Abstract
Background
We evaluated the importance of high‐density lipoprotein (HDL) functionality for target‐lesion revascularization in patients treated with coronary stents using a rapid cell‐free assay system to evaluate the functional capacity of HDL to accept additional cholesterol (cholesterol‐uptake capacity; CUC).
Methods and Results
From an optical coherence tomography (OCT) registry of patients treated with coronary stents, 207 patients were enrolled and their HDL was functionally evaluated by measuring the CUC. Follow‐up OCT was performed (median duration, 24.5 months after stenting) to evaluate the presence of neoatherosclerosis. Clinical follow‐up was performed to assess target‐lesion revascularization for a median duration of 42.3 months after stent implantation. Neoatherosclerosis was identified in 37 patients (17.9%). Multivariate logistic regression analysis revealed that a decreased CUC was independently associated with neoatherosclerosis (odds ratio, 0.799; P<0.001). The CUC showed a significant inverse correlation with incidence of target‐lesion revascularization (odds ratio, 0.887; P=0.003) and with lipid accumulation inside stents, suggesting that neoatherosclerosis contributes to the association between CUC and target‐lesion revascularization.
Conclusions
Impaired HDL functionality, detected as decreased CUC, might lead to future stent failure by provoking atherogenic changes of the neointima within stents. Both quantitative and qualitative assessments of HDL might enable the improved prediction of clinical outcomes after stent implantation.
Keywords: cholesterol‐uptake capacity, high‐density lipoprotein, neoatherosclerosis, optical coherence tomography, target‐lesion revascularization
Subject Categories: Lipids and Cholesterol, Optical Coherence Tomography (OCT), Revascularization
Clinical Perspective
What Is New?
This is the first study that reveals the potential relation between high‐density lipoprotein functionality evaluated by cholesterol‐uptake capacity, neoatherosclerosis, and clinical outcome of target‐lesion revascularization long term after stent implantation in human optical coherence tomography registry.
What Are the Clinical Implications?
We believe that more‐active secondary prevention will lead to improved outcomes by measuring cholesterol‐uptake capacity, which identifies patients with increased risk of neoatherosclerosis and residual risk for target‐lesion revascularization.
Intracoronary stent implantation has markedly reduced the incidence of restenosis in patients with coronary artery disease compared with that of percutaneous old balloon angioplasty.1 However, in‐stent restenosis requiring target‐lesion revascularization (TLR) occurs even with the use of drug‐eluting stents. Emerging evidence suggests that among various potential risk factors, atherogenic progression within the neointima, that is, neoatherosclerosis (NA), is one of the major contributors to TLR, and the lipid profile is one of the key risk factors for the development of NA.2, 3, 4
Recent animal and human studies have demonstrated the importance of high‐density lipoprotein (HDL) functionality, rather than HDL‐cholesterol (HDL‐C) levels, in the development of de novo coronary artery disease.5, 6 Cholesterol‐efflux capacity, a measure of the ability of HDL to promote cholesterol removal from lipid‐laden macrophages,7 is inversely correlated with incidence of cardiovascular events and improves cardiovascular risk prediction beyond that using traditional coronary risk factors.7, 8, 9 Therefore, we hypothesized that the ability of HDL to promote cholesterol removal from lipid‐laden macrophages could be associated with TLR through its effect on NA progression within stents.
Recently, we developed a rapid cell‐free assay system to directly evaluate the capacity of HDL to accept additional cholesterol; the measurement of the cholesterol‐uptake capacity (CUC) enables HDL functionality to be readily evaluated in our daily practice.10 Thus, in this study, we aimed to clarify the relationships among CUC, NA, and TLR using the novel cell‐free assay system, CUC measurements, and optical coherence tomography (OCT).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Population
The Kobe University Hospital OCT registry is a single‐center registry of consecutive patients who have undergone coronary artery OCT.11 All patients who have undergone OCT are eligible for inclusion in this registry. OCT was performed for the following reasons: (1) planned follow‐up coronary angiography and OCT, which includes any case at the time of stent implantation or routine stent follow‐up regardless of symptoms; (2) evidence of myocardial ischemia, such as silent myocardial ischemia, stable angina, or acute coronary syndrome. Exclusion criteria for OCT were: (1) an anatomically unsuitable target artery for OCT, according to previously described criteria12; (2) apparent congestive heart failure; (3) renal insufficiency with a baseline creatinine level of ≥2.0 mg/dL except under hemodialysis; or (4) written informed consent was not obtained from the patient.
From the OCT registry, consecutive patients were enrolled who (1) had undergone percutaneous coronary intervention for de novo coronary artery disease; (2) had been treated with bare‐metal stents, sirolimus‐eluting stents (Cypher; Cordis, Miami Lakes, FL), paclitaxel‐eluting stents (Taxus; Boston Scientific, Natick, MA), biolimus‐eluting stents (Nobori; Terumo Corporation, Tokyo, Japan), or everolimus‐eluting stents (XIENCE V; Abbott Vascular, Santa Clara, CA); or (3) had successfully undergone follow‐up OCT for the target stents >6 months after stenting. Exclusion criteria were as follows: (1) patients in whom the stent was implanted in the left main trunk and (2) patients whose OCT images were of insufficient quality. In the case of patients with stents implanted in ≥2 lesions, the stent that was implanted first was selected as the target stent. Patients were enrolled in the study, and CUC was measured at the time of follow‐up OCT by a clinical follow‐up.
The percutaneous coronary intervention procedure was performed by intravascular ultrasound (Boston Scientific Corporation; Volcano Corporation, Rancho Cordova, CA) or OCT guidance (C7 or C8 Dragonfly; St. Jude Medical, St. Paul, MN). All patients were treated with aspirin (100 mg/day) and clopidogrel (75 mg/day), ticlopidine (200 mg/day), or prasugrel (3.75 mg/day) for at least 3 months after stent implantation. This study was approved by the ethics committee of Kobe University (Kobe, Japan).
