Abstract
Background
Sodium (Na+) in saline water may increase blood pressure (BP), but potassium (K+), calcium (Ca2+), and magnesium (Mg2+) may lower BP. We assessed the association between drinking water salinity and population BP.
Methods and Results
We pooled 6487 BP measurements from 2 cohorts in coastal Bangladesh. We used multilevel linear models to estimate BP differences across water salinity categories: fresh water (electrical conductivity, <0.7 mS/cm), mild salinity (electrical conductivity ≥0.7 and <2 mS/cm), and moderate salinity (electrical conductivity ≥2 and <10 mS/cm). We assessed whether salinity categories were associated with hypertension using multilevel multinomial logistic models. Models included participant‐, household‐, and community‐level random intercepts. Models were adjusted for age, sex, body mass index (BMI), physical activity, smoking, household wealth, alcohol consumption, sleep hours, religion, and salt consumption. We evaluated the 24‐hour urinary minerals across salinity categories, and the associations between urinary minerals and BP using multilevel linear models. Compared with fresh water drinkers, mild‐salinity water drinkers had lower mean systolic BP (−1.55 [95% CI: −3.22–0.12] mm Hg) and lower mean diastolic BP (−1.26 [95% CI: −2.21–−0.32] mm Hg) adjusted models. The adjusted odds ratio among mild‐salinity water drinkers for stage 1 hypertension was 0.60 (95% CI: 0.43–0.84) and for stage 2 hypertension was 0.56 (95% CI: 0.46–0.89). Mild‐salinity water drinkers had high urinary Ca2+, and Mg2+, and both urinary Ca2+ and Mg2+ were associated with lower BP.
Conclusions
Drinking mild‐salinity water was associated with lower BP, which can be explained by higher intake of Ca2+ and Mg2+ through saline water.
Keywords: blood pressure, calcium, drinking water salinity, magnesium, potassium, sodium, water salinity
Subject Categories: Epidemiology, Diet and Nutrition, Hypertension
Short abstract
See Editorial Bispham and Nowak
Clinical Perspective
What Is New?
Higher drinking water salinity or mineral contents are associated with higher urinary sodium, calcium, and magnesium concentrations.
Blood pressure lowering effects of calcium and magnesium overweighed the blood pressure increasing effects of sodium, reflecting an overall inverse association between drinking water salinity, and blood pressure.
What Are the Clinical Implications?
High sodium or low calcium or magnesium content in patients’ drinking water can increase their blood pressure and risks for hypertension.
Adding calcium and magnesium to drinking water may be a useful strategy for reducing the population burden of hypertension when drinking water sources have low levels of these minerals.
Introduction
Globally, >1 billion people living in coastal areas rely on groundwater as their principal water source.1 Nearly 204 million of them reside in areas that are affected by seawater intrusion,2 a process that increases groundwater salinity because of movement of the fresh‐saline groundwater interface towards the inland along the shores.3 Seawater intrusion will affect more coastal regions in the future because of increased volume of groundwater extraction to meet the population demand and global climate change such as change in precipitation patterns affecting groundwater recharge, decreased upstream river flow, frequent cyclones and sea‐level rise.4
Seawater intrusion causes mineralization of the groundwater.5 Communities in seawater intrusion affected areas drink brackish groundwater, rainwater, surface water (eg, pond water), or desalinated water.6 The salinity of these water sources varies as does the mineral concentrations; however, limited data exist on drinking water salinity, mineral intake, and cardiovascular health of the population. Drinking saline water has been associated with high sodium (Na+) intake,7 high blood pressure (BP),8 and high incidence of preeclampsia in seawater intrusion affected southwest coastal Bangladesh.9
Water salinity often refers to sodium chloride concentration, but in hydrogeology water salinity is measured as electrical conductivity (EC)—the ability of water to conduct electrical current or electrons where all dissolved ions are the conductors.10 The major cations contributing to water EC are Na+, calcium (Ca2+), potassium (K+), and magnesium (Mg2+)11—these are also the main macro‐minerals influencing human cardiovascular health. Most published studies from Bangladesh considered Na+ intake and urinary Na+ as a result of exposure to water salinity (Table 1),7, 8, 9, 12, 13 and therefore could not assess the health effects of other minerals present in brackish or saline water. Epidemiological studies, however, suggest that K+,14 Mg2+,15 and Ca2+,16 intake have inverse associations with BP and cardiovascular diseases. Drinking high‐salinity water may increase BP because of high Na+ concentration but may also lower BP if saline water contains high concentrations of K+, Mg2+, and Ca2+. In contrast, low‐salinity drinking water can reduce the intake of harmful Na+, but can also reduce intake of salubrious K+, Mg2+, and Ca2+. Data are limited on how all minerals together in saline water contribute to BP. We analyzed data from 2 studies to determine the association between drinking water salinity with BP, urinary Na+, K+, Ca2+, and Mg2+ excretion.
