Abstract
Background
Secondary prevention after acute myocardial infarction (AMI) requires long‐term guideline‐directed medical therapy. However, the level of medication adherence, factors associated with poor adherence, and extent to which good adherence can reduce adverse events after AMI in China remain uncertain.
Methods and Results
In 2013 to 2014, 4001 AMI patients aged ≥18 years were discharged alive from 53 hospitals across China (mean age 60.5±11.7 years; 22.7% female). Good adherence was defined as taking medications (aspirin, β‐blockers, statins, clopidogrel, or angiotensin‐converting enzyme inhibitors/angiotensin‐receptor blockers) ≥90% of the time as prescribed. Cox models assessed the association between good adherence (a time‐varying covariate) and 1‐year cardiovascular events after AMI. The most common medications were aspirin (82.2%) and statins (80.5%). There were 243 patients who were not prescribed any medications during follow‐up; 1‐year event rates were higher for these patients (25.1%, 95% CI 19.7–30.6%) versus those taking ≥1 medications (6.6%, 95% CI 5.76–7.34%). The overall rate of good adherence was 52.9%. Good adherence was associated with lower risk of 1‐year events (adjusted hazard ratio 0.61, 95% CI 0.49–0.77). The most common reason for poor adherence was belief that one's condition had improved/no longer required medication. More comorbidities and lower education level were associated with poor adherence.
Conclusions
Good adherence reduced 1‐year cardiovascular event risk after AMI. About half of our cohort did not have good adherence. National efforts to improve AMI outcomes in China should focus on medication adherence and educating patients on the importance of cardiovascular medications for reducing risk of recurrent events.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01624909.
Keywords: acute myocardial infarction, cardiovascular adverse events, medication adherence, patient‐reported outcomes
Subject Categories: Clinical Studies, Quality and Outcomes, Secondary Prevention, Cardiovascular Disease
Clinical Perspective
What Is New?
Good medication adherence was associated with lower risk of 1‐year events and about half of our cohort did not have good medication adherence.
The most common reason for poor medication adherence was belief that one's condition had improved or no longer required medication.
More comorbidities and lower education level were associated with poor medication adherence.
What Are the Clinical Implications?
Good medication adherence may reduce 1‐year cardiovascular event risk after acute myocardial infarction.
National efforts to improve acute myocardial infarction outcomes in China should focus on medication adherence and educating patients on the importance of cardiovascular medications for reducing risk of recurrent events.
Introduction
Patients who survive an acute myocardial infarction (AMI) remain at high risk for major cardiovascular adverse events, including recurrent AMI, heart failure, stroke, and death.1, 2, 3, 4, 5, 6 Appropriate postdischarge treatment with guideline‐directed medical therapy is an important secondary preventive measure to reduce the risk of recurrent events for cardiovascular disease.7, 8, 9, 10, 11, 12, 13 For secondary prevention treatment after AMI, dual antiplatelet therapy with aspirin and either clopidogrel or prasugrel, β‐blockers, statins, and angiotensin‐converting enzyme inhibitors/angiotensin‐receptor blockers (ACEIs/ARBs) are recommended for at least 12 months, unless contraindicated.6
Adherence is a key factor associated with the effectiveness of all pharmacological therapies, and it is particularly critical for medications prescribed for chronic conditions. Increasing adherence may have a greater effect on health than improvements in specific medical therapies.14 Patients with good adherence to their prescribed medications should have better outcomes than those who are poorly adherent.7, 8, 9, 10, 11, 12, 13 However, despite its benefits, improving patient adherence to guideline‐recommended medication remains a challenge. For example, a review of 127 articles published from January 1, 1990 through March 31, 2010 reported that ≈50% of patients with cardiovascular disease did not take their medications as prescribed.14
Greater focus on medication adherence after AMI and the factors associated with adherence is needed to capture the complete healthcare experience of AMI patients in China, which can provide an indication of the proper target for interventions. Corresponding data from China, however, are scarce. Prior studies on secondary prevention medication adherence in China were limited by specific communities, hospitals, populations, or conditions.15, 16, 17, 18 Further evaluation of adherence in China has become increasingly important, given that heart disease is the second leading cause of death19 and ≈700 000 new cases of AMI occur each year.20
Accordingly, we used data from the China PEACE Prospective AMI study (China Patient‐centered Evaluative Assessment of Cardiac Events Prospective Study of AMI) 21 to identify factors associated with poor adherence and estimate the level of patient‐reported medication adherence for patients discharged for AMI in China. We also evaluated the extent to which medication adherence was associated with risk of 1‐year major cardiovascular adverse events. This study, which included medical record–abstracted data and detailed collection of longitudinal information on patients with AMI, is ideally positioned to generate knowledge to improve post‐acute care for AMI in China.
