Abstract
Background
Silent brain infarcts (SBI) are increasingly being recognized as an important complication of cardiac procedures as well as a potential surrogate marker for studies on brain injury. The extent of subclinical brain injury is poorly defined.
Methods and Results
We conducted a systematic review and meta‐analysis utilizing studies of SBIs and focal neurologic deficits following cardiac procedures. Our final analysis included 42 studies with 49 separate intervention groups for a total of 2632 patients. The prevalence of SBIs following transcatheter aortic valve implantation was 0.71 (95% CI 0.64‐0.77); following aortic valve replacement 0.44 (95% CI 0.31‐0.57); in a mixed cardiothoracic surgery group 0.39 (95% CI 0.28‐0.49); coronary artery bypass graft 0.25 (95% CI 0.15‐0.35); percutaneous coronary intervention 0.14 (95% CI 0.10‐0.19); and off‐pump coronary artery bypass 0.14 (0.00‐0.58). The risk ratio of focal neurologic deficits to SBI in aortic valve replacement was 0.22 (95% CI 0.15‐0.32); in off‐pump coronary artery bypass 0.21 (95% CI 0.02‐2.04); with mixed cardiothoracic surgery 0.15 (95% CI 0.07‐0.33); coronary artery bypass graft 0.10 (95% CI 0.05‐0.18); transcatheter aortic valve implantation 0.10 (95% CI 0.07‐0.14); and percutaneous coronary intervention 0.06 (95% CI 0.03‐0.14). The mean number of SBIs per patient was significantly higher in the transcatheter aortic valve implantation group (4.58 ± 2.09) compared with both the aortic valve replacement group (2.16 ± 1.62, P=0.03) and the percutaneous coronary intervention group (1.88 ± 1.02, P=0.03).
Conclusions
SBIs are a very common complication following cardiac procedures, particularly those involving the aortic valve. The high frequency of SBIs compared with strokes highlights the importance of recording this surrogate measure in cardiac interventional studies. We suggest that further work is required to standardize reporting in order to facilitate the use of SBIs as a routine outcome measure.
Keywords: cardiac surgery, magnetic resonance imaging, silent brain infarction, transapical aortic valve implantation
Subject Categories: Ischemic Stroke, Cardiovascular Surgery, Catheter-Based Coronary and Valvular Interventions, Cognitive Impairment, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
Silent brain infarcts are strongly associated with cardiac procedures, particularly those involving the aortic valve.
What Are the Clinical Implications?
Silent brain infarcts present as a potentially important surrogate marker for brain injury in studies of cardiac procedures.
Introduction
Stroke after cardiac surgery is one of the most devastating outcomes for both patients and doctors. It is considered one of the most significant complications perioperatively, but the risk of clinically evident stroke remains low. In conventional coronary artery bypass graft (CABG) the rate of stroke approaches 2%, whereas in anaortic off‐pump coronary artery bypass (OPCAB), the rate has been reported at less than 0.4%.1 For aortic valve replacement procedures, stroke rates are higher, recently reported as 5.1% for surgical aortic valve replacement (AVR) and 5.3% in transcatheter aortic valve implantation (TAVI).2 There is, however, significant variability related to the risk level of individual study populations.3
Acute brain injury after cardiac surgery exists on a broad spectrum ranging from major stroke to subclinical brain injury, which includes postoperative cognitive dysfunction (POCD) and silent brain infarcts (SBI). SBIs are clinically silent, radiologically diagnosed infarcts, and although they can be defined by a number of magnetic resonance imaging (MRI) sequences, density‐weighted imaging (DWI) is the preferred technique due to its ability to demonstrate small ischemic lesions as bright hyperintensities that are evident within a few hours of onset of ischemia and generally disappear within 14 days.4, 5
Although the initial insult is clinically silent, SBIs have been linked to significant morbidity. The risk of subsequent stroke has been shown to increase more than 5 times when SBIs are present,6 which may simply reflect associated undescribed risk factors or possibly indicate an associated reduced threshold for further cerebral ischemic injury. Additional associated sequelae include cognitive dysfunction, increased risk of dementia,7 psychiatric disturbances,8 and reduced quality of life.
Subtle POCD has been a known but poorly defined complication of cardiac procedures, and although an association with SBIs has been hypothesized, the evidence to date is inconclusive, with conflicting data presented across a number of relatively small studies.9 A difficulty faced in quantifying the true incidence of POCD and SBI is the wide variation in both the definitions used for POCD and the radiological diagnostic techniques used to measure SBIs, respectively.10, 11
SBIs may have a role as a tool to measure brain injury post–cardiac surgery as the incidence of SBIs postoperatively is far greater than that of overt stroke.9 SBIs may therefore have a role as a standard outcome measure in cardiovascular interventional trials. The purpose of this systematic study and meta‐analysis was: (1) to report the prevalence of SBI following common cardiac procedures using standardized criteria, with prevalence defined as the proportion of this postoperative population who are noted to have DWI evidence of SBIs within the first 14 days; (2) to evaluate the effect of procedural and patient‐related risk factors; (3) to test if SBI frequency is proportionally related to the incidence of postoperative stroke, thus adding weight to the use of SBI as a more sensitive marker for procedure‐related brain injury; and (4) to assess the association between POCD and SBIs.
Methods
The authors declare that all supporting data are available within the article.
Search Strategy
Electronic databases PubMed and Google Scholar were searched for relevant studies. Search terms included “cardiac surgery,” “cardiac surgical procedures,” “coronary artery bypass,” “aortic valve,” “replantation,” “transcatheter aortic valve replacement,” “percutaneous coronary intervention,” “silent,” “brain infarction,” “DWI lesions,” “magnetic resonance imaging,” and “brain injury.” Reference lists of appropriate studies were also examined for relevant literature.
Inclusion Criteria
Studies were included that specifically utilized DWI in the early postoperative period following cardiac procedures to assess for acute cerebral ischemic lesions. Inclusion criteria included (1) DWI following open cardiac surgical procedures (CABG, OPCAB, AVR, mitral valve repair (MVR), and mixed procedures), TAVI, and percutaneous coronary intervention (PCI); (2) MRI performed within 14 days of the procedure; (3) assessment for focal neurologic deficits indicating stroke or transient ischemic attack (TIA), performed postoperatively; (4) age >18 years; and (5) English language studies.
Of the included studies, only patients who had postoperative DWI imaging were included in the analysis.
Studies comparing the use of embolic protection devices during TAVI were excluded, as were studies utilizing other imaging techniques such as susceptibility‐weighted MRI or gradient‐echo MRI.
Data Extraction
Extraction included first author and first author/corresponding author's institution to avoid the potential of including a single cohort twice. Data points included baseline characteristics, total number of patients scanned, total number of patients with new postoperative SBIs, number of SBIs per patient, and early postoperative focal neurologic deficit. When reported, the volume of individual lesions and the total lesion load per patient were collected. Neurocognitive testing and subsequent results were also included to assess for a potential correlation between imaging findings and neurocognitive decline.
Statistical Analyses
For studies that reported data as median and range,12, 13, 14, 15, 16, 17, 18 a technique described by Hozo et al19 was utilized to calculate an estimate of the mean and SD. For data reported as median and IQR,20, 21 the mean and SD were estimated according to calculations per Luo et al22 and Wan et al,23 respectively. For comparison of means 1‐way ANOVA was performed with post hoc Tukey honestly significant difference (HSD) utilized for specific significance values reported. SPSS v23 (IBM, Armonk, NY) and interactive statistics24 were used for descriptive statistics and comparison of means.
A meta‐analysis of prevalence was performed for both SBI and focal neurologic deficits (FND) in addition to a meta‐analysis estimating the risk ratio between SBI and FND. Both were performed on the Microsoft Excel plug‐in, MetaXL (www.epigear.com; Sunrise Beach, Queensland, Australia). Because the multiple procedural groups in our analysis displayed a high heterogeneity, the inverse variance heterogeneity estimate was utilized. This method of meta‐analysis has been proposed as a replacement for the random effects method due to its ability to more accurately estimate statistical error and to provide more valid CIs.25 Publication bias is demonstrated by Funnel plots.
Author B.I. had full access to the collected study data and takes responsibility for data integrity and integrity of the data analysis. The requirement for informed consent by subjects was waived for this study type.
Results
A total of 902 studies were initially identified, from which 42 studies were included in this analysis (Figure 1). These comprised 49 separate intervention groups: 7 AVR, 9 CABG, 2 OPCAB, 5 mixed cardiothoracic surgery (CTSx; studies that did not differentiate among CABG, AVR, mitral valve repair, Tricuspid valve repair, and combined valve replacement and coronary artery bypass procedures), 16 TAVI, and 10 PCI. Taken together they accounted for a total of 2632 patients, of whom 951 patients were identified to have new postoperative SBI, and 67 patients were found to have an early postoperative FND.
Figure 1.
