Abstract
Background
The purpose of this study was to investigate the association between kidney function and arterial calcification in major vascular beds and to establish whether arterial calcification mediates the relation between kidney function measures and cardiovascular disease (CVD) incidence.
Methods and Results
In 2241 participants from the Rotterdam Study (mean age 69 years, 52% female), kidney function was assessed using the estimated glomerular filtration rate and urine albumin‐to‐creatinine ratio. All participants underwent noncontrast computed tomography to quantify the amount of arterial calcification in the coronary arteries, aortic arch, extracranial, and intracranial internal carotid arteries. We used linear regression models, adjusted for age, sex, and cardiovascular risk factors, to evaluate the association between kidney function and arterial calcification volume in the 4 vessel beds. Incidence rate of CVD was calculated in 3 groups of participants based on their kidney function and presence of arterial calcification. We conducted mediation analysis to evaluate whether arterial calcification mediates this association. We found that in age‐ and sex‐adjusted models, lower estimated glomerular filtration rate and higher albumin‐to‐creatinine ratio were associated with larger calcification volumes in all 4 vascular beds. Adjusting for cardiovascular risk factors attenuated the effect estimates. CVD incidence was higher in participants with estimated glomerular filtration rate <60 mL/min per 1.73 m2 and presence of arterial calcification compared with individuals with estimated glomerular filtration rate >60 and no calcification. After adjusting for cardiovascular risk factors, arterial calcification did not mediate the association between kidney function measures and CVD incidence.
Conclusions
The association of impaired kidney function and larger volumes of arterial calcification is partly explained by cardiovascular risk factors. Arterial calcification does not mediate the association between kidney function and CVD beyond cardiovascular risk factors.
Keywords: atherosclerosis, calcification, computed tomography, imaging, kidney
Subject Categories: Epidemiology, Cardiovascular Disease, Risk Factors, Atherosclerosis
Clinical Perspective
What Is New?
Impaired kidney function, assessed through estimated glomerular filtration rate for cystatin C and albumin‐to‐creatinine ratio, is associated with higher volumes of calcification in the coronary arteries, the aortic arch, and the extra‐ and intracranial internal carotid arteries, but this association is partly explained by the presence of traditional cardiovascular risk factors.
There was no evidence for a mediating role for arterial calcification in the association between kidney function and cardiovascular events beyond traditional cardiovascular risk factors.
What Are the Clinical Implications?
In the general population with mild‐to‐moderate kidney impairment, a substantial portion of the association between worse kidney function and arterial calcification is explained by cardiovascular risk factors.
Hence, these findings emphasize the need for novel investigations into other mechanisms underlying the increased risk of cardiovascular events in these individuals.
Introduction
Impaired kidney function is accompanied by a higher risk of cardiovascular events, such as myocardial infarctions and stroke.1 One of the potential factors that can explain this excess risk is arterial calcification,2 which seems to be more prevalent in arteries of patients with chronic kidney disease compared with healthy individuals.2, 3
Arterial calcification is one of the strongest risk factors for cardiovascular events and mortality.4 The calcification burden differs considerably across different vessel beds and carries differential predictive value for various manifestations of cardiovascular events.5, 6, 7, 8 Hence, it is important to understand whether the association of impaired kidney function with various forms of cardiovascular disease is through arterial calcification in the relevant (or adjunct) vessels.6, 7, 8, 9
In a large study of community‐dwelling middle‐aged and elderly people, we investigated the association between kidney function and arterial calcification in 4 major vessel beds, including coronary artery, aortic arch, and extracranial and intracranial carotid artery calcification. We also studied the incidence of cardiovascular disease in subgroups of participants based on their kidney function level and calcification burden. Finally, we conducted a mediation analysis to evaluate whether arterial calcification mediates the association between impaired kidney function and higher incidence of cardiovascular disease.
Methods
Study Population
The present study is embedded within the Rotterdam Study, a prospective population‐based study among individuals 45 years and older living in Ommoord, a district of Rotterdam. Between 2003 and 2006, all participants who visited the research center were invited to undergo nonenhanced multidetector computed tomography. In total, 2524 individuals were scanned (response rate 78%). Among them, 2241 participants had serum creatinine measurements, 2195 serum cystatin C, and 1767 urinary albumin‐to‐creatinine ratio.10 The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272‐159521‐PG). The Rotterdam Study has been entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the World Health Organization International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalogue number NTR6831.10 All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians. Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Department of Epidemiology, Erasmus MC University Medical Center at f.vanrooij@erasmusmc.nl.