Methods
Study Sample
The China PEACE Prospective AMI study consecutively enrolled 5901 AMI patients aged ≥18 years across 53 acute‐care hospitals between January 1, 2013 and July 17, 2014.22 Patient information was collected at baseline (ie, the index AMI hospitalization) and postdischarge at 1, 6, and 12 months. Baseline data included medical chart–abstracted information and in‐hospital patient interviews. All postdischarge interviews were conducted either in‐person or via telephone when an in‐person interview was not feasible. Patients were classified as nonresponders after 5 telephone interview attempts with no response. The overall response rate was 97.8%. Real Data Medical Research, Inc., in Ningbo, China, abstracted all medical records, with an agreement rate between repeat abstractions of 98% for key data elements. During the 1‐year follow‐up period, trained staff interviewed patients and systematically sought information on all clinical events, including recurrent AMI, angina, stroke, heart failure, transient ischemic attack, repeated angiography procedures, revascularization, bleeding, and rehospitalizations. For patients who died during follow‐up, death information was obtained from the family or physician. For patients who were unable to attend an onsite interview, information was obtained by telephone through direct correspondence with the patient, patient's family members, or patient's physician. It is our goal to share the China PEACE Prospective AMI study data; however, at this time, we are unable to do so.
We excluded patients who did not agree to participate in the follow‐up interviews (n=1206), were transferred to another acute‐care hospital (n=113), who died before discharge (n=287), or were lost to follow‐up immediately after discharge (n=68). We further excluded those patients whose postdischarge interviews were conducted via telephone with a proxy answering on the patient's behalf (n=226) because the interview responses may not have accurately reflected that patient's adherence. Overall, these excluded patients were older (age ≥65 years, 54.8% versus 38.0%), more female (31.9% versus 22.7%), and more had no job at baseline (94.9% versus 59.2%) than those analyzed. The Human Investigation Committee of China's National Clinical Research Center of Cardiovascular Diseases approved this study. All patients provided written informed consent.
Patient Characteristics
Patient characteristics included demographics (age, sex), risky behaviors (alcohol drinking, cigarette smoking), socioeconomic status (income, education, occupation, insurance), medical history/comorbidities (hypertension, diabetes mellitus, angina, coronary heart disease, prior ventricular tachycardia/fibrillation, atrial fibrillation, heart failure, prior AMI, prior percutaneous coronary intervention, prior coronary artery bypass grafting), lifestyle factors (snoring during natural sleep), >4 hours between symptom onset and hospital admission, pre‐arrival medical assistance, in‐hospital diagnoses (ST‐segment elevation, inferior/anterior AMI, Killip class 3 or 4, left ventricular ejection fraction <40%), renal dysfunction (blood urea nitrogen >40 mg/dL or creatinine >2.5 mg/dL), patient's admission temperature in °C, heart rate >90 beats per minute, blood glucose >12 mmol/L, systolic blood pressure <100 mm Hg, white blood cell count 6 to 12 or >12 per mL, in‐hospital treatments (coronary artery bypass grafting, percutaneous coronary intervention, medications), and number of in‐hospital complications (recurrent angina, recurrent AMI, pericardial effusion, atrial fibrillation, cardiopulmonary resuscitation, ventricular tachycardia/fibrillation, new heart failure, infection, stroke, and major bleeding; range from 0 to 10 complications). Characteristics with missing values were imputed using the multiple imputation method with 10 imputations.23 The final imputed value was an average of the 10 imputations. Rates of missing ranged from 0.2% (blood pressure and white blood cell count) to 2.7% (blood glucose).
Outcome
Our outcome was 1‐year major cardiovascular adverse events, a binary variable defined as the occurrence of recurrent AMI, stroke (ischemic or hemorrhagic), heart failure, or death, within 1 year of discharge from the index AMI hospitalization. For patients with multiple events, the first event was selected for statistical modeling. Information on the outcome was obtained through interviews (Data S1).
Medications and Adherence
We targeted 5 medications that were commonly prescribed to patients discharged for AMI, including aspirin, β‐blockers, statins, clopidogrel, and ACEIs/ARBs. These medications could be prescribed either at the time of discharge or any time during the 1‐year follow‐up period; not every patient was prescribed all 5 medications, and some were not even prescribed 1 of the medications. Medication adherence was composited by individual medications prescribed to a patient. Specifically, in a follow‐up interview, each patient was asked a set of questions regarding his/her medications, including the corresponding adherence and the main reason for not taking medications as prescribed. Patients were asked, “In the past month, have you ever inadvertently forgot to take the medicines that your doctor prescribed and you are required to take daily?” A patient was assigned an adherence score of 1.0 for a response of “never,” 0.90 for “once,” 0.80 for “2 to 3 times,” 0.60 for “once per week,” 0.40 for “2 to 5 times per week,” and 0.0 for “every day or almost every day.” An adherence score of 0.0 was assigned if the medication was not prescribed at discharge. We repeated this assignment for each patient and each medication prescribed to that patient. A patient's overall adherence score was the average of the scores for the individual medications. An overall score ≥0.90 was considered good adherence,24, 25 and a score <0.90 was considered poor adherence. We repeated this procedure for the 6‐ and 12‐month interviews.
