy+implantable cardioverter defibrillator; HF, heart failure; MI, myocardial infarction; ref., reference; SAC/VAL, sacubitril/valsartan.
Unadjusted results for HF hospitalization: hazard ratio=0.56; 95% CI, 0.33 to 0.94; P=0.030.
Guideline therapies include ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor antagonist, hydralazine+isosorbide dinitrate, digoxin, and ivabradine.
Health plan–dependent quintiles; quintiles are ordered from 1 (lowest cost) to 5 (highest cost).
Table 3.
Multivariable Proportional Hazards Model of Follow‐up All‐Cause Hospitalization
| Independent Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| SAC/VAL cohort (ref.: ACEI/ARB cohort)a | 0.51 (0.36–0.71) | <0.001 |
| Health plan type, patient age (ref.: commercial, 18–64 y) | ||
| Medicare Advantage, 18 to 64 y | 2.12 (1.13–3.98) | 0.020 |
| Medicare Advantage, ≥65 y | 1.96 (1.08–3.56) | 0.028 |
| Male (ref.: female) | 0.89 (0.62–1.28) | 0.536 |
| Baseline comorbid conditions | ||
| Dyslipidemia | 0.92 (0.58–1.47) | 0.723 |
| Ischemic heart disease other than MI | 1.69 (1.01–2.81) | 0.044 |
| Diabetes mellitus (including complications) | 0.99 (0.66–1.47) | 0.944 |
| Atrial fibrillation | 1.16 (0.82–1.63) | 0.414 |
| Renal disease | 1.66 (1.12–2.45) | 0.012 |
| Chronic obstructive pulmonary disease | 0.74 (0.50–1.11) | 0.142 |
| Peripheral artery disease | 0.75 (0.46–1.22) | 0.241 |
| Cerebrovascular disease | 0.95 (0.60–1.49) | 0.812 |
| Pulmonary edema | 0.81 (0.49–1.35) | 0.422 |
| Asthma | 1.24 (0.80–1.94) | 0.338 |
| Pulmonary vascular disease | 1.00 (0.60–1.67) | 0.992 |
| Primary malignancy | 1.60 (0.98–2.62) | 0.059 |
| Liver disease | 0.84 (0.46–1.55) | 0.580 |
| Baseline symptoms | ||
| Shortness of breath | 2.10 (1.11–3.98) | 0.023 |
| Altered consciousness | 1.07 (0.75–1.52) | 0.717 |
| Tachycardia | 1.19 (0.83–1.71) | 0.340 |
| Edema and fluid overload | 1.52 (1.01–2.29) | 0.047 |
| Palpitations | 0.71 (0.39–1.30) | 0.264 |
| Baseline number of HF guideline‐recommended therapiesb (ref.: 0–1) | ||
| 2 | 1.31 (0.72–2.38) | 0.382 |
| 3 | 0.99 (0.52–1.86) | 0.963 |
| ≥4 | 1.45 (0.76–2.77) | 0.259 |
| Baseline CRT/ICD | 1.18 (0.80–1.73) | 0.408 |
| Baseline revascularization | 1.32 (0.78–2.23) | 0.300 |
| Baseline HF hospitalizations (ref.: 0) | ||
| 1 | 1.24 (0.75–2.07) | 0.402 |
| ≥2 | 2.59 (1.37–4.91) | 0.004 |
| Baseline all‐cause medical costsb (ref.: quintile 1) | ||
| Quintile 2 | 1.48 (0.75–2.90) | 0.259 |
| Quintile 3 | 1.18 (0.64–2.17) | 0.607 |
| Quintile 4 | 1.10 (0.56–2.16) | 0.791 |
| Quintile 5 | 1.45 (0.72–2.93) | 0.296 |
| Baseline all‐cause outpatient pharmacy costsc (ref.: quintile 1) | ||
| Quintile 2 | 0.88 (0.46–1.69) | 0.701 |
| Quintile 3 | 1.11 (0.62–2.01) | 0.721 |
| Quintile 4 | 1.63 (0.91–2.94) | 0.102 |
| Quintile 5 | 1.49 (0.79–2.84) | 0.221 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CRT/ICD, cardiac resynchronization therapy+implantable cardioverter defibrillator; HF, heart failure; MI, myocardial infarction; ref., reference; SAC/VAL, sacubitril/valsartan.
Unadjusted results for all‐cause hospitalization: hazard ratio=0.57; 95% CI, 0.42 to 0.77; P<0.001.
