Abstract
Background
People living with HIV (PLWH) have an increased risk of myocardial infarction (MI). Changes in the gut microbiota that occur with chronic HIV infection could play a role in HIV‐associated atherosclerosis. Choline, carnitine, betaine, and trimethylamine N‐oxide are small molecules that are, in part, metabolized or produced by the gut microbiome. We hypothesized that these metabolites would be associated with carotid artery intima‐media thickness and MI in PLWH.
Methods and Results
Carotid artery intima‐media thickness was measured at baseline and at a median interval of 4 years in 162 PLWH from the SCOPE (Study of the Consequences of the Protease Inhibitor Era) cohort in San Francisco, CA. Separately, 105 PLWH (36 cases with type I adjudicated MI and 69 controls without MI) were selected from the Center for AIDS Research Network of Integrated Clinical Systems, a multicenter clinic‐based cohort. Controls were matched by demographics, CD4 cell count, and duration of viral suppression. In the SCOPE cohort, higher carnitine levels had a significant association with presence of carotid plaque and greater baseline and progression of mean carotid artery intima‐media thickness after adjusting for traditional cardiovascular disease risk factors. In the treated and suppressed subgroup, these associations with carnitine remained significant after adjustment for cardiovascular disease risk factors. In the Center for AIDS Research Network of Integrated Clinical Systems cohort, the risk of MI was significantly increased in subjects with carnitine levels in the highest quartile after adjustment for cardiovascular disease risk factors.
Conclusions
In PLWH, including the treated and suppressed subgroup, carnitine is independently associated with carotid artery intima‐media thickness, carotid plaque, and MI in 2 separate cohorts. These results emphasize the potential role of gut microbiota in HIV‐associated atherosclerosis and MI, especially in relation to carnitine metabolism.
Keywords: atherosclerosis, carnitine, gut microbiota, HIV, myocardial infarction
Subject Categories: Biomarkers, Translational Studies, Coronary Artery Disease, Pathophysiology
Clinical Perspective
What Is New?
Whether gut microbiota–associated metabolites have an effect on cardiovascular disease in people living with HIV is not yet clear.
This study found that the metabolite, carnitine, was independently associated with atherosclerosis, as measured by carotid artery intima‐media thickness, and adjudicated myocardial infarction in 2 separate longitudinal clinic‐based cohorts of people living with HIV; this finding remained present in individuals who were virally suppressed on antiretroviral therapy.
Unlike in the general population, trimethylamine N‐oxide was not associated with atherosclerosis or myocardial infarction in these 2 cohorts of people living with HIV.
What Are the Clinical Implications?
This study provides support to the hypothesis that changes in the gut microbiota and certain associated metabolites have an effect on cardiovascular disease in people living with HIV.
Thus, targeting changes in the gut microbiota that suppress serum carnitine levels can serve as potential novel therapeutic options in this population.
Introduction
Atherosclerotic cardiovascular disease (CVD) is a leading cause of mortality in people living with HIV (PLWH).1, 2, 3, 4 PLWH have a 2‐fold increased risk of myocardial infarction (MI) and a 4‐fold increased risk of sudden cardiac death compared with the general population.5, 6, 7, 8 Although early antiretroviral therapy (ART) decreases the risk of CVD by 7‐fold, PLWH receiving ART with normalized CD4 cell counts and suppressed viral load continue to have an increased risk of MI, even after adjusting for traditional CVD risk factors.7, 9, 10, 11, 12 Thus, other mechanisms involved in the development of atherosclerotic CVD in PLWH need to be identified.
Multiple factors, such as increased inflammation, thrombotic activity, immune activation, and endothelial activation, play a part in CVD in PLWH.13, 14, 15, 16, 17, 18 However, the role of the gut microbiota in the increased burden of atherosclerotic CVD in chronic HIV infection is unclear. Despite treatment with ART, significant changes occur in the gut mucosa and microbiota in chronic HIV infection.19, 20, 21 This appears to be attributable to latent viral reservoirs in lymphoid tissues that continue to have ongoing viral replication.14, 22 One of the primary reservoirs is the gut‐associated lymphoid tissue, where HIV infects CD4+ T cells, leading to changes in the gut mucosa and microbiota.18, 19, 20, 21 Although the changes in the gut cause downstream inflammation and immune activation, how the secretory function of the gut microbiota is affected is not known.
The gut microbiota is involved in the metabolism of various macromolecules that lead to generation of metabolites that have local and systemic effects. Metabolites generated from the breakdown of dietary phosphatidylcholine by gut microbiota have been studied in uninfected individuals. These metabolites, such as trimethylamine N‐oxide (TMAO), carnitine, betaine, and choline, are independently associated with atherosclerosis and MI in the general population.23, 24, 25, 26, 27 In uninfected individuals, bacteria from the genus Prevotella are thought to play a role in generating these specific metabolites.26 Interestingly, multiple studies using different sampling techniques have shown that the gut microbiota in HIV‐infected adults, even those receiving effective ART, is also enriched in bacteria from the genus Prevotella and depleted in bacteria from the genus Bacteroides.20, 21 Given these changes in the gut microbiota in chronic HIV infection, we hypothesized that these metabolites play a role in CVD in PLWH.
The association between these metabolites and CVD in PLWH has been evaluated in a few studies, but the results have been inconsistent. One study showed a U‐shaped association between TMAO levels and coronary artery stenosis.28 A more recent study showed an association between TMAO and progression of carotid artery atherosclerosis.27 However, other studies have shown no association with plaque burden or MI.29, 30, 31 Moreover, some of the studies have not evaluated all 4 of the metabolites. Thus, the objective of our study was to evaluate associations of TMAO, carnitine, betaine, and choline with atherosclerosis and MI in PLWH. We measured metabolite levels in 2 separate cohorts. Using an observational single‐center cohort, we modeled the association of these metabolites with baseline and progression of carotid artery intima‐media thickness (cIMT) as well as carotid plaque. Furthermore, using a multicenter, nested, case‐control cohort, we modeled the associations of these metabolites with adjudicated type I MI.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Study Cohorts
SCOPE cohort
A total of 162 individuals were randomly chosen from the SCOPE (Study of the Consequences of the Protease Inhibitor Era) cohort. SCOPE is an observational prospective cohort based on the HIV/AIDS clinics at the Zuckerberg San Francisco General Hospital and the San Francisco Veterans Affairs Medical Center (San Francisco, CA). PLWH included the following: (1) treated and suppressed, receiving ART with an undetectable HIV RNA level; (2) treated and unsuppressed, receiving ART with a detectable HIV RNA level; (3) untreated and unsuppressed, untreated with a detectable HIV RNA level; and (4) untreated and suppressed, not receiving ART but with undetectable HIV RNA levels (elite controllers). Participants with a viral load of <75 copies/mL were considered to be suppressed.