Assessment of Kidney Function Markers
Serum creatinine was measured with an enzymatic assay method and standardized to isotope–dilution mass spectrometry–traceable measurements.11 Serum cystatin C was measured with a particle‐enhanced immunonephelometric assay.12 Estimated glomerular filtration rate (eGFR) was calculated for creatinine (eGFRcr) and cystatin C (eGFRcys) on the basis of the Chronic Kidney Disease Epidemiology Collaboration equation.13 Participants collected the first morning urine before arriving at the research center. Urine albumin and creatinine were determined by a turbidimetric method and measured by a Hitachi MODULAR P Analyzer (Roche/Hitachi Diagnostics, Mannheim, Germany). Albumin‐to‐creatinine ratio (milligrams per gram) was estimated by dividing albumin by creatinine. We used log‐transformed values to obtain values per doubling of the albumin‐to‐creatinine ratio. Before transformation, we added 1 to the values to account for those who did not have albuminuria. On average, there was a 6‐ and 5‐year difference between the time of computed tomography (CT) scans and eGFR and albumin‐to‐creatinine ratio measurements, respectively. Hence, all analyses were adjusted for the time interval between kidney function measurement and the time of the CT scans.
Assessment of Arterial Calcification
Noncontrast CT images were obtained using 16‐slice (n=785) or 64‐slice (n=1739) multidetector CT scanners (Somatom Sensation 16 or 64; Siemens, Forchheim, Germany). The acquisition‐protocol has been described in detail before.5, 6, 7 In short, using a cardiac scan and a scan that reached from the aortic arch to the intracranial vasculature (1 cm above the sella turcica), we scanned the following vessels: coronary arteries, aortic arch, extracranial carotid arteries, and intracranial carotid arteries. We visualized and quantified the amount of arterial calcification in these vessel beds. Coronary artery calcification, aortic arch calcification, and extracranial carotid artery calcification were quantified using commercially available software (Syngo CalciumScoring; Siemens). Intracranial carotid artery calcification was evaluated using a semiautomatic scoring method that is described in detail elsewhere (Figure 1).5 All calcification volumes are expressed in cubic millimeters. Because calcification scores were not normally distributed, we used natural log‐transformed values after adding 1 mm3 to the nontransformed values to account for zero values. To correct for the size of the vessels and be able to compare the effect estimates, we used standardized values of log‐transformed calcification scores.
Figure 1.

Visual representations of the vascular beds under study. AAC indicates aortic arch calcification; CAC, coronary artery calcification; ECAC, extracranial carotid artery calcification; ICAC, intracranial carotid artery calcification.
Other Measurements
All covariables were assessed at the time of kidney function measurements.10 Body mass index was calculated by dividing participants’ weight in kilograms by the square of one's height in meters. Blood pressure was measured twice in a single visit and the average of 2 measurements was used in the analyses. Information on antihypertensive medication use was based on home interview. Serum total and high‐density lipoprotein cholesterol levels were measured using an automated enzymatic method. Diabetes mellitus was ascertained using general practitioners’ records (including laboratory glucose measurements), hospital discharge letters, and serum glucose measurements from the Rotterdam Study visits, which take place approximately every 4 years. Information on smoking was gathered using questionnaires.
Incidence of Cardiovascular Events
Cardiovascular events included fatal or nonfatal acute myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, and stroke. Information on incident cases was obtained from general practitioners and from letters and discharge reports of medical specialists. Follow‐up data until January 1, 2012 were used (median follow‐up=5.5 years). All participants with a history of cardiovascular events at time of CT scanning were excluded from the analyses involving incidence of cardiovascular events.