Table 1.
Summary of Published Articles Examining Salinity and Blood Pressure in Southwest Coastal Bangladesh
| Studies From Southwest Coastal Bangladesh | Salinity Measurement | Outcomes | Study Design | Study Duration | Geographical Coverage |
|---|---|---|---|---|---|
| Al Nahian et al12 | Electrical conductivity | Hypertension | Longitudinal |
Feb 2014 to Feb 2015 3 visits, 4 months apart |
9 districts of coastal Bangladesh |
| Scheelbeek et al13 | Na+ in water | Blood pressure of adult population | Cohort study | March 2013, March 2014, May 2014 | 3 subdistricts of same district |
| Talukder et al8 | Electrical conductivity | Blood pressure | Cross‐sectional | May to June 2014 | 1 subdistrict |
| Khan et al9 | Na+ in water | Preeclampsia | Case‐control | October 2009 to April 2011 | 1 subdistrict |
| Khan et al7 | Electrical conductivity | Hypertension in pregnancy | Observational | October 2009 to March 2010 | 1 subdistrict |
Methods
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. We pooled data from 2 studies led by the International Centre for Diarrhoeal Disease Research, Bangladesh across 3 seawater intrusion affected districts in southwest coastal Bangladesh (Figure 1). We pooled 6487 BP measurements and mineral concentrations of 6391 urine samples (Figure 2). The studies were implemented as part of a health impact evaluation of a drinking water salinity lowering intervention called managed aquifer recharge, a technology of artificially recharging brackish aquifers with rainwater and pond water to lower salinity.17 The first was an observational study that followed 383 participants from 166 households from 4 communities and visited each twice when participants drinking water salinity was low: once during the pre‐monsoon (May 10, 2016–June 20, 2016) and subsequently during the monsoon (July 20, 2016–August 20, 2016). The second study was a stepped‐wedge randomized trial (n=1191 from 542 households, followed for 5 visits) to assess the health impacts of water access across 16 communities during the dry season from December 2016 to April 2017 when participants drinking water salinity was high.17 The interval of both visits in the first study was 2 months and the interval between each successive visit of the second study was 1 month (Figure 2).
Figure 1.
Map of the study sites. RCT indicates randomized controlled trial.
Figure 2.
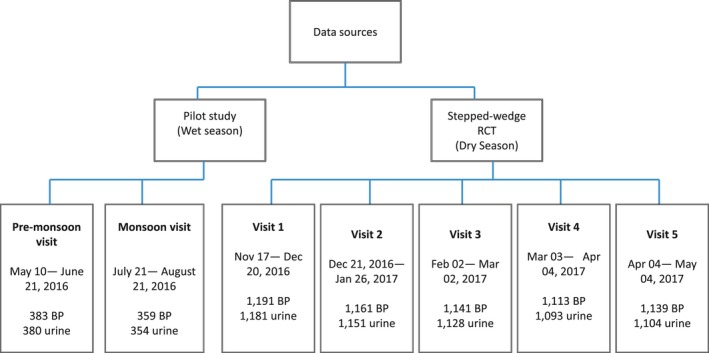
Data sources and study profiles. BP indicates blood pressure; RCT, randomized controlled trial.