Each participant undergoes an in‐depth questionnaire interview detailing their HIV disease history and medical history, including cardiac risk factors. Participants in the SCOPE cohort are seen every 4 months for blood tests and are given questionnaires about medications, health‐related behaviors, and symptoms. The University of California, San Francisco, Committee on Human Research approved the study, and all subjects provided written informed consent. Subjects were identified as having hypertension, having diabetes mellitus, or being a current smoker if those diagnoses were present before the baseline cIMT scan. HIV duration and ART history, including class and duration of ART, were recorded at the timing of the baseline cIMT scan.
CNICS cohort
A total of 105 individuals were chosen from the Center for AIDS Research Network of Integrated Clinical Systems (CNICS), which is an 8‐site practice‐based network in the United States (including University of California, San Francisco) consisting of >31 000 PLWH who are followed up in primary care clinics devoted to HIV/AIDS (see Acknowledgments). Of the 105 participants, 36 were cases and 69 were controls. Cases were PLWH who developed a type I MI (atherothrombotic, as opposed to type II, which is demand related) between 2001 and 2012 (hereafter referred to as the “index diagnosis date”).32 Cases were prescribed ART, had a plasma HIV RNA level of <500 copies/mL within 7 days of the index specimen date, and had no history of MI. Controls were selected for each case using incidence density sampling from those who had no known history of MI up to the index diagnosis date. Controls were matched by age at index diagnosis date (±5 years), birth sex, race, CD4+ T‐cell count taken within 14 days of the index specimen date (±50 cells/mm3 for cases that had CD4 cell counts <500 cells/mm3 or simply >500 cells/mm3 for cases with CD4 cell counts >500 cells/mm3), and duration of ART‐mediated virologic suppression <500 copies/mL before the specimen date (±1‐year duration for cases with duration <2 years and any duration >2 years for cases with a duration >2 years). Up to 2 controls for each case were selected. Although University of California, San Francisco is a CNICS site, the SCOPE cohort and CNICS are mostly distinct.
As a clinic‐based cohort, patients are evaluated at regular intervals (typically every 1–3 months), and most of the available data are derived from routine clinical care, as captured by electronic medical records. Each site as well as the CNICS consortium have validated instruments to monitor data quality and accuracy. Institutional review boards at each site approved the study protocol, and all study participants provided informed consent to be included in the cohort.
Laboratory Assays
SCOPE cohort
All blood samples were collected after a 12‐hour period of fasting within 6 months of the first cIMT measurement. Whole blood was centrifuged, and plasma was saved and frozen at −80°C until use. The same collected sample was used to measure total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, HIV RNA level, and current CD4+ and CD8+ T‐cell count. Nadir CD4+ T‐cell count was defined as the lowest value before the baseline IMT scan. Soluble markers of inflammation (interleukin‐6 and hs‐CRP [high‐sensitivity C‐reactive protein]) and coagulopathy (D‐dimer) were measured in cryopreserved plasma samples using a multiplex electrochemiluminescence assay (Meso Scale Discovery, MD).
CNICS cohort
Patients at each site undergo plasma/serum collection every 6 months. The median time interval between case samples before MI was 8.8 months; for controls, it was 11.1 months. Type I MI events were determined on the basis of a validated adjudication protocol adapted from the MESA (Multi‐Ethnic Study of Atherosclerosis).32
For both cohorts, plasma samples were sent to the Cleveland Clinic (Cleveland, OH) for measurement of TMAO, betaine, carnitine, and choline levels. The plasma levels were determined using stable isotope dilution liquid chromatography–tandem mass spectrometry. The accuracy of this technique has been described previously.33
Study End Points
SCOPE cohort
cIMT measurements were made with a GE Vivid 7 system using a 10‐MHz linear array probe, according to the standardized protocol of the ARIC (Atherosclerosis Risk in Communities) Study34 (see Data S1 for details). The median overall follow‐up time between baseline and the last available cIMT measurement was 3.1 years (interquartile range, 2.0–4.7 years). Each participant underwent cIMT measurement at the beginning and end of the study. We defined carotid plaque as a focal region of IMT >1.5 mm, which is recommended by the Society for Vascular Medicine (American Society of Echocardiography guidelines).35 We have previously reported on the reproducibility of cIMT performed in our vascular laboratory, including a variation coefficient of 3.4% and an intraclass correlation coefficient of 0.98.36, 37
CNICS cohort
MI events were determined on the basis of a validated adjudication protocol. The criteria used to classify MI were adapted from the MESA and included history of cardiac chest pain, elevated cardiac enzymes, and appropriate electrocardiographic changes. Only patients with type 1 MI were included in this study (see Data S1 for details).