Cholesterol‐Uptake Assay
CUC was measured at the time of the follow‐up OCT as per our previous methods.10 Briefly, apolipoprotein (apo) B–containing lipoproteins were precipitated from serum using polyethylene glycol 4000, and 1 μL of this apoB‐depleted serum sample, whose apoA1 concentration was adjusted to 5 μg/mL using apoA1 in PBS, was incubated with 100 μL of 5 μmol/L of BODIPY‐cholesterol (Avanti Polar Lipids, Alabaster, AL) at 37°C for 2 hours. Subsequently, the apoB‐depleted serum mixture was transferred into wells of 96‐well black‐bottom microplates coated with an anti‐apoA1 antibody (clone 1C5; Sanbio B.V., Uden, Netherlands), and plates were incubated at 25°C for 1 hour. After washing the wells 5 times with PBS, 100 μL of 20 mmol/L of cyclodextrin (Sigma‐Aldrich, St. Louis, MO) in PBS was added to wells to enhance the fluorescence signal derived from BODIPY‐cholesterol, and the plate was incubated at 25°C for 1 hour. Fluorescence intensity was measured at 535 nm with excitation at 485 nm using an Infinite 200 Pro microplate reader (Tecan, Männedorf, Switzerland). Intensity values were standardized using a calibration curve generated from serial dilutions of recombinant apoA1 (Sigma‐Aldrich) for each microplate and adjusted by multiplying by the apoA1 concentrations in each apoB‐depleted serum sample.
OCT Examination
The OCT procedure was performed as reported.13 Briefly, a 0.014‐inch standard guide wire was positioned distally in the target vessel, and the OCT catheter (C7 and C8 Dragonfly; St. Jude Medical) was advanced to the distal end of the target lesion. For image acquisition, blood in the lumen area was replaced with an iodine contrast medium that was continuously flushed using a power injector. The entire length of the region of interest was scanned using the integrated automated pullback device at 20 mm/s.
OCT Analysis
Offline OCT analysis was performed using dedicated software (Light Lab Imaging Inc, Westford, MA). All images were analyzed at every frame in the stents by 2 independent investigators, who were blinded to the angiographic and clinical findings. NA was defined as a neointima containing a diffuse border and a signal‐poor region, with the struts underneath invisible because of the marked signal attenuation (Figure 1A).4, 14, 15 To quantify the circumferential extent of NA, the lipid‐core arc was measured at a 0.2‐mm interval throughout the segments showing NA. The mean lipid‐core arc was calculated for each lesion, and the lipid index was computed by multiplying the mean lipid‐core arc by the lipid‐core longitudinal length.16 Thin‐cap fibroatheroma‐like neointima was defined as a neointima characterized by a fibrous cap thickness at the thinnest part of <65 μm and an angle of lipid‐laden neointima of >180 degrees (Figure 1B).4, 14 Macrophage accumulation sites were defined as confluent or punctate highly backscattering focal regions in the artery wall (Figure 1C).17 Macrophage grading was also performed to quantify the extent of macrophage accumulation based on the axial and circumferential distribution. In OCT images showing NA, the macrophage arc was measured at 0.2‐mm intervals and divided into 5 groups: grade 0, no macrophages; grade 1, localized macrophage accumulation, <30 degrees; grade 2, clustered accumulation, ≥30 and <90 degrees; grade 3, clustered accumulation, ≥90 and <270 degrees; and grade 4, clustered accumulation, ≥270 degrees. Macrophage grade was evaluated as the summation of grades 0 to 4 across all cross‐sections in NA.17 In‐stent thrombus was defined as a mass protruding beyond the stent strut into the lumen, with marked attenuation behind the mass (Figure 2A).18 A peri‐strut low‐intensity area was defined as a neointima containing a region around stent struts displaying a homogeneous lower‐intensity appearance relative to the surrounding tissue without notable signal attenuation behind the area in at least 1 cross‐section (Figure 2B).19 The vasa vasorum was defined as well‐delineated low‐backscattering structures, <200 μm in diameter, showing a trajectory within the vessel (Figure 2C).20
Figure 1.
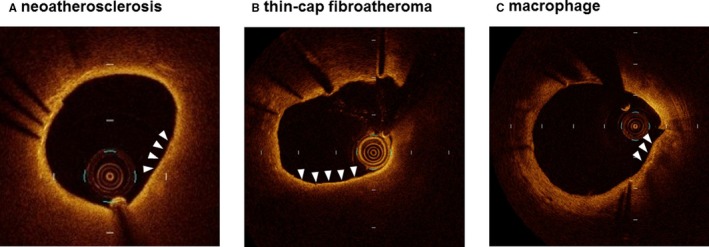
Representative optical coherence tomography images showing (A) neoatherosclerosis, (B) thin‐cap fibroatheroma, and (C) macrophage.
Figure 2.
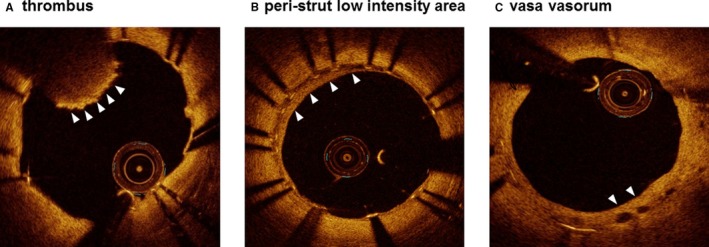
Representative optical coherence tomography images showing a thrombus (A), peri‐strut low‐intensity area (B), and vasa vasorum (C).
Outcome Variables and Definitions
Clinical outcome data (median duration between stent implantation and clinical follow‐up, 42.3 months) were obtained by reviewing outpatient records or telephone interviews for death, myocardial infarction, and TLR. The primary outcome was ischemia‐driven TLR during the follow‐up period. All deaths were considered cardiac related unless unequivocal noncardiac enzyme involvement could be established. Myocardial infarction was considered in cases where the cardiac enzyme levels (troponin or myocardial band fraction of creatine kinase) were greater than the upper limit of the normal value. All events were carefully verified and adjudicated by independent clinicians.
Statistical Analysis
Statistical analyses were conducted using SPSS software (version 25; SPSS Inc, Chicago, IL). Qualitative data are presented with frequencies and are shown as means±SD. For continuous variables, comparisons between 2 groups were performed using a 2‐tailed, unpaired t test, Welch test, or Wilcoxon test, according to the data of normal or non‐normal distribution and equal variance, respectively. Discrete variables are presented as percentages, and comparisons were performed using the chi‐squared analysis or Fisher's exact test. Logistic regression analysis were performed to identify independent predictors of the presence of NA and TLR. Age, sex, and the variables achieving a P<0.05 in above tests were entered in the uni‐ and multivariate analysis to determine the independent risk factor for NA and TLR. Survival curves were constructed using Kaplan–Meier estimates and compared using the log‐rank test. Odds ratio and 95% CIs were calculated using the logistic regression model. The high‐sensitivity C‐reactive protein (hsCRP) value was multiplied by 100 before the logistic regression analysis. A receiver operating characteristic curve analysis was performed to determine the predictability (sensitivity and specificity) of the presence of NA from CUC level.
