Abstract
Background
Patients with a Fontan circulation achieve lower peak heart rates (HR) during exercise. Whether this impaired chronotropic response reflects pathology of the sinoatrial node or is a consequence of altered cardiac hemodynamics is uncertain. We evaluated the adequacy of HR acceleration throughout exercise relative to metabolic demand and cardiac output in patients with a Fontan circulation relative to healthy controls.
Methods and Results
Thirty subjects (20 healthy controls and 10 Fontan patients) underwent cardiac magnetic resonance imaging with simultaneous invasive pressure recording via a pulmonary and radial artery catheter during supine bicycle exercise to near maximal exertion. Adequacy of cardiac index, stroke volume, and HR reserve was assessed by determining the exercise‐induced increase (∆) in cardiac index, stroke volume, and HR relative to the increase in oxygen consumption (VO 2). HR reserve was lower in Fontan patients compared with controls (71±21 versus 92±15 bpm; P=0.001). In contrast, increases in HR relative to workload and VO 2 were higher than in controls. The change in cardiac index relative to the change in VO 2 (∆cardiac index/∆VO 2) was similar between groups, but Fontan patients had increased ∆HR/∆VO 2 and reduced ∆ stroke volume/∆VO 2 compared with controls. There was an early and marked reduction in stroke volume during exercise in Fontan patients corresponding with a plateau in cardiac output at a low peak HR.
Conclusions
In Fontan patients, the chronotropic response is appropriate relative to exercise intensity, implying normal sinoatrial function. However, premature reductions in ventricular filling and stroke volume cause an early plateau in cardiac output beyond which further increases in HR would be physiologically implausible. Thus, abnormal cardiac filling rather than sinoatrial node dysfunction explains the diminished HR reserve in Fontan patients.
Keywords: cardiac magnetic resonance imaging, chronotropic incompetence, exercise physiology, Fontan procedure, heart rate
Subject Categories: Congenital Heart Disease, Electrophysiology, Magnetic Resonance Imaging (MRI), Imaging, Physiology
Clinical Perspective
What Is New?
The determinants of cardiac output at rest and during exercise are very different in healthy controls and Fontan patients.
In normal individuals, cardiac output augmentation is achieved by an increase in heart rate and stroke volume, whereas in Fontan patients cardiac output only augments by increases in heart rate.
The increase in heart rate relative to workload or metabolic demand is robust or even enhanced in Fontan patients but then ceases to increase further at a lower peak exercise value, corresponding to a point at which stroke volume is falling abruptly and cardiac output is starting to plateau.
What Are the Clinical Implications?
Chronotropic constraint in well‐functioning Fontan patients is not a primary electrical phenomenon, but more likely a secondary feature caused by inadequate systemic ventricular filling, inappropriate ventricular contraction, and impaired cardiac output augmentation.
In Fontan patients, medications resulting in relative bradycardia during exercise may improve exercise capacity because of enhanced diastolic filling, whereas chronotropic medications or pacing would be expected to have negative effects.
Introduction
Chronotropic incompetence, broadly defined as the inability of the heart to increase its rate commensurate with increased activity or demand, is commonly observed in patients with a Fontan circulation and is associated with exercise limitation. The term “chronotropic incompetence” implies that the ability of the sinus node to respond appropriately to varying physical workload is impaired, suggesting that increasing heart rate (HR) reserve might improve exercise tolerance.1, 2 This assumes, however, that cardiac filling and stroke volume are replete throughout exercise, which may not be valid in patients with a Fontan circulation, in whom flow through the pulmonary vasculature may be challenged during exercise because of a lack of a presystemic pump.3
Despite the reported association between exercise intolerance and chronotropic incompetence,4, 5 the appropriateness of the HR response throughout exercise relative to exercise level has not been specifically studied in Fontan patients. This is clinically important because therapies that affect the chronotropic response to exercise are being actively investigated in these patients (ClinicalTrials.gov identifier: NCT02946892), and it could be argued that these are unlikely to be successful if the chronotropic response is not the primary abnormality. We hypothesized that the lower peak HR is a secondary phenomenon associated as consequence rather than cause of premature exercise exhaustion. To investigate the relationship between HR, ventricular filling, and exercise fatigue, we used exercise cardiac magnetic resonance (CMR) imaging in stable patients with a Fontan circulation as compared with healthy controls.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
Twenty healthy subjects volunteered to participate after responding to local advertisements. All subjects were (1) healthy; (2) had no history of cardiovascular disease, symptoms, or risk factors; and (3) had a normal ECG and transthoracic echocardiogram.
The Fontan group consisted of 10 patients followed in the pediatric cardiology or adult congenital heart disease clinic.6 All patients were clinically stable, which was defined as (1) the absence of a history of atrial arrhythmias or sinus node dysfunction, (2) stable exercise capacity over time, and (3) long‐term stable Fontan palliation. Two patients reported mild exercise intolerance consistent with a New York Heart Association class II, whereas the remaining patients were asymptomatic (New York Heart Association class I). None of the Fontan patients had significant atrioventricular valve regurgitation, a residual fenestration, or significant aorta‐pulmonary collaterals on their last invasive evaluation; all had normal sinus rhythm. Five patients had an intracardiac total cavopulmonary connection, and 5 had an extracardiac total cavopulmonary connection. Eight patients had a systemic left ventricle, and 2 had a systemic right ventricle.
The study protocol conformed to the Declaration of Helsinki and was approved by the local ethics committee. All patients provided informed consent.