Statistical Analysis
Associations between kidney function markers and arterial calcification in the 4 vessel beds were evaluated using linear regression models. Betas and 95% CIs for difference in arterial calcification volume were estimated per SD decrease of eGFR measures and doubling of albumin‐to‐creatinine ratio. We performed analyses in 2 models. In the first model, analyses were adjusted for age, sex, and cohort. In the second model, we additionally adjusted for cardiovascular risk factors including body mass index, hypertension, diabetes mellitus, smoking, total cholesterol, and high‐density lipoprotein cholesterol. To investigate a possible nonlinear association between kidney function markers and arterial calcification, we applied ordinary least squares linear regression models with restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles. Additionally, we also created standard multivariate linear regression models with a quadratic term to provide effect estimates for eGFRcr. Moreover, we adjusted the associations of eGFRcr with arterial calcification for age (represented as spline) and interactions of age (as a spline) and sex. Since nonlinear associations with eGFRcr could be reflective of non‐GFR determinants, such as loss of muscle mass, we used eGFRcys for later analyses. We further compared the incidence rates of cardiovascular disease per 1000 person‐years in different categories of kidney function (based on eGFRcys) and arterial calcification.14 We used the globally accepted cutoff of 60 mL/min per 1.73 m2 for eGFR to define CKD.15, 16 Next, the categories were defined as (1) eGFRcys >60 mL/min per 1.73 m2 and no calcification, (2) presence of either eGFRcys <60 mL/min per 1.73 m2 or calcification, and (3) eGFRcys <60 mL/min per 1.73 m2 and presence of calcification. For this analysis, we excluded those with previous history of cardiovascular disease (sample size=2091). We conducted a mediation analysis using the approach developed by Valeri and Van der Weele.17, 18 We presented total effect as well as natural direct effect (exposure effect that is not mediated by the mediator) and natural indirect effect (exposure effect that is mediated by the mediator). To explore the presence of interaction between worse kidney function and arterial calcification, we also evaluated all models after including an interaction term between kidney measures and volumes of arterial calcification.
Results
Table 1 presents the characteristics of the study participants at the time of kidney function measurement. Mean age of the population was 69.5±6.7 years and 52% were female. Eighty percent of the population had an eGFRcys that was >60 mL/min per 1.73 m2; in 10% this was between 45 and 60 mL/min per 1.73 m2, and in 2% the eGFRcys was <45 mL/min per 1.73 m2. The associations between markers of kidney function and calcification in different vascular beds are presented in Table 2. In the age‐ and sex‐adjusted models, lower eGFRcys was associated with larger volumes of aortic arch calcification (difference in Ln‐transformed calcification volume per 1 SD lower eGFRcys: 0.07, 95% CI: 0.02, 0.12), extracranial carotid artery calcification (difference: 0.05, 95% CI: 0.01, 0.09), and intracranial carotid artery calcification (difference: 0.06, 95% CI: 0.02, 0.11). A higher albumin‐to‐creatinine ratio was associated with larger volumes of coronary artery calcification (difference in Ln‐transformed calcification volume per doubling of albumin‐to‐creatinine ratio: 0.06, 95% CI: 0.04, 0.09), aortic arch calcification (difference: 0.05, 95% CI: 0.02, 0.08), extracranial carotid artery calcification (difference: 0.04, 95% CI: 0.01, 0.07), and intracranial carotid artery calcification (difference: 0.05, 95% CI: 0.02, 0.07). After adjustment for cardiovascular risk factors, the effect estimates attenuated and were no longer statistically significant for eGFR. The association between albumin–creatinine ratio and calcification in coronary artery calcification and aortic arch calcification remained statistically significant (Table 2). We noted a U‐shaped association between eGFRcr and calcification in all 4 vascular beds (all P<0.05) (Figure 2 and Figure S1; results from standard linear regression models including the quadratic term are shown in Table S1). Figure S2 compares the model including eGFRcr as nonlinear term and the model using both eGFRcr and covariables as spline.
Table 1.