Guideline therapies include ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor antagonist, hydralazine+isosorbide dinitrate, digoxin, and ivabradine.
Health plan–dependent quintiles; quintiles are ordered from 1 (lowest cost) to 5 (highest cost).
Postindex Healthcare Costs
Patients treated with SAC/VAL had lower postindex PPPM HF hospital costs (P=0.048), all‐cause total costs (P=0.033), all‐cause medical costs (P=0.004), and all‐cause hospital costs (P<0.001) compared with patients treated with ACEI/ARB (Figure 4, health plan–paid plus patient‐paid). Patients treated with ACEI/ARB versus SAC/VAL had mean (SD) PPPM costs that were >4 times higher for HF hospitalization ($1122 [$7290] versus $248 [$1588]; P=0.048) and >3 times higher for all‐cause hospitalization ($2770 [$9189] versus ($810 [$2921]; P<0.001). Mean (SD) PPPM all‐cause outpatient pharmacy costs were higher for SAC/VAL compared with ACEI/ARB ($947 [$1282] versus $515 [$1041]; P<0.001). After adjusting for preindex demographics and characteristics, risk of incurring increased total PPPM costs was lower for patients treated with SAC/VAL compared with ACEI/ARB (cost ratio [95% CI] 0.74 [0.59–0.94]; P=0.013; Table 4). When a sensitivity analysis excluding signs and symptoms from the multivariable model was performed, adjusted risk remained lower for patients treated with SAC/VAL compared with ACEI/ARB (cost ratio [95% CI] 0.71 [0.56–0.90]; P=0.004; full results not shown).
Figure 4.
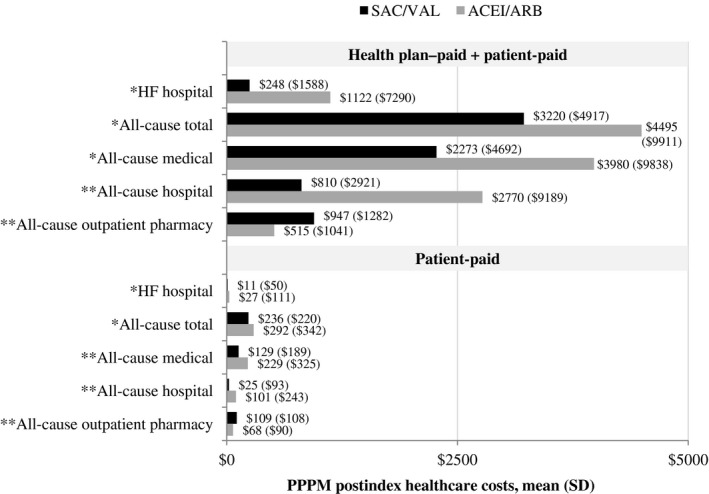
Per‐patient per‐month (PPPM) postindex healthcare costs. Combined health plan–paid+patient‐paid amounts (top) and patient‐paid amounts (bottom) are shown. *P<0.05; **P<0.001. ACEI/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker; HF, heart failure; SAC/VAL, sacubitril/valsartan.
Table 4.