Exercise Cardiac Magnetic Resonance Protocol
Before exercise, a 7F pulmonary artery catheter was inserted in the internal jugular vein and guided under fluoroscopy or pressure curve monitoring to the proximal right main pulmonary artery. A 20‐gauge arterial catheter was placed in the radial artery. In the cardiac magnetic resonance (CMR) suite, these catheters were attached to CMR‐compatible pressure transducers that were connected to a PowerLab recording system (AD Instruments, Oxford, United Kingdom).
Patients then underwent CMR at rest, and at low, moderate, and peak exercise using an electronically controlled supine CMR ergometer (Lode, Groningen, The Netherlands).7 Exercise intensities were determined by prior measurement of maximum exercise power using cardiopulmonary exercise testing, as determined previously.6, 7 Peak exercise power corresponded to the maximal exercise intensity that could be sustained for ≈120 seconds of data acquisition in the CMR bore. Pulmonary and systemic arterial pressures were continuously recorded throughout exercise and analyzed off‐line using LabChart v6.1.1 (AD Instruments). Using an in‐house developed software program (RightVol, Leuven, Belgium), end‐diastolic and end‐systolic volumes (EDVi, ESVi) of the systemic ventricle (left ventricle in controls) were calculated by a summation of disks and indexed for body‐surface area. From these measurements, stroke volume (SVi), cardiac index (CI), and ejection fraction were inferred.
At rest and at peak exercise, arterial and central venous blood samples were collected and analyzed for oxygen saturation (SaO2, ScvO2), oxygen tension (PaO2, PcvO2), and carbon dioxide tension (PaCO2, PcvCO2) using an automated blood gas analyzer (ABL 700, Radiometer; Copenhagen, Denmark). Arteriovenous O2 content difference C(a‐cv)O2 was measured directly as the difference between arterial and central venous oxygen content. Oxygen consumption (VO2) was calculated according to the Fick principle from CMR‐derived cardiac output, arterial and central venous oxygen saturations, and hemoglobin.
HR reserve (HRR) was defined as the difference between peak exercise and resting HR. Age‐predicted maximal HR was defined as 220−age. Adjusted HRR was determined as HRR divided by the difference between age‐predicted maximal HR and resting HR.4 Furthermore, similar to the approach by Wilkoff and Miller,8 adequacy of cardiac output, stroke volume, and HRR was assessed by determining the exercise‐induced increase in CI, SVi, and HR relative to the increase in VO2 (in mL/min per kg).
Statistical Analysis
Data were analyzed using IBM SPSS statistics 22 software. Gaussian distribution of all continuous variables was tested using a Kolmogorov–Smirnov test. Descriptive data for continuous variables are presented as means±SD or as medians (25th and 75th percentile) when appropriate. Categorical data were reported as numbers (percentage) and compared using the Fisher exact test. Comparisons between groups were performed using unpaired 2‐sample t tests or the Mann–Whitney test, as appropriate. The impact of exercise on biventricular volumes (EDVi, ESVi, SVi, and CI) and HR was evaluated by a linear mixed model that included workload and group (controls versus Fontan patients) and their interaction as fixed effects. To account for the repeated nature of the data, an unstructured variance–covariance matrix was included in the model and the Bonferroni post hoc test was used to correct for multiple comparisons. The interaction between group and workload was included to assess differences between groups. Individual slope coefficients of the relationships between HR and absolute workload (in watts) were derived from serial measurements of HR and exercise power during incremental exercise using linear regression analysis. Differences in HR‐workload slope coefficients between groups were compared using independent samples t test. The relationship between cardiac filling (SVi) and HR was estimated using a quadratic regression analysis. Differences in curves for each group (control versus Fontan patients) were compared using the extra sum‐of‐squares F test. Tests were 2‐sided and a P<0.05 was considered significant.
Results
Clinical Characteristics
The clinical characteristics and resting hemodynamic data are depicted in Table 1. Fontan patients were younger than the controls (20±4 versus 35±11 years; P<0.001). None of the subjects were receiving pulmonary vasodilator therapy and none was treated with bradycardic drugs. Fontan patients had a slightly higher resting HR than controls. Systemic ventricular volumes, ejection fraction (Figure 1), as well as CI were similar between both groups.
Table 1.
Baseline Characteristics
| Healthy Controls (n=20) | Fontan Patients (n=10) | P Value | |
|---|---|---|---|
| Clinical | |||
| Age, y | 35±11 | 20±4 | <0.0001 |
| BSA, m2 | 1.96±0.09 | 1.68±0.14 | <0.0001 |
| BMI, kg/m2 | 23.9±3.3 | 22.0±2.0 | 0.118 |
| Male sex, n (%) | 19 (95) | 6 (60) | 0.031 |
| Resting systemic ventricular volumes and hemodynamics | |||
| Heart rate, bpm | 62±10 | 72±14 | 0.030 |
| EDVi, mL/m2 | 107±17 | 100±17 | 0.336 |
| ESVi, mL/m2 | 43±9 | 44±16 | 0.967 |
| SVi, mL/m2 | 63±12 | 57±10 | 0.134 |
| Ejection fraction, % | 59.4±5.7 | 57.3±10.5 | 0.482 |
| CI, L/min per m2 | 3.9±0.7 | 4.1±0.9 | 0.546 |
| mPAP, mm Hg | 13±3 | 9±3 | 0.007 |
| mSAP, mm Hg | 93±9 | 81±7 | 0.001 |
| tPVR, dynes·s/cm5 | 140±44 | 119±69 | 0.306 |
| tSVR, dynes·s/cm5 | 1017±226 | 1003±234 | 0.875 |
| SatO2, % | 98.2±1.0 | 97.2±0.5 | 0.011 |
| ScvO2, % | 73.5±7.8 | 75.1±4.8 | 0.581 |
BMI indicates body mass index; BSA, body surface area; CI, cardiac index; EDVi, end‐diastolic volume index; ESVi, end‐systolic volume index; mPAP, mean pulmonary artery pressure; mSAP, mean systemic arterial pressure; ScvO2, central venous oxygen saturation; SatO2, arterial oxygen saturation; SVi, stroke volume index; tPVR, total pulmonary vascular resistance; tSVR, total systemic vascular resistance.