Statistical Analysis
We compared characteristics between patients who had at least 1 major cardiovascular adverse event after discharge and those who had no events using the χ2 test for categorical variables and the Kruskal–Wallis test for continuous variables. To assess the association between 1‐year postdischarge major cardiovascular adverse events and medication adherence, we fitted 3 Cox extended regression models with good adherence (yes/no) as a time‐varying covariate: (1) unadjusted; (2) adjusted for patient demographics (age, sex); and (3) adjusted for the risk factors that predicted 1‐year major cardiovascular adverse events in our previous study,22 including demographics (age), socioeconomic status (college degree), comorbidities (prior AMI, prior ventricular tachycardia/fibrillation, hypertension, angina), hospital diagnoses/tests (ejection fraction <40%, ejection fraction unable to measure, renal dysfunction, heart rate >90 beats per minute, blood glucose >12 mmol/L, systolic blood pressure <100 mm Hg, white blood cell count 6–12 or >12 per mL), access to care (pre‐arrival medical assistance, time from symptoms to admission >4 hours), and number of in‐hospital complications. This sequence of assessments allowed us to control for individual patient characteristics and make inferences at the patient level regarding the impact of medication adherence on the outcome. To remove the potential bias of assigning patients an adherence score of 0 if none of the 5 targeted medications were prescribed, we conducted a sensitivity analysis by refitting the above models but excluding patients who were not prescribed at least 1 of the 5 targeted medications during the follow‐up period. Additionally, we fit a logistic model to assess the association between patient characteristics and medication adherence (overall score ≥0.9, yes/no) at 1 month. Analyses were conducted using SAS version 9.4, 64‐bit Windows (SAS Institute Inc., Cary, NC). All statistical testing was 2‐sided, and P<0.05 was considered statistically significant.
Results
Study Sample
The final sample included 4001 patients with AMI who were discharged alive and not transferred to another acute‐care hospital. The mean (SD) age was 60.5 (11.7) years, and 22.7% were female. The most common comorbidities were hypertension (55.9%), coronary heart disease (42.6%), and dyslipidemia (30.8%). The median (interquartile range) hospital length of stay was 11 (8–14) days. Patient characteristics differed between those who had ≥1 cardiovascular adverse events during follow‐up and those who had no events (Table 1).
Table 1.
Patient Characteristics According to Cardiovascular Adverse Events Within 1 Year
| Patient Characteristics | Aggregate (n=4001) | No Event (n=3693) | ≥1 Events (n=308) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), y | 60.5 (11.7) | 59.9 (11.7) | 68.0 (10.9) | <0.001 |
| Age category, n (%) | <0.001 | |||
| 45–64 y | 2108 (52.7) | 2011 (54.5) | 97 (31.5) | |
| 65–74 y | 997 (24.9) | 885 (24.0) | 112 (36.4) | |
| 75–84 y | 482 (12.1) | 404 (10.9) | 78 (25.3) | |
| ≥85 y | 43 (1.1) | 31 (0.8) | 12 (3.9) | |
| Female, n (%) | 909 (22.7) | 804 (21.8) | 105 (34.1) | <0.001 |
| Employed, n (%) | 1637 (40.8) | 1564 (42.4) | 68 (22.1) | <0.001 |
| Owned car, n (%) | 2182 (54.5) | 2047 (55.4) | 135 (43.8) | <0.001 |
| No college degree, n (%) | 3454 (86.3) | 3173 (85.9) | 281 (91.2) | 0.009 |
| Farmer, n (%) | 882 (22.0) | 807 (21.9) | 75 (24.4) | 0.310 |
| Worker, n (%) | 1188 (29.7) | 1103 (29.9) | 85 (27.6) | 0.400 |
| Farmer with insurance, n (%) | 1384 (34.6) | 1271 (34.4) | 113 (36.7) | 0.421 |
| Medical history and comorbidities | ||||
| Family history of acute myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft, n (%) | 437 (10.9) | 401 (10.9) | 36 (11.7) | 0.653 |
| History of smoking, n (%) | 798 (20.0) | 720 (19.5) | 78 (25.3) | 0.014 |
| Never smoked, n (%) | 1110 (27.7) | 998 (27.0) | 112 (36.4) | <0.001 |
| Never drink, n (%) | 2137 (53.4) | 1933 (52.3) | 204 (66.2) | <0.001 |
| History of angina, n (%) | 159 (4.0) | 136 (3.7) | 23 (7.5) | 0.001 |
| History of acute myocardial infarction, n (%) | 313 (7.8) | 259 (7.0) | 54 (17.5) | <0.001 |
| History of percutaneous coronary intervention, n (%) | 264 (6.6) | 235 (6.4) | 29 (9.4) | 0.038 |
| History of coronary heart disease, n (%) | 1703 (42.6) | 1542 (41.8) | 161 (52.3) | <0.001 |
| History of ventricular fibrillation or ventricular tachycardia, n (%) | 95 (2.4) | 85 (2.3) | 10 (3.3) | 0.295 |