Statistical Analysis
SCOPE cohort
We summarized baseline clinical characteristics for the entire sample and for the subset of treated and suppressed subjects. Continuous variables were summarized as median (with interquartile range), and categorical variables were summarized as percentage. We used both unadjusted and multivariable‐adjusted robust linear regression models using Huber's M‐estimation to examine relationships of each metabolite and inflammatory biomarker (interleukin‐6, hs‐CRP, or D‐dimer) with baseline cIMT; and we used linear mixed models with random intercepts to model progression of cIMT. Multivariable models were adjusted for demographic characteristics (age, sex, and race/ethnicity), traditional cardiac risk factors (diabetes mellitus, hypertension, hypercholesterolemia, and smoking), and current use of cholesterol medications at the time of enrollment. We used a logistic regression model to examine the associations between each metabolite and baseline carotid plaque, using the same adjustment variables. In separate analyses, we further adjusted for potential HIV‐specific confounding factors, such as CD4+ T‐cell count, HIV viral load, current ART, hepatitis C virus infection, opportunistic infections, and current antibiotic use. We constructed separate models for the entire HIV‐infected cohort and for the subset of treated and suppressed subjects. We log transformed only the dependent variables that were right skewed to normalize their distributions. In the models with ≈10% randomly missing values in low‐density lipoprotein cholesterol and inflammatory markers, multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 10 repetitions.38, 39 The variables used to create the imputation model included demographics, cardiovascular risk factors, HIV‐related factors, and inflammatory markers. Multiple imputation estimates of model parameters were computed by averaging the estimates from 10 repetitions of the imputation algorithm, and the variance and CI of these estimates were computed using Rubin's combining formula.40
CNICS cohort
Differences between cases and controls were summarized using univariable conditional logistic regression analyses. The metabolites were divided into quartiles based on the cutoffs of the median, 25th percentile, and 75th percentile values. We also performed the analyses using other approaches to represent the metabolites, such as continuous variables, tertiles, and partitioned regressions. Traditional cardiovascular risk factors, such as current smoking, diabetes mellitus, hypertension, and total cholesterol, were entered into a multivariable adjustment model. Multivariable conditional logistic regression models were also used to examine the associations between each metabolite and MI. The interaction effect between each of the metabolites and various HIV‐specific risk factors on the risk of MI was also assessed. All analyses were performed using the SAS system, version 9.4.
Results
Clinical Characteristics (SCOPE Cohort)
As shown in Table 1, the SCOPE cohort included 162 PLWH with a median age of 49 years at baseline. They were predominantly men (91%) and white (62%). The median HIV duration was 14 years, and the median CD4 cell count was 524 cells/mm3. Eighty subjects were treated with a suppressed viral load. Overall, 39% had hypertension and 35% were active smokers. The median 10‐year atherosclerotic CVD risk for this group was 5.5%. The initial unadjusted regression analysis showed that carnitine and betaine had significant positive associations with both baseline and annual progression of mean cIMT. TMAO and choline did not have a significant association with either baseline or annual progression of mean cIMT (Table S1).
Table 1.
Summary of Demographics and Baseline Clinical Characteristics (SCOPE Cohort)
| Variable | All HIV+ (N=162) | Treated and Suppressed (N=80) |
|---|---|---|
| Demographics | ||
| Age, y | 49 (42–55) | 50 (45–56) |
| White | 62 | 73 |
| Black | 23 | 10 |
| Hispanic | 10 | 13 |
| Men | 91 | 94 |
| Cardiovascular risk factors | ||
| Body mass index, kg/m2 | 22 (20–25) | 22 (20–26) |
| Hypertension | 39 | 45 |
| Diabetes mellitus | 11 | 11 |
| Current smoking | 35 | 19 |
| Smoking pack years | 4 (0–17) | 2 (0–20) |
| Low‐density lipoprotein, mg/dL | 104 (83–127) | 108 (87–132) |
| High‐density lipoprotein, mg/dL | 44 (36–51) | 44 (36–51) |
| Total cholesterol, mg/dL | 176 (156–209) | 184 (170–223) |
| Triglycerides, mg/dL | 122 (81–193) | 98 (152–263) |
| 10‐y ASCVD risk, % | 5.5 (3.2–9.3) | 5.3 (3.2–9.3) |
| HIV‐related factors | ||
| Current CD4 cell count, cells/mm3 | 524 (342–713) | 516 (325–667) |
| Nadir CD4 cell count, cells/mm3 | 212 (75–330) | 130 (24–230) |
| Viral load, copies/mL | 75 (50–982) | 50 (40–75) |
| CD4/CD8 ratio | 0.5 (0.3–0.8) | 0.5 (0.3–0.8) |
| HIV duration, y | 14 (7–19) | 16 (10–19) |
| NNRTI use | 26 | 51 |
| NRTI use | 56 | 99 |
| Protease inhibitor use | 35 | 61 |
| Hepatitis C coinfection | 13 | 6 |
| Soluble markers | ||
| Interleukin‐6, pg/mL | 1.1 (0.7–1.8) | 1.1 (0.7–1.8) |
| D‐dimer, mg/L | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) |
| hs‐CRP, mg/L | 2.1 (0.9–5.1) | 2.3 (0.9–4.8) |
| Carotid IMT | ||
| Mean, mm | 0.9 (0.8–1.1) | 0.9 (0.8–1.2) |
Continuous variables are summarized as median (interquartile range), and categorical variables are summarized as percentage. ASCVD indicates atherosclerotic cardiovascular disease; hs‐CRP, high‐sensitivity C‐reactive protein; IMT, intima‐media thickness; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; SCOPE, Study of the Consequences of the Protease Inhibitor Era.
Carnitine and Betaine Are Associated With Higher Baseline Carotid IMT
Among all HIV+ subjects, greater carnitine levels had a significant association with higher mean baseline cIMT (8.2% per 10‐μmol/L increase of carnitine; P<0.01) after multivariable adjustment for demographics and traditional CVD risk factors (Table 2). Similarly, greater betaine levels had a significant association with higher mean baseline cIMT (2.6% per doubling 10‐μmol/L increase of betaine; P<0.01). Both carnitine and betaine remained significantly associated with mean baseline cIMT after further adjustment for CD4+ T‐cell count, viral load, and current ART (Table S2). The associations remained largely unchanged when hepatitis C virus coinfection, opportunistic infections, and antibiotic use were also adjusted for (Table S3).
Table 2.