Figure 1.
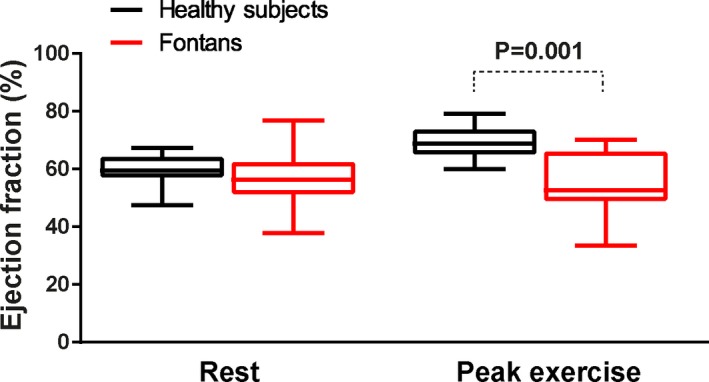
Distribution of systemic ventricular EF at rest and at peak exercise. At rest, EF was similar between groups, whereas at peak exercise EF was lower in the Fontan patients. EF indicates ejection fraction.
HR Reserve
As depicted in Table 2, augmentation of HR from rest to peak exercise was lower in Fontan compared with controls as expressed by impaired HRR (71±21 versus 92±15 bpm; P=0.001) and adjusted HRR (56±14 versus 76±11; P<0.001). Peak exercise HR was lower in Fontan patients than in controls (144±15 versus 155±13 bpm; 72±8 versus 84±8% of predicted; both P<0.05), whereas peak lactate levels were similar (4.4±1.3 versus 4.2±1.3 mmol/L), confirming exercise to a similarly intense level beyond ventilatory threshold. In contrast, for any given value of exercise power or oxygen consumption, HR was actually higher in the Fontan group than in controls (Figure 2A). Similarly, ∆HR/∆VO2 (∆HR 6.0±3.2 versus 3.7±2.5/mL/kg oxygen consumption; P=0.054) and ∆HR/∆W (∆HR 0.83±0.35 versus 0.51±0.09 /W; P=0.018) were higher in Fontan patients than in controls. The slope of the relationship between HR and workload was higher in Fontan patients than controls despite lower peak HR (Figure 2B), whereas the opposite was observed when HR was expressed as a percentage of maximal exercise power (Figure 2C).
Table 2.
Heart Rate Reserve
| Healthy Controls (n=20) | Fontan Patients (n=10) | P Value | |
|---|---|---|---|
| Resting HR, bpm | 62±10 | 72±14 | 0.030 |
| Peak HR, bpm | 155±13 | 144±15 | 0.047 |
| HRR, bpm | 71±21 | 92±15 | 0.001 |
| Adjusted HRR, bpm | 56±14 | 76±11 | <0.001 |
| ∆CI/∆VO2 | 0.26±0.09 | 0.26±0.10 | 0.925 |
| ∆HR/∆VO2 | 6.0±3.2 | 3.7±2.5 | 0.054 |
| ∆HR/∆W | 0.83±0.35 | 0.51±0.09 | 0.018 |
∆CI indicates change in cardiac index from rest to peak exercise; HR, heart rate; ∆HR, change in heart rate from rest to peak exercise; HRR, heart rate reserve; ∆VO2, change in oxygen consumption (in mL/min per kg) from rest to peak exercise; ∆W, change in exercise power (workload) from rest to peak exercise.
Figure 2.
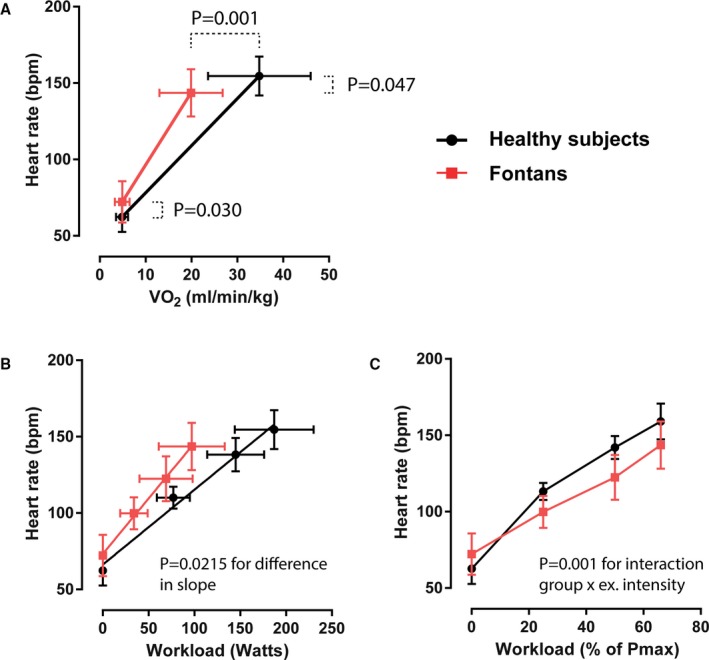
Heart rate response to exercise. Heart rate is expressed vs (A) metabolic demand (oxygen consumption [VO 2], (B) absolute power (in watts), and (C) percentage of maximal obtained exercise capacity determined by prior upright exercise testing. ex. intensity indicates exercise intensity. Data are presented as means and SD.