Multivariable Generalized Linear Model of Follow‐up All‐Cause Healthcare Costs
| Independent Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| SAC/VAL cohort (ref.: ACEI/ARB cohort) | 0.74 (0.59–0.94) | 0.013 |
| Health plan type, patient age (ref.: commercial, 18–64 y) | ||
| Medicare Advantage, 18 to 64 y | 0.97 (0.59–1.59) | 0.890 |
| Medicare Advantage, ≥65 y | 0.72 (0.50–1.04) | 0.081 |
| Male (ref.: female) | 0.95 (0.71–1.26) | 0.705 |
| Baseline comorbid conditions | ||
| Dyslipidemia | 0.90 (0.58–1.42) | 0.660 |
| Ischemic heart disease other than MI | 1.24 (0.86–1.78) | 0.256 |
| Diabetes mellitus (including complications) | 0.73 (0.55–0.97) | 0.031 |
| Atrial fibrillation | 1.02 (0.77–1.34) | 0.902 |
| Renal disease | 1.13 (0.82–1.55) | 0.451 |
| Chronic obstructive pulmonary disease | 1.01 (0.73–1.40) | 0.948 |
| Peripheral artery disease | 0.91 (0.67–1.22) | 0.514 |
| Cerebrovascular disease | 0.90 (0.68–1.19) | 0.456 |
| Pulmonary edema | 0.80 (0.47–1.36) | 0.404 |
| Asthma | 0.81 (0.57–1.15) | 0.228 |
| Pulmonary vascular disease | 1.12 (0.81–1.56) | 0.499 |
| Primary malignancy | 1.06 (0.74–1.52) | 0.745 |
| Liver disease | 0.95 (0.66–1.38) | 0.789 |
| Baseline symptoms | ||
| Shortness of breath | 1.25 (0.87–1.79) | 0.232 |
| Altered consciousness | 1.00 (0.76–1.32) | 0.987 |
| Tachycardia | 0.98 (0.77–1.26) | 0.886 |
| Edema and fluid overload | 1.33 (0.91–1.93) | 0.139 |
| Palpitations | 1.33 (0.78–2.26) | 0.300 |
| Baseline number of HF guideline recommended therapiesa (ref.: 0–1) | ||
| 2 | 1.23 (0.74–2.05) | 0.425 |
| 3 | 1.12 (0.71–1.77) | 0.637 |
| ≥4 | 1.54 (0.94–2.54) | 0.090 |
| Baseline CRT/ICD | 0.98 (0.76–1.27) | 0.885 |
| Baseline revascularization | 0.76 (0.50–1.15) | 0.195 |
| Baseline HF hospitalizations (ref.: 0) | ||
| 1 | 1.35 (0.93–1.98) | 0.119 |
| ≥2 | 1.17 (0.70–1.96) | 0.554 |
| Baseline all‐cause medical costsb (ref.: quintile 1) | ||
| Quintile 2 | 1.33 (0.88–2.01) | 0.181 |
| Quintile 3 | 1.48 (0.98–2.24) | 0.066 |
| Quintile 4 | 1.72 (1.07–2.77) | 0.025 |
| Quintile 5 | 2.12 (1.25–3.58) | 0.005 |
| Baseline all‐cause outpatient pharmacy costsb (ref.: quintile 1) | ||
| Quintile 2 | 1.23 (0.79–1.94) | 0.362 |
| Quintile 3 | 0.95 (0.62–1.46) | 0.824 |
| Quintile 4 | 1.30 (0.82–2.04) | 0.263 |
| Quintile 5 | 1.96 (1.21–3.19) | 0.007 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CRT/ICD, cardiac resynchronization therapy+implantable cardioverter defibrillator; HF, heart failure; MI, myocardial infarction; ref., reference; SAC/VAL, sacubitril/valsartan.
Guideline therapies include ACEI/ARB, evidence‐based beta blocker, mineralocorticoid receptor antagonist, hydralazine+isosorbide dinitrate, digoxin, ivabradine.
Health plan–dependent quintiles; quintiles are ordered from 1 (lowest cost) to 5 (highest cost).
The impact on patient‐paid costs was similar to that observed for overall costs. Compared with ACEI/ARB–treated patients, those treated with SAC/VAL had lower postindex PPPM patient‐paid HF hospital costs (P=0.023), all‐cause total costs (P=0.023), all‐cause medical costs (P<0.001), and all‐cause hospital costs (P<0.001), but higher postindex PPPM all‐cause outpatient pharmacy costs (P<0.001; Figure 4).
Discussion
In this real‐world analysis, patients with HFrEF treated with SAC/VAL in clinical practice were less likely to be hospitalized and incurred lower total healthcare costs than those treated with ACEI/ARB. Our results provide an important complement to those of PARADIGM‐HF, in which SAC/VAL was superior to enalapril for reducing cardiovascular death and first HF hospitalization in a clinical trial setting.6 Real‐world data regarding longitudinal health economic outcomes among patients with HFrEF who were treated with SAC/VAL have been reported in only 1 other article to date, a single‐arm report by Antol et al.13 The substantial reduction in hospitalization observed in our study's SAC/VAL cohort was congruent with findings from the Antol et al study, which involved enrollees in health plans managed by Humana (primarily Medicare Advantage). After initiating SAC/VAL, patients had a decrease in 3‐month all‐cause hospitalization, from 27.5% to 17.0%.13 Hospitalization is an important outcome in HFrEF; it is widespread,14 and patients who are hospitalized are highly likely to be readmitted.8, 15 In a 2017 analysis, more than 40% of Medicare beneficiaries hospitalized for HF were readmitted within 90 days.15 Given that hospitalization is a robust predictor of increased mortality in the HFrEF population,7 reduction of inpatient admissions is essential to improving patient morbidity and mortality outcomes.