Baseline Characteristics of Study Participants
| Sample size, n | 2241 |
| Age, y | 69.5 (6.7) |
| Women | 1163 (51.9) |
| Systolic blood pressure, mm Hg | 141.7 (20.8) |
| Diastolic blood pressure, mm Hg | 78.3 (10.8) |
| Body mass index, kg/m2 | 26.9 (3.7) |
| Current smoking | 402 (18.0) |
| Total cholesterol, mmol/L | 5.8 (0.9) |
| HDL cholesterol, mmol/L | 1.4 (0.4) |
| Hypertension | 1334 (59.5) |
| Diabetes mellitus | 214 (9.5) |
| eGFR creatinine, mL/min per 1.73 m2 | 79.3 (13.8) |
| eGFR cystatin C, mL/min per 1.73 m2 | 80.2 (17.0) |
| Albumin‐to‐creatinine ratio, mg/g | 4.5 (2.4–10.4) |
| Coronary artery calcification, mm3 | 52.1 (1.8–271.4) |
| Aortic arch calcification, mm3 | 250.7 (42.8–827.2) |
| Extracranial carotid artery calcification, mm3 | 22.5 (0–113.6) |
| Intracranial carotid artery calcification, mm3 | 42.3 (6.7–140.5) |
Categorical variables are presented as numbers (percentages), continuous variables as means (SD), and albumin‐to‐creatinine ratio and calcification markers are presented as medians (interquartile ranges). The following variables had missing data: body mass index (n=7), systolic and diastolic blood pressure (n=5), hypertension (n=12), smoking (n=9), and history of diabetes mellitus (n=9). eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein.
Table 2.
Association Between Kidney Function and Calcification in Major Arteries
| CAC | AAC | ECAC | ICAC | |||||
|---|---|---|---|---|---|---|---|---|
| Difference (95% CI)a P Value | ||||||||
| eGFRcys (n=2195)a | ||||||||
| Model 1 | 0.04 (−0.01, 0.08) | 0.086 | 0.07 (0.02, 0.12) | 0.003 | 0.05 (0.01, 0.09) | 0.040 | 0.06 (0.02, 0.11) | 0.009 |
| Model 2 | 0.01 (−0.03, 0.05) | 0.663 | 0.04 (−0.00, 0.09) | 0.062 | 0.03 (−0.02, 0.08) | 0.195 | 0.04 (−0.01, 0.09) | 0.078 |
| ACR (n=1767)a | ||||||||
| Model 1 | 0.06 (0.04, 0.09) | 0.001 | 0.05 (0.02, 0.08) | 0.001 | 0.04 (0.01, 0.07) | 0.012 | 0.05 (0.02, 0.07) | 0.002 |
| Model 2 | 0.04 (0.01, 0.07) | 0.004 | 0.03 (0.01, 0.06) | 0.042 | 0.02 (−0.01, 0.05) | 0.214 | 0.03 (−0.00, 0.06) | 0.063 |
Model 1: Adjusted for age, sex, and cohort effect. Model 2: Adjusted for model 1 and additionally for body mass index, hypertension, diabetes mellitus, smoking, total cholesterol, and HDL cholesterol. AAC indicates aortic arch calcification; ACR, albumin‐to‐creatinine ratio; CAC, coronary artery calcification; ECAC, extracranial carotid artery calcification; eGFR, estimated glomerular filtration rate; eGFRcys, eGFR calculated for creatinine; HDL, high‐density lipoprotein; ICAC, intracranial carotid artery calcification.
Reported beta and CIs are standardized log increase in calcification per 1 SD decrease in eGFR estimates or doubling in ACR.
Figure 2.

Nonlinear association of eGFR based on creatinine and arterial calcification in different vascular beds. X‐axes represent eGFR values based on creatinine and Y‐axes represent logarithm of arterial calcification values. AAC indicates aortic arch calcification; CAC, coronary artery calcification; ECAC, extracranial carotid artery calcification; eGFR, estimated glomerular filtration rate; ICAC, intracranial carotid artery calcification.
Participants with both eGFRcys <60 mL/min per 1.73 m2 and calcification in their vascular beds had higher incidence of cardiovascular disease compared with individuals with eGFRcys >60 and without calcification (all P<0.05) (Figure 3).
Figure 3.