Exercise Hemodynamics
Changes in cardiac volumes and pressures are depicted in Table 3. In both controls and Fontan patients, there was a decrease in systemic ventricular EDVi during exercise. However, in controls EDVi was maintained through moderate‐intensity exercise and fell modestly at peak intensity (P=0.001; Figure 3). In Fontan patients, EDVi fell quite markedly from low‐ to moderate‐intensity exercise and then fell further at peak exercise (P=0.035). Systemic ventricular ESVi decreased progressively from rest to peak exercise in controls, whereas in Fontan patients ESVi did not change (P=0.001 for interaction). As a result, whereas controls had an increase in SVi during exercise, SVi decreased during exercise in the Fontan patients (P<0.001 for interaction; Figure 3). Figure 4 depicts the individual changes in CI, HR, and SVi from rest to peak exercise. Figure 5 compares the SVi response to exercise in the 5 youngest controls versus the 5 oldest Fontan patients (age 24±4 versus 22±4 years; P=0.612). The differences in the SVi pattern during exercise in these age‐matched groups were similar to those observed in the total study population.
Table 3.
Exercise Hemodynamics
| Healthy Controls (n=20) | Fontan Patients (n=10) | P Value | |
|---|---|---|---|
| Heart rate, bpm | 155±13 | 144±15 | 0.047 |
| EDVi, mL/m2 | 104±18 | 96±16 | 0.237 |
| ESVi, mL/m2 | 32±9 | 44±17 | 0.013 |
| SVi, mL/m2 | 72±12 | 52±9 | <0.001 |
| Ejection fraction, % | 69.7±5.4 | 54.8±10.9 | 0.002 |
| CI, L/min per m2 | 11.0±2.0 | 7.4±1.7 | <0.001 |
| mPAP, mm Hg | 27±7 | 21±5 | 0.022 |
| mSAP, mm Hg | 121±13 | 106±5 | <0.001 |
| tPVR, dynes·s/cm5 | 104±43 | 137±45 | 0.068 |
| tSVR, dynes·s/cm5 | 467±126 | 710±164 | <0.001 |
| SatO2, % | 97.0±2.7 | 94.3±1.2 | 0.005 |
| SatcvO2, % | 41.0±10.7 | 49.1±8.7 | 0.056 |
CI indicates cardiac index; EDVi, end‐diastolic volume index; ESVi, end‐systolic volume index; mPAP, mean pulmonary artery pressure; mSAP, mean systemic arterial pressure; SatcvO2, central venous oxygen saturation; SatO2, arterial oxygen saturation; SVi, stroke volume index; tPVR, total pulmonary vascular resistance; tSVR, total systemic vascular resistance.
Figure 3.
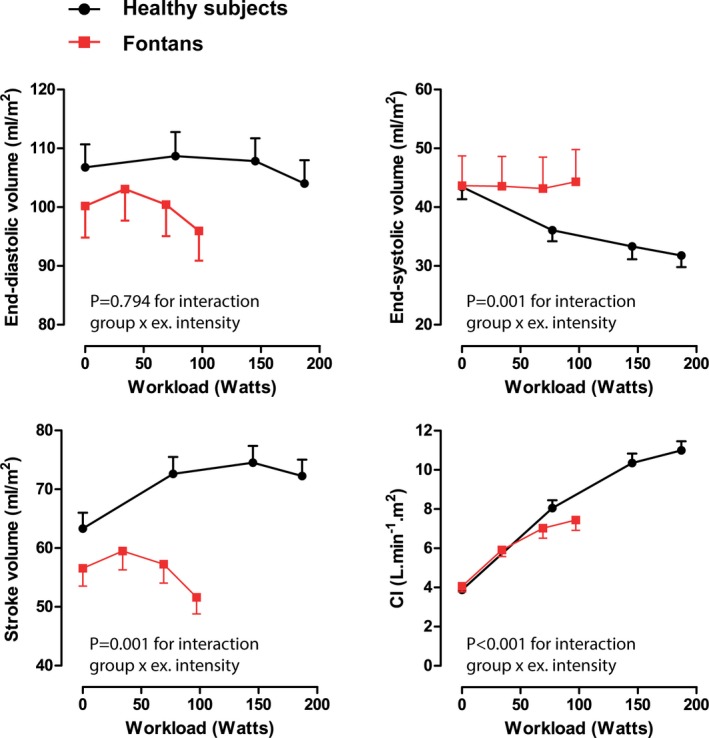
Changes in systemic ventricular volume and output during exercise. Changes in end‐diastolic volume, end‐systolic volume, stroke volume, and CI from rest to peak exercise in controls and Fontan patients. Data are presented as means and SEM at each time point. CI indicates cardiac index; ex. intensity, exercise intensity.
Figure 4.