Association of Metabolites With Baseline and Annual Progression of Mean cIMT (SCOPE Cohort)
| Parameter (per 10‐μmol/L Increase of Metabolite) | HIV+ All Groups | HIV+ Treated and Suppressed | ||
|---|---|---|---|---|
| Baseline Mean cIMT | Annual Progression of Mean cIMT | Baseline Mean cIMT | Annual Progression of Mean cIMT | |
| TMAO |
0.0 (−3.2 to 3.11) P=0.96 |
0.1 (−0.8 to 1.0) P=0.82 |
−3.0 (−8.7 to 3.1) P=0.34 |
−0.4 (−2.1 to 1.5) P=0.66 |
| Carnitine |
8.2 (4.2 to 12.3) P<0.01 |
1.3 (0.2 to 2.4) P=0.02 |
8.2 (3.2 to 13.5) P<0.01 |
1.4 (0 to 3.0) P=0.05 |
| Betaine |
2.6 (0.7 to 4.5) P<0.01 |
0.6 (0.1 to 1.1) P=0.04 |
2.1 (−0.8 to 5.1) P=0.17 |
0.6 (−0.2 to 1.5) P=0.15 |
| Choline |
−2.5 (−16.3 to 13.6) P=0.74 |
1.4 (−2.7 to 6.2) P=0.52 |
−4.9 (−13.5 to 18.2) P=0.65 |
1.9 (−4.3 to 10) P=0.58 |
Data are given as percentage estimate (95% CI). Relative difference in baseline and annual progression of mean cIMT associated with a 10‐μmol/L increase in each metabolite in all HIV+ and treated and suppressed subjects after adjusting for age, sex, race, hypertension, diabetes mellitus, smoking, hypercholesterolemia, and current use of cholesterol‐lowering medications. cIMT indicates carotid artery intima‐media thickness; SCOPE, Study of the Consequences of the Protease Inhibitor Era; TMAO, trimethylamine N‐oxide.
Results were similar when the analysis was restricted to participants who were effectively treated and virally suppressed (Table 2). Carnitine remained significantly associated with higher mean baseline cIMT (8.2% per 10‐μmol/L increase of carnitine; P<0.01). Betaine also maintained the similar effect size on cIMT but failed to reach statistical significance (2.5%; P=0.17). In contrast, TMAO and choline showed little association with baseline cIMT levels at any segment in either the entire cohort or the treated and suppressed group.
Carnitine and Betaine Are Associated With Carotid IMT Progression
Both betaine and carnitine were also associated with faster cIMT progression. Among all HIV+ subjects, carnitine was associated with significantly greater progression of mean cIMT (1.3% per year per 10‐μmol/L increase of carnitine; P=0.02) after multivariable adjustment for demographics and traditional CVD risk factors (Table 2). Betaine was associated with significantly greater progression of mean cIMT (0.6% per year for each 10‐μmol/L increase of betaine; P=0.04) after multivariable adjustment for demographics and traditional CVD risk factors.
The analysis was then restricted to the treated and suppressed group only (Table 2). Carnitine was significantly associated with greater annual progression of mean cIMT (1.4% per year per 10‐μmol/L increase of carnitine; P=0.05). Betaine also remained associated with greater annual progression of mean cIMT with a similar effect size as the entire sample, but the association was no longer significant (0.6% per year per 10‐μmol/L increase of betaine; P=0.15). In contrast, choline and TMAO showed no statistically significant associations with cIMT progression in either the entire cohort or the treated and suppressed group.
Carnitine Is Associated With Baseline Carotid Plaque
Similar findings were demonstrated when the relationship between these metabolites and carotid plaque was evaluated. Carnitine was independently associated with baseline carotid plaque in multivariate analysis adjusted for demographic and CVD risk factors (odds ratio [OR]=1.06 per 1‐μmol/L increase of carnitine; P=0.02) in all HIV+ subjects (Table 3). When the analysis was restricted to the treated and suppressed group, carnitine remained significantly associated with baseline carotid plaque (OR=1.08 per 1‐μmol/L increase of carnitine; P=0.02). Betaine, TMAO, and choline did not have a significant association with carotid plaque.
Table 3.
Association of Metabolites With Baseline Carotid Plaque (SCOPE Cohort)
| Parameter (per 1‐μmol/L Increase of Metabolite) | HIV+ All Groups | HIV+ Treated and Suppressed |
|---|---|---|
| TMAO |
1.0 (0.96–1.03) P=0.96 |
0.98 (0.92–1.05) P=0.56 |
| Carnitine |
1.06 (1.01–1.11) P=0.02 |
1.08 (1.01–1.16) P=0.02 |
| Betaine |
1.02 (1.00–1.04) P=0.12 |
1.02 (0.98–1.05) P=0.38 |
| Choline |
1.08 (0.92–1.28) P=0.35 |
1.06 (0.84–1.35) P=0.61 |
Data are given as odds ratio (95% CI). Association of 1‐μmol/L increase in each metabolite with odds of baseline carotid plaque in all HIV+ and treated and suppressed subjects after adjusting for age, sex, race, hypertension, diabetes mellitus, hypercholesterolemia, smoking, and current use of cholesterol‐lowering medications. SCOPE indicates Study of the Consequences of the Protease Inhibitor Era; TMAO, trimethylamine N‐oxide.
Interleukin‐6 and D‐Dimer Are Associated With Higher Baseline Carotid IMT
We next evaluated the association between hs‐CRP, interleukin‐6, D‐dimer, and baseline mean cIMT. Among all HIV+ subjects, interleukin‐6 and D‐dimer were associated with significantly higher baseline mean cIMT after adjustment for demographics and traditional CVD risk factors (Table 4). When the analysis was restricted to the treated and suppressed group, interleukin‐6 and D‐dimer remained significantly associated with higher baseline mean cIMT (Table 4). In contrast, hs‐CRP showed weak associations with baseline mean cIMT in all HIV+ subjects as well as in the treated and suppressed subgroup.
Table 4.