In this study, total postindex HF hospital costs and all‐cause total healthcare costs were lower for patients treated with SAC/VAL versus ACEI/ARB. Despite strong evidence of superior efficacy, adoption of SAC/VAL in clinical practice after US market approval has been relatively slow, with reports ranging from 2%16 to 13%17 of patients with HFrEF. Cost has often been implicated as a barrier to use of novel pharmacological therapy, because both health plan– and patient‐paid costs for new drugs are considerably higher than those for established HFrEF guideline therapies.16, 18, 19 In 2016, the estimated monthly retail cost of treatment with SAC/VAL was $425 compared with $9 and $25 for lisinopril and enalapril, respectively,19 and in a retrospective analysis using 2015–2016 data from the OptumLabs Data Warehouse, patient‐paid monthly costs for SAC/VAL remained high—a mean of $71 compared with $2 to $3 for lisinopril, losartan, carvedilol, and spironolactone—despite the majority of monthly costs being covered by payers.16 Our findings offer new evidence that SAC/VAL provides value in terms of healthcare costs (HF hospital, all‐cause total, all‐cause medical, and all‐cause hospital). Perceptions surrounding drug cost should be balanced with clinical and total cost benefits. Similar to other analyses of real‐world data for SAC/VAL, our results reflect an early view (the first 15 months after US Food and Drug Administration approval) of patient access to this therapy. As additional evidence of clinical practice outcomes becomes available and SAC/VAL is increasingly accepted by the healthcare community, its accessibility and affordability may change markedly.
In our analysis, and congruent with existing data, both combined and patient‐paid outpatient pharmacy costs were higher for SAC/VAL compared with ACEI/ARB. Higher drug costs were offset by reduced monthly medical costs; in particular, hospital costs and total healthcare costs were lower in the SAC/VAL cohort. Our real‐world results provide further evidence that the economic burden of HF is dominated by inpatient costs. In 1 report, inpatient costs accounted for as much as 62% of total healthcare costs.15 Economic modeling analyses using data from PARADIGM‐HF consistently indicated that treating HFrEF with SAC/VAL compared with enalapril would be cost‐effective from a US payer perspective.20, 21, 22, 23 The present report expands on current knowledge by examining cost from a patient perspective. Interestingly, our finding that total healthcare costs were lower for SAC/VAL initiators contrasts with findings from modeling analyses, in which researchers suggested that increased quality‐adjusted life‐years associated with SAC/VAL use among patients with HFrEF would come with higher healthcare costs.20, 21, 22, 23 Possible explanations for this discrepancy include differences in event rates between our patient sample and the PARADIGM‐HF study sample, as well as our use of actual paid amounts rather than modeled cost data. Our results contribute to the body of literature and can be utilized to help make future cost‐effectiveness analyses more reflective of real‐world patient outcomes.
Data regarding real‐world characteristics among patients with HFrEF who were treated with SAC/VAL were found in only 3 articles: by Antol et al,13 a single‐arm, retrospective, medical record–augmented data claims study of 200 US patients initiating SAC/VAL between August 2015 and March 2016; by Wachter et al,24 an electronic medical records–based study of 1041 German patients initiating SAC/VAL in 2016; and from the authors of the Change in Management of Patients with Heart Failure (CHAMP‐HF) registry,17 a prospective longitudinal study of 3497 US patients, 452 of which were prescribed SAC/VAL. Patients in claims‐ and electronic medical records–based samples, compared with patients from trials and registries, were older (mean age, 68, 72, 73, 64, and 66 years for the current study, Antol et al, Wachter et al, the PARADIGM‐HF clinical trial, and the CHAMP‐HF registry, respectively) and had a higher comorbidity burden, including higher percentages with hypertension (92%, 94%, 56%, 71%, and 82%), diabetes mellitus (56%, 53%, 31% [exception, lower percentage], 34%, and 41%), and atrial fibrillation (45%, 48%, 29% [atrial fibrillation/flutter; exception, lower percentage], 37%, and 36%).