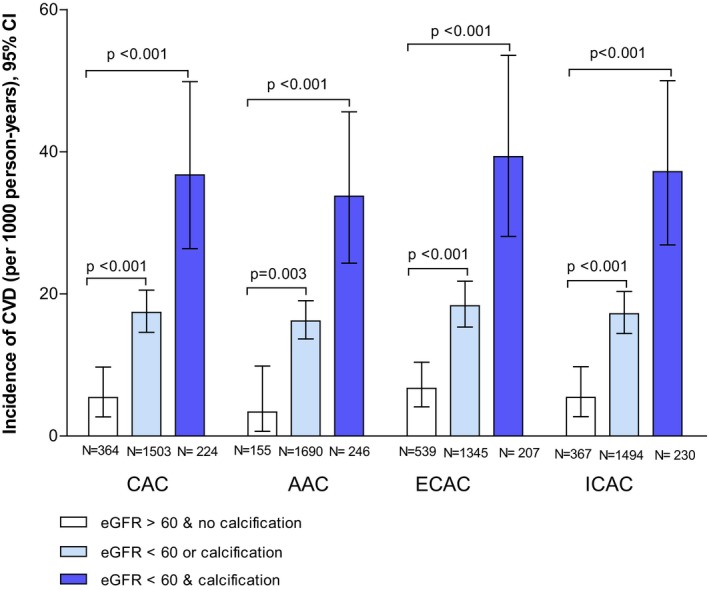
Incidence rates of cardiovascular disorders in 3 categories of participants based on eGFR level and presence of calcification. AAC indicates aortic arch calcification; CAC, coronary artery calcification; CVD, cardiovascular disease; ECAC, extracranial carotid artery calcification; eGFR, estimated glomerular filtration rate; ICAC, intracranial carotid artery calcification.
Table 3 presents the results of the mediation analysis. Adjusted for age, sex, and cardiovascular risk factors, percent mediated via arterial calcification in the observed association between kidney function measures and incident cardiovascular disease was small and not statistically significant (from 0% to 23%). Including an interaction term between kidney measures and volumes of arterial calcification did not statistically significantly improve the models (data not shown).
Table 3.
Estimates of Direct and Indirect Effects of Kidney Function Measures on Cardiovascular Disease and Percentage Mediated by Arterial Calcification
| Cardiovascular Disease | Percent Mediateda | |||
|---|---|---|---|---|
| Natural Direct Effect | Natural Indirect Effect | Total Effect | ||
| Odds Ratio (95% CI) | % | |||
| eGFR (per 1 SD decrease) | ||||
| CAC | 1.19 (0.98, 1.44) | 1.00 (0.98, 1.03) | 1.20 (0.99, 1.45) | 0 |
| AAC | 1.17 (0.97, 1.42) | 1.01 (0.99, 1.03) | 1.19 (0.99, 1.44) | 6 |
| ECAC | 1.17 (0.97, 1.42) | 1.01 (0.99, 1.03) | 1.18 (0.98, 1.43) | 6 |
| ICAC | 1.18 (0.97, 1.42) | 1.02 (0.99, 1.04) | 1.19 (0.99, 1.44) | 11 |
| ACR (per doubling) | ||||
| CAC | 1.07 (0.95, 1.21) | 1.02 (1.00, 1.03) | 1.09 (0.96, 1.23) | 23 |
| AAC | 1.07 (0.95, 1.21) | 1.01 (0.99, 1.02) | 1.08 (0.96, 1.22) | 13 |
| ECAC | 1.08 (0.96, 1.22) | 1.00 (0.99, 1.01) | 1.08 (0.96, 1.22) | 0 |
| ICAC | 1.08 (0.95, 1.21) | 1.01 (0.99, 1.03) | 1.09 (0.97, 1.23) | 12 |
Models are adjusted for age, sex, cohort effect and body mass index, hypertension, diabetes mellitus, smoking, total cholesterol, and HDL cholesterol. AAC indicates aortic arch calcification; ACR, albumin‐to‐creatinine ratio; CAC, coronary artery calcification; ECAC, extracranial carotid artery calcification; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; ICAC, intracranial carotid artery calcification.
The portion of the total effects mediated by arterial calcification is defined as the natural indirect effect over the total effect.
Discussion
In this population‐based study, we found that a lower eGFRcys and a higher albumin‐to‐creatinine ratio were associated with higher volumes of calcification in the coronary arteries, the aortic arch, and the extra‐ and intracranial internal carotid arteries. The order of magnitude of these associations was similar across all 4 vessels. The effect estimates attenuated after adjustment for cardiovascular risk factors. We observed a nonlinear association between eGFRcr and calcifications in all vessel beds. Adjusting for age, sex, and cardiovascular risk factors, arterial calcification did not mediate the association between kidney function and cardiovascular events.