Association of Inflammatory Markers With Baseline and Annual Progression of Mean cIMT (SCOPE Cohort)
| Parameter | HIV+ All Groups | HIV+ Treated and Suppressed | ||
|---|---|---|---|---|
| Baseline Mean cIMT | Annual Progression of Mean cIMT | Baseline Mean cIMT | Annual Progression of Mean cIMT | |
| Interleukin‐6 (per 10‐pg/mL increase) |
1.9 (0.2 to 3.6) P=0.03 |
0.6 (0 to 1.1) P=0.03 |
6.3 (0.2 to 10.5) P<0.01 |
1.3 (0 to 2.6) P=0.05 |
| D‐dimer (per 0.1‐mg/L increase) |
0.9 (0.4 to 1.4) P<0.01 |
0.1 (−0.1 to 0.2) P=0.24 |
0.8 (0.2 to 1.4) P<0.01 |
0.1 (−0.1 to 0.2) P=0.47 |
| hs‐CRP (per 1‐mg/L increase) |
0.3 (−0.3 to 0.8) P=0.32 |
0.01 (−0.2 to 0.2) P=0.85 |
0.5 (−0.3 to 1.3) P=0.23 |
0.1 (−0.2 to 0.3) P=0.53 |
Data are given as percentage estimate (95% CI). Relative difference in baseline and annual progression of mean cIMT associated with a specified increase in each inflammatory marker in all HIV+ and treated and suppressed subjects after adjusting for age, sex, race, hypertension, diabetes mellitus, smoking, hypercholesterolemia, and current use of cholesterol‐lowering medications. cIMT indicates carotid artery intima‐media thickness; hs‐CRP, high‐sensitivity C‐reactive protein; SCOPE, Study of the Consequences of the Protease Inhibitor Era.
Interleukin‐6 Is Associated With Carotid IMT Progression
Among all HIV+ subjects, interleukin‐6 was significantly associated with greater annual progression of mean cIMT after adjustment for demographics and traditional CVD risk factors (Table 4). When the analysis was restricted to the treated and suppressed subgroup, interleukin‐6 remained significantly associated with greater annual progression of mean cIMT (Table 4). However, an association between D‐dimer and progression of cIMT was not observed. Like with baseline cIMT, hs‐CRP showed weak associations with cIMT progression among all HIV+ subjects and the treated and suppressed subgroup.
Effect of Interleukin‐6 and D‐Dimer on the Association Between Carnitine and cIMT
Given the findings above, we evaluated whether interleukin‐6 and D‐dimer were potential mediators of the association between carnitine and carotid IMT in the treated and suppressed group. The analysis was only done for the treated and suppressed subgroup because of the heterogeneity of the entire cohort. Because only carnitine was significantly associated with baseline and annual progression of mean cIMT in the treated and suppressed subgroup, the other metabolites were excluded from this analysis. After adjusting for demographics, neither interleukin‐6 nor D‐dimer had a significant association with carnitine.
The association between carnitine and baseline mean cIMT was somewhat weakened when interleukin‐6 or D‐dimer was added to the adjustment model (from 8.2% [P=0.001] to 6.6% [P=0.002]) but remained highly significant. The association between carnitine and annual progression of mean cIMT did change when interleukin‐6 was added to the adjustment model (from 1.3% [P=0.02] to 0.9% [P=0.11]). D‐dimer was not added to the adjustment model because it was not significantly associated with annual progression of mean cIMT.
Clinical Characteristics of MI Cases and Controls (CNICS Cohort)
There were 36 cases and 69 controls in the study (Table 5). The median age for the cases was 50 years, and the median age for the controls was 49 years. Approximately a quarter of the subjects in each group were women, 46% were white, and 51% were black. Both groups had a median CD4 cell count >500 cells/mm3, a viral load of <500 copies/mL, and a similar duration of viral load suppression. The prevalences of diabetes mellitus (5%) and hypertension (30%) were similar in each group. Cases had greater triglyceride levels and a higher proportion of active smokers.
Table 5.
Clinical Characteristics of Cases and Controls (CNICS)
| Characteristic | Cases (N=36) | Controls (N=69) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, ya | 50 (47–58) | 49 (46–57) | 0.98 (0.84–1.15) | 0.84 |
| Race | ||||
| White | 17 (47) | 31 (45) | ··· | |
| Black | 18 (50) | 36 (52) | 0.91 (0.40–2.07) | 0.83 |
| Other | 1 (3) | 2 (3) | 0.91 (0.08–10.8) | 0.94 |
| Men | 28 (78) | 53 (77) | 1.06 (0.4–2.77) | 0.91 |
| Cardiovascular risk factors | ||||
| Hypertension | 11 (31) | 21 (30) | 0.88 (0.37–2.09) | 0.77 |
| Diabetes mellitus | 2 (6) | 3 (4) | 1.26 (0.2–8.03) | 0.81 |
| Active smoking | 13 (36) | 13 (19) | 2.08 (0.76–5.67) | 0.15 |
| Hepatitis C | 3 (8) | 11 (16) | 0.22 (0.02–1.93) | 0.17 |
| CKDb | 1 (3) | 3 (4) | 0.65 (0.07–6.60) | 0.72 |
| Triglycerides, mg/dLb | 184 (131–273) | 146 (99–249) | 1.08 (1.0–1.17) | 0.05 |
| LDL, mg/dLb | 117 (86–157) | 102 (76–118) | 1.12 (0.98–1.28) | 0.11 |
| HDL, mg/dLb | 48 (35–52) | 49 (34–59) | 0.94 (0.82–1.07) | 0.37 |
| TC, mg/dLb | 182 (162–240) | 178 (155–208) | 1.14 (0.94–1.38) | 0.19 |
| HIV‐related factors | ||||
| Current CD4 cell count, cells/mm3 c | 536 (348–688) | 616 (420–839) | 0.39 (0.12–1.31) | 0.13 |
| CD4/CD8 ratioc | 0.40 (0.30–0.90) | 0.60 (0.40–1.0) | 0.65 (0.26–1.17) | 0.15 |
| Viral load, copies/mLc | 48 (48–50) | 48 (40–65) | 0.85 (0.49–1.47) | 0.56 |
| Duration of viral load suppression, moc | 2.8 (1–4.7) | 2.5 (1.2–6.6) | 0.89 (0.68–1.15) | 0.37 |
Data are shown as number (percentage) or median (interquartile range). CKD indicates chronic kidney disease; CNICS, Center for AIDS Research Network of Integrated Clinical Systems; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; TC, total cholesterol.