Results
Patient and Lesion Characteristics
Five‐hundred two patients (613 lesions) who were enrolled into the OCT registry between April 2011 and January 2017 were screened. Among them, 249 patients who met the inclusion criteria were identified as candidates for this study. Next, 42 of the 249 patients were excluded based on (1) implantation of the stent in the left main trunk (n=27) and (2) an insufficient quality of the OCT images (n=15). Finally, 207 consecutive patients (207 lesions) were enrolled, and their HDL was functionally evaluated by measuring the CUC at the time of follow‐up OCT. Median duration between stent implantation and follow‐up OCT was 24.5 months.
Overall, NA was identified in 37 patients (NA+ group), and the remaining 170 patients did not exhibit NA (NA− group) (Figure 3). In most of the NA+ group patients, NA was identified at the late phase (after 1 year but within 3 years; n=13) and at the very late phase (beyond 3 years; n=21), but NA was detected in 3 patients at the early phase (<1 year). Incidence of TLR was significantly higher in the NA+ group than in the NA− group (Figure 4). With regard to patient characteristics (Table 1), the duration between stent implantation and follow‐up OCT was significantly longer in the NA+ group than in the NA− group. hsCRP levels at baseline, and the hsCRP and low‐density lipoprotein‐cholesterol (LDL‐C) levels at follow‐up OCT were significantly higher in the NA+ group than in the NA− group. CUCs at follow‐up OCT were significantly lower in the NA+ group than in the NA− group (NA+ versus NA−: 18.4±4.30 versus 24.4 ± 6.26 arbitrary units; P<0.001). The change in serum hsCRP levels was greater in the NA+ group than in the NA– group. The 2 groups showed no other statistically significant differences in baseline patient and lesion characteristics.
Figure 3.
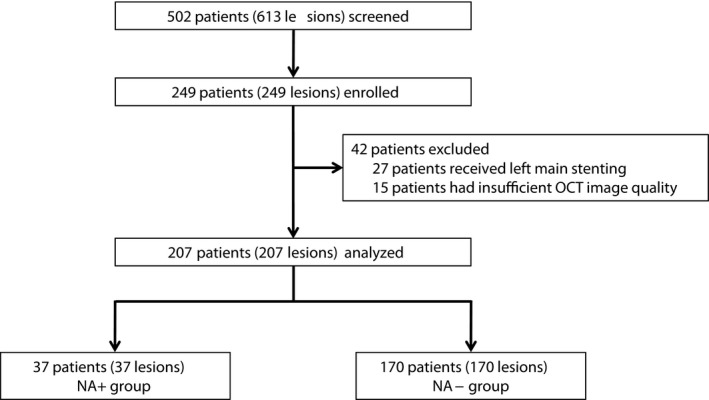
Study population. NA indicates neoatherosclerosis; OCT, optical coherence tomography.
Figure 4.
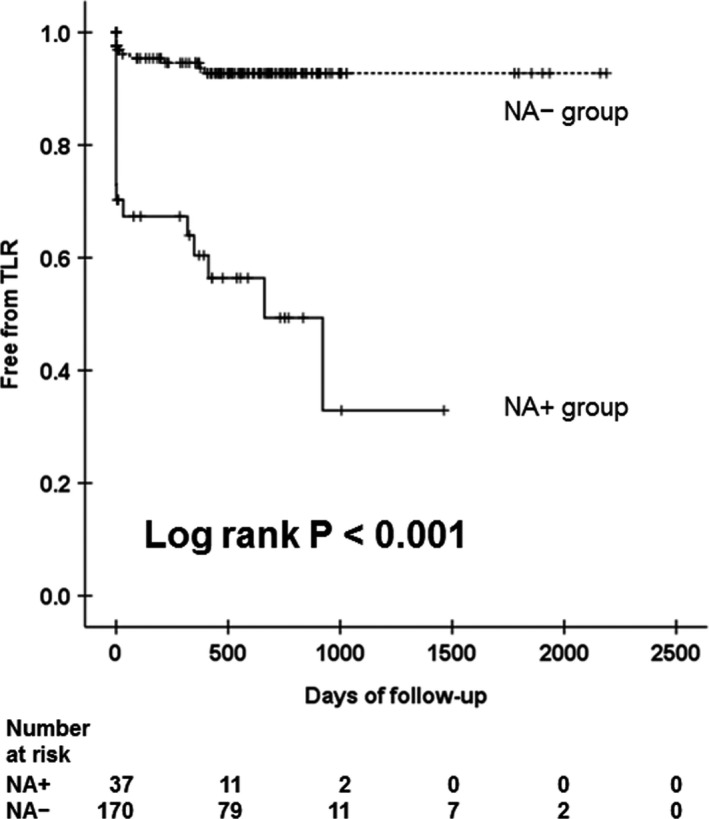
Association between neoatherosclerosis (NA) and target‐lesion revascularization (TLR). Kaplan–Meier survival curve of TLR stratified according to NA.
Table 1.