It was not surprising that the PARADIGM‐HF sample was younger and healthier than our real‐world sample of patients with HFrEF, given that clinical trials are often conducted among individuals with stable non‐HF preindex characteristics. For example, in 2 reports of hospitalized adults, a retrospective review of patient characteristics showed that only 55%25 and 45%26 of patients who were US Food and Drug Administration‐eligible for SAC/VAL would have met the PARADIGM‐HF inclusion criteria. Retrospectively obtained real‐world data from patients initiating SAC/VAL have been similar to those in a contemporary non–SAC/VAL real‐world report of patients with HFrEF,15 and reflect that real‐world patients with chronic HF may have more age‐related and medical challenges that could influence clinical outcomes over time. Thus, there is a need to examine data from clinically complex cohorts that are representative of patients treated in clinical practice following publication of clinical trial results.
Study Limitations
Our findings should be interpreted in light of several limitations. Because this was a retrospective observational study, causal relationships cannot be inferred between the outcomes of interest and treatment with SAC/VAL or ACEI/ARB. Furthermore, the potential impact of concomitant HF‐related therapies was not examined, and other unmeasured variables, such as drug dosage, patient socioeconomic status, or provider performance metrics, may have also affected the results. This study was conducted in a US managed‐care sample, and the mean length of the postindex period was 6 months. Results may not be generalizable to other populations, such as patients who are uninsured or on fee‐for‐service healthcare plans, and may be most relevant in the short‐term period following SAC/VAL (versus ACEI/ARB) initiation. Patients in the cohorts were matched at a 1:1 ratio, which limited the precision of estimators; however, a 1:1 ratio was chosen to reduce bias in estimators to the greatest possible extent. In addition, some small between‐cohort differences in several preindex patient characteristics remained after matching. Although the list of match variables was extensive, it is also possible that there were between‐cohort imbalances in disease severity that were not evident in the observed patient characteristics, particularly given that the diagnosis codes used to identify signs and symptoms can be imprecise and do not capture stability or severity, and patients with more‐persistent symptoms may have been more likely to initiate SAC/VAL. Because this study used claims data, we recognize inherent limitations; most important, pharmacy claims reflected medication fills, not over‐the‐counter drug use, physician‐provided samples, fills not processed through the primary insurer (eg, medications available through low‐cost generic programs), or medications prescribed but not filled. In addition, presence of a diagnosis code on a medical claim does not assure the presence of disease, given that diagnoses may be coded incorrectly or included as rule‐out criteria. Conversely, identification of conditions and signs and symptoms based on diagnosis codes requires that they were actually coded. Some information is not available in claims data, such as New York Heart Association functional class, ejection fraction percentage, blood pressure, and results from testing of serum electrolytes and biomarkers (eg, potassium, renal function markers, and N‐terminal pro‐B‐type natriuretic peptide). Finally, a proxy algorithm was used to help identify patients with HFrEF.
Conclusion
Among patients with HFrEF, treatment with SAC/VAL in clinical practice was associated with lower short‐term postindex hospitalization risk and total healthcare costs compared with treatment with ACEI/ARB. Increased pharmacy costs for SAC/VAL–treated patients were mitigated by lower medical costs, including HF and all‐cause hospital costs. These findings underscore the need to consider clinical benefit and total costs in addition to drug costs when making treatment decisions. Treatment of real‐world patients with SAC/VAL has the potential to improve clinical outcomes and reduce the economic burden of HFrEF.
Sources of Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Disclosures
Albert is a consultant for Novartis Pharmaceuticals Corporation (modest relationship). Swindle and Buysman are employees of Optum, which was contracted by Novartis Pharmaceuticals Corporation to conduct this study and provide medical writing services (modest relationship). Chang is an employee of Novartis Pharmaceuticals Corporation (significant relationship) and owns stock in Novartis AG (modest relationship).
Acknowledgments
Medical writing services were provided by Yvette Edmonds, PhD, an employee of Optum. Programming of the claims data was provided by Feng Cao, PhD; Randall Gerdes; and Damon Van Voorhis, BS, employees of Optum. Analytical support was provided by Bret Gitar, MA, an employee of Optum.