| History of atrial fibrillation, n (%) | 117 (2.9) | 102 (2.8) | 15 (4.9) | 0.035 |
| History heart failure, n (%) | 1015 (25.4) | 895 (24.2) | 120 (39.0) | <0.001 |
| History of dyslipidemia, n (%) | 1233 (30.8) | 1121 (30.4) | 112 (36.4) | 0.028 |
| History of chronic renal failure, n (%) | 43 (1.1) | 38 (1.0) | 5 (1.6) | 0.331 |
| History of hypertension, n (%) | 2235 (55.9) | 2020 (54.7) | 215 (69.8) | <0.001 |
| Diabetes mellitus, n (%) | 959 (24.0) | 856 (23.2) | 103 (33.4) | <0.001 |
| Major surgery in past 4 wks, n (%) | 82 (2.1) | 75 (2.0) | 7 (2.3) | 0.774 |
| Pneumonia, n (%) | 438 (11.0) | 381 (10.3) | 57 (18.5) | <0.001 |
| Anemia, n (%) | 533 (13.3) | 467 (12.7) | 66 (21.4) | <0.001 |
| No prior medical assistance, n (%) | 2509 (62.7) | 2293 (62.1) | 216 (70.1) | 0.005 |
| Prior aspirin, n (%) | 556 (13.9) | 526 (14.2) | 30 (9.7) | 0.028 |
| Coexisting conditions | ||||
| Killip 3 or 4, n (%) | 174 (4.4) | 146 (4.0) | 28 (9.1) | <0.001 |
| Current smoking, n (%) | 2331 (58.3) | 2192 (59.4) | 139 (45.1) | <0.001 |
| Non‐ST elevation, n (%) | 366 (9.2) | 341 (9.2) | 25 (8.1) | 0.514 |
| ST depression, n (%) | 948 (23.7) | 873 (23.6) | 75 (24.4) | 0.778 |
| Acute inferior myocardial infarction, n (%) | 1514 (37.8) | 1417 (38.4) | 97 (31.5) | 0.017 |
| Acute anterior myocardial infarction, n (%) | 725 (18.1) | 682 (18.5) | 43 (14.0) | 0.049 |
| Admission heart failure, n (%) | 1008 (25.2) | 889 (24.1) | 119 (38.6) | <0.001 |
| Ischemia symptoms >20 min, n (%) | 2900 (72.5) | 2693 (72.9) | 207 (67.2) | 0.031 |
| Ejection fraction <40%, n (%) | 285 (7.1) | 224 (6.1) | 61 (19.8) | <0.001 |
| Ejection fraction unmeasured, n (%) | 556 (13.9) | 485 (13.1) | 71 (23.1) | <0.001 |
| Coronary artery bypass graft surgery, n (%) | 32 (0.8) | 31 (.0.8) | 1 (0.3) | 0.330 |
| Primary percutaneous coronary intervention, n (%) | 1197 (29.9) | 1132 (30.7) | 65 (21.1) | <0.001 |
| Symptoms‐to‐admission >4 h, n (%) | 2298 (57.4) | 2115 (57.3) | 183 (59.4) | 0.465 |
| Systolic blood pressure <100 mm Hg, n (%) | 314 (7.9) | 283 (7.7) | 31 (10.1) | 0.132 |
| White blood cell count 6–12×103/μL, n (%) | 2805 (70.1) | 2597 (70.3) | 208 (67.5) | 0.304 |
| White blood cell count >12×103/μL, n (%) | 323 (8.1) | 291 (7.9) | 32 (10.4) | 0.120 |
| Fasting blood glucose >216 mg/dL, n (%) | 229 (5.7) | 203 (5.5) | 26 (8.4) | 0.033 |
| Renal dysfunction, n (%) | 828 (20.7) | 693 (18.8) | 135 (43.8) | <0.001 |
| Heart rate >90/min, n (%) | 554 (13.9) | 470 (12.7) | 84 (27.3) | <0.001 |
| Liver disease, n (%) | 62 (1.6) | 60 (1.6) | 2 (0.7) | 0.183 |
| Hypothyroidism, n (%) | 46 (1.2) | 40 (1.1) | 6 (2.0) | 0.171 |
| In‐hospital complications, mean (SD) | 0.85 (1.01) | 0.82 (0.99) | 1.25 (1.24) | <0.001 |
| Length of stay, d, median (interquartile range) | 11 (6) | 11 (6) | 12 (8) | <0.001 |
One‐Year Major Cardiovascular Adverse Events
The observed rate of 1‐year cardiovascular adverse events was 7.7% (95% CI 6.85–8.50). Among patients who had ≥1 cardiovascular adverse events during follow‐up, the total number of events per patient ranged from 1 to 7; 37.0% of patients had ≥2 events. The most common event was heart failure (5.7%), followed by recurrent AMI (3.2%) and stroke (1.0%). The 1‐year cardiovascular‐related and noncardiovascular‐related mortality rates were 2.4% and 0.3%, respectively. The median (interquartile range) days from discharge to an event was 100 (29–198); recurrent AMI occurred earlier than the other events (median 73 [6–194] days; Figure S1).
Medications
Among the 4001 patients who were discharged alive, 243 (6.1%) were not prescribed any of the 5 targeted medications at discharge or during the follow‐up period. For patients who survived the first month after discharge, aspirin (82.2%) was the most frequently prescribed medication, followed by statins (80.5%). This pattern did not change substantially over the follow‐up period, though medication rates were lower overall at 6 months (Figure 1). For aspirin, β‐blockers, statins, clopidogrel, and ACEIs/ARBs, respectively, 70.1% (n=2596), 68.5% (n=1565), 79.7% (n=2510), 75.3% (n=2347), and 61.8% (n=1056) of patients reported that they continued to take the medication between the 1‐ and 12‐month interviews. Among patients who reported taking medications as prescribed at the 1‐month interview, 1.7% were taking 1 medication and 70.9% were taking 4 to 5 medications (Figure 2). By 12 months, 6.1% were taking 1 medication and 60.1% were taking 4 to 5 medications.
Figure 1.