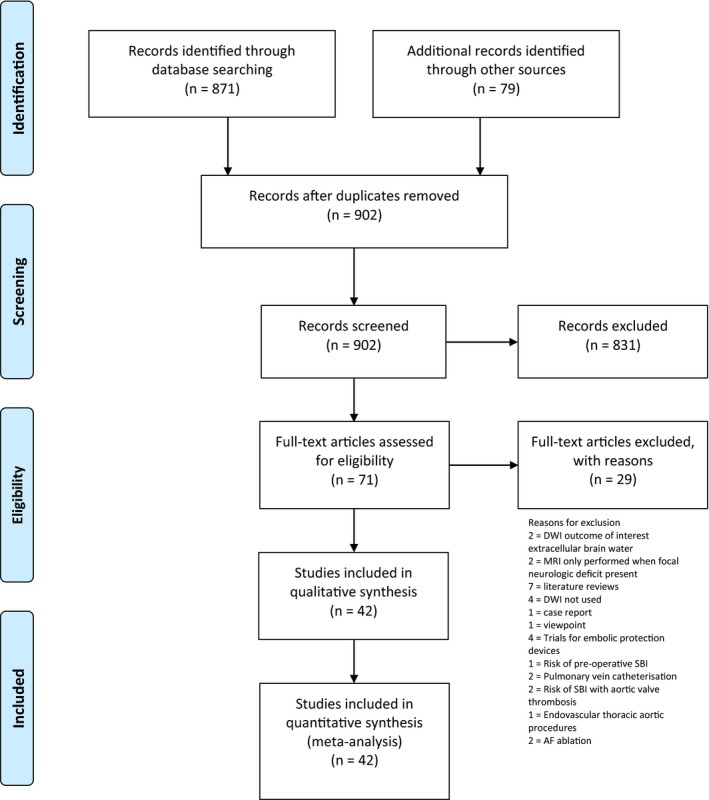
PRISMA flow diagram. AF indicates atrial fibrillation; DWI, density‐weighted imaging; MRI, magnetic resonance imaging; SBI, silent brain infarct.
All included studies were prospective cohort studies and used DWI to identify new cerebral ischemic lesions (Table 1).26, 27, 28, 29, 30, 31, 32, 33 Imaging was performed within the first 14 postoperative days in all patients, with the majority of patients being scanned within the first postoperative week. Thirty‐one studies were performed with 1.5T MRI, and 4 studies with 3T MRI; 7 studies did not report on the MRI magnetic field strength.
Table 1.
Included Study Characteristics
| Author | Year | Procedure | Institution | Country | Study Design | No. Participants (Postoperative MRI) | SBI | FND | MRI Strength | MRI Follow‐Up (Postoperative Day) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kahlert13 | 2010 | TAVI | University hospital, Essen | Germany | Prospective cohort compared with retrospective cohort | 32 | 27 | 0 | 1.5T | 2 to 5 |
| Ghanem14 | 2010 | TAVI | University of Bonn | Germany | Prospective cohort | 22 | 16 | 2 | 1.5T | 2.2±0.4 |
| Arnold26 | 2010 | TAVI | University Hospital Erlangen | Germany | Prospective cohort | 25 | 17 | 2 | 1.5T | 6±2 |
| Rodés‐Cabau27 | 2011 | TAVI | Laval University | Canada | Prospective cohort | 60 | 41 | 2 | 1.5T | 4±1 |
| Astarci28 (group 1) | 2011 | TAVI | University Hospital Saint‐Luc | Belgium | Prospective cohort | 35 | 32 | 0 | 3T | 2 to 5 |
| Fairbarin29 | 2012 | TAVI | University of Leeds | UK | Prospective cohort | 31 | 24 | 2 | 1.5T | 5±1.55 |
| Ghanem30 | 2012 | TAVI | University of Bonn | Germany | Prospective cohort | 39 | 28 | 4 | 1.5T | 3±1 |
| Ghanem16 | 2013 | TAVI | University of Bonn | Germany | Prospective cohort | 56 | 36 | 0 | 1.5T | 3±1 |
| Alassar12 (group 2) | 2015 | TAVI | St Georges Hospital, London | UK | Prospective cohort | 62 | 47 | 1 | NA | 6 |
| Samim31 | 2015 | TAVI | University medical centre, Utrecht | The Netherlands | Prospective cohort | 42 | 38 | 1 | 3T | 1 to 5 |
| Uddin17 (group 2) | 2015 | TAVI | University of Leeds | UK | Prospective cohort | 70 | 54 | 2 | 1.5T | 1 to 7 |
| Altisent18 (group 2) | 2016 | TAVI | Universitat Autònoma de Barcelona | Spain | Prospective cohort | 40 | 18 | 0 | 1.5T | 6.5±3.5 |
| Lansky20 | 2016 | TAVI | Yale University School of Medicine | USA | Prospective cohort | 34 | 32 | 8 | NA | 4±2 |
| Fanning21 | 2016 | TAVI | The Prince Charles Hospital | Australia | Prospective cohort | 30 | 18 | 1 | 1.5T | 3±1 |
| Ghanem32 | 2017 | TAVI | University Hospital Bonn | Germany | Prospective cohort | 27 | 17 | 0 | NA | 1 to 3 |
| Knipp33 | 2013 | TAVI | University Hospital, Essen | Germany | Prospective cohort | 28 | 7 | 0 | 1.5T | Predischarge |
| Hamon34 | 2007 | PCI | University Hospital of Caen | France | Prospective cohort | 41 | 2 | 0 | 1.5T | 1 |
| Murai35 | 2008 | PCI | Osaka Medical College | Japan | Prospective cohort | 101 | 26 | 0 | 3T | 3±1 |
| Schwarz15 (Group 1) | 2011 | PCI | Justus Liebig University Giessen | Germany | Prospective cohort | 75 | 1 | 0 | 1.5T | 2 to 4 |
| Deveci36 | 2016 | PCI | Cukurova University | Turkey | Prospective cohort | 30 | 12 | 0 | 1.5T | 1 |
| Lund37 | 2005 | PCI | Rikshospitalet University Hospital | Norway | Prospective cohort | 42 | 5 | 1 | 1.5T | 1 |
| Busing38 | 2005 | PCI | University Hospital Mannheim | Germany | Prospective cohort | 48 | 7 | 0 | 1.5T | 1 to 2 |
| Ohi39 | 2013 | PCI | Gifu Uni versity Graduate School of Medicine | Japan | Prospective cohort | 111 | 20 | 0 | 1.5T | 1 to 7 |
| Hamon40 | 2012 | PCI | University Hospital of Caen | France | Prospective cohort | 160 | 24 | 2 | 1.5T | 1 to 2 |
| Kim41 | 2012 | PCI | University of Ulsan College of Medicine | Republic of Korea | Retrospective cohort | 272 | 45 | 0 | NA | 1 to 7 |
| Kim42 | 2011 | PCI | Keimyung University Dongsan Medical Center | Republic of Korea | Prospective cohort | 197 | 20 | 0 | 3T | 1 to 1 |
| Friday43 | 2005 | OPCAB | Lankenau Hospital | USA | Prospective cohort | 16 | 5 | 0 | 1.5T | 4 to 14 |
| Djaiani44 (group 2) | 2006 | OPCAB | University of Toronto | Canada | Case‐control | 13 | 0 | 0 | NA | 3 to 7 |
| Folyd45 (group 2) | 2006 | Mixed CTSx | Hospital of the University of Pennsylvania | USA | Prospective cohort | 34 | 0 | 0 | 1.5T | 6±2 |
| Cook46 | 2007 | Mixed CTSx | Hospital of the University of Pennsylvania | USA | Prospective cohort | 50 | 16 | 4 | 1.5T | 4.5±1.5 |
| Barber47 | 2008 | Mixed CTSx | University of Auckland | New Zealand | Prospective cohort | 36 | 15 | 1 | 1.5T | 1 to 5 |
| Knipp48 | 2017 | Mixed CTSx | University hospital, Essen | Germany | Prospective cohort compared with retrospective cohort | 36 | 19 | 0 | 1.5T | Predischarge |
| Knipp49 | 2005 | Mixed CTSx | University Clinic of Essen | Germany | Prospective cohort | 30 | 14 | 0 | 1.5T | 5.0±1.4 |
| Bendszus50 | 2002 | CABG | University of Wurzburg | Germany | Prospective cohort | 35 | 9 | 0 | 1.5T | 3 |
| Restrepo51 | 2002 | CABG | Johns Hopkins | USA | Prospective cohort | 13 | 4 | 1 | NA | 4±1.6 |
| Knipp52 | 2004 | CABG | University Hospital, Essen | Germany | Prospective cohort | 29 | 13 | 0 | 1.5T | Predischarge |
| Djaiani53 | 2004 | CABG | Toronto General Hospital | Canada | Prospective cohort | 50 | 8 | 1 | NA | 3 to 7 |
| Djaiani44 (group 1) | 2006 | CABG | University of Toronto | Canada | Case‐control | 13 | 8 | 1 | NA | 3 to 7 |
| Knipp54 | 2008 | CABG | University clinic of Essen | Germany | Prospective cohort | 39 | 20 | 0 | 1.5T | Predischarge |
| Schwarz15 (group 2) | 2011 | CABG | Justus Liebig University Giessen | Germany | Prospective cohort | 39 | 7 | 0 | 1.5T | 2 to 4 |
| Nah55 | 2014 | CABG | University of Ulsan College of Medicine | South Korea | Prospective cohort | 127 | 35 | 4 | 1.5T | 3 |
| Gerriets56 | 2010 | CABG | Justus Liebig University Giessen | Germany | Prospective cohort | 86 | 13 | 0 | 1.5T | 1 to 3 |
| Stolz57 | 2004 | AVR | Kerckhoff Klinik | Germany | Prospective cohort | 14 | 3 | NA | 1 to 6 | |
| Folyd45 (group 1) | 2006 | AVR | Hospital of the University of Pennsylvania | USA | Prospective cohort | 37 | 6 | 2 | 1.5T | 6±2 |
| Astarci28 (group 2) | 2011 | AVR | University Hospital Saint‐Luc | Belgium | Prospective cohort | 13 | 1 | 0 | 3T | 2 to 5 |
| Alassar12 (Group 1) | 2015 | AVR | St Georges Hospital, London | UK | Prospective cohort | 32 | 23 | 1 | NA | 6 |
| Uddin17 (group 1) | 2015 | AVR | University of Leeds | UK | Prospective cohort | 38 | 17 | 1 | 1.5T | 1 to 7 |
| Altisent18 (group 1) | 2016 | AVR | Universitat Autònoma de Barcelona | Spain | Prospective cohort | 27 | 11 | 0 | 1.5T | 9±3 |
| Messe58 | 2015 | AVR | Hospital of the University of Pennsylvania | USA | Prospective cohort | 129 | 79 | 20 | 1.5T | 6.35±2.25 |
AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; FND, focal neurological deficit; Mixed CTSx, mixed cardiothoracic surgical group; MRI, magnetic resonance imaging; NA, not available; OPCAB, off‐pump coronary artery bypass; PCI, percutaneous coronary intervention; SBI, silent brain infarct; TAVI, transcatheter aortic valve implantation.