Previous studies including kidney disease patients investigated the link between kidney function and calcification in the coronary arteries and the aorta.19, 20, 21, 22, 23, 24, 25 Kidney impairment has been associated with coronary calcification in different populations.19, 21, 23, 25 Reports on patients with chronic kidney disease found an independent association between kidney function and vascular calcification,19, 21 whereas the association was not independent of traditional cardiovascular risk factors in the general population.23 Similarly, among predialysis patients, lower eGFR was found to be prominently related to the presence of abdominal aortic calcification.20 In contrast, a report from the population‐based Multi‐Ethnic Study of Atherosclerosis showed that mild impairment in kidney function is only modestly associated with aortic valve calcification.24 We observed that, similar to other population‐based studies, the effect estimates attenuated after adjustment for cardiovascular risk factors, suggesting that in the general population at least part of this association is explained by cardiovascular risk factors. Vascular calcification can be the consequence of a wide range of different biological processes. In patients with more severe stages of kidney disease, mechanisms directly related to lower glomerular filtration, such as disturbed calcium–phosphate metabolism, or medical therapies to treat renal osteodystrophy, have also been implicated in the pathophysiology of vascular calcification.2, 3, 26 This could explain the association between kidney function markers and calcification independent of cardiovascular risk factors in a population with more advanced stages of kidney impairment. Future studies investigating this association in populations with lower levels of eGFR are needed to explore mechanisms beyond cardiovascular risk factors.
The present study extends the literature by including calcification in vessels other than the coronary arteries or the aorta. Calcification can have different anatomical and histological locations. Given that correlations between calcifications in different vessel beds are only moderate27, 28 (r from our study=0.4–0.5), the association between kidney function and calcification could differ across various vessel beds with potential implications for clinical events. For example, calcification in intra‐ and extracranial arteries has been shown be a strong predictor of stroke.5, 7 Patients with kidney impairment also have excess risk for stroke and one of the proposed mechanisms is a higher load of arterial calcification in these patients.29 Similar to coronary and aortic calcification, we observed a trend showing an association between eGFR and albuminuria with calcification in intracranial carotid arteries. The strength of the association as well as the incidence rate of cardiovascular disorders were similar across different vessels, suggesting that the association between kidney function and arterial calcification is not restricted to a particular type of a vessel or region but rather a more systemic phenomenon. We found that, in adjusted models, a small portion of the association between kidney measures and cardiovascular events was mediated through arterial calcification and were not statistically significant. Our population‐based cohort is relatively healthy with lower burden of calcification, and the mediating role of calcification is likely more prominent in patients with advanced chronic kidney disease.
We noted a nonlinear association between creatinine‐based eGFR and calcification. Nonlinear associations of creatinine in relation to cardiovascular outcomes have been reported previously.12, 30 Individuals with very low creatinine levels (high eGFR) are also at increased risk of cardiovascular disorders, possibly because of malnutrition and loss of muscle mass.31 Unlike creatinine, levels of serum cystatin C are reported to be independent of muscle mass.31 Nevertheless, inflammation and cardiovascular risk factors such as obesity and diabetes mellitus can also influence serum cystatin C levels.31, 32 Another reason for the nonlinear association of eGFRcr and calcification could be because of the glomerular hyperfiltration. Hyperfiltration has been associated with vascular dysfunction because it may represent an adaptive mechanism to maintain glomerular perfusion.33, 34 Given that the same trend was not observed with eGFR based on cystatin C, this observation should be confirmed in future studies. We found more prominent associations when using albuminuria as a marker of kidney function than when using eGFR. This finding has been reported in different studies, with albuminuria being a marker of generalized endothelial dysfunction as the most appealing explanation.35, 36 In addition, albuminuria may better capture the influence of other calcifying mechanisms such as inflammation in the arterial system.37
We included a relatively large sample of community‐dwelling individuals with available standardized image‐based measures of calcification in 4 major vascular beds, and different markers of kidney function that enabled 1‐on‐1 comparisons of kidney function measures and arterial calcification in different vessel beds within this population. However, limitations of our study should be acknowledged. Given the relatively preserved eGFR in our population, the findings are difficult to extrapolate to older individuals and people with more severe kidney disease. One of the main assumptions of mediation analysis is to rule out confounding; we adjusted the analyses for main cardiovascular risk factors and demographic characteristics but there is the possibility of residual confounding and reverse causation. One important potential confounder that we did not take into account is the level of serum phosphate. Serum phosphate has been found to be an independent risk factor for cardiovascular events in both the general population and in patients with kidney disease. Future studies are needed to study the role of phosphate in this association. Another important limitation of this study is the time interval between measuring kidney function and CT scan. Although adjustment for the time interval did not change the results, we acknowledge that during this interval there may have been changes in kidney function measures.