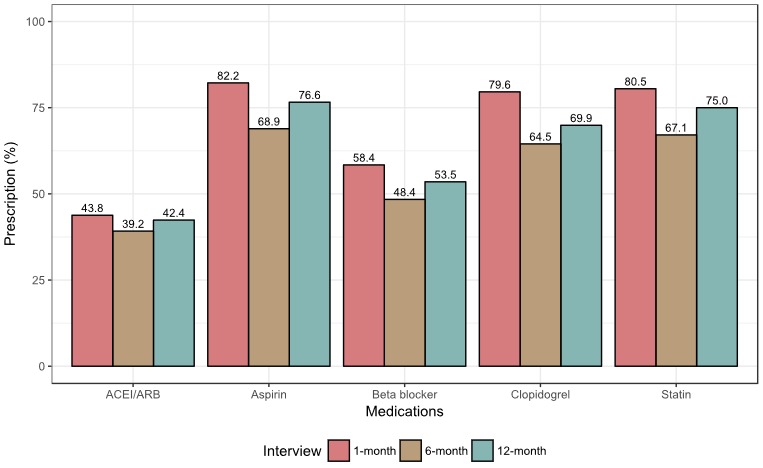
Distribution of prescribed medications over the study period. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker.
Figure 2.
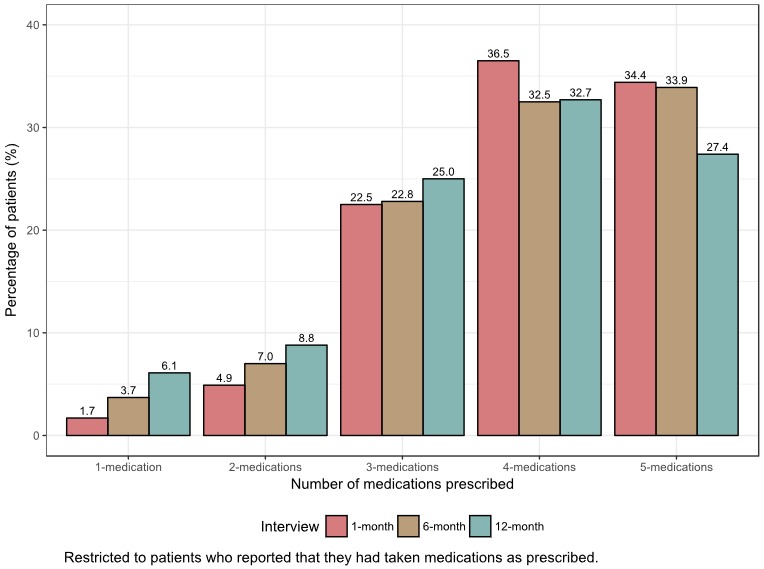
Number of prescribed medications per patient over the study period.
Association Between Adherence and Major Cardiovascular Adverse Events
The rate of good adherence (score ≥0.90) for all prescribed medications was 73.5% at 1 month, 55.0% at 6 months, and 54.6% at 12 months. The overall rate for all eligible months was 52.9%. Among patients who were prescribed ≥1 of the targeted medications, the rate of postdischarge major cardiovascular adverse events within 1 year was 6.6% (95% CI 5.76–7.34) and the median (interquartile range) days to the first event was 100 (31–195). The event rate was higher (25.1%, 95% 19.7–30.6) and the median (interquartile range) time to the first event was shorter (13 [3–59] days) for patients who reported having none of the targeted medications. Patients with good adherence had a lower risk of 1‐year major cardiovascular adverse events than patients with poor adherence (adjusted hazard ratio 0.61, 95% CI 0.49–0.77) (Table 2, Figure 3). This finding did not change substantially after excluding the patients who reported having none of the targeted medications (adjusted hazard ratio 0.76, 95% CI 0.59–0.98).
Table 2.
Results From Extended Cox Regression Models
| Model | Hazard Ratio | 95% CI |
|---|---|---|
|
Model #1 (unadjusted) Good adherence |
0.57 | 0.45–0.71 |
|
Model #2 (adjusted for patient demographics [age, sex]) Good adherence |
0.60 | 0.47–0.75 |
|
Model #3 (adjusted for the risk factors that predicted 1‐y major cardiovascular adverse events in previous study)a
Good adherence |
0.61 | 0.49–0.77 |
Model #3 was adjusted for patient demographics (age), socioeconomic status (college degree), comorbidities (prior acute myocardial infarction, prior ventricular tachycardia/fibrillation, hypertension, angina), hospital diagnoses/tests (ejection fraction [EF] <40%, EF unable to measure, renal dysfunction, heart rate >90 beats per minute, blood glucose >12 mmol/L, systolic blood pressure <100 mm Hg, white blood cell count 6–12 or >12 per mL), access to care (pre‐arrival medical assistance, time from symptoms to admission >4 h), and number of in‐hospital complications.
Figure 3.