Baseline characteristics of each procedural group are demonstrated in Table 2. The TAVI group was significantly older than all other groups, as was the AVR group (with the exception of the TAVI group being older still). The mean age of patients with new postoperative DWI lesions was older than those without, although this difference was nonsignificant (71.94 ± 7.27 years versus 66.51 ± 9.48 years, P=0.08).
Table 2.
Baseline Characteristics
| 1. CABG | 2. OPCAB | 3. AVR | 4. TAVI | 5. Mixed CTSx | 6. PCI | P‐Value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 vs 2 | 1 vs 3 | 1 vs 4 | 1 vs 5 | 1 vs 6 | 2 vs 3 | 2 vs 4 | 2 vs 5 | 2 vs 6 | 3 vs 4 | 3 vs 5 | 3 vs 6 | 4 vs 5 | 4 vs 6 | 5 vs 6 | |||||||
| Age, y | 65.51±8.06 | 70.3±6.4 | 70.76±9.31 | 81.76±5.76 | 66.44±10.54 | 67.13±9.8 | 0.05 | <0.01 | <0.01 | 0.84 | 0.02 | >0.99 | 0.00 | 0.23 | 0.39 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.92 |
| Male, % | 79.6 | 78.37 | 62.76 | 49.97 | 69.61 | 70.3 | >0.99 | 0.24 | <0.01 | 0.74 | 0.06 | 0.62 | 0.03 | 0.95 | 0.83 | 0.41 | 0.96 | 0.99 | 0.06 | 0.03 | >0.99 |
| Smoking, % | 42.9 | 12.5 | 36.6 | 21.91 | 44.41 | 41.17 | NR | 0.99 | 0.35 | >0.99 | >0.99 | NR | NR | NR | NR | 0.82 | 0.99 | >0.99 | 0.37 | 0.26 | >0.99 |
| HTN, % | 75.25 | 68.27 | 71.93 | 79.5 | 52.6 | 57.6 | >0.99 | >0.99 | >0.99 | 0.45 | 0.52 | >0.99 | 0.97 | 0.93 | 0.98 | 0.99 | 0.76 | 0.87 | 0.19 | 0.18 | >0.99 |
| Prior CVA, % | 9.36 | 12.5 | 11.23 | 19.78 | 11.18 | 9.75 | NR | 0.53 | >0.99 | >0.99 | >0.99 | NR | NR | NR | NR | 0.7 | >0.99 | >0.99 | 0.56 | 0.19 | >0.99 |
| Diabetes mellitus, % | 34.72 | 40.38 | 22.37 | 28.77 | 14.4 | 35.18 | 0.98 | 0.53 | 0.86 | 0.44 | >0.99 | 0.94 | 0.68 | 0.06 | 0.99 | 0.93 | 0.9 | 0.44 | 0.19 | 0.76 | 0.02 |
| Cholesterol, % | 65 | 67.31 | 62.75 | 63.54 | 52.19 | 45.31 | >0.99 | >0.99 | >0.99 | 0.98 | 0.75 | >0.99 | >0.99 | 0.98 | 0.87 | >0.99 | >0.99 | 0.91 | 0.98 | 0.67 | >0.99 |
| Preexisting AF, % | 5.81 | NA | 21.93 | 37.17 | 24.75 | 16.62 | NR | 0.71 | 0.05 | 0.49 | 0.86 | NR | NR | NR | NR | 0.56 | >0.99 | 0.99 | 0.6 | 0.11 | 0.91 |
AF indicates atrial fibrillation; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CVA, cerebrovascular accident; HTN, hypertension; Mixed CTSx, mixed cardiothoracic surgical group; NA, not available; OPCAB, off‐pump coronary artery bypass; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Prevalence of preexisting atrial fibrillation was significantly higher in the TAVI group as compared with the CABG group (37.17% versus 5.81%, P=0.05); however, only 2 CABG study groups reported on the presence of preexisting atrial fibrillation. Diabetes mellitus was more common in the PCI group as compared with the mixed CTSx group (45.31% versus 4.4%, P=0.02). The proportion male was significantly less in the TAVI group as compared with the CABG, OPCAB, and PCI groups (49.97% versus 79.60%, P≤0.01; 49.97% versus 78.37%, P=0.03; 49.97% versus 70.3%, P≤0.01, respectively). No other significant differences in baseline characteristics were identified among groups.
The pooled postoperative prevalence rate of SBIs in the early postoperative period is shown in Figure 2. Rates of new postoperative SBIs varied from 0.14 (95% CI 0.0‐0.58) for the OPCAB group to 0.71 (95% CI 0.64‐0.77) for the TAVI group. The pooled postoperative prevalence of stroke varied from 0.00 (95% CI 0.00‐0.001) for PCI, to 0.09 (95% CI 0.03‐0.16) for AVR (Figure 3).
Figure 2.
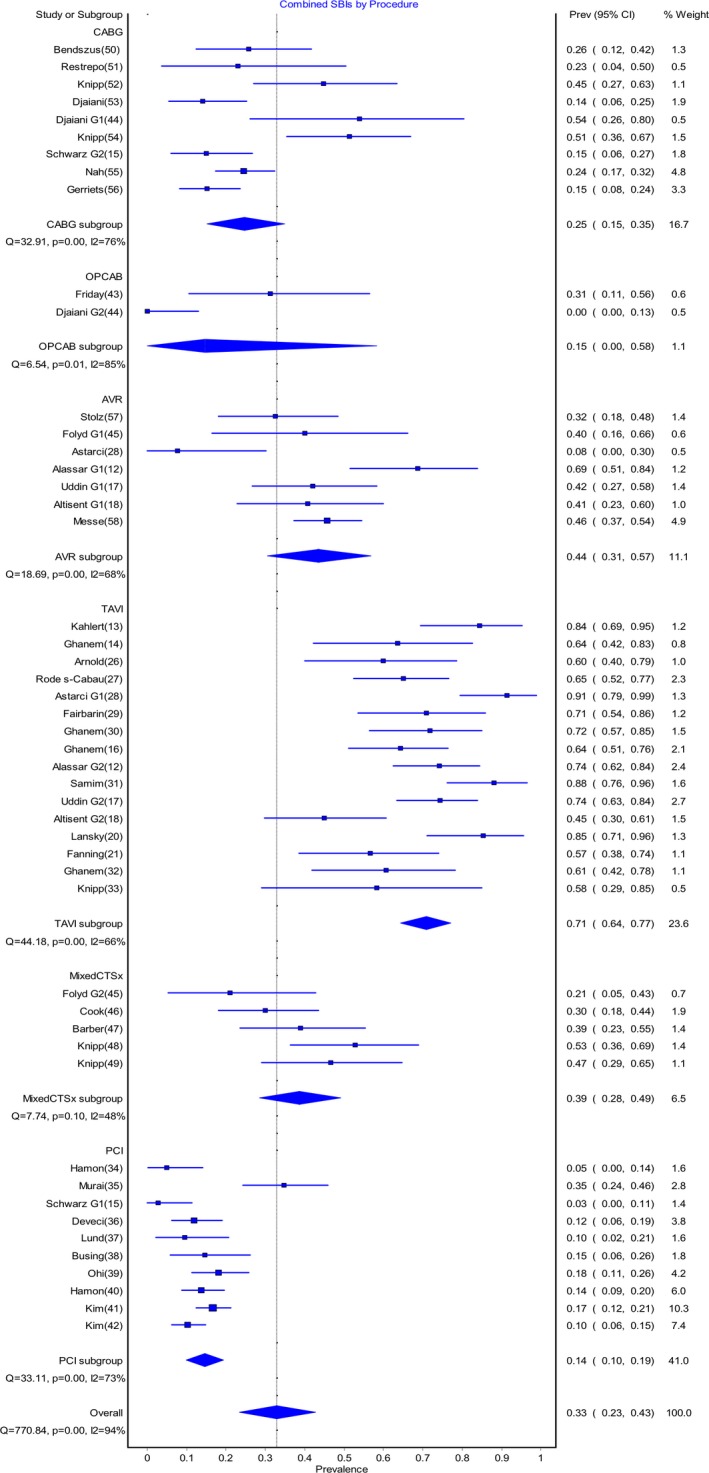
Pooled prevalence of silent brain infacts (SBIs) post–cardiac procedures. AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; MixedCTSx, mixed cardiothoracic surgical group; OPCAB, off‐pump coronary artery bypass; PCI, percutaneous coronary intervention; Prev, prevalence; TAVI, transcatheter aortic valve implantation.
Figure 3.