Baseline Patient and Lesion Characteristics
| Variable | NA+ (n=37) | NA− (n=170) | P Value |
|---|---|---|---|
| Clinical characteristics at follow‐up OCT | |||
| Age, y | 71.4±9.72 | 69.9±9.64 | 0.393 |
| Male | 29 (78.4) | 139 (81.8) | 0.633 |
| Duration between stent implantation and follow‐up OCT (mo) | 64.9±52.0 | 43.2±54.2 | 0.001 |
| Diabetes mellitus | 22 (59.5) | 82 (48.2) | 0.216 |
| Hypertension | 29 (78.4) | 138 (81.2) | 0.696 |
| Dyslipidemia | 34 (91.9) | 158 (92.9) | 0.523 |
| Smoking | 21 (56.8) | 89 (52.4) | 0.627 |
| Hemodialysis | 1 (2.7) | 5 (2.9) | 0.708 |
| Angina status | |||
| Stable angina pectoris | 21 (56.8) | 103 (60.6) | 0.667 |
| Unstable angina pectoris or acute coronary syndrome | 16 (43.2) | 67 (39.4) | |
| Medication at follow‐up OCT | |||
| Dual antiplatelet therapy | 24 (64.9) | 122 (71.8) | 0.404 |
| Statin | 34 (91.9) | 158 (92.9) | 0.523 |
| Rosuvastatin | 23 (67.6) | 87 (55.1) | |
| 2.5 mg | 15 (65.2) | 51 (58.6) | 0.742 |
| 5.0 mg | 5 (21.8) | 26 (29.9) | |
| 10 mg | 3 (13.0) | 10 (11.5) | |
| Atorvastatin | 4 (11.8) | 31 (19.6) | |
| 5.0 mg | 1 (25.0) | 5 (16.1) | 0.608 |
| 10 mg | 3 (75.0) | 17 (54.9) | |
| 20 mg | 0 (0) | 9 (29.0) | |
| Pitavastatin | 5 (14.7) | 35 (22.1) | |
| 1.0 mg | 2 (40.0) | 9 (25.7) | 0.789 |
| 2.0 mg | 3 (60.0) | 22 (62.9) | |
| 4.0 mg | 0 (0) | 4 (11.4) | |
| Pravastatin | 2 (5.9) | 5 (3.2) | |
| 5.0 mg | 2 (100) | 4 (80.0) | 0.714 |
| 10 mg | 0 (0) | 1 (20.0) | |
| ACE‐I and/or ARB | 27 (73.0) | 107 (62.9) | 0.247 |
| Beta‐blocker | 22 (59.5) | 100 (58.8) | 0.943 |
| EPA | 6 (16.2) | 20 (11.8) | 0.308 |
| Laboratory data at baseline | |||
| hsCRP, mg/dL | 0.21±0.23 | 0.18±0.34 | 0.007 |
| LDL‐C, mg/dL | 108.5±35.5 | 106.8±29.7 | 0.787 |
| Laboratory data at follow‐up OCT | |||
| hsCRP, mg/dL | 0.32±0.72 | 0.07±0.09 | <0.001 |
| Creatinine, mg/dL | 1.18±1.05 | 0.91±0.19 | 0.086 |
| HbA1c, % | 6.50±1.01 | 6.28±0.86 | 0.175 |
| Total cholesterol, mg/dL | 149.3±36.2 | 148.4±33.6 | 0.927 |
| HDL‐C, mg/dL | 43.4±10.6 | 47.3±12.9 | 0.092 |
| LDL‐C, mg/dL | 97.6±27.3 | 83.7±26.9 | 0.005 |
| Triglyceride, mg/dL | 143.9±87.0 | 134.9±72.1 | 0.509 |
| CUC, A.U. | 18.4±4.30 | 24.4±6.26 | <0.001 |
| The change of laboratory data (follow‐up OCT—baseline) | |||
| hsCRP, mg/dL | 0.12±0.80 | −0.11±0.31 | 0.043 |
| LDL‐C, mg/dL | −15.5±33.1 | −23.7±36.6 | 0.067 |
| Lesion and stent characteristics at index procedure | |||
| Lesion location | |||
| Left anterior descending artery | 19 (51.4) | 90 (52.9) | 0.229 |
| Left circumflex artery | 3 (8.1) | 30 (17.6) | |
| Right coronary artery | 15 (40.5) | 50 (29.5) | |
| Type of stent | |||
| Bare‐metal stent | 7 (18.9) | 21 (12.4) | 0.166 |
| Sirolimus‐eluting stent | 6 (16.2) | 13 (7.6) | |
| Paclitaxel‐eluting stent | 3 (8.1) | 7 (4.1) | |
| Biolimus‐eluting stent | 4 (10.8) | 28 (16.5) | |
| Everolimus‐eluting stent | 17 (46.0) | 101 (59.4) | |
| Mean stent size, mm | 3.12±0.31 | 3.09±0.40 | 0.619 |
| Total stent length, mm | 24.2±9.17 | 23.8±6.97 | 0.914 |
Values are presented as means±SD or absolute numbers (%). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; A.U., arbitrary units; CUC, cholesterol‐uptake capacity; EPA, eicosapentaenoic acid; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; NA, neoatherosclerosis; OCT, optical coherence tomography.
In terms of OCT findings (Table 2), the minimum lumen and stent areas did not differ significantly between groups. However, the macrophage accumulation was significantly higher in the NA+ group than in the NA− group. Other qualitative OCT findings, such as the detection of a thin‐cap fibroatheroma, thrombus, peri‐strut low‐intensity area, and vasa vasorum, were not significantly different between the 2 groups.
Table 2.
OCT Findings
| Variable | NA+ (n=37) | NA− (n=170) | P Value |
|---|---|---|---|
| Minimum lumen area, mm2 | 4.32±1.75 | 4.64±1.82 | 0.395 |
| Minimum stent area, mm2 | 6.13±2.02 | 6.09±2.20 | 0.926 |
| Incomplete stent apposition | 6 (16.2) | 24 (14.1) | 0.742 |
| Thin‐cap fibroatheroma | 1 (2.7) | 0 (0.0) | 0.179 |
| Macrophage accumulation | 37 (100) | 10 (5.9) | <0.001 |
| Thrombus | 1 (2.7) | 2 (1.2) | 0.448 |
| Peri‐strut low‐intensity area | 2 (5.4) | 26 (15.3) | 0.111 |
| Vasa vasorum | 5 (13.5) | 15 (8.8) | 0.273 |
Values are presented as means±SD or absolute numbers (%). NA indicates neoatherosclerosis; OCT, optical coherence tomography.
Clinical Risk Factors for NA
All patient and lesion characteristics were analyzed using uni‐ and multivariate logistic regression analyses to clarify the risk factors for NA (Table 3). A univariate analysis revealed that the duration between stent implantation and follow‐up OCT, hsCRP, and LDL‐C levels at follow‐up OCT were positively associated with NA, whereas CUC was negatively associated with NA. Moreover, a multivariate logistic regression analysis showed that increased hsCRP levels at follow‐up OCT, LDL‐C levels at follow‐up OCT, and decreased CUC were independently associated with NA (Table 3). Based on the receiver operating characteristic curve analysis, the optimal cut‐off value of CUC for the presence of NA was 20.89 arbitrary units, with a sensitivity of 81.1% and a specificity of 70.0% (area under the curve, 0.784; 95% CI, 0.710–0.859; Figure 5).
Table 3.