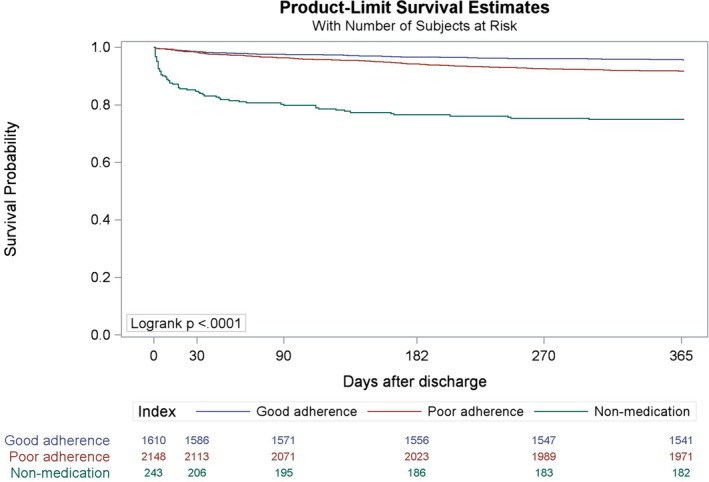
Kaplan–Meier estimator to show the observed probability of being free from 1‐year major cardiovascular adverse events in the good adherence (score ≥0.90), poor adherence (score <0.90), and no medication groups.
Patient Characteristics Associated With Adherence
The most frequently reported reason for patients not taking medications as prescribed was, “I think that my condition has improved and do not need to take it.” The proportion of patients reporting this reason increased over time (Figure 4). Other common reasons for poor adherence were cost and concern about side effects, which both varied by medications. Patients with more comorbidities and lower levels of education were more likely to poorly adhere to their medications (Figure S2).
Figure 4.
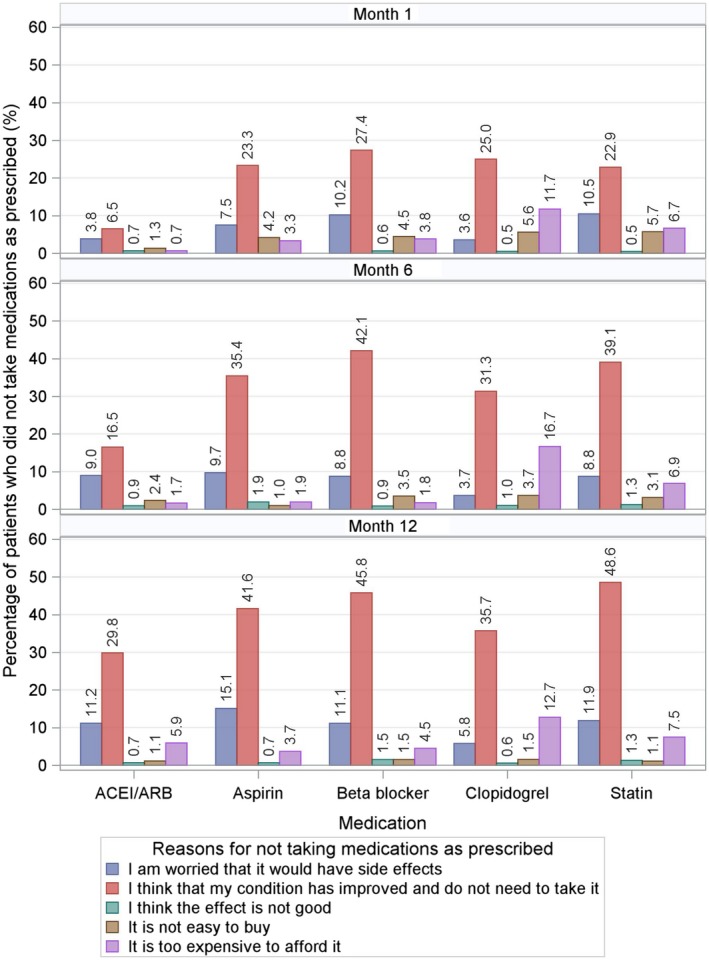
Reasons for not taking medications as prescribed by interview period (top panel: 1 month after discharge, middle panel: 6 months after discharge, and bottom panel: 12 months after discharge). The most frequently reported reason for patients not taking medications as prescribed was, “I think that my condition has improved and do not need to take it.” The proportion of patients reporting this reason increased over time. Take β‐blocker for example; the percentage of patients reporting this reason was 27.4% at 1 month, 42.1% at 6 months, and 45.8% at 12 months. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker.
Discussion
In this large, comprehensive investigation of the association between medication adherence and 1‐year major cardiovascular adverse events among patients discharged with AMI in China, we found that good adherence was significantly associated with lower risk of 1‐year major cardiovascular adverse events. We also observed that nearly 30% of AMI patients in our Chinese cohort did not take medications as prescribed during the first month after discharge. Given the important role of patient medication adherence in the effectiveness of guideline‐directed medical therapy for the prevention of postdischarge cardiovascular adverse events, physicians should ensure intensive follow‐up of their patients regarding medication adherence and modify prescription regimens when required.
The reasons for poor adherence are diverse. Although we found that age, education level, and comorbidities were associated with poor adherence, the low c‐statistic of 0.64 for our prediction model suggests that these characteristics are only partially responsible for poor adherence. Another factor that could contribute to poor adherence is health literacy. We found that the primary reason for patients failing to take their medications as prescribed was the belief that their condition had improved and that the medication was no longer needed. Such a decision, against physician guidance, was common in our Chinese patient population throughout the 1‐year follow‐up period. Cost was less of a concern when it came to poor adherence because <10% of patients reported that they stopped their medications because of the cost. National efforts should focus on improving patient health literacy, with an emphasis on the importance of following physician guidelines for medication use.