In conclusion, we found that in the general population, impairment of kidney function, particularly albumin‐to‐creatinine ratio, is associated with larger volumes of arterial calcification in different major vascular beds. At least part of this association is explained by cardiovascular risk factors. The effect estimates did not seem to be more prominent with 1 vessel bed and was rather similar across all 4 vessels. Arterial calcification did not mediate the association between kidney function and cardiovascular disease above cardiovascular risk factors in the general population.
Sources of Funding
The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly; the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission; and the Municipality of Rotterdam. The research leading to these results has received funding from the European Union Seventh Framework Programma (FP7/2007‐2013) under grant agreement no. 601055, VPH‐DARE@IT. Oscar H. Franco works in Erasmus‐AGE, a center for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd), Metagenics Inc, and AXA. Maryam Kavousi is supported by the VENI grant (91616079) from The Netherlands Organization for Health Research and Development (ZonMw). Nestlé Nutrition (Nestec Ltd), Metagenics Inc, and AXA had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1. The Association Between GFRcr/GFRcr Squared and Calcification in Different Arteries
Figure S1. Nonlinear association of kidney measures and arterial calcification in different vascular beds.
Figure S2. Nonlinear association of eGFRcr and arterial calcification in different vascular beds.
Acknowledgments
The Rotterdam Study investigators are grateful to the participants and staff from the Rotterdam Study, the participating general practitioners, and the pharmacists. We thank Dr Magda Meester for her assistance.
(J Am Heart Assoc. 2019;8:e010930 DOI: 10.1161/JAHA.118.010930.)
References
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 2. Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 2017;91:808–817. [DOI] [PubMed] [Google Scholar]
- 3. Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. [DOI] [PubMed] [Google Scholar]
- 4. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta‐analysis. Vasc Health Risk Manag. 2009;5:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bos D, Ikram MA, Elias‐Smale SE, Krestin GP, Hofman A, Witteman JC, van der Lugt A, Vernooij MW. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol. 2011;31:2331–2337. [DOI] [PubMed] [Google Scholar]
- 6. Bos D, Leening MJ, Kavousi M, Hofman A, Franco OH, van der Lugt A, Vernooij MW, Ikram MA. Comparison of atherosclerotic calcification in major vessel beds on the risk of all‐cause and cause‐specific mortality: the Rotterdam Study. Circ Cardiovasc Imaging. 2015;8:e003843. [DOI] [PubMed] [Google Scholar]
- 7. Bos D, Portegies ML, van der Lugt A, Bos MJ, Koudstaal PJ, Hofman A, Krestin GP, Franco OH, Vernooij MW, Ikram MA. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam Study. JAMA Neurol. 2014;71:405–411. [DOI] [PubMed] [Google Scholar]
- 8. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140–146. [DOI] [PubMed] [Google Scholar]
- 10. Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, Klaver CCW, Nijsten TEC, Peeters RP, Stricker BH, Tiemeier H, Uitterlinden AG, Vernooij MW, Hofman A. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sedaghat S, de Vries PS, Boender J, Sonneveld MA, Hoorn EJ, Hofman A, de Maat MP, Franco OH, Ikram MA, Leebeek FW, Dehghan A. von Willebrand factor, ADAMTS13 activity, and decline in kidney function: a population‐based cohort study. Am J Kidney Dis. 2016;68:726–732. [DOI] [PubMed] [Google Scholar]
- 12. Sedaghat S, Cremers LG, de Groot M, Hofman A, van der Lugt A, Niessen WJ, Franco OH, Dehghan A, Ikram MA, Vernooij MW. Lower microstructural integrity of brain white matter is related to higher mortality. Neurology. 2016;87:927–934. [DOI] [PubMed] [Google Scholar]
- 13. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; Investigators C‐E . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin DO, Austin H. Exact estimates for a rate ratio. Epidemiology. 1996;7:29–33. [DOI] [PubMed] [Google Scholar]