Odds ratio calculated on the basis of increment per year.
Odds ratio calculated on the basis of increment per 10% change.
Odds ratio calculated on the basis of increment per doubling.
Higher Carnitine Levels Are Associated With Increased Odds of MI
When analyzed as continuous variables, none of the metabolites showed statistically significant associations with greater odds of MI status (Table S4). Because we observed nonlinear associations with MI status, we also examined quartiles of each metabolite (Table S5).
In unadjusted analysis, there was no significant increase in the OR for MI in subjects with higher levels of any of the metabolites (Figure). Because triglyceride level was the only CVD risk factor that was significantly associated with an increased OR for MI, we adjusted for it to minimize potential confounding. After adjustment, HIV‐infected adults with high carnitine levels (quartile 4) had a significantly increased OR for MI compared with those with lower carnitine levels (OR, 3.6; 95% CI, 1.1–12.0; P=0.035). There was no such significantly increased OR for MI seen in subjects with high levels of betaine, TMAO, or choline after adjustment. When we further adjusted for other traditional CVD risk factors, such as current smoking, diabetes mellitus, hypertension, and total cholesterol, HIV‐infected adults with carnitine levels in the highest quartile continued to have a significantly increased OR for MI, with a similar effect size compared with those with lower carnitine levels (OR, 3.8; 95% CI, 1.0–14.7; P=0.050).
Figure 1.
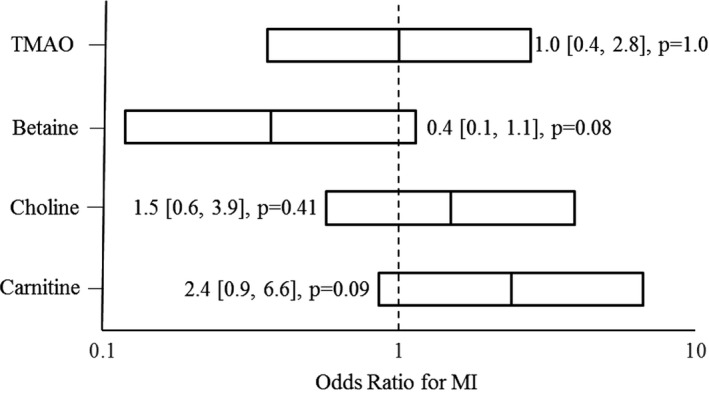
Association of highest quartile of each metabolite with odds of myocardial infarction (MI) in the Center for AIDS Research Network of Integrated Clinical Systems cohort. The odds ratio for MI was obtained from a conditional logistic regression model and shown with 95% CI. TMAO indicates trimethylamine N‐oxide.
Discussion
This study used 2 separate cohorts to investigate the role of gut microbiota–associated metabolites in atherosclerotic disease in PLWH. The single‐center observational study, using the SCOPE cohort, assessed atherosclerotic burden by measuring baseline cIMT and carotid plaque as well as progression of cIMT. After adjusting for traditional CVD risk factors, both betaine and carnitine remained significantly associated with baseline and progression of cIMT in the entire cohort, whereas only carnitine was strongly associated with baseline carotid plaque. Among the effectively treated and suppressed individuals, carnitine remained significantly associated with baseline cIMT and carotid plaque as well as progression of cIMT, whereas the association between betaine and cIMT lost statistical significance. We then determined the association between the metabolites and adjudicated type I MI using a nested case‐control study from the multicenter CNICS cohort of treated and suppressed PLWH. After adjustment for CVD risk factors, individuals with carnitine levels in the highest quartile were found to be at a significantly increased risk of MI. To our knowledge, this is the first study to show an independent association between high carnitine levels and atherosclerotic burden, as measured by cIMT and type I MI in PLWH who are effectively treated and suppressed.
Studies in the general population have shown that TMAO largely mediates the effects of betaine and carnitine on CVD.24, 26, 27, 41, 42 Despite this well‐established relationship, we did not find a significant association between TMAO and cIMT or MI in our cohorts. This finding is supported by several previous studies that have shown an inconsistent relationship between TMAO and CVD among PLWH.28, 29, 30, 31, 43 A recent study by Shan et al found an association between incident carotid artery plaque and TMAO in PLWH; however, they did not evaluate carnitine or cardiovascular events.27 Alterations to gut mucosa and microbiota secondary to HIV infection likely alter these metabolites, which could help explain the lack of association seen between TMAO and CVD in PLWH compared with the general population.19, 20, 21 Adding to the growing evidence for the role of gut microbiota in HIV‐associated CVD, Kehrmann et al found signature divergences in the gut microbiota of individuals with and without coronary artery disease in a cohort of PLWH.44 In the SCOPE cohort, we observed a significant negative association between betaine and HIV‐related factors, such as CD4 cell count and ART. Carnitine did not have a significant association with HIV‐specific factors in either of the 2 cohorts (Data S1). This finding could explain why a significant association was not seen between betaine and cIMT or MI in individuals who were treated and suppressed.