Our study, based on chart‐abstracted information and patient interviews, represents a unique, large, and contemporary investigation of the association between medication adherence and 1‐year major cardiovascular adverse events following discharge for AMI in China. In contrast to prior studies that treated adherence as a static variable, we considered adherence as a time‐varying variable, allowing it to vary over the study period. This approach is important because we found that adherence declined over time. Although the overall 1‐year mortality rate for patients surviving an AMI is important, many patients experience nonfatal adverse events that can dramatically affect their quality of life. Medication adherence may help prevent these nonfatal cardiovascular adverse events, which are important from a patient perspective. Educating patients regarding their long‐term risk of both fatal and nonfatal adverse events after AMI might provide an even stronger incentive to follow up with their physicians and adhere to medications.
Our study also identified potential for improved quality of care at the hospital level. There were 6.1% of patients who were not prescribed any of the 5 targeted medications at discharge or during the follow‐up period. The observed rate of postdischarge major cardiovascular adverse events was 3 times higher for these patients when compared with those prescribed at least 1 of the 5 targeted medications. Although many of the patients who were not prescribed medications died shortly after discharge, the 75th percentile of survival time was 59 days postdischarge for these patients, indicating potential to prescribe secondary preventive medications up to 2 months after discharge. It is possible that some of the events could have been delayed or prevented if the patients had been provided with guideline‐directed medications. Overall, the process of care can be improved for patients with AMI in China. For example, a study of 1663 AMI patients discharged from 13 hospitals in Beijing in 2005 found that in‐hospital mortality was 8.2%; the rates of guideline‐based medications for AMI at discharge were 93.6% for aspirin, 78.7% for β‐blockers, 85.4% for low molecular weight heparin, 85.1% for ACEIs/ARBs, and 74.7% for statins.26 Another study that included 2128 AMI patients across 20 tertiary hospitals in Heilongjiang Province in 2009 to 2010 found that in‐hospital mortality was 7.7%, and the rates of guideline‐based medications for AMI were 71.6% for aspirin, 41.3% for β‐blockers, 82.5% for clopidogrel, 63.5% for ACEIs/ARBs, and 80.4% for statins.27 It is clear that national efforts are needed to improve quality of care and increase the rates of guideline‐recommended medications at the hospital level.
Our study has several limitations. Medication adherence was measured according to patient self‐report. The data were drawn from large, urban hospitals, which provide elite‐level care in China. Thus, our findings may not generalize to patients treated at lower‐tier institutions. Poor medication adherence could also be the result of physician factors, such as prescription of complex medication regimens, communication barriers, ineffective communication of information about adverse effects, and provision of care by multiple physicians. Our data set does not include information on physician behaviors, and we were therefore unable to assess the association between physician characteristics and patient medication adherence. Further research is needed in this important area to better understand the challenge of medication adherence in China.
In conclusion, medication adherence was associated with reduced risk of 1‐year major cardiovascular adverse events for patients discharged alive with AMI in China. To improve outcomes after AMI, national efforts should focus on encouraging patients to follow physician guidelines for postdischarge medications and improving quality of care at the hospital level.
Sources of Funding
This project was supported by the Major Public Health Service Project from the Ministry of Finance and National Health and Family Planning Commission of China, the National Key Research and Development Program (2017YFC1310801, 2017YFC1310803) from the Ministry of Science and Technology of China, the CAMS Innovation Fund for Medical Science (2017‐I2M‐2‐002, 2017‐I2M‐B&R‐02, 2016‐I2M‐1‐006, 2016‐I2M‐2‐004), and the 111 Project from the Ministry of Education of China (B16005).
Disclosures
Drs Krumholz and Normand work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures that are publicly reported. Dr Krumholz was a recipient of a research grant, through Yale, from Medtronic and the US Food and Drug Administration to develop methods for postmarket surveillance of medical devices; is a recipient of research agreements with Medtronic and Johnson & Johnson (Janssen), through Yale, to develop methods of clinical trial data sharing; chairs a Cardiac Scientific Advisory Board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science and the Physician Advisory Board for Aetna; and is the founder of Hugo, a personal health information platform. Dr Ho serves on a Steering Committee for a clinical trial on medication adherence sponsored by Janssen. He receives grant funding from NHLBI and VA Health Services Research and Development (HSR&D). The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental methods.
Figure S1. Median days to 1‐year major cardiovascular adverse events.
Figure S2. Patient characteristics associated with poor adherence.
Acknowledgments
We appreciate the multiple contributions made by study teams at the China Oxford Centre for International Health Research and the Yale‐New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operation, particularly the data collection efforts. We thank the hospitals that participated in the China Patient‐centered Evaluative Assessment of Cardiac Events Prospective Study of AMI, for their contributions to this work.
(J Am Heart Assoc. 2019;8:e011793 DOI: 10.1161/JAHA.118.011793.)