- 15. Bash LD, Coresh J, Kottgen A, Parekh RS, Fulop T, Wang Y, Astor BC. Defining incident chronic kidney disease in the research setting: the ARIC Study. Am J Epidemiol. 2009;170:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, Levey AS, Riella MC, Shlipak MG, Wang H, White CT, Winearls CG. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1. [DOI] [PubMed] [Google Scholar]
- 17. VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 18. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure‐mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. (PMID: 23379553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El Barzouhi A, Elias‐Smale S, Dehghan A, Vliegenthart‐Proenca R, Oudkerk M, Hofman A, Witteman JC. Renal function is related to severity of coronary artery calcification in elderly persons: the Rotterdam Study. PLoS One. 2011;6:e16738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanada S, Ando R, Naito S, Kobayashi N, Wakabayashi M, Hata T, Sasaki S. Assessment and significance of abdominal aortic calcification in chronic kidney disease. Nephrol Dial Transplant. 2010;25:1888–1895. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura S, Ishibashi‐Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parikh NI, Hwang SJ, Larson MG, Hoffmann U, Levy D, Meigs JB, O'Donnell CJ, Fox CS. Indexes of kidney function and coronary artery and abdominal aortic calcium (from the Framingham Offspring Study). Am J Cardiol. 2008;102:440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ix JH, Katz R, Kestenbaum B, Fried LF, Kramer H, Stehman‐Breen C, Shlipak MG. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ix JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL, Takasu J, Detrano R, O'Brien KD. Kidney function and aortic valve and mitral annular calcification in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2007;50:412–420. [DOI] [PubMed] [Google Scholar]
- 25. Fox CS, Larson MG, Keyes MJ, Levy D, Clouse ME, Culleton B, O'Donnell CJ. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: the Framingham Heart Study. Kidney Int. 2004;66:2017–2021. [DOI] [PubMed] [Google Scholar]
- 26. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon‐Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. [DOI] [PubMed] [Google Scholar]
- 28. Odink AE, van der Lugt A, Hofman A, Hunink MG, Breteler MM, Krestin GP, Witteman JC. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam Study. Atherosclerosis. 2007;193:408–413. [DOI] [PubMed] [Google Scholar]
- 29. Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney‐brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–719. [DOI] [PubMed] [Google Scholar]
- 30. Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. [DOI] [PubMed] [Google Scholar]
- 31. Seronie‐Vivien S, Delanaye P, Pieroni L, Mariat C, Froissart M, Cristol JP; SFBC “Biology of renal function and renal failure” working group . Cystatin C: current position and future prospects. Clin Chem Lab Med. 2008;46:1664–1686. [DOI] [PubMed] [Google Scholar]
- 32. Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre‐diabetes and pre‐hypertension. Nephrol Dial Transplant. 2012;27:1708–1714. [DOI] [PubMed] [Google Scholar]
- 34. Cherney DZ, Sochett EB, Lai V, Dekker MG, Slorach C, Scholey JW, Bradley TJ. Renal hyperfiltration and arterial stiffness in humans with uncomplicated type 1 diabetes. Diabetes Care. 2010;33:2068–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suh‐Chiou C, Moyses RM, Bittencourt MS, Bensenor IM, Lotufo PA. Chronic kidney disease and coronary artery calcification in the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Clin Cardiol. 2017;40:1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedrinelli R, Giampietro O, Carmassi F, Melillo E, Dell'Omo G, Catapano G, Matteucci E, Talarico L, Morale M, De Negri F, Di Bello V, Melilo E. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 1994;344:14–18. [DOI] [PubMed] [Google Scholar]
- 37. Trimarchi H, Muryan A, Dicugno M, Young P, Forrester M, Lombi F, Pomeranz V, Iriarte R, Rana MS, Alonso M. Proteinuria: an ignored marker of inflammation and cardiovascular disease in chronic hemodialysis. Int J Nephrol Renovasc Dis. 2012;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The Association Between GFRcr/GFRcr Squared and Calcification in Different Arteries
Figure S1. Nonlinear association of kidney measures and arterial calcification in different vascular beds.
Figure S2. Nonlinear association of eGFRcr and arterial calcification in different vascular beds.