Electrical Conductivity Measurement
During each visit, we recorded household‐reported primary drinking water sources used in the previous 24 hours and asked whether they had stored drinking water in their households. We collected available household stored drinking water samples and measured the temperature‐adjusted EC at 25°C during the visit using a Hanna Salinity meter (model: H198192, accuracy: ±1%). We calibrated the Salinity meters every 10 days.
Blood Pressure Risk Factors
We collected data on demographics (age, sex, body mass index [BMI]), household assets, participant‐reported smoking status (never, current, and former smoker), and work‐related physical activity (vigorous, moderate, and sedentary). We also collected data on use of table salt during cooking (yes or no), consumption of additional table salt with food (yes or no), alcohol consumption (yes or no), hours of sleep (<6, ≥6 to <9, and ≥9 hours), and self‐reported disease status (hypertension, diabetes mellitus, and chronic kidney diseases) using a structured questionnaire. We used the World Health Organization (WHO) Global Physical Activity Questionnaire for determining participants physical activity status.18 Participants’ weight was measured in all visits using a Seca weight machine (model: 874‐1321009; accuracy: 0.05–0.1 kg, Hamburg, Germany) and height in 1 visit using a Shorr board (accuracy: 1/8” or 0.1 cm; Olney, Maryland).
Outcomes
Blood pressure
During the same visit, participants’ systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at each visit day (between 7.30 am and 2.00 pm) using an Omron HEM–907 (accuracy: within ±4 mm Hg, Kyoto, Japan) digital monitor usually by research staff of the same sex.19 Blood pressure was measured following WHO guidelines for BP measurement20 and the recommendations described by Pickering et al.21 Caffeine (tea, coffee, carbonated beverages), eating, heavy physical activities, and smoking were prohibited for 30 minutes before measuring BP. The blood pressure measurement procedure was described to participants who rested for at least 5 minutes on a chair in the sitting position with arms supported. An appropriately sized cuff based on mid‐upper arm circumference was used (small size cuff if mid‐upper arm circumference <22 cm; medium size cuff if mid‐upper arm circumference ≥22 to <32 cm; and large size if cuff ≥32 cm). BP was measured 3 times while in the sitting position; first left arm, then right arm, then again left arm—the arithmetic mean was used for analyses.
24‐hour urine collection
We measured 24‐hour urine volume of the participants in all visits to measure the daily urinary excretions of minerals relevant to cardiovascular health, creatinine, and total protein. Twenty‐four‐hour urine volume also provided the information on daily water consumption by the participants. All participants received a 4‐L plastic container for 24‐hour urine collection and a mug to transfer the voided urine to the 4‐L plastic container. We instructed the participants to discard the first morning urine and start collecting from the second void,22 and to transfer all other voids of the day, and the next first morning.23 Volume of 24‐hour urine samples was measured at the household, and 15‐mL samples from the 4‐L plastic container were taken after stirring. We transported urine samples to a field laboratory at 2 to 8°C for processing, aliquoting, and analysis on the same day.
Twenty‐four‐hour urinary Na+, K+, Ca2+, Mg2+, creatinine, and total protein
Direct ion selective electrode methods, commonly used in clinical biochemistry laboratories with high agreement with the conventional flame photometer,24 were used to measure the urinary Na+ and K+ in all samples with a semi‐auto electrolyte analyzer (Biolyte 2000, Bio‐care Corporation, Taiwan, coefficient of variation: ±5%). Urinary Ca2+ and Mg2+ were measured by photometric titration methods using a semi‐auto biochemistry analyzer (Evolution 3000, BSI, Italy, coefficient of variation: <1%). Laboratory staff followed the manufacturer's guidelines for conditioning and calibration. We measured urine creatinine by a colorimetric method (Jaffe reaction). Urine total protein was measured using a colorimetric method by a semi‐auto biochemistry analyzer (Evolution 3000, BSI, Italy, coefficient of variation: <1%).