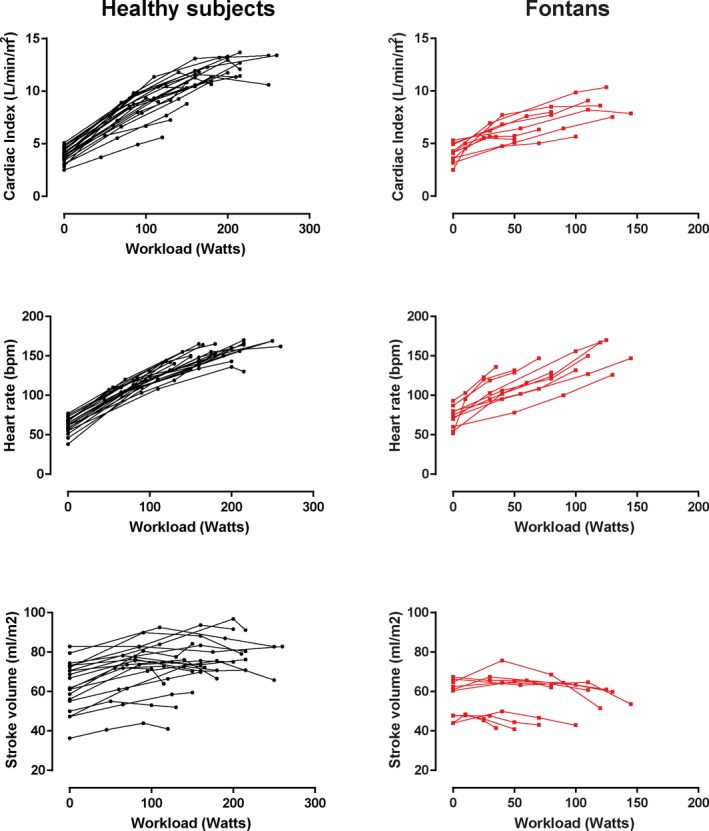
Individual changes in cardiac hemodynamics during exercise. Changes in cardiac index, heart rate, and stroke volume from rest to peak exercise are depicted in controls (left panels) and Fontan patients (right panels).
Figure 5.
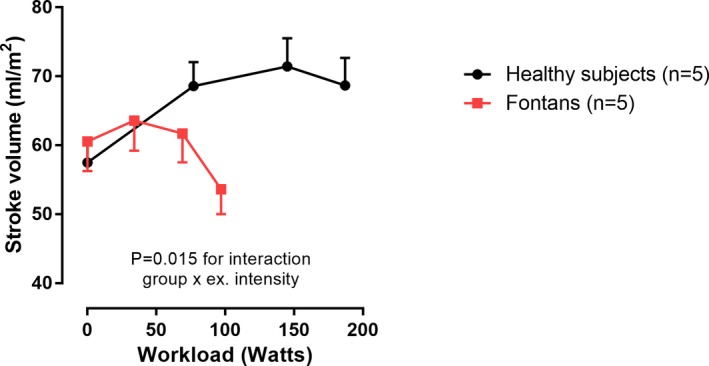
Stroke volume response to exercise in a subset of age‐matched Fontan patients and controls. Changes in stroke volume from rest to peak exercise in controls and Fontan patients. ex. intensity indicates exercise intensity. Data are presented as means and SEM at each time point.
Figure 6 depicts quadratic regression analysis of the mean values for SVi and cardiac output versus the average values of HR. A significantly different pattern of SVi augmentation is observed with a peak stroke volume occurring at a HR of ≈140 bpm in control subjects as compared with 95 bpm in Fontan patients. For the purpose of this regression analysis, the pattern of SVi and cardiac output in the higher HR ranges is assumed to follow the quadratic trend.
Figure 6.
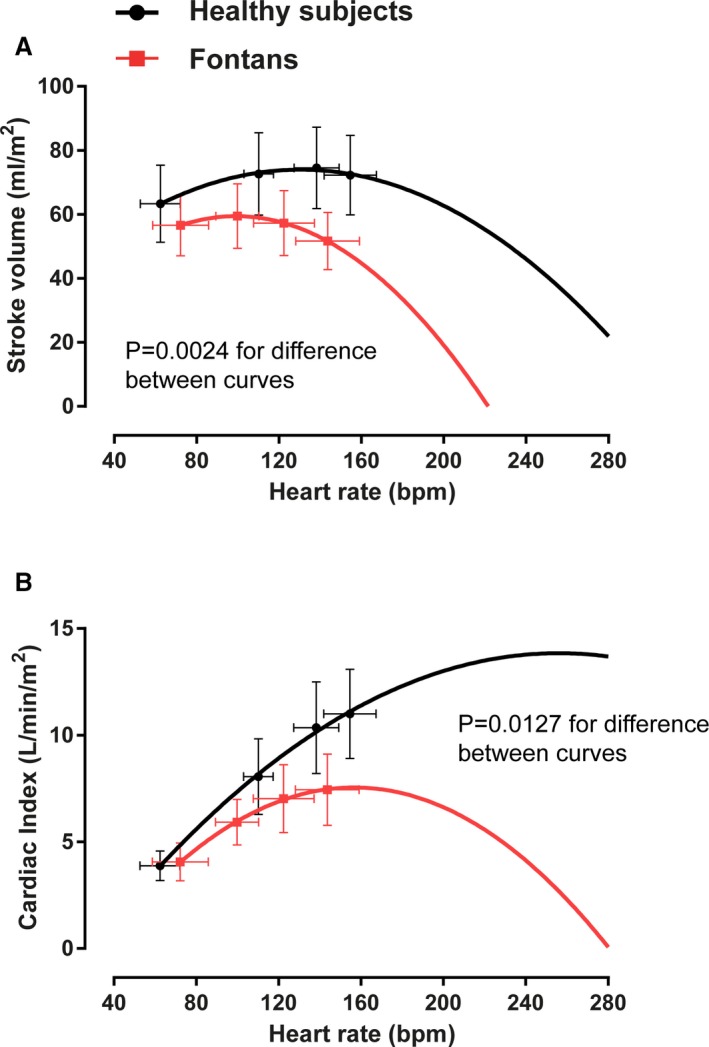
Quadratic regression analysis of mean stroke volume and cardiac output vs average heart rate values. In the Fontan patients, an additional increase in heart rate beyond peak exercise values would result in (A) a disproportionate fall in stroke volume such that (B) cardiac output cannot increase further.