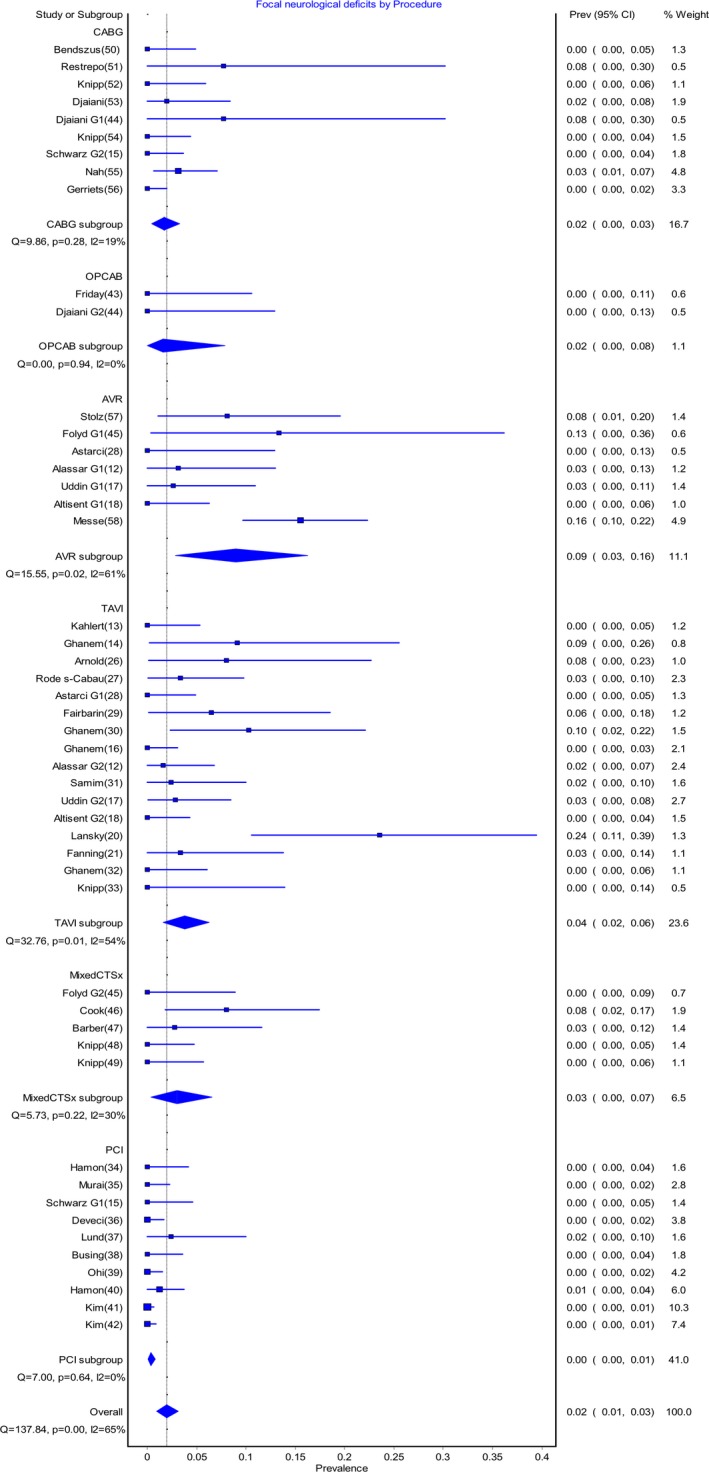
Pooled prevalence of focal neurologic deficits post–cardiac procedures. AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; Mixed CTSx, mixed cardiothoracic surgical group; OPCAB, off‐pump coronary artery bypass; PCI, percutaneous coronary intervention; Prev, prevalence; TAVI, transcatheter aortic valve implantation.
The funnel plots for the pooled prevalence meta‐analysis of SBIs are shown in Figure 4, and that for the pooled prevalence meta‐analysis of FNDs is shown in Figure 5. Funnel plots were not utilized for the OPCAB group because only 2 studies were included.
Figure 4.
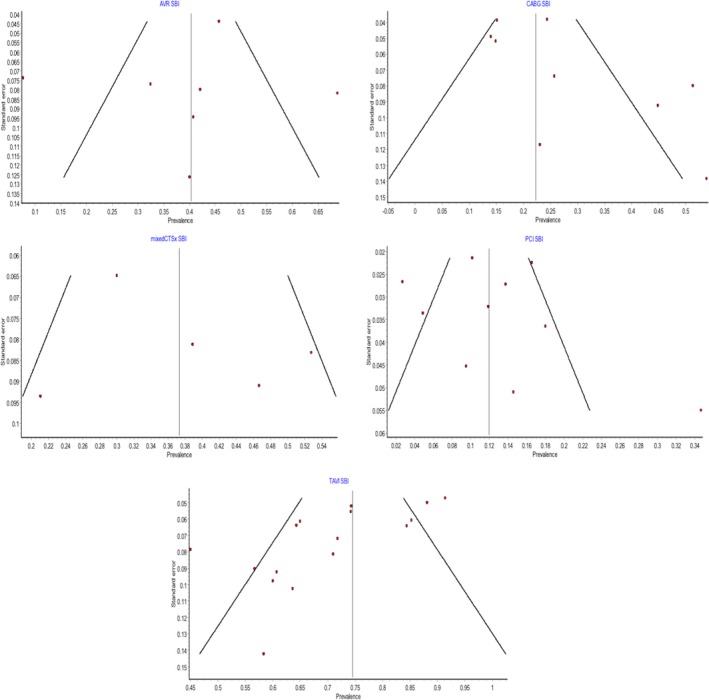
Funnel plots assessing interstudy bias for pooled prevalence of silent brain infarcts. AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; mixedCTSx, mixed cardiothoracic surgical group; PCI, percutaneous coronary intervention; SBI, silent brain infarct; TAVI, transcatheter aortic valve implantation.
Figure 5.
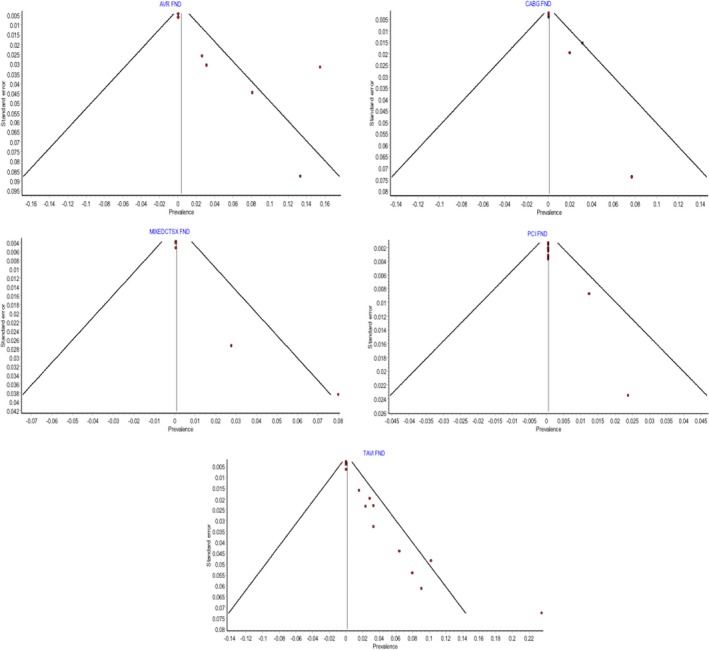
Funnel plots assessing interstudy bias for pooled prevalence of focal neurologic deficits. AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; FND, focal neurologic deficits; MIXEDCTSX, mixed cardiothoracic surgical group; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Of the patients with new postoperative DWI lesions, the mean number of lesions was 3.38 ± 2.01 per patient across all procedural groups (Table 3). There was a statistically significant difference in mean lesion size among groups as determined by 1‐way ANOVA (F[4,30] = 4.473, P=0.006). The results of the post hoc Tukey HSD are demonstrated in Table 3.
Table 3.
Mean Number of SBIs Per Patient for Procedural Groups
| Procedural Group | Number of SBIs (Mean±SD) | P‐Value | ||||
|---|---|---|---|---|---|---|
| AVR | CABG | Mixed CTSx | PCI | TAVI | ||
| AVR | 2.16±1.62 | ··· | >0.99 | 0.77 | >0.99 | 0.09 |
| CABG | 2.11±0.25 | >0.99 | ··· | 0.82 | >0.99 | 0.88 |
| Mixed CTSx | 3.38±0.72 | 0.77 | 0.82 | ··· | 0.67 | 0.7 |
| PCI | 1.88±1.02 | >0.99 | >0.99 | 0.67 | ··· | 0.33 |
| TAVI | 4.58±2.09 | 0.03 | 0.09 | 0.7 | 0.03 | ··· |
| Total | 3.38±2.01 | ··· | ··· | ··· | ··· | ··· |
AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; Mixed CTSx, mixed cardiothoracic surgical group; PCI, percutaneous coronary intervention; SBI, silent brain infarct; TAVI, transcatheter aortic valve implantation.
TAVI patients had significantly more lesions than AVR (4.58 ± 2.09 versus 2.16 ± 1.62, P=0.03) and PCI patients (4.58 ± 2.09 versus 1.88 ± 1.02, P=0.03). The mean volume of individual SBI lesions was reported in only 9 studies with a mean single‐lesion size of 114 mm3 (range 24‐760 mm3). The mean volume of total SBI lesion load per patient was also reported in only 9 studies with a mean volume of 1585.87 mm3 (range 132‐8830 mm3). Of the 9 studies reporting individual lesion volume and total lesion load per patient, 6 were TAVI, 1 CABG, 1 AVR, and 1 mixed CTSx.
In the meta‐analysis (Figure 6) comparing the risk ratio of FND to SBI, the overall risk ratio was 0.13 (95% CI 0.11‐0.16) across all procedures. There was a significant difference between the risk ratio for AVR and that for PCI (risk ratio 0.22, 95% CI 0.15‐0.32 versus 0.06, 95% CI 0.03‐0.14; P=0.02). Of the other procedural groups, the risk ratio for CABG was 0.10 (95% CI 0.05‐0.18), TAVI 0.10 (95% CI 0.07‐0.14), and mixed CTSx 0.15 (95% CI 0.07‐0.33). There were no other significant differences in risk ratios among these groups.
Figure 6.