Because carnitine and betaine have distinct physiological roles, there are plausible TMAO‐independent mechanisms for CVD. Carnitine is primarily an intracellular metabolite that plays a crucial role in long‐chain fatty acid oxidation and carbohydrate metabolism.45 Studies using inhibitors of carnitine have shown that carnitine can lead to mitochondrial dysfunction, which can play a role in atherosclerosis.46 Betaine is a methyl donor in one‐carbon metabolism, which is crucial in homocysteine metabolism and in DNA methylation.47 Epigenetic changes, such as aberrant DNA methylation, play an important part in development of atherosclerosis.48
Gut microbial dysbiosis in chronic HIV infection has a wide range of effects, including creation of a “leaky gut syndrome,” which leads to increased microbial translocation and chronic inflammation.15, 49 Given that markers of inflammation are strong predictors of CVD in PLWH, it is important to understand where these metabolites stand in the pathway involving gut microbiota changes, gut mucosa breakdown, and chronic inflammation. Thus, we evaluated the association of carnitine and betaine with markers of inflammation. We measured interleukin‐6, D‐dimer, and hs‐CRP, which are known to be strongly predictive of CVD in PLWH.14, 50, 51 We confirmed that interleukin‐6 and D‐dimer were independently associated with atherosclerosis, as measured by cIMT in our SCOPE cohort. We next evaluated whether interleukin‐6 and D‐dimer were potential mediators of the association observed between cIMT and the metabolites, carnitine and betaine. Our analysis showed that neither interleukin‐6 nor D‐dimer was associated with carnitine or betaine. Furthermore, addition of interleukin‐6 or D‐dimer did not change the association between carnitine and baseline mean cIMT. However, addition of interleukin‐6 to the adjustment model did modestly weaken the association between carnitine and annual progression of mean cIMT.
The primary limitation of this study was the small sample size of the 2 cohorts. We attempted to overcome these sample size limitations by using 2 distinctive cohorts and end points, which allowed us to demonstrate the independent association of carnitine with cIMT and carotid plaque along with a nearly 4‐fold higher odds of MI among individuals with carnitine in the highest quartile. Another limitation of our study was the lack of uninfected control groups. Given the observational nature of both cohorts, there might be confounding factors that were not accounted for by the multivariable adjustment or the case‐control design. However, the case‐control design of the CNICS study led to careful matching of demographics and HIV‐related factors, which allows us to better identify the predictive role of the metabolites on the risk of MI. Comparing these 2 groups is likely more biologically meaningful versus comparison to uninfected individuals.
In conclusion, we found that carnitine is independently associated with atherosclerosis, as measured by cIMT and carotid plaque, and that higher carnitine levels are strongly associated with subsequent MI in a study of 2 cohorts of PLWH. Our results suggest that changes in gut microbiota not only contribute to increased inflammation but also influence metabolites, like carnitine, leading to an increased burden of CVD. Additional studies are needed to confirm these findings and determine if high carnitine levels can be used to identify PLWH at risk for developing CVD.52 Future studies designed to target carnitine should delineate the downstream pathways that lead to HIV‐associated atherosclerosis and clinical events.
Sources of Funding
This study was funded by the National Institutes of Health (K24AI112393 to Hsue.)
Disclosures
Hsue has received honoraria from Gilead and Merck, outside of the submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplementary methods and results.
Table S1. Unadjusted Association of Metabolites With Carotid IMT (SCOPE)
Table S2. Multivariable Adjusted Association of Metabolites With Baseline Carotid IMT in 162 HIV+ Subjects (SCOPE)
Table S3. Additional Multivariable Adjusted Association of Metabolites With Baseline Carotid IMT in 162 HIV+ Subjects (SCOPE)
Table S4. Metabolite Levels in Cases and Controls (CNICS)
Table S5. Quartile Cutoffs for Each Metabolite (CNICS)
Acknowledgments
The authors acknowledge the following Center for AIDS Research Network of Integrated Clinical Systems sites: Case Western Reserve University, University of Alabama at Birmingham, University of California, San Francisco, University of Washington, University of California, San Diego, Fenway Community Health Center of Harvard University, University of North Carolina, and University of North Carolina at Chapel Hill.
(J Am Heart Assoc. 2019;8:e011037 DOI: 10.1161/JAHA.118.011037.)
References
- 1. Lang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D. Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. [DOI] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Kowalska JD, de Wit S, Law M, el Sadr W, Kirk O, Friis‐Moller N, Monforte A, Phillips AN, Sabin CA, Lundgren JD. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. [DOI] [PubMed] [Google Scholar]
- 3. Kearns A, Gordon J, Burdo TH, Qin X. HIV‐1‐associated atherosclerosis: unraveling the missing link. J Am Coll Cardiol. 2017;69:3084–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gili S, Grosso Marra W, D'Ascenzo F, Lonni E, Calcagno A, Cannillo M, Ballocca F, Cerrato E, Pianelli M, Barbero U, Mancone M, DiNicolantonio JJ, Lavie CJ, Omede P, Montefusco A, Bonora S, Gasparini M, Biondi‐Zoccai G, Moretti C, Gaita F. Comparative safety and efficacy of statins for primary prevention in human immunodeficiency virus‐positive patients: a systematic review and meta‐analysis. Eur Heart J. 2016;37:3600–3609. [DOI] [PubMed] [Google Scholar]
- 5. Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, Burkholder GA, Mathews WC, Silverberg MJ, Sterling TR, Heckbert SR, Budoff MJ, Van Rompaey S, Delaney JAC, Wong C, Tong W, Palella FJ, Elion RA, Martin JN, Brooks JT, Jacobson LP, Eron JJ, Justice AC, Freiberg MS, Klein DB, Post WS, Saag MS, Moore RD, Crane HM. Increased risk of myocardial infarction in HIV‐infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta‐analysis. PLoS One. 2017;12:e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2011;8:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, Girard PM, Grund B, Law M, Losso MH, Palfreeman A, Wood R. Major clinical outcomes in antiretroviral therapy (ART)‐naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. [DOI] [PubMed] [Google Scholar]
- 11. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–741. [DOI] [PubMed] [Google Scholar]