Discussion
Our study demonstrates that the determinants of cardiac output at rest and during exercise are very different in healthy controls and Fontan patients. In normal individuals, cardiac output augmentation is achieved by an increase in HR and stroke volume, which in turn is determined by increased contractility and nonlimited preload. On the contrary, in Fontan patients, the exercise‐induced increase in cardiac output is primarily achieved by increases in HR. We show that the increase in HR relative to workload or metabolic demand is robust or even enhanced in Fontan patients but then ceases to increase further at a lower peak exercise value. This corresponds to a point at which stroke volume is falling abruptly and cardiac output is starting to plateau, which suggests that further increases in HR would not be physiologically possible. In other words, this suggests that the chronotropic constraint in Fontan patients is more likely a secondary phenomenon caused by inadequate systemic ventricular filling, inappropriate ventricular contraction, and impaired cardiac output augmentation rather than true “chronotropic incompetence.”
HR during exercise is determined by the sinus node function, the local effect of autonomic innervation, circulating catecholamines, and increased ventricular preload. Previous studies have used the term “chronotropic incompetence” when peak exercise HR failed to reach a lower arbitrary percentage (either 85%, 80%, or, less commonly, 70%) of the age‐predicted maximal HR.4 However, chronotropic “incompetence” implies that the ability of the sinus node to respond appropriately to varying physical workload is impaired. Therefore, Wilkoff and Miller utilized the expired gas analysis technique to calculate the metabolic–chronotropic relationship from the ratio of the HRR to the metabolic reserve during submaximal exercise.8 This physiological concept enables evaluation of whether a single HR value at any point during the exercise protocol is consistent with normal chronotropic function.8 Using this approach, previous investigators demonstrated that patients with sick sinus syndrome were unable to accelerate their HR to the level of normal subjects at a comparable exercise‐induced VO2 value.9, 10 In Fontan patients, it has been shown that the sinus node may be dysfunctional, either congenital or by damage caused by multiple surgeries (direct damage or indirectly by damaging the arterial supply or innervation). In this study, however, we demonstrate that in a selected cohort of well‐functioning Fontan patients, HR was actually higher at any given value of VO2 than in healthy subjects, indicating that the capacity to increase HR during exercise was intact in these patients.
The question then arises as to why HRR is reduced in these patients with Fontan physiology. Although the increase in HR relative to metabolic load is increased relative to controls, the HR augmentation in Fontan patients stops abruptly at a low peak value. This lower peak exercise HR might constitute a “brake” in Fontan patients that serves to prevent the precipitous falls in stroke volume and cardiac output that would be expected if further increases in HR were possible. The extrapolated relationships between cardiac output and HR in Figure 6 provide a hypothetical illustration of this principle and depict a premature inflection point beyond which continued increases in exercise would not be considered possible because of a falling cardiac output.
This attenuated increase in cardiac output can be explained by the lower stroke volume in Fontan patients relative to controls, and this is further exacerbated by quite profound reductions in stroke volume that occur at only mild‐to‐moderate exercise intensities. The cause of these early and profound reductions in stroke volume during exercise may be explained by abnormalities in cardiac filling, reductions in systolic contractility, or both. Stroke volume represents the amount of blood ejected during systole and is often equated with contractile function. However, it is also important to remember that stroke volume also represents the amount of ventricular filling that is possible during diastole and may therefore be constrained by diastolic factors. The typical Fontan physiology is defined by blunted transpulmonary blood flow because of a lower transpulmonary pressure gradient in the absence of a presystemic pump. This is expected to result in reduced left‐sided filling.6 The connection between this phenomenon and a physiological chronotropic limitation might be explained by the Bainbridge reflex. In 1915, Bainbridge conducted elegant canine studies in which he observed an immediate and marked augmentation in HR following increases in venous pressures and cardiac filling. This phenomenon appeared to be independent of arterial blood pressure and autonomic innervation.11 Bainbridge concluded that an intrinsic cardiac reflex enabled increases in venous pressure to be transferred to the arterial system without excessive dilation of the heart by means of increased HR and, conversely, that reductions in HR enabled adequate cardiac filling when venous pressures fell.
Consistent with the premise that the diminished HR reserve in Fontan patients may be explained by inadequate ventricular filling, previous studies have demonstrated that atrial pacing at rest in Fontan patients does not augment output,12 and that pacing beyond maximal HRs does not improve aerobic capacity.13, 14 Therefore, as demonstrated in Figure 3, systemic ventricular preload is impaired at low‐intensity exercise such that SVi fails to augment normally, and in fact decreases during moderate and intensive exercise. Further increases in exercise intensity require a commensurate increase in cardiac output, which is not possible in the Fontan circuit: transpulmonary flow cannot be augmented, and a further increase of HR will result in a disproportionate fall in SVi, as illustrated in Figure 6. This would explain the clinical observation that supraventricular arrhythmias are poorly tolerated by Fontan patients.