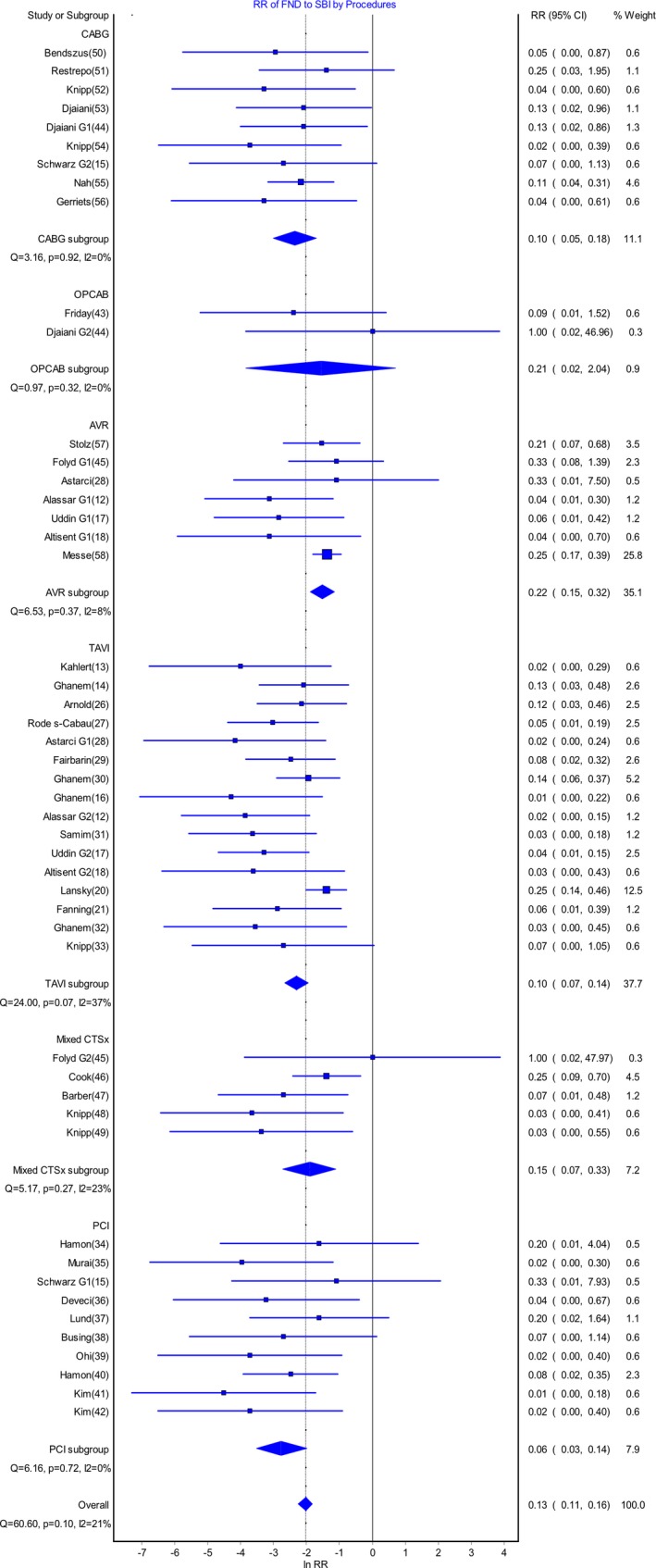
Meta‐analysis demonstrating risk ratio of focal neurologic deficits (FNDs) to silent brain infarcts (SBIs) for cardiac procedures. AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; Mixed CTSx, mixed cardiothoracic surgical group; OPCAB, off‐pump coronary artery bypass; PCI, percutaneous coronary intervention; RR, risk ratio; TAVI, transcatheter aortic valve implantation.
The funnel plots for the meta‐analysis assessing the risk ratio of FND to SBIs are shown in Figure 7. Again funnel plots were not utilized for the OPCAB group because only 2 studies are included.
Figure 7.
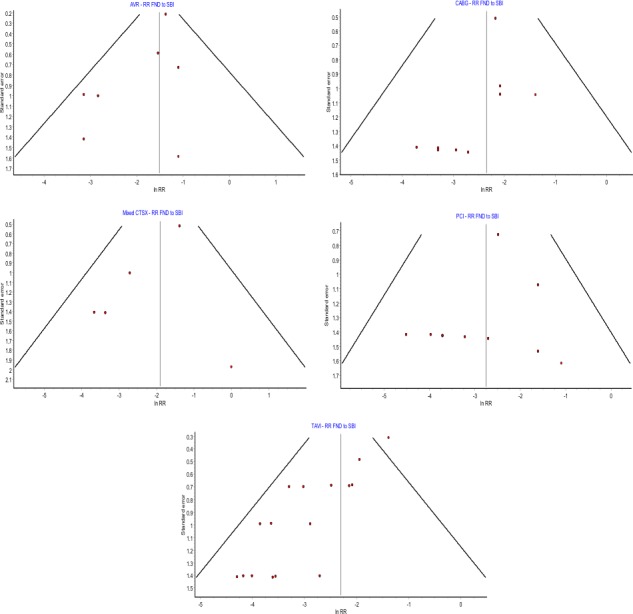
Funnel plots assessing interstudy bias meta‐analysis comparing risk ratio of focal neurologic deficits (FNDs) to silent brain infarcts (SBIs). AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; Mixed CTSX, mixed cardiothoracic surgical group; PCI, percutaneous coronary intervention; RR, risk ratio; TAVI, transcatheter aortic valve implantation.
A total of 13 studies reported on a variable array of clinical neurocognitive measures pre‐ and postoperatively to assess for correlation with DWI lesions (Table 4). Only 3 studies37, 46, 47 utilized all 4 core neuropsychological tests (Rey auditory verbal learning test, trail‐making A, trail‐making B, and grooved‐pegboard test) as recommended in a previously published consensus statement of POCD after cardiac surgery.59 All studies performed preoperative cognitive testing at baseline, but only 8 studies performed postoperative testing at least 3 months following the operation.
Table 4.
Summary of Reported Cognitive Testing Batteries for Postoperative Cognitive Dysfunction and Association With SBIs
| Study | Neurocognitive Domains Tested | Timing of Testing | Cognitive Decline | Association Between DWI Lesions and POCD |
|---|---|---|---|---|
| Bendszus (2002)50 |
|
|
Yes but no details given | Nil |
| Knipp (2004)52 |
|
|
Nil | |
| Cook (2007)46 |
|
|
Nil | |
| Knipp (2008)54 |
|
|
Nil | |
| Barber (2008)47 | Manual dexterity; Psychomotor speed; Executive function; Memory |
|
63% had decline in 1 domain; 34% declined in 2 | Yes, (OR 37.49, 95% CI 4.01‐350.18) |
| Kahlert (2010)13 | MMSE |
|
Nil significant changes | NA |
| Schwarz (2011)15 |
|
|
As compared with controlsa |
Yes, SBI correlated with reduced performance in tests of verbal and visual memory |
| Ghanem (2013)16 |
|
|
|
Nil |
| Alassar (2015)12 |
Overall cognition; Executive function; Processing speed MemoryaSpecific tests not mentioned |
|
No improvement in cognitive function seen in AVR and TAVI groups at 3 mo | Nil |
| Ghanem (2017)32 | MMSE |
|
Nonsignificant overall | Nonsignificant (P=0.067) |
| Lund (2005)37 |
|
|
Cognitive impairment seen in 16.7% defined as decline ≥20% in test scores in at least 2 of 12 tests | Yes, significant difference in 2 tests assessing learning and attention |
| Gerriets (2010)56 |
|
|
|
Presence of postoperative SBIs correlated with decreased scores in letter‐interference test and attention domain at postoperative day 2‐4. Nil association of SBIs and POCD at 3 mo |
AVLT indicates auditory verbal learning test; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CD, cognitive dysfunction; DWI, diffusion‐weighted imaging; MMSE, Mini‐Mental State Exam; NVLT, non‐verbal learning test; PCI, percutaneous coronary intervention; POCD, postoperative cognitive dysfunction; SBI, silent brain infarct; SKT, Syndrom Kurztest attention test; TAVI, transcatheter aortic valve implantation; VLMT, verbal learning and memory test; WAIS, Wechsler Adult Intelligence Scale; WAIS‐R, revised WAIS.
Tests recommended by Murkin et al59 in the consensus statement for diagnosis of POCD.
Knipp et al (2004)52 demonstrated a significant decline in the verbal learning test (p=0.012) at 3 months post‐CABG, however this was not correlated to the presence of new SBI. Similarly, in another study of CABG patients, this same group demonstrated a 23% decline in cognitive function after 3 months, not correlated to new SBIs.52 Gerriets et al56 demonstrated a significant correlation between SBIs and cognitive decline in the attention domain at postoperative days 2 to 4, and although there was a decline in verbal and visual memory at 3 months, this was not correlated to new SBIs. Barber et al47 demonstrated a significant association to postperative cognitive decline and the presence of postoperative SBIs at 6 weeks following AVR (OR 37.49 95% CI 4.01‐350.18), with an additional association shown between the ischemic burden and the degree of cognitive dysfunction. In a study of both CABG and PCI by Schwarz et al,15 the presence of postoperative SBI was correlated with a reduced performance in verbal and visual memory at 3 months. Two studies12, 49 did not demonstrate the presence of POCD at 3 months postoperatively. Although Lund et al37 demonstrated a significant association between SBI and POCD following left heart catheterization, this was demonstrated only in the first postoperative day.
Discussion
Brain injury associated with cardiac surgery exists on a spectrum from clinically overt stroke and TIA to subtle POCD and SBI. The latter 2 categories have been shown to occur at much higher rates than the former ones, and although not as acutely catastrophic for patients, they can significantly reduce quality of life and predispose to longer‐term neurologic dysfunction. The importance of these subtle brain injuries is 2‐fold: they are a significant surgical complication that, combined, may affect more than 50% of patients postoperatively with long‐term consequences, and they present an important outcome measure for studies of neuroprotective techniques due to high rates of occurrence.