References
- 1. Chaudhry SI, Khan RF, Chen J, Dharmarajan K, Dodson JA, Masoudi FA, Wang Y, Krumholz HM. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in Medicare beneficiaries: 1999–2010. J Am Heart Assoc. 2014;3:e001197 DOI: 10.1161/JAHA.114.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Lichtman JH, Dharmarajan K, Masoudi FA, Ross JS, Dodson JA, Chen J, Spertus JA, Chaudhry SI, Nallamothu BK, Krumholz HM. National trends in stroke after acute myocardial infarction among Medicare patients in the United States: 1999 to 2010. Am Heart J. 2015;169:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sampson UK, Pfeffer MA, McMurray JJ, Lokhnygina Y, White HD, Solomon SD. Predictors of stroke in high‐risk patients after acute myocardial infarction: insights from the VALIANT Trial. Eur Heart J. 2007;28:685–691. [DOI] [PubMed] [Google Scholar]
- 5. Tu JV, Austin PC, Walld R, Roos L, Agras J, Mcdonald KM. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37:992–997. [DOI] [PubMed] [Google Scholar]
- 6. Jneid H, Addison D, Bhatt DL, Fonarow GC, Gokak S, Grady KL, Green LA, Heidenreich PA, Ho PM, Jurgens CY, King ML, Kumbhani DJ, Pancholy S. 2017 AHA/ACC clinical performance and quality measures for adults with ST‐elevation and non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2017;70:2048–2090. [DOI] [PubMed] [Google Scholar]
- 7. Kim S, Shin DW, Yun JM, Hwang Y, Park SK, Ko YJ, Cho B. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension. 2016;67:506–512. [DOI] [PubMed] [Google Scholar]
- 8. Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta‐analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Q, Chang A, Ritchey MD, Loustalot F. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population‐based cohort study. J Am Heart Assoc. 2017;6:e006056 DOI: 10.1161/JAHA.117.006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. [DOI] [PubMed] [Google Scholar]
- 11. De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Hoffman E, Goto S, Ohman EM, Bhatt DL; REduction of Atherothrombosis for Continued Health Registry Investigators . Adherence to secondary prevention medications and four‐year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126:693–700.e1. [DOI] [PubMed] [Google Scholar]
- 13. Kuepper‐Nybelen J, Hellmich M, Abbas S, Ihle P, Griebenow R, Schubert I. Association of long‐term adherence to evidence‐based combination drug therapy after acute myocardial infarction with all‐cause mortality. A prospective cohort study based on claims data. Eur J Clin Pharmacol. 2012;68:1451–1460. [DOI] [PubMed] [Google Scholar]
- 14. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han X, Zhou F, Li H, Xing X, Chen L, Wang Y, Zhang C, Liu X, Suo L, Wang J, Yu G, Wang G, Yao X, Yu H, Wang L, Liu M, Xue C, Liu B, Zhu X, Li Y, Xiao Y, Cui X, Li L, Purdy JE, Cao B; CAP‐China Network . Effects of age, comorbidity and adherence to current antimicrobial guidelines on mortality in hospitalized elderly patients with community‐acquired pneumonia. BMC Infect Dis. 2018;18:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kipsang J, Chen J, Tang C, Li X, Wang H. Self reported adherence to antiretroviral treatment and correlates in Hunan province, the Peoples Republic of China. Int J Nurs Sci. 2018;5:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei L, Champman S, Li X, Li X, Li S, Chen R, Bo N, Chater A, Horne R. Beliefs about medicines and non‐adherence in patients with stroke, diabetes mellitus and rheumatoid arthritis: a cross‐sectional study in China. BMJ Open. 2017;7:e017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yue Z, Bin W, Weilin Q, Aifang Y. Effect of medication adherence on blood pressure control and risk factors for antihypertensive medication adherence. J Eval Clin Pract. 2015;21:166–172. [DOI] [PubMed] [Google Scholar]
- 19. He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. [DOI] [PubMed] [Google Scholar]
- 20. Group WB . Toward a healthy and harmonious life in China: stemming the rising tide of non‐communicable diseases. 2011. Available at: http://www.worldbank.org/en/news/feature/2011/07/26/toward-health-harmonious-life-china-stemming-rising-tide-of-non-communicable-diseases. Accessed March 27, 2014.
- 21. Li J, Dreyer RP, Li X, Du X, Downing NS, Li L, Zhang HB, Feng F, Guan WC, Xu X, Li SX, Lin ZQ, Masoudi FA, Spertus JA, Krumholz HM, Jiang LX; China PEACE Collaborative Group . China patient‐centered evaluative assessment of cardiac events prospective study of acute myocardial infarction: study design. Chin Med J (Engl). 2016;129:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Li J, Zheng X, Jiang ZH, Hu S, Wadhera RK, Bai XK, Lu JP, Wang QY, Li YT, Wu CQ, Xing C, Normand S‐L, Krumholz HM, Jiang LX. Risk factors associated with major cardiovascular events 1 year after acute myocardial infarction. JAMA Netw Open. 2018;1:e181079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–2310. [DOI] [PubMed] [Google Scholar]
- 26. Liu HX, Gao W, Zhao D, Shang JJ. Survey on the hospitalization treatment status of acute myocardial infarction patients in 13 hospitals of western medicine and traditional Chinese medicine in Beijing. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:306–310. [PubMed] [Google Scholar]
- 27. Wang Y, Fu R, Wang Z, Bao H, Chen Y, Yang F, Luo X, Liu M. Assessing the quality of care for patients with acute myocardial infarction in China. Clin Cardiol. 2015;38:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Figure S1. Median days to 1‐year major cardiovascular adverse events.
Figure S2. Patient characteristics associated with poor adherence.