It cannot be distinguished whether the compromised systemic ventricular filling during exercise and the lower maximum HR are intrinsically linked or whether there is just an indirect relationship. The Bainbridge reflex could be a way to explain causality. However, it is also possible that maximal exercise capacity and maximal HR are reached prematurely because cardiac output cannot be maintained, rather than implying a direct feedback mechanism. Furthermore, although inadequate ventricular filling may explain the impaired SVi response to exercise in Fontan patients, the lack of a change in end‐systolic volume also suggests that contractile reserve may be impaired. This is in line with previous studies showing a diminished contractile response with elevated HR in Fontan physiology.15
Clinical Implications
The novel and important clinical implication of this study is that a lower HRR in Fontan patients is not a primary electrical phenomenon and that any increases in HR during exercise would be expected to reduce exercise performance. Just as for other conditions associated with systemic ventricular inflow restriction (Mustard,16 Senning,17 and mitral stenosis), a HR inappropriate for cardiac output is dangerous and may result in hypotension and death. The term “chronotropic limitation” may be more appropriate to describe the reduced HRR rather than chronotropic incompetence, which has previously been associated with impairment of the sinoatrial pacemaker apparatus. The suggestion that reduced HRR, in certain patient populations, may be the consequence of impaired exercise hemodynamics rather than constituting a primary electrical phenomenon is clinically relevant because therapies that affect the chronotropic response to exercise, such as β‐blockers and ivabradine, are being actively investigated as a novel therapeutic target in Fontan patients.18 Our data would support the hypothesis that medications resulting in relative bradycardia during exercise may improve exercise capacity because of enhanced diastolic filling, whereas chronotropic medications or pacing would be expected to have negative effects.
Limitations
The small sample size was an expected limitation of this study, given the constraints of recruiting healthy subjects for an invasive study protocol and the low community prevalence of patients with a Fontan circulation. Because of its low prevalence, the Fontan population was relatively mixed including both patients with intracardiac and extracardiac conduits as well as different ventricular geometry. However, the dominant feature of all patients was Fontan physiology. Moreover, previous studies did not reveal any association between ventricular geometry and exercise capacity or outcome.19 The Fontan patients were significantly younger, more likely to be female, and had lower body surface area, but this is unlikely to explain the observed differences in HRR. Rather, the younger age would be expected to diminish the observed differences. Furthermore, the overall cardiovascular response to exercise has been shown to be similar in men and women.20
VO2 was calculated by the direct Fick method as the product of cardiac output derived by CMR and C(a‐cv)O2 measured by blood gas collection rather than direct measurement of expiratory gases because of incompatibilities between the cardiopulmonary testing equipment and the CMR environment. Central venous gases rather than true mixed venous gases were obtained because of the complex geometry of the Fontan circulation (eg, tubing offset and connection angles). The development of CMR‐compatible ergospirometry systems may enable simultaneous evaluation of cardiac volumes and expiratory gases, thereby avoiding the need for invasive blood gas measurements in future studies.21 It should be pointed out that the exercise CMR protocol consisted of supine cycling exercise. It is well known that changes in HR and hemodynamics depend on body position (supine versus upright) because of the difference in venous return.22, 23 During supine posture, passive left ventricular filling is enhanced by the effects of gravity. Therefore, the observed reduction in EDVi and SVi during exercise may be even more apparent during exercise in the upright position. Although sinus node dysfunction is a known complication after Fontan operations,24 this study specifically focused on well‐functioning Fontan patients without established sinus node dysfunction. The slope of HR versus workload or VO2 is affected by the individuals’ exercise capacity, which may partly explain the attenuated slope of HR to VO2 (∆HR/∆VO2) in the control subjects. Nevertheless, ∆HR/∆VO2 in the Fontan population was 2 times higher than that observed in a prior study in patients with sick sinus syndrome,9 thereby arguing against overt sinoatrial dysfunction in our cohort of well‐functioning Fontan patients.
Because of the small, but potentially serious, potential for adverse events in performing pulmonary capillary wedge pressure measurements during exercise, no wedge pressure measurements were obtained as part of the exercise protocol. Thus, we cannot directly validate our assertion that reduced left atrial pressures were the prequel to impaired ventricular filling and reduced left ventricular EDVi during exercise. We did not find an association between HRR and pulmonary artery pressures. However, the latter do not only depend on left‐sided filling pressures but also on the characteristics of the pulmonary circulation (resistance and compliance) and flow. Finally, peak HRs in the CMR bore were slightly lower than true peak values in all groups because peak exercise power needed to be sustained for ≈120 seconds of data acquisition during supine cycling.
Conclusion
At any given exercise intensity, chronotropic responsiveness is preserved, and even increased, in Fontan patients, indicating normal sinoatrial function. However, exercise capacity and maximum HR are attenuated, likely as a consequence of reduced systemic ventricular filling, falling stroke volume, and a premature plateau in cardiac output. Thus, it would appear that the diminished HRR observed in Fontan patients is a secondary phenomenon rather than an incompetence of the sinoatrial pacemaker apparatus.
Sources of Funding
This study was funded by a grant from the Fund for Scientific Research Flanders (FWO) and by The Van Itterbeek and the Eddy Merckx Research grant. Claessen is supported by a postdoctoral research grant from the Frans Van De Werf Fund for Clinical Cardiovascular Research, the UZ Leuven Future Fund, and from the Mathilde Horlait‐Dapsens Scholarship. La Gerche is supported by the Fund for Scientific Research Flanders (FWO) and from the National Health and Medical Research Council (NHMRC) of Australia; Willems is supported as a postdoctoral clinical researcher by the Fund for Scientific Research Flanders (FWO).
Disclosures
None.
Acknowledgments
First and foremost, we would like to thank all patients for their participation in the study. We would also like to thank Stefan Ghysels, Guido Putzeys, and Chris Byloos for their help in performing the exercise MRI studies, and Astrid Vloemans and Sonia Rens for their help with the logistics.
(J Am Heart Assoc. 2019;8:e012008 DOI: 10.1161/JAHA.119.012008.)