This systematic review and meta‐analysis primarily reports on pooled prevalence rates of early postoperative SBIs and FND following common cardiac surgical and interventional procedures.
In our study the early postoperative stroke/TIA rates are in line with commonly reported figures for procedural groups, although the AVR group rate of 9% was higher than expected. This finding could be traced back to a specific AVR study that had been included, which reported a stroke rate of 17%58 and carried a weighting of 44% in the meta‐analysis. Excluding this study from the analysis resulted in the prevalence rate of FND following AVR decreasing to 5%.
There is significant variability in the postoperative prevalence rate of SBIs with regard to the procedural group. The TAVI and AVR groups report the highest rates, 74% and 58%, respectively, demonstrating that SBIs are very common following procedures involving the aortic valve and manipulation of the aorta. This is in accordance with the suspected etiology of SBIs being in part due to microemboli as a result of direct disruption of atherosclerotic plaque in the ascending aorta. Increased levels of proximal thoracic aortic atheroma have been shown to be associated with higher rates of intraoperative cerebral embolism as evident on transcranial Doppler and higher rates of SBIs postoperatively following AVR.53 TAVI presents a particularly high‐risk procedure for embolism due to a number of factors. Most studies to date have focused on high‐risk surgical populations with severe aortic stenosis. This population is thus likely to have increased proximal aortic atherosclerosis beause this is well correlated with the amount of aortic valve calcium and stenosis severity.60 For TAVI, the intra‐aortic catheter and in‐situ valve expansion inside calcific aortic valves pose individual risks for embolic phenomenon.61
With this considered, it can be seen why neurologic injury has been the Achilles heel of TAVI to date, with the high rate of embolic events mostly limiting its application in clinical practice to either inoperable or high–surgical risk patients.61
The PARTNER trial reported an increased rate of stroke postoperatively and at 1 year for TAVI as compared with AVR.62 When all neurologic injuries (stroke and TIA combined) were compared, there was a further separation in the rate of reported neurologic injury between TAVI and AVR at 1 year (8.7% versus 4.3%, respectively) and 2 years (11.2% versus 6.5%, respectively).63 More recent studies, likely due to the advent of newer‐generation devices and more collective procedural experience report similar rates of perioperative stroke between these interventions64; however, the rates of SBIs remain significantly higher following TAVI. We hypothesize this may increase the vulnerability of this population to medium‐ and long‐term stroke and TIA. Although the majority of clinically overt neurologic injuries occur within the first 30 days of TAVI, there is evidence of an ongoing stroke and TIA risk long term.61
For the mixed CTSx group, which consisted of a mixture of CABG only, valve only, or simultaneous valve and CABG operations, the prevalence rate of SBIs of 36% may reflect the decreased risk of cerebral embolism seen in the coronary artery bypass operations because they involve less manipulation of the ascending aorta. Moreover, prevalence of SBIs fell further for isolated CABG (26%), OPCAB (14%), and PCI (15%). This is supported by evidence that anaortic OPCAB, in which there is no manipulation or cross‐clamping of the aorta, reduces the rates of clinically overt neurologic injury as compared with conventional CABG.65 In this analysis only 2 studies reporting on OPCAB were available, and neither was performed with the anaortic technique.43, 44 Further studies are required to evaluate the risk of SBIs in anaortic OPCAB as compared with techniques involving clamping of the aorta or cardiopulmonary bypass.
Although the stroke rate following PCI has been shown to be negligible, the rate of SBI that we report remains not insignificant at 15%. The main mechanism is again thought to relate to atheroma disruption by guide wires in the ascending aorta resulting in cerebral embolism.66 Subsequently, procedural time is a predictor of SBI risk in this population.38
SBIs have been shown to occur at significantly higher rates than strokes or TIAs, which has resulted in their becoming a potential surrogate measure of brain injury associated with cardiac procedures. Obtaining adequate statistical power remains a challenge in trials studying postoperative stroke and TIA due to low rates of occurrence. This is highlighted by Aggarwal et al,67 who performed a large registry trial that included more than 700 000 patients and evaluated the incidence and risk of stroke following PCI. They reported a stroke rate of 0.22%—an event rate too low for the data to be utilized in developing a predictive model.
This can be further highlighted by calculating the sample size that would be required of a hypothetical cohort study to compare the risk of brain injury associated with traditional CABG versus anaortic OPCAB. Data from a network meta‐analysis recently published by 1 of our authors (M.P.V.) showed the rate of stroke following CABG to be 1.8% versus 0.4% for anaortic OPCAB.65 With these stroke rates, a randomized controlled trial comparing these techniques would require a total sample size of 1744, with a power of 80% and an absolute error of 5%. Maintaining these parameters but changing the outcome measure to SBI—utilizing a hypothetical rate of SBI for anaortic OPCAB of 10% and our reported SBI rate in CABG of 25%— would require the total sample size to be 200.
The overall stroke‐to‐SBI risk ratio for cardiac procedures we report of 0.13 is similar to that previously reported by Cho et al,68 who report an overall risk ratio of 0.10 for cardiac procedures and cerebral angiography combined. Although the only significant difference in risk ratio was between AVR and PCI, the trend was toward a higher risk ratio for more invasive open surgical valve procedures, which made up the majority of the mixed CTSx group. Although they are useful as a guide to demonstrate some consistency between the occurrence of stroke and SBI, these rates do not take into account the number or size of DWI lesions.
Unfortunately, at present the utility of SBIs as a common outcome measure for surgical brain injury is limited by the current variability in definition and reporting. Of the 927 patients with SBI postoperatively, in only 427 patients were the actual number of lesions reported. Furthermore, the volume of individual lesions and/or the total lesion load was only reported in 9 studies. The size and number of SBIs are likely to be important factors in determining the increased patient risk of future neurologic complications. In patients with acute ischemic stroke, DWI lesion volume in the middle cerebral artery territory has been shown to correlate with higher scores in the National Institutes of Health Stroke Scale69 as well as with poorer long‐term outcomes and increased risk of hemorrhagic transformation.70 More closely related, severe strokes have been associated with the presence of multiple coexisting SBIs.71 Therefore, merely stating the presence of post‐procedural SBIs gives little indication of the extent of neurologic injury sustained.
For SBIs to be utilized as a surrogate measure of brain injury, the number, volume, and locations of lesions should be reported. Additionally, the application of specific imaging criteria of SBIs, as has previously been suggested,72 would increase the reproducibility for subsequent trials and thus comparability. Specific location of SBIs is also relevant as it may give clues as to the etiology of the lesions. A diffuse pattern of cerebral involvement would be consistent with an embolic source, whereas when present in watershed zones, cerebral hypoperfusion may be the likely factor involved.
We additionally performed a systematic review of POCD and its association with SBIs following cardiac procedures. Problems with the significant variability in the definitions of POCD led to the development of a consensus statement being published in 1995 outlining specific criteria that should be utilized when assessing for POCD.59 A systematic review in 2010 found that there was poor uptake of this proposed criterion, with significant heterogeneity seen particularly in the definition of what constituted POCD and the contents of the neurocognitive test batteries performed.10 Of the 13 studies that assessed for correlation between POCD and SBI in our review, there was low uptake of the consensus statement recommendations, making these results difficult to compare. Of the 4 studies that did report an association between POCD and DWI lesions, the measurement of POCD within 4 days of the procedure largely nullifies the significance of these results without repeat testing at 3 months (or more) postoperatively. Early POCD is difficult to diagnose due to multiple confounders such as anesthetic agents, analgesia, and delirium impacting on the cognitive state acutely. Without the application of consistent criteria and strict definitions, the prevalence and risk factors of POCD will be unable to be accurately measured; nor will treatment options nor preventative techniques be able to be proven.
SBIs are shown to be very common complication of cardiac procedures. Increased understanding of these lesions demonstrates that they are likely not as “silent” as their name would suggest. The common occurrence of lesions perioperatively also presents them as a potential surrogate marker for neuroprotective studies. At present, however, inconsistencies in how these are defined and reported limit their clinical and research applicability.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e010920 DOI: 10.1161/JAHA.118.010920.)