Uni‐ and Multivariate Logistic Regression Analysis of Clinical Parameters for Neoatherosclerosis
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 1.017 | 0.979 to 1.057 | 0.392 | 1.015 | 0.966 to 1.066 | 0.564 |
| Male | 0.808 | 0.337 to 1.938 | 0.634 | 0.523 | 0.172 to 1.588 | 0.253 |
| Duration between stent implantation and follow‐up OCT | 1.006 | 1.001 to 1.012 | 0.033 | 1.007 | 1.000 to 1.014 | 0.065 |
| hsCRP at follow‐up OCT | 1.064 | 1.027 to 1.103 | 0.001 | 1.044 | 1.010 to 1.080 | 0.011 |
| LDL‐C at follow‐up OCT | 1.017 | 1.004 to 1.030 | 0.009 | 1.018 | 1.002 to 1.035 | 0.025 |
| CUC | 0.814 | 0.749 to 0.886 | <0.001 | 0.799 | 0.725 to 0.879 | <0.001 |
CUC indicates cholesterol‐uptake capacity; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; OCT, optical coherence tomography; OR, odds ratio.
Figure 5.
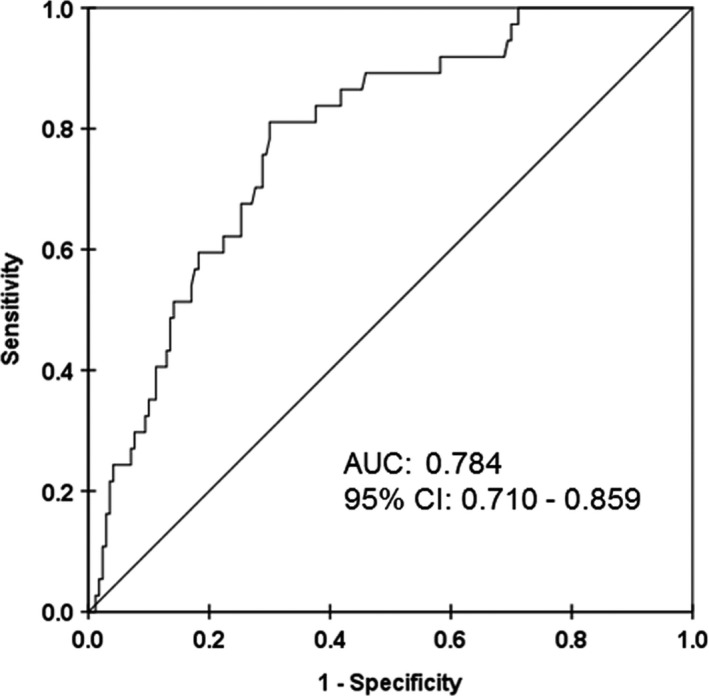
Receiver operating characteristic curve of the association between cholesterol‐uptake capacity and neoatherosclerosis. AUC indicates area under the curve.
Relationships Among CUC, NA, and TLR
CUC was lower in patients who suffered from TLR (n=27) than in patients who did not (n=180; TLR+ versus TLR−: 19.8±4.34 versus 23.9±6.47 arbitrary units; P<0.001). To examine potential risk factors for TLR, we evaluated the associations among patient characteristics, local OCT findings, and TLR by performing uni‐ and multivariate logistic regression analyses. A univariate analysis revealed that the duration between stent implantation and follow‐up OCT and bare‐metal stent use was positively associated with TLR and that increased CUC was negatively associated with TLR. A multivariate logistic regression analysis showed that CUC and bare‐metal stent use were independently associated with TLR (Table 4). With respect to OCT findings, a univariate analysis revealed that the presence of NA was positively associated with TLR. A multivariate logistic regression analysis showed that the presence of NA was the only finding independently associated with TLR (Table 5). Furthermore, the CUC exhibited significant negative associations with the lipid index and the macrophage grade in patients presenting NA (Figure 6).
Table 4.
Uni‐ and Multivariate Logistic Regression Analysis of Patient Characteristics for Target‐Lesion Revascularization
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 0.991 | 0.951 to 1.033 | 0.664 | 0.978 | 0.935 to 1.022 | 0.322 |
| Male | 1.388 | 0.451 to 4.271 | 0.568 | 1.204 | 0.363 to 3.993 | 0.762 |
| Duration between stent implantation and follow‐up OCT | 1.009 | 1.003 to 1.015 | 0.004 | 1.006 | 0.996 to 1.017 | 0.208 |
| CUC | 0.889 | 0.823 to 0.960 | 0.003 | 0.887 | 0.819 to 0.961 | 0.003 |
| Bare‐metal stent | 4.237 | 1.670 to 10.75 | 0.002 | 4.211 | 1.601 to 11.08 | 0.004 |
CUC indicates cholesterol‐uptake capacity; OCT, optical coherence tomography; OR, odds ratio.
Table 5.
Uni‐ and Multivariate Logistic Regression Analysis of OCT Findings for Target‐Lesion Revascularization
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Minimum lumen area | 0.760 | 0.556 to 1.039 | 0.086 | 0.653 | 0.412 to 1.037 | 0.071 |
| Minimum stent area | 0.955 | 0.778 to 1.172 | 0.660 | 1.212 | 0.845 to 1.738 | 0.297 |
| Incomplete stent apposition | 0.708 | 0.199 to 2.517 | 0.594 | 1.562 | 0.332 to 7.352 | 0.572 |
| Neoatherosclerosis | 13.60 | 5.480 to 33.75 | <0.001 | 12.78 | 4.521 to 36.12 | <0.001 |
| Peri‐strut low‐intensity area | 0.775 | 0.217 to 2.766 | 0.695 | 0.857 | 0.151 to 4.862 | 0.862 |
| Vasa vasorum | 1.783 | 0.548 to 5.798 | 0.337 | 1.241 | 0.235 to 6.564 | 0.799 |
OCT indicates optical coherence tomography; OR, odds ratio.
Figure 6.
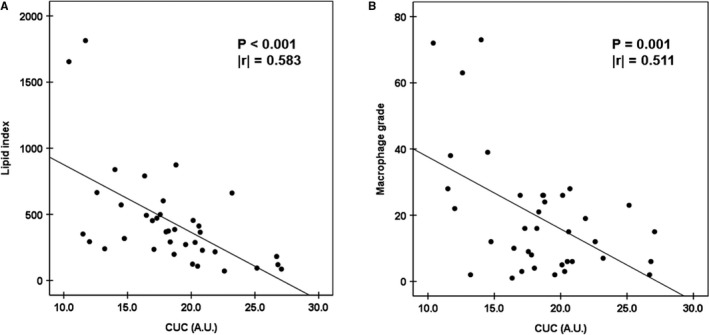
Statistical correlation between (A) cholesterol‐uptake capacity (CUC) and lipid index and (B) CUC and macrophage grade. A.U. indicates arbitrary units.