(J Am Heart Assoc. 2019;8:e011089 DOI: 10.1161/JAHA.118.011089.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biglane JB, Becnel MF, Ventura HO, Krim SR. Pharmacologic therapy for heart failure with reduced ejection fraction: closing the gap between clinical guidelines and practice. Prog Cardiovasc Dis. 2017;60:187–197. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 5. FDA approves new drug to treat heart failure [press release]. Silver Spring, MD: US Food and Drug Administration; 08 July 2015. Available at: https://wayback.archive-it.org/7993/20171102201703/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm. Accessed June 22, 2018. [Google Scholar]
- 6. Mogensen UM, Kober L, Kristensen SL, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray JJV; PARADIGM‐HF Investigators and Committees . The effects of sacubitril/valsartan on coronary outcomes in PARADIGM‐HF. Am Heart J. 2017;188:35–41. [DOI] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 8. Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol. 2014;37:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL; HF‐ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. [DOI] [PubMed] [Google Scholar]
- 11. Consumer Price Index . Medical care. Series ID: SUUR0000SAM. Washington, DC: US Bureau of Labor Statistics; 2016. Available at: https://www.bls.gov/cpi/data.htm. Accessed June 15, 2017. [Google Scholar]
- 12. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antol DD, Casebeer AW, DeClue RW, Stemkowski S, Russo PA. An early view of real‐world patient response to sacubitril/valsartan: a retrospective study of patients with heart failure with reduced ejection fraction. Adv Ther. 2018;35:785–795. [DOI] [PubMed] [Google Scholar]
- 14. Bress AP, King JB, Brixner D, Kielhorn A, Patel HK, Maya J, Lee VC, Biskupiak J, Munger M. Pharmacotherapy treatment patterns, outcomes, and health resource utilization among patients with heart failure with reduced ejection fraction at a U.S. academic medical center. Pharmacotherapy. 2016;36:174–186. [DOI] [PubMed] [Google Scholar]
- 15. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sangaralingham LR, Sangaralingham SJ, Shah ND, Yao X, Dunlay SM. Adoption of sacubitril/valsartan for the management of patients with heart failure. Circ Heart Fail. 2018;11:e004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeVore AD, Mi X, Thomas L, Sharma PP, Albert NM, Butler J, Hernandez AF, Patterson JH, Spertus JA, Williams FB, Duffy CI, McCague K, Fonarow GC. Characteristics and treatments of patients enrolled in the CHAMP‐HF registry compared with patients enrolled in the PARADIGM‐HF trial. J Am Heart Assoc. 2018;7:e009237 DOI: 10.1161/JAHA.118.009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hubers SA, Brown NJ. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation. 2016;133:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chavey WE, Hogikyan RV, Van Harrison R, Nicklas JM. Heart failure due to reduced ejection fraction: medical management. Am Fam Physician. 2017;95:13–20. [PubMed] [Google Scholar]
- 20. Zueger PM, Kumar VM, Harrington RL, Rigoni GC, Atwood A, DiDomenico RJ, Touchette DR. Cost‐effectiveness analysis of sacubitril/valsartan for the treatment of heart failure with reduced ejection fraction in the United States. Pharmacotherapy. 2018;38:520–530. [DOI] [PubMed] [Google Scholar]
- 21. Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux‐Voinet C, Turner SJ, Rouleau JL, Zile MR, McMurray JJ, Solomon SD. Cost‐effectiveness analysis of sacubitril/valsartan vs enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016;1:666–672. [DOI] [PubMed] [Google Scholar]
- 22. Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD, Heidenreich PA. Cost‐effectiveness of sacubitril/valsartan in patients with heart failure with reduced ejection fraction. Ann Intern Med. 2016;165:681–689. [DOI] [PubMed] [Google Scholar]
- 23. King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost‐effectiveness of sacubitril/valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2016;4:392–402. [DOI] [PubMed] [Google Scholar]
- 24. Wachter R, Viriato D, Klebs S, Grunow SS, Schindler M, Engelhard J, Proenca CC, Calado F, Schlienger R, Dworak M, Balas B, Bruce Wirta S. Early insights into the characteristics and evolution of clinical parameters in a cohort of patients prescribed sacubitril/valsartan in Germany. Postgrad Med. 2018;130:308–316. [DOI] [PubMed] [Google Scholar]
- 25. Parikh KS, Lippmann SJ, Greiner M, Heidenreich PA, Yancy CW, Fonarow GC, Hernandez AF. Scope of sacubitril/valsartan eligibility after heart failure hospitalization: findings from the GWTG‐HF Registry (Get With The Guidelines‐Heart Failure). Circulation. 2017;135:2077–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez AL, Kittipibul V, Tang WHW, Starling RC. Patients not meeting PARADIGM‐HF enrollment criteria are eligible for sacubitril/valsartan on the basis of FDA approval: the need to close the gap. JACC Heart Fail. 2017;5:460–463. [DOI] [PubMed] [Google Scholar]