References
- 1. Edelman JJ, Yan TD, Bannon PG, Wilson MK, Vallely MP. Coronary artery bypass grafting with and without manipulation of the ascending aorta–a meta‐analysis. Heart Lung Circ. 2011;20:318–324. [DOI] [PubMed] [Google Scholar]
- 2. Indraratna P, Tian DH, Yan TD, Doyle MP, Cao C. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a meta‐analysis of randomized controlled trials. Int J Cardiol. 2016;224:382–387. [DOI] [PubMed] [Google Scholar]
- 3. Miller DC, Blackstone EH, Mack MJ, Svensson LG, Kodali SK, Kapadia S, Rajeswaran J, Anderson WN, Moses JW, Tuzcu EM, Webb JG, Leon MB, Smith CR; PARTNER Trial Investigators and Patients, PARTNER Stroke Substudy Writing Group and Executive Committee . Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg. 2012;143:832–843.e13 [DOI] [PubMed] [Google Scholar]
- 4. Burdette JH, Ricci PE, Petitti N, Elster AD. Cerebral infarction: time course of signal intensity changes on diffusion‐weighted MR images. AJR Am J Roentgenol. 1998;171:791–795. [DOI] [PubMed] [Google Scholar]
- 5. Provenzale JM, Sorensen AG. Diffusion‐weighted MR imaging in acute stroke: theoretic considerations and clinical applications. AJR Am J Roentgenol. 1999;173:1459–1467. [DOI] [PubMed] [Google Scholar]
- 6. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, Rotterdam Scan S. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 7. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 8. Avdibegovic E, Becirovic E, Selimbasic Z, Hasanovic M, Sinanovic O. Cerebral cortical atrophy and silent brain infarcts in psychiatric patients. Psychiatr Danub. 2007;19:49–55. [PubMed] [Google Scholar]
- 9. Sun X, Lindsay J, Monsein LH, Hill PC, Corso PJ. Silent brain injury after cardiac surgery: a review: cognitive dysfunction and magnetic resonance imaging diffusion‐weighted imaging findings. J Am Coll Cardiol. 2012;60:791–797. [DOI] [PubMed] [Google Scholar]
- 10. Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, Marcantonio ER. Measurement of post‐operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010;54:663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. [DOI] [PubMed] [Google Scholar]
- 12. Alassar A, Soppa G, Edsell M, Rich P, Roy D, Chis Ster I, Joyce R, Valencia O, Barrick T, Howe F, Moat N, Morris R, Markus HS, Jahangiri M. Incidence and mechanisms of cerebral ischemia after transcatheter aortic valve implantation compared with surgical aortic valve replacement. Ann Thorac Surg. 2015;99:802–808. [DOI] [PubMed] [Google Scholar]
- 13. Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al‐Rashid F, Weber M, Johansson U, Wendt D, Jakob HG, Forsting M, Sack S, Erbel R, Eggebrecht H. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion‐weighted magnetic resonance imaging study. Circulation. 2010;121:870–878. [DOI] [PubMed] [Google Scholar]
- 14. Ghanem A, Muller A, Nahle CP, Kocurek J, Werner N, Hammerstingl C, Schild HH, Schwab JO, Mellert F, Fimmers R, Nickenig G, Thomas D. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion‐weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427–1432. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz N, Schoenburg M, Mollmann H, Kastaun S, Kaps M, Bachmann G, Sammer G, Hamm C, Walther T, Gerriets T. Cognitive decline and ischemic microlesions after coronary catheterization. A comparison to coronary artery bypass grafting. Am Heart J. 2011;162:756–763. [DOI] [PubMed] [Google Scholar]
- 16. Ghanem A, Kocurek J, Sinning JM, Wagner M, Becker BV, Vogel M, Schroder T, Wolfsgruber S, Vasa‐Nicotera M, Hammerstingl C, Schwab JO, Thomas D, Werner N, Grube E, Nickenig G, Muller A. Cognitive trajectory after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2013;6:615–624. [DOI] [PubMed] [Google Scholar]
- 17. Uddin A, Fairbairn TA, Djoukhader IK, Igra M, Kidambi A, Motwani M, Herzog B, Ripley DP, Musa TA, Goddard AJ, Blackman DJ, Plein S, Greenwood JP. Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement: effect on health‐related quality of life. Circ Cardiovasc Interv. 2015;8:e001913. [DOI] [PubMed] [Google Scholar]
- 18. Abdul‐Jawad Altisent O, Ferreira‐Gonzalez I, Marsal JR, Ribera A, Auger C, Ortega G, Cascant P, Urena M, Del Blanco BG, Serra V, Sureda C, Igual A, Rovira A, Gonzalez‐Alujas MT, Gonzalez A, Puri R, Cuellar H, Tornos P, Rodes‐Cabau J, Garcia‐Dorado D. Neurological damage after transcatheter aortic valve implantation compared with surgical aortic valve replacement in intermediate risk patients. Clin Res Cardiol. 2016;105:508–517. [DOI] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lansky AJ, Brown D, Pena C, Pietras CG, Parise H, Ng VG, Meller S, Abrams KJ, Cleman M, Margolis P, Petrossian G, Brickman AM, Voros S, Moses J, Forrest JK. Neurologic complications of unprotected transcatheter aortic valve implantation (from the Neuro‐TAVI Trial). Am J Cardiol. 2016;118:1519–1526. [DOI] [PubMed] [Google Scholar]
- 21. Fanning JP, Wesley AJ, Walters DL, Eeles EM, Barnett AG, Platts DG, Clarke AJ, Wong AA, Strugnell WE, O'Sullivan C, Tronstad O, Fraser JF. Neurological Injury in Intermediate‐Risk Transcatheter Aortic Valve Implantation. J Am Heart Assoc. 2016;5:e004203 DOI: 10.1161/JAHA.116.004203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2018;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- 23. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pezzullo J. Interactive Stats, Stats pages: statspages. 2017. Available at: http://statpages.info/index.html. Accessed June 23, 2018.
- 25. Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta‐analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials. 2015;45:130–138. [DOI] [PubMed] [Google Scholar]
- 26. Arnold M, Schulz‐Heise S, Achenbach S, Ott S, Dorfler A, Ropers D, Feyrer R, Einhaus F, Loders S, Mahmoud F, Roerick O, Daniel WG, Weyand M, Ensminger SM, Ludwig J. Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. JACC Cardiovasc Interv. 2010;3:1126–1132. [DOI] [PubMed] [Google Scholar]
- 27. Rodes‐Cabau J, Dumont E, Boone RH, Larose E, Bagur R, Gurvitch R, Bedard F, Doyle D, De Larochelliere R, Jayasuria C, Villeneuve J, Marrero A, Cote M, Pibarot P, Webb JG. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011;57:18–28. [DOI] [PubMed] [Google Scholar]
- 28. Astarci P, Glineur D, Kefer J, D'Hoore W, Renkin J, Vanoverschelde JL, El Khoury G, Grandin C. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans‐apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg. 2011;40:475–479. [DOI] [PubMed] [Google Scholar]
- 29. Fairbairn TA, Mather AN, Bijsterveld P, Worthy G, Currie S, Goddard AJ, Blackman DJ, Plein S, Greenwood JP. Diffusion‐weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012;98:18–23. [DOI] [PubMed] [Google Scholar]
- 30. Ghanem A, Muller A, Sinning JM, Kocurek J, Becker BV, Vogel M, Vasa‐Nicotera M, Hammerstingl C, Schwab JO, Nahle CP, Thomas D, Wagner M, Grube E, Werner N, Nickenig G. Prognostic value of cerebral injury following transfemoral aortic valve implantation. EuroIntervention. 2013;8:1296–1306. [DOI] [PubMed] [Google Scholar]
- 31. Samim M, Hendrikse J, van der Worp HB, Agostoni P, Nijhoff F, Doevendans PA, Stella PR. Silent ischemic brain lesions after transcatheter aortic valve replacement: lesion distribution and predictors. Clin Res Cardiol. 2015;104:430–438. [DOI] [PubMed] [Google Scholar]
- 32. Ghanem A, Dorner J, Schulze‐Hagen L, Muller A, Wilsing M, Sinning JM, Lutkens J, Frerker C, Kuck KH, Graff I, Schild H, Werner N, Grube E, Nickenig G. Subacute subclinical brain infarctions after transcatheter aortic valve implantation negatively impact cognitive function in long‐term follow‐up. PLoS One. 2017;12:e0168852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knipp SC, Kahlert P, Jokisch D, Schlamann M, Wendt D, Weimar C, Jakob H, Thielmann M. Cognitive function after transapical aortic valve implantation: a single‐centre study with 3‐month follow‐up. Interact Cardiovasc Thorac Surg. 2013;16:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamon M, Gomes S, Clergeau MR, Fradin S, Morello R, Hamon M. Risk of acute brain injury related to cerebral microembolism during cardiac catheterization performed by right upper limb arterial access. Stroke. 2007;38:2176–2179. [DOI] [PubMed] [Google Scholar]
- 35. Murai M, Hazui H, Sugie A, Hoshiga M, Negoro N, Muraoka H, Miyamoto H, Kobata H, Fukumoto H, Ishihara T, Morita H, Hanafusa T. Asymptomatic acute ischemic stroke after primary percutaneous coronary intervention in patients with acute coronary syndrome might be caused mainly by manipulating catheters or devices in the ascending aorta, regardless of the approach to the coronary artery. Circ J. 2008;72:51–55. [DOI] [PubMed] [Google Scholar]
- 36. Deveci OS, Celik AI, Ikikardes F, Ozmen C, Cagliyan CE, Deniz A, Bicakci K, Bicakci S, Evlice A, Demir T, Kanadasi M, Demir M, Demirtas M. The incidence and the risk factors of silent embolic cerebral infarction after coronary angiography and percutaneous coronary interventions. Angiology. 2016;67:433–437. [DOI] [PubMed] [Google Scholar]
- 37. Lund C, Nes RB, Ugelstad TP, Due‐Tonnessen P, Andersen R, Hol PK, Brucher R, Russell D. Cerebral emboli during left heart catheterization may cause acute brain injury. Eur Heart J. 2005;26:1269–1275. [DOI] [PubMed] [Google Scholar]
- 38. Busing KA, Schulte‐Sasse C, Fluchter S, Suselbeck T, Haase KK, Neff W, Hirsch JG, Borggrefe M, Duber C. Cerebral infarction: incidence and risk factors after diagnostic and interventional cardiac catheterization—prospective evaluation at diffusion‐weighted MR imaging. Radiology. 2005;235:177–183. [DOI] [PubMed] [Google Scholar]