Subgroup Analysis of Patients Treated With Drug‐Eluting Stents
We conducted a subgroup analysis of patients treated with drug‐eluting stents (n=179, NA+: n=30, NA−: n=149) by excluding patients treated with bare‐metal stents (n=28). In this subgroup, the duration between stent implantation and follow‐up OCT was significantly longer in the NA+ group than in the NA− group. hsCRP levels at follow‐up OCT were significantly higher in the NA+ group than in the NA− group. CUC at follow‐up OCT was significantly lower in the NA+ group than in the NA− group (Table S1). In terms of OCT findings, macrophage accumulation was significantly higher in the NA+ group than in the NA− group (Table S2). A univariate analysis revealed that the duration between stent implantation and follow‐up OCT, hsCRP were positively associated with NA, whereas CUC was negatively associated with NA. A multivariate logistic regression analysis showed that duration between stent implantation and follow‐up OCT, increased hsCRP, and decreased CUC was independently associated with NA (Table S3). Additionally, decreased CUC and the presence of NA were independently associated with TLR (TLR+: n=18, TLR−: n=161) in this subgroup (Tables S4 and S5).
Subgroup Analysis for Cases With Matched Follow‐up Durations
To adjust follow‐up duration between the NA+ and NA– groups, we conducted a subgroup analysis of patients whose follow‐up period was between 1 and 5 years after stent implantation (n=97, NA+: n=16, NA−: n=81). In this subgroup, as observed using the full sample, hsCRP levels at follow‐up OCT were significantly higher in the NA+ group than in the NA− group. CUC at follow‐up OCT was significantly lower in the NA+ group than in the NA− group. Macrophage accumulation was significantly higher in the NA+ group than in the NA− group. On the other hand, there was no significant difference in follow‐up duration between the NA+ and NA− groups (27.1±10.9 versus 27.0±11.8, respectively; P=0.972; Tables S6 and S7). Furthermore, increased hsCRP levels and decreased CUC were independent risk factors for NA (Table S8). Additionally, decreased CUC and the presence of NA were independently associated with TLR (TLR+: n=8, TLR−: n=89) in this subgroup (Tables S9 and S10).
Discussion
Numerous epidemiological studies have shown that a decreased HDL‐C level is a key risk factor for coronary artery disease.21, 22, 23 However, previous randomized studies have failed to demonstrate the clinical efficacy of increasing plasma HDL‐C concentrations to reduce cardiovascular events.24 Recently, the importance of HDL functionality has become increasingly clear, particularly for development of coronary artery disease. However, no study to date has specifically evaluated the relationship between HDL‐C and in‐stent NA, although HDL‐C is an ideal agent for prevention of the initiation and progression of in‐stent NA because of to its atheroprotective effects through its ability to efflux cholesterol from plaque macrophages, inhibit low‐density lipoprotein oxidation, reduce expression of cell adhesion molecules, suppress chemokine expression, and reduce monocyte infiltration.25, 26 Our study, for the first time, reveals the potential relation between HDL functionality and NA in human long‐term OCT analysis. In addition, we demonstrated the relation between not only CUC and NA, but also between CUC and clinical outcome of TLR long term after stent implantation in a large‐scale OCT registry. Several key findings were obtained. (1) A decreased CUC was independently associated with the presence of NA (odds ratio, 0.799; P<0.001) in addition to LDL‐C and hsCRP, which have been reported as the risk factors for NA. (2) CUC showed significant inverse relationships with extent of NA and macrophage accumulation. (3) A decreased CUC was an independent risk factor for TLR in patients treated with coronary stents. (4) Detection of NA in follow‐up OCT images was independently associated with TLR. (5) These findings were consistent, irrespective of stent type (bare‐metal stents or drug‐eluting stents) and follow‐up duration.
HDL has various cardioprotective functions; for example, HDL mediates reverse cholesterol transport and exerts anti‐inflammatory and ‐oxidative effects.27, 28 Reverse cholesterol transport by cholesterol efflux from macrophages is regarded as one of the primary HDL functions that protects against atherosclerosis. The current standard index for assessing HDL functionality is cholesterol‐efflux capacity, which is inversely related to incidence of cardiovascular events in population‐based studies.7, 8 It improves the prediction of cardiovascular disease risk beyond that using conventional risk factors.9 So, as an alternative, we have developed the CUC assay, a novel plate‐assay system for assessing the CUC of HDL without using radioisotopes or cells. A recent validation study demonstrated the high reproducibility (coefficient of variation, 0.3–7.0%) and short processing time (<6 hour) of the assay as well as a significant correlation with the cholesterol‐efflux capacity using apoB‐depleted serum from human serum samples (n=30; r 2=0.47; P<0.001).10 Therefore, we propose that CUC can serve as a useful alternative functional index for evaluating the capacity of HDL to accept additional cholesterol.