- 13. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton NI, Prineas RJ, Neaton JD. Inflammation, coagulation and cardiovascular disease in HIV‐infected individuals. PLoS One. 2012;7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr, Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M, Tracy RP, Brown TT, Post WS. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185:456–462. [DOI] [PubMed] [Google Scholar]
- 18. Krikke M, van Lelyveld SF, Tesselaar K, Arends JE, Hoepelman IM, Visseren FL. The role of T cells in the development of cardiovascular disease in HIV‐infected patients. Atherosclerosis. 2014;237:92–98. [DOI] [PubMed] [Google Scholar]
- 19. Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, Norman JM, Keller BC, Luevano JM, Wang D, Boum Y, Martin JN, Hunt PW, Bangsberg DR, Siedner MJ, Kwon DS, Virgin HW. Altered virome and bacterial microbiome in human immunodeficiency virus‐associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC. An altered intestinal mucosal microbiome in HIV‐1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV‐induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes. 2014;5:562–570. [DOI] [PubMed] [Google Scholar]
- 22. Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254:326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shan Z, Clish CB, Hua S, Scott JM, Hanna DB, Burk RD, Haberlen SA, Shah SJ, Margolick JB, Sears CL, Post WS, Landay AL, Lazar JM, Hodis HN, Anastos K, Kaplan RC, Qi Q. Gut microbial‐related choline metabolite trimethylamine‐N‐oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis. 2018;218:1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller PE, Haberlen SA, Brown TT, Margolick JB, DiDonato JA, Hazen SL, Witt MD, Kingsley LA, Palella FJ Jr, Budoff M, Jacobson LP, Post WS, Sears CL. Brief report: intestinal microbiota‐produced trimethylamine‐N‐oxide and its association with coronary stenosis and HIV serostatus. J Acquir Immune Defic Syndr. 2016;72:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, Abbara S, Gauguier D, Capeau J, Boccara F, Grinspoon SK. Plaque burden in HIV‐infected patients is associated with serum intestinal microbiota‐generated trimethylamine. AIDS. 2015;29:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haissman JM, Knudsen A, Hoel H, Kjaer A, Kristoffersen US, Berge RK, Katzenstein TL, Svardal A, Ueland T, Aukrust P, Lebech AM, Nielsen SD, Troseid M. Microbiota‐dependent marker TMAO is elevated in silent ischemia but is not associated with first‐time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr. 2016;71:130–136. [DOI] [PubMed] [Google Scholar]
- 31. Knudsen A, Christensen TE, Thorsteinsson K, Ghotbi AA, Hasbak P, Lebech AM, Nielsen SD, Hov JR, Berge R, Ripa RS, Kjaer A, Troseid M. Microbiota‐dependent marker TMAO is not associated with decreased myocardial perfusion in well‐treated HIV‐infected patients as assessed by 82Rubidium PET/CT. J Acquir Immune Defic Syndr. 2016;72:e83–e85. [DOI] [PubMed] [Google Scholar]
- 32. Crane HM, Heckbert SR, Drozd DR, Budoff MJ, Delaney JA, Rodriguez C, Paramsothy P, Lober WB, Burkholder G, Willig JH, Mugavero MJ, Mathews WC, Crane PK, Moore RD, Napravnik S, Eron JJ, Hunt P, Geng E, Hsue P, Barnes GS, McReynolds J, Peter I, Grunfeld C, Saag MS, Kitahata MM; Centers for AIDS Research Network of Integrated Clinical Systems Cohort Investigators . Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. Am J Epidemiol. 2014;179:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine‐N‐oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. [DOI] [PubMed] [Google Scholar]
- 35. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima‐Media Thickness Task Force: endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 36. Post WS, Budoff M, Kingsley L, Palella FJ Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, Maka K, Martin JN, Ganz P, Deeks SG. Carotid intima‐media thickness progression in HIV‐infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1:e000422 DOI: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bodner TE. What improves with increased missing data imputations? Struct Equ Modeling. 2008;15:651–675. [Google Scholar]
- 39. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 40. Rubin DB. Multiple Imputation for Nonresponse in Surveys (Wiley Series in Probability and Statistics). 1987. [Google Scholar]
- 41. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Luscher TF. Gut microbiota‐dependent trimethylamine N‐oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota‐generated metabolite trimethylamine‐N‐oxide. Eur Heart J. 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haissman JM, Haugaard AK, Ostrowski SR, Berge RK, Hov JR, Troseid M, Nielsen SD. Microbiota‐dependent metabolite and cardiovascular disease marker trimethylamine‐N‐oxide (TMAO) is associated with monocyte activation but not platelet function in untreated HIV infection. BMC Infect Dis. 2017;17:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kehrmann J, Menzel J, Saeedghalati M, Obeid R, Schulze C, Holzendorf V, Farahpour F, Reinsch N, Klein‐Hitpass L, Streeck H, Hoffmann D, Buer J, Esser S. Gut microbiota in HIV‐infected individuals linked to coronary heart disease. J Infect Dis. 2019;219:497–508. [DOI] [PubMed] [Google Scholar]
- 45. Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond). 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dambrova M, Makrecka‐Kuka M, Vilskersts R, Makarova E, Kuka J, Liepinsh E. Pharmacological effects of meldonium: biochemical mechanisms and biomarkers of cardiometabolic activity. Pharmacol Res. 2016;113:771–780. [DOI] [PubMed] [Google Scholar]
- 47. Day CR, Kempson SA. Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta. 2016;1860:1098–1106. [DOI] [PubMed] [Google Scholar]
- 48. Hai Z, Zuo W. Aberrant DNA methylation in the pathogenesis of atherosclerosis. Clin Chim Acta. 2016;456:69–74. [DOI] [PubMed] [Google Scholar]
- 49. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV‐infected adults. J Infect Dis. 2012;205(suppl 3):S375–S382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3:e000844 DOI: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D'Agostino RB Sr. Cardiovascular risk estimation in 2012: lessons learned and applicability to the HIV population. J Infect Dis. 2012;205(suppl 3):S362–S367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary methods and results.
Table S1. Unadjusted Association of Metabolites With Carotid IMT (SCOPE)
Table S2. Multivariable Adjusted Association of Metabolites With Baseline Carotid IMT in 162 HIV+ Subjects (SCOPE)
Table S3. Additional Multivariable Adjusted Association of Metabolites With Baseline Carotid IMT in 162 HIV+ Subjects (SCOPE)
Table S4. Metabolite Levels in Cases and Controls (CNICS)
Table S5. Quartile Cutoffs for Each Metabolite (CNICS)