- 39. Ohi Y, Uno Y, Oohira T, Itakura K, Nishigaki K, Minatoguchi S. Cerebral microembolism following coronary angiography—a prospective comparative study between left cardiac catheterization and multidetector computed tomography. Intern Med. 2013;52:1869–1874. [DOI] [PubMed] [Google Scholar]
- 40. Hamon M, Lipiecki J, Carrie D, Burzotta F, Durel N, Coutance G, Boudou N, Colosimo C, Trani C, Dumonteil N, Morello R, Viader F, Claise B, Hamon M. Silent cerebral infarcts after cardiac catheterization: a randomized comparison of radial and femoral approaches. Am Heart J. 2012;164:449–454.e1. [DOI] [PubMed] [Google Scholar]
- 41. Kim YH, Park DW, Ahn JM, Yun SC, Song HG, Lee JY, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, Jang Y, Jeong MH, Kim HS, Hur SH, Rha SW, Lim DS, Her SH, Seung KB, Seong IW, Park SJ; PRECOMBAT‐2 Investigators . Everolimus‐eluting stent implantation for unprotected left main coronary artery stenosis. The PRECOMBAT‐2 (Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus‐Eluting Stent in Patients with Left Main Coronary Artery Disease) study. JACC Cardiovasc Interv. 2012;5:708–717. [DOI] [PubMed] [Google Scholar]
- 42. Kim IC, Hur SH, Park NH, Jun DH, Cho YK, Nam CW, Kim H, Han SW, Choi SY, Kim YN, Kim KB. Incidence and predictors of silent embolic cerebral infarction following diagnostic coronary angiography. Int J Cardiol. 2011;148:179–182. [DOI] [PubMed] [Google Scholar]
- 43. Friday G, Sutter F, Curtin A, Kenton E, Caplan B, Nocera R, Siddiqui A, Goldman S. Brain magnetic resonance imaging abnormalities following off‐pump cardiac surgery. Heart Surg Forum. 2005;8:E105–E109. [DOI] [PubMed] [Google Scholar]
- 44. Djaiani G, Fedorko L, Cusimano RJ, Mikulis D, Carroll J, Poonawala H, Beattie S, Karski J. Off‐pump coronary bypass surgery: risk of ischemic brain lesions in patients with atheromatous thoracic aorta. Can J Anaesth. 2006;53:795–801. [DOI] [PubMed] [Google Scholar]
- 45. Floyd TF, Shah PN, Price CC, Harris F, Ratcliffe SJ, Acker MA, Bavaria JE, Rahmouni H, Kuersten B, Wiegers S, McGarvey ML, Woo JY, Pochettino AA, Melhem ER. Clinically silent cerebral ischemic events after cardiac surgery: their incidence, regional vascular occurrence, and procedural dependence. Ann Thorac Surg. 2006;81:2160–2166. [DOI] [PubMed] [Google Scholar]
- 46. Cook DJ, Huston J III, Trenerry MR, Brown RD Jr, Zehr KJ, Sundt TM III. Postcardiac surgical cognitive impairment in the aged using diffusion‐weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83:1389–1395. [DOI] [PubMed] [Google Scholar]
- 47. Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion‐weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–1433. [DOI] [PubMed] [Google Scholar]
- 48. Knipp SC, Weimar C, Schlamann M, Schweter S, Wendt D, Thielmann M, Benedik J, Jakob H. Early and long‐term cognitive outcome after conventional cardiac valve surgery. Interact Cardiovasc Thorac Surg. 2017;24:534–540. [DOI] [PubMed] [Google Scholar]
- 49. Knipp SC, Matatko N, Schlamann M, Wilhelm H, Thielmann M, Forsting M, Diener HC, Jakob H. Small ischemic brain lesions after cardiac valve replacement detected by diffusion‐weighted magnetic resonance imaging: relation to neurocognitive function. Eur J Cardiothorac Surg. 2005;28:88–96. [DOI] [PubMed] [Google Scholar]
- 50. Bendszus M, Reents W, Franke D, Mullges W, Babin‐Ebell J, Koltzenburg M, Warmuth‐Metz M, Solymosi L. Brain damage after coronary artery bypass grafting. Arch Neurol. 2002;59:1090–1095. [DOI] [PubMed] [Google Scholar]
- 51. Restrepo L, Wityk RJ, Grega MA, Borowicz L Jr, Barker PB, Jacobs MA, Beauchamp NJ, Hillis AE, McKhann GM. Diffusion‐ and perfusion‐weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke. 2002;33:2909–2915. [DOI] [PubMed] [Google Scholar]
- 52. Knipp SC, Matatko N, Wilhelm H, Schlamann M, Massoudy P, Forsting M, Diener HC, Jakob H. Evaluation of brain injury after coronary artery bypass grafting. A prospective study using neuropsychological assessment and diffusion‐weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25:791–800. [DOI] [PubMed] [Google Scholar]
- 53. Djaiani G, Fedorko L, Borger M, Mikulis D, Carroll J, Cheng D, Karkouti K, Beattie S, Karski J. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356–e358. [DOI] [PubMed] [Google Scholar]
- 54. Knipp SC, Matatko N, Wilhelm H, Schlamann M, Thielmann M, Losch C, Diener HC, Jakob H. Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion‐weighted magnetic resonance imaging. Ann Thorac Surg. 2008;85:872–879. [DOI] [PubMed] [Google Scholar]
- 55. Nah HW, Lee JW, Chung CH, Choo SJ, Kwon SU, Kim JS, Warach S, Kang DW. New brain infarcts on magnetic resonance imaging after coronary artery bypass graft surgery: lesion patterns, mechanism, and predictors. Ann Neurol. 2014;76:347–355. [DOI] [PubMed] [Google Scholar]
- 56. Gerriets T, Schwarz N, Bachmann G, Kaps M, Kloevekorn WP, Sammer G, Tschernatsch M, Nottbohm R, Blaes F, Schonburg M. Evaluation of methods to predict early long‐term neurobehavioral outcome after coronary artery bypass grafting. Am J Cardiol. 2010;105:1095–1101. [DOI] [PubMed] [Google Scholar]
- 57. Stolz E, Gerriets T, Kluge A, Klovekorn WP, Kaps M, Bachmann G. Diffusion‐weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: implications for future neuroprotective trials? Stroke. 2004;35:888–892. [DOI] [PubMed] [Google Scholar]
- 58. Messe SR, Acker MA, Kasner SE, Fanning M, Giovannetti T, Ratcliffe SJ, Bilello M, Szeto WY, Bavaria JE, Hargrove WC III, Mohler ER III, Floyd TF; Determining Neurologic Outcomes From Valve Operations (DeNOVO) Investigators . Stroke after aortic valve surgery: results from a prospective cohort. Circulation. 2014;129:2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. [DOI] [PubMed] [Google Scholar]
- 60. Adler Y, Vaturi M, Wiser I, Shapira Y, Herz I, Weisenberg D, Sela N, Battler A, Sagie A. Nonobstructive aortic valve calcium as a window to atherosclerosis of the aorta. Am J Cardiol. 2000;86:68–71. [DOI] [PubMed] [Google Scholar]
- 61. Kahlert P, Al‐Rashid F, Plicht B, Hildebrandt H, Patsalis P, Chilali KE, Wendt D, Thielmann M, Bergmann L, Kottenberg E, Schlamann M, Eggebrecht H, Jakob H, Heusch G, Konorza T, Erbel R. Incidence, predictors, origin and prevention of early and late neurological events after transcatheter aortic valve implantation (TAVI): a comprehensive review of current data. J Thromb Thrombolysis. 2013;35:436–449. [DOI] [PubMed] [Google Scholar]
- 62. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators . Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 63. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB; PARTNER Trial Investigators . Two‐year outcomes after transcatheter or surgical aortic‐valve replacement. N Engl J Med. 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 64. Carnero‐Alcazar M, Maroto LC, Cobiella‐Carnicer J, Vilacosta I, Nombela‐Franco L, Alswies A, Villagran‐Medinilla E, Macaya C. Transcatheter versus surgical aortic valve replacement in moderate and high‐risk patients: a meta‐analysis. Eur J Cardiothorac Surg. 2017;51:644–652. [DOI] [PubMed] [Google Scholar]
- 65. Zhao DF, Edelman JJ, Seco M, Bannon PG, Wilson MK, Byrom MJ, Thourani V, Lamy A, Taggart DP, Puskas JD, Vallely MP. Coronary artery bypass grafting with and without manipulation of the ascending aorta: a network meta‐analysis. J Am Coll Cardiol. 2017;69:924–936. [DOI] [PubMed] [Google Scholar]
- 66. Eggebrecht H, Oldenburg O, Dirsch O, Haude M, Baumgart D, Welge D, Herrmann J, Arnold G, Schmid KW, Erbel R. Potential embolization by atherosclerotic debris dislodged from aortic wall during cardiac catheterization: histological and clinical findings in 7,621 patients. Catheter Cardiovasc Interv. 2000;49:389–394. [DOI] [PubMed] [Google Scholar]
- 67. Aggarwal A, Dai D, Rumsfeld JS, Klein LW, Roe MT; American College of Cardiology National Cardiovascular Data Registry . Incidence and predictors of stroke associated with percutaneous coronary intervention. Am J Cardiol. 2009;104:349–353. [DOI] [PubMed] [Google Scholar]
- 68. Cho SM, Deshpande A, Pasupuleti V, Hernandez AV, Uchino K. Radiographic and clinical brain infarcts in cardiac and diagnostic procedures: a systematic review and meta‐analysis. Stroke. 2017;48:2753–2759. [DOI] [PubMed] [Google Scholar]
- 69. Schroder J, Cheng B, Ebinger M, Kohrmann M, Wu O, Kang DW, Liebeskind DS, Tourdias T, Singer OC, Christensen S, Campbell B, Luby M, Warach S, Fiehler J, Fiebach JB, Gerloff C, Thomalla G; STIR and VISTA Imaging Investigators . Validity of acute stroke lesion volume estimation by diffusion‐weighted imaging—Alberta Stroke Program Early Computed Tomographic Score depends on lesion location in 496 patients with middle cerebral artery stroke. Stroke. 2014;45:3583–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP; DEFUSE Investigators . Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 71. Chen DW, Wang YX, Shi J, Zhang WQ, Yang F, Yin YW, Ma LN. Multiple silent brain infarcts are associated with severer stroke in patients with first‐ever ischemic stroke without advanced leukoaraiosis. J Stroke Cerebrovasc Dis. 2017;26:1988–1995. [DOI] [PubMed] [Google Scholar]
- 72. Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke. 2014;45:3461–3471. [DOI] [PubMed] [Google Scholar]