In this study, we demonstrated that a decreased CUC is an independent risk factor for NA and TLR after stent implantation. Moreover, CUC remained statistically significant in a multivariate analysis, suggesting that the functional evaluation of HDL is important for risk stratification of patients treated with coronary stents. Although drug‐eluting stents markedly reduce the incidence of mid‐term TLR, in‐stent restenosis requiring TLR still occurs and remains an issue, particularly during the long‐term follow‐up of patients treated with coronary stents. Based on a recent postmortem study, one of the primary mechanisms underlying this adverse clinical event is NA progression within the neointima. Recently, high‐resolution OCT imaging has enabled the in vivo detection of NA. We have demonstrated that presence of OCT‐detected NA is a significant risk factor for TLR occurrence and major adverse cardiac events during a mean follow‐up of 4.24±2.31 years after stenting.3 Consistent with these previous findings, the results of this study showed that presence of NA is significantly associated with an increased incidence of TLR. Moreover, we found that in addition to increased LDL‐C and hsCRP levels, a decreased CUC was positively and independently associated with presence of NA. Although the detailed mechanisms are unclear, we believe that the reverse cholesterol transport mediated by HDL by cholesterol efflux from macrophages plays a central role in the relationships among decreased CUC, NA, and TLR. LDL‐C accumulation in the arterial wall is generally regarded as the initial step of atherosclerosis. The positive association between an increased LDL‐C and lipid index observed here are in accord with this view. Accumulated LDL‐C is then oxidized, which promotes the differentiation of monocytes into macrophages, and the macrophages take up the modified LDL‐C using scavenger receptors, followed by transformation into lipid‐filled foam cells, a hallmark of the initial stage of atherosclerosis. Conversely, HDL opposes this process by removing excess lipids from macrophages.5, 29, 30 Therefore, when HDL function declines, accumulation of foam cells proceeds, which can be detected by OCT. Accordingly, a significant negative relationship was detected between longitudinal extent of NA (assessed using the lipid index) and CUC. Moreover, a detailed OCT examination revealed a significant negative association between CUC and macrophage grade. Recent studies reported that removal of cholesterol from the plasma membrane by HDL might modulate the proinflammatory properties of macrophages. Furthermore, it has been reported that lipid rafts are cholesterol‐rich membrane microdomains and function as platforms for signal transduction, so disorganization of lipid rafts on macrophages can lead to attenuation of their inflammatory activation.31, 32 Therefore, we currently consider that dysfunction of cholesterol efflux from macrophage caused by impaired HDL functionality leads to weakening of the anti‐inflammatory effect and contributes to occurrence of NA in patients treated with coronary stents. Unfortunately, we cannot find the improvement method of HDL functionality yet, and it is one of the important future aims of our study. However, our results extend these previous findings to patients treated with coronary stents and suggest that decreased CUC could affect atherosclerotic progression within the neointima. Furthermore, we can measure CUC in a relatively short time, leading to the discovery of patients with the risk of occurrence of NA and residual risk for TLR. We believe that more‐active secondary prevention will lead to improved outcomes.
Our study had various limitations. First, this was a single‐center, nonrandomized study with a limited sample size. Thus, a potential risk of patient‐selection bias exists. Especially, potential impact of age and sex on our results remain uncertain. Although we performed a multivariate analysis, there was insufficient power to detect independent predictors for TLR owing to the limited sample size. Thus, we performed a receiver operating characteristic curve analysis to determine the predictability of the presence of NA based on CUC. Additionally, we performed a subgroup analysis to exclude the impact of bare‐metal stents and the difference in follow‐up duration. We found consistent associations among a decreased CUC, NA, and TLR, even in these subgroups. Second, we excluded patients for whom OCT examination was performed <6 months after stenting owing to insufficient neointimal proliferation for assessing atherogenic changes at this early stage. Therefore, our data were limited to evaluation of the association between CUC and OCT findings in the mid‐to‐late phase after stenting (>6 months). Moreover, because it takes a certain period of time from stent implantation to NA occurrence,33 it is difficult to confirm that the patients in the NA− arm by early‐phase OCT could have been diagnosed if the follow‐up OCT was done at a later time. Third, we did not validate the OCT findings with histological findings; therefore, the regions identified as NA were not confirmed to contain atherosclerotic changes and to be within the neointima. However, because previous pathological studies have clearly demonstrated that OCT exhibits high diagnostic accuracy for detection of lipidic components,2 we believe that our findings provide valuable information. Fourth, given that this is an experimental observational study, it is difficult to identify the underlying mechanisms of four findings. Further study is required to elucidate the underlying mechanisms of our findings. Finally, although we found a significant relation among CUC, NA, and TLR, we still do not know whether CUC truly regulates TLR. A prospective, randomized study is warranted to see whether improving CUC can truly reduce TLR.
In conclusion, impaired HDL functionality, which promotes cholesterol efflux from macrophages, evaluated based on a decreased CUC, was independently associated with NA and TLR. A larger‐scale, prospective, randomized study is warranted to further clarify whether improving CUC can truly regulate TLR. Also, identification of the underlying mechanism of these associations is warranted.
Funding of Sources
Research funding is provided by Ken‐ichi Hirata, Sysmex Corporation, and Grant‐in‐Aid for Scientific Research (C) 25461085.
Disclosures
Harada, Murakami, and Kiriyama belong to Sysmex Corporation. Toh and Irino belong to The Division of Evidence‐based Laboratory Medicine, Kobe University Graduate School of Medicine, which is established by the endowment fund from the Sysmex Corporation. There is no declaration of consultant or advisory role. There is no declaration of stock ownership and honoraria. There is no declaration of expert testimony and patents.
Supporting information
Table S1. Baseline Patient and Lesion Characteristics in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S2. OCT Findings in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S3. Uni‐ and Multivariate Logistic Regression Analysis of Clinical Parameters for Neoatherosclerosis in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S4. Uni‐ and Multivariate Logistic Regression Analysis of Patient Characteristics for Target‐Lesion Revascularization in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S5. Uni‐ and Multivariate Logistic Regression Analysis of OCT Findings for Target‐Lesion Revascularization in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S6. Baseline Patient and Lesion Characteristics in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S7. OCT Findings in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S8. Uni‐ and Multivariate Logistic Regression Analysis of Clinical Parameters for Neoatherosclerosis in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S9. Uni‐ and Multivariate Logistic Regression Analysis of Patient Characteristics for Target‐Lesion Revascularization in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S10. Uni‐ and Multivariate Logistic Regression Analysis of OCT Findings for Target‐Lesion Revascularization in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
(J Am Heart Assoc. 2019;8:e011975 DOI: 10.1161/JAHA.119.011975.)