References
- 1. Diller GP, Dimopoulos K, Okonko D, Uebing A, Broberg CS, Babu‐Narayan S, Bayne S, Poole‐Wilson PA, Sutton R, Francis DP, Gatzoulis MA. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol. 2006;48:1250–1256. [DOI] [PubMed] [Google Scholar]
- 2. Diller GP, Okonko DO, Uebing A, Dimopoulos K, Bayne S, Sutton R, Francis DP, Gatzoulis MA. Impaired heart rate response to exercise in adult patients with a systemic right ventricle or univentricular circulation: prevalence, relation to exercise, and potential therapeutic implications. Int J Cardiol. 2009;134:59–66. [DOI] [PubMed] [Google Scholar]
- 3. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimiaie J, Sherez J, Aviram G, Megidish R, Viskin S, Halkin A, Ingbir M, Nesher N, Biner S, Keren G, Topilsky Y. Determinants of effort intolerance in patients with heart failure: combined echocardiography and cardiopulmonary stress protocol. JACC Heart Fail. 2015;3:803–814. [DOI] [PubMed] [Google Scholar]
- 6. Van De Bruaene A, La Gerche A, Claessen G, De Meester P, Devroe S, Gillijns H, Bogaert J, Claus P, Heidbuchel H, Gewillig M, Budts W. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging. 2014;7:265–273. [DOI] [PubMed] [Google Scholar]
- 7. La Gerche A, Claessen G, Van de Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: a new gold standard for ventricular volume quantification during high‐intensity exercise. Circ Cardiovasc Imaging. 2013;6:329–338. [DOI] [PubMed] [Google Scholar]
- 8. Wilkoff BL, Miller RE. Exercise testing for chronotropic assessment. Cardiol Clin. 1992;10:705–717. [PubMed] [Google Scholar]
- 9. Holden W, McAnulty JH, Rahimtoola SH. Characterisation of heart rate response to exercise in the sick sinus syndrome. Br Heart J. 1978;40:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbott JA, Hirschfeld DS, Kunkel FW, Scheinman MM, Modin G. Graded exercise testing in patients with sinus node dysfunction. Am J Med. 1977;62:330–338. [DOI] [PubMed] [Google Scholar]
- 11. Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol. 1915;50:65–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barber G, Di Sessa T, Child JS, Perloff JK, Laks H, George BL, Williams RG. Hemodynamic responses to isolated increments in heart rate by atrial pacing after a Fontan procedure. Am Heart J. 1988;115:837–841. [DOI] [PubMed] [Google Scholar]
- 13. Paridon SM, Karpawich PP, Pinsky WW. The effects of rate responsive pacing on exercise performance in the postoperative univentricular heart. Pacing Clin Electrophysiol. 1993;16:1256–1262. [DOI] [PubMed] [Google Scholar]
- 14. Karpawich PP, Paridon SM, Pinsky WW. Failure of rate responsive ventricular pacing to improve physiological performance in the univentricular heart. Pacing Clin Electrophysiol. 1991;14:2058–2061. [DOI] [PubMed] [Google Scholar]
- 15. Senzaki H, Masutani S, Ishido H, Taketazu M, Kobayashi T, Sasaki N, Asano H, Katogi T, Kyo S, Yokote Y. Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol. 2006;47:2528–2535. [DOI] [PubMed] [Google Scholar]
- 16. Derrick GP, Narang I, White PA, Kelleher A, Bush A, Penny DJ, Redington AN. Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load‐independent indexes of right ventricular performance after the Mustard operation. Circulation. 2000;102:III154–III159. [DOI] [PubMed] [Google Scholar]
- 17. Helsen F, Claus P, Van De Bruaene A, Claessen G, La Gerche A, De Meester P, Gabriels C, Claeys M, Petit P, Santens B, Troost E, Voigt JU, Bogaert J, Budts W. Advanced imaging to phenotype patients with a systemic right ventricle. J Am Heart Assoc. 2018;7:e009185 DOI: 10.1161/JAHA.118.009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruijsink JB, Duong P, Pushparajah K, Frigiola A, Nordsletten D, Razavi R. 6014 selective heart rate inhibition improves inadequate exercise response in Fontan circulation. Eur Heart J. 2018;39:ehy566.6014–ehy6566.6014. [Google Scholar]
- 19. Atz AM, Zak V, Mahony L, Uzark K, D'Agincourt N, Goldberg DJ, Williams RV, Breitbart RE, Colan SD, Burns KM, Margossian R, Henderson HT, Korsin R, Marino BS, Daniels K, McCrindle BW; Pediatric Heart Network Investigators . Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol. 2017;69:2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu Q, Levine BD. Cardiovascular response to exercise in women. Med Sci Sports Exerc. 2005;37:1433–1435. [DOI] [PubMed] [Google Scholar]
- 21. Barber NJ, Ako EO, Kowalik GT, Steeden JA, Pandya B, Muthurangu V. MR augmented cardiopulmonary exercise testing‐a novel approach to assessing cardiovascular function. Physiol Meas. 2015;36:N85–N94. [DOI] [PubMed] [Google Scholar]
- 22. Bevegard S, Holmgren A, Jonsson B. The effect of body position on the circulation at rest and during exercise, with special reference to the influence on the stroke volume. Acta Physiol Scand. 1960;49:279–298. [DOI] [PubMed] [Google Scholar]
- 23. Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation. 1980;62:528–534. [DOI] [PubMed] [Google Scholar]
- 24. Bae EJ, Lee JY, Noh CI, Kim WH, Kim YJ. Sinus node dysfunction after Fontan modifications—influence of surgical method. Int J Cardiol. 2003;88:285–291. [DOI] [PubMed] [Google Scholar]