References
- 1. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, Belardi J, Sigwart U, Colombo A, Goy JJ, van den Heuvel P, Delcan J, Morel MA. A comparison of balloon‐expandable‐stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36:2147–2159. [DOI] [PubMed] [Google Scholar]
- 3. Kuroda M, Otake H, Shinke T, Takaya T, Nakagawa M, Osue T, Taniguchi Y, Iwasaki M, Nishio R, Kinutani H, Konishi A, Hirata K. The impact of in‐stent neoatherosclerosis on long‐term clinical outcomes: an observational study from the Kobe University Hospital optical coherence tomography registry. EuroIntervention. 2016;12:e1366–e1374. [DOI] [PubMed] [Google Scholar]
- 4. Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, Lee SW, Kim YH, Whan Lee C, Park SW, Park SJ. Optical coherence tomographic analysis of in‐stent neoatherosclerosis after drug‐eluting stent implantation. Circulation. 2011;123:2954–2963. [DOI] [PubMed] [Google Scholar]
- 5. Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan‐Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(suppl):S189–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothblat GH, de la Llera‐Moya M, Atger V, Kellner‐Weibel G, Williams DL, Phillips MC. Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- 8. Khera AV, Cuchel M, de la Llera‐Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high‐density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harada A, Toh R, Murakami K, Kiriyama M, Yoshikawa K, Miwa K, Kubo T, Irino Y, Mori K, Tanaka N, Nishimura K, Ishida T, Hirata K. Cholesterol uptake capacity: a new measure of HDL functionality for coronary risk assessment. J Appl Lab Med. 2017;2:186–200. [DOI] [PubMed] [Google Scholar]
- 11. Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. [DOI] [PubMed] [Google Scholar]
- 12. Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review: clinical and research applications. JACC Cardiovasc Interv. 2009;2:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsumoto D, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, Sawada T, Paredes OL, Hirata K, Yokoyama M. Neointimal coverage of sirolimus‐eluting stents at 6‐month follow‐up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–967. [DOI] [PubMed] [Google Scholar]
- 14. Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, Mizuno K. Appearance of lipid‐laden intima and neovascularization after implantation of bare‐metal stents: extended late‐phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol. 2009;55:26–32. [DOI] [PubMed] [Google Scholar]
- 15. Lee SY, Shin DH, Mintz GS, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Optical coherence tomography‐based evaluation of in‐stent neoatherosclerosis in lesions with more than 50% neointimal cross‐sectional area stenosis. EuroIntervention. 2013;9:945–951. [DOI] [PubMed] [Google Scholar]
- 16. Xie Z, Tian J, Ma L, Du H, Dong N, Hou J, He J, Dai J, Liu X, Pan H, Liu Y, Yu B. Comparison of optical coherence tomography and intravascular ultrasound for evaluation of coronary lipid‐rich atherosclerotic plaque progression and regression. Eur Heart J Cardiovasc Imaging. 2015;16:1374–1380. [DOI] [PubMed] [Google Scholar]
- 17. Tahara S, Morooka T, Wang Z, Bezerra HG, Rollins AM, Simon DI, Costa MA. Intravascular optical coherence tomography detection of atherosclerosis and inflammation in murine aorta. Arterioscler Thromb Vasc Biol. 2012;32:1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otake H, Shite J, Ako J, Shinke T, Tanino Y, Ogasawara D, Sawada T, Miyoshi N, Kato H, Koo BK, Honda Y, Fitzgerald PJ, Hirata K. Local determinants of thrombus formation following sirolimus‐eluting stent implantation assessed by optical coherence tomography. JACC Cardiovasc Interv. 2009;2:459–466. [DOI] [PubMed] [Google Scholar]
- 19. Otake H, Shite J, Ikeno F, Shinke T, Teramoto T, Miyoshi N, Ako J, Honda Y, Fitzgerald PJ, Hirata K. Evaluation of the peri‐strut low intensity area following sirolimus‐and paclitaxel‐eluting stents implantation: Insights from an optical coherence tomography study in humans. Int J Cardiol. 2012;157:38–42. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia‐Garcia HM, van Soest G, van der Giessen W, Regar E. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–293. [DOI] [PubMed] [Google Scholar]
- 21. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. [DOI] [PubMed] [Google Scholar]
- 22. Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA. High‐density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. [DOI] [PubMed] [Google Scholar]
- 23. Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB; Veterans Affairs High‐Density Lipoprotein Intervention Trial . Relation of gemfibrozil treatment and lipid levels with major coronary events: VA‐HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 25. Wu BJ, Li Y, Ong KL, Sun Y, Shrestha S, Hou L, Johns D, Barter PJ, Rye KA. Reduction of In‐stent restenosis by cholesteryl ester transfer protein inhibition. Arterioscler Thromb Vasc Biol. 2017;37:2333–2341. [DOI] [PubMed] [Google Scholar]
- 26. Vanags LZ, Wong NKP, Nicholls SJ, Bursill CA. High‐Density Lipoproteins and Apolipoprotein A‐I Improve Stent Biocompatibility. Arterioscler Thromb Vasc Biol. 2018;38:1691–1701. [DOI] [PubMed] [Google Scholar]
- 27. deGoma EM, deGoma RL, Rader DJ. Beyond high‐density lipoprotein cholesterol levels: evaluating high‐density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eren E, Yilmaz N, Aydin O. High density lipoprotein and it's dysfunction. Open Biochem J. 2012;6:78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhatt A, Rohatgi A. HDL cholesterol efflux capacity: cardiovascular risk factor and potential therapeutic target. Curr Atheroscler Rep. 2016;18:2. [DOI] [PubMed] [Google Scholar]
- 30. Terasaka N, Wang N, Yvan‐Charvet L, Tall AR. High‐density lipoprotein protects macrophages from oxidized low‐density lipoprotein‐induced apoptosis by promoting efflux of 7‐ketocholesterol via ABCG1. Proc Natl Acad Sci USA. 2007;104:15093–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yvan‐Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter‐deficient macrophages: free cholesterol accumulation, increased signaling via toll‐like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Parks JS. Macrophage ABCA1 reduces MyD88‐dependent toll‐like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare‐metal and drug‐eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Patient and Lesion Characteristics in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S2. OCT Findings in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S3. Uni‐ and Multivariate Logistic Regression Analysis of Clinical Parameters for Neoatherosclerosis in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S4. Uni‐ and Multivariate Logistic Regression Analysis of Patient Characteristics for Target‐Lesion Revascularization in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S5. Uni‐ and Multivariate Logistic Regression Analysis of OCT Findings for Target‐Lesion Revascularization in a Subgroup of 179 Patients Treated With Drug‐Eluting Stents
Table S6. Baseline Patient and Lesion Characteristics in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S7. OCT Findings in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S8. Uni‐ and Multivariate Logistic Regression Analysis of Clinical Parameters for Neoatherosclerosis in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S9. Uni‐ and Multivariate Logistic Regression Analysis of Patient Characteristics for Target‐Lesion Revascularization in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation
Table S10. Uni‐ and Multivariate Logistic Regression Analysis of OCT Findings for Target‐Lesion Revascularization in a Subgroup of 97 Patients Whose Follow‐up Period Was Between 1 and 5 Years After Stent Implantation