Statistical Analyses
Descriptive statistics
We calculated mean and SD of approximately normally distributed variables, median and interquartile range of skewed variables, and proportions for categorical variables. We used the 2‐sample test of proportions or the Wilcoxon rank‐sum test, as applicable, to compare the proportions or medians with respect to reference group. We derived the household wealth score by principal component analysis using data for ownership of a refrigerator, television, mobile phones, motorcycle, bicycle, sewing machine, chair, table, wristwatch, wardrobe, wooden cot, motor pump, rice husking machine, motorized rickshaw, car, and access to electricity. We then categorized the wealth score into household wealth quintiles. We calculated pairwise Spearman correlations between drinking water EC and SBP.
Water salinity and blood pressure associations
The associations of concurrent water EC categories with mean SBP and DBP were modeled using multilevel linear models. EC categories were defined by the Food and Agricultural Organization of the United Nations: fresh water (EC <0.7 mS/cm), mild salinity (EC ≥0.7 and <2 mS/cm), and moderate salinity (EC ≥2 and <10 mS/cm).25 All regression models included 3‐level random intercepts to account for multilevel clustering of longitudinal visits within participants, participants within households, and households within communities. We estimated models using the maximum likelihood and reported cluster robust standard errors. We reported findings of unadjusted models (model 1); models adjusted for age, sex, BMI (model 2); and models that additionally adjusted for smoking, physical activities, alcohol consumption, consumption of additional table salt with food, sleep hours categories, religion, and household wealth (model 3). Age and BMI were used as continuous variables in the models, but other covariates were used as categorical variables (Table 2). Addition of table salt during cooking was not used in the model since 100% households reported to add salt during cooking; however, we adjusted models for the consumption of additional table salt with food.
Table 2.
Characteristics of the Participants and Households at Enrollment
| Characteristics | Drinking Water Electrical Conductivity (EC) Categories | |||||
|---|---|---|---|---|---|---|
| Fresh Water (EC <0.7 mS/cm, n=547) | P Value | Mild‐Salinity Water (EC: 0.7 to <2 mS/cm, n=523) | P Value | Moderate‐Salinity Water (EC: 2–10 mS/cm, n=503) | P Value | |
| Age (y), median (IQR) | 40 (31–54) | Ref | 41 (30–54) | 0.900 | 40 (30–54) | 0.672 |
| Age categories, % (n) | ||||||
| 20 to <30 y | 21 (117) | Ref | 23 (122) | 0.709 | 22 (110) | 0.855 |
| 30 to <40 y | 27 (150) | Ref | 25 (130) | 0.704 | 27 (137) | 1.000 |
| 40 to <50 y | 20 (112) | Ref | 20 (105) | 1.000 | 21 (105) | 0.855 |
| 50 to <60 y | 15 (82) | Ref | 16 (82) | 0.860 | 17 (87) | 0.723 |
| 60 to <70 y | 11 (58) | Ref | 10 (54) | 0.863 | 9 (43) | 0.742 |
| ≥70 y | 5 (28) | Ref | 6 (30) | 0.868 | 4 (21) | 0.868 |
| Male sex, % (n) | 41 (226) | Ref | 41 (214) | 1.000 | 40 (203) | 0.833 |
| BMI, median (IQR) | 22.3 (19.5–25) | Ref | 21.6 (19.4–23.9) | 0.006 | 21.4 (18.9–23.9) | <0.001 |
| WHO BMI categories, % (n) | ||||||
| Underweight (<18.5) | 15 (79) | Ref | 16 (81) | 0.861 | 19 (94) | 0.487 |
| Normal weight (18.5 to <25) | 59 (317) | Ref | 67 (339) | 0.034 | 64 (321) | 0.194 |
| Overweight (≥25 to <30) | 22 (118) | Ref | 15 (75) | 0.229 | 14 (71) | 0.175 |
| Obese (≥30) | 4 (23) | Ref | 3 (14) | 0.875 | 3 (13) | 0.877 |
| Smoking categories, % (n) | ||||||
| Never | 54 (294) | Ref | 49 (258) | 0.241 | 53 (267) | 0.813 |
| Former | 9 (47) | Ref | 12 (61) | 0.617 | 8 (40) | 0.868 |
| Current | 38 (206) | Ref | 39 (204) | 0.835 | 39 (196) | 0.837 |
| WHO work‐related physical activity, % (n) | ||||||
| Sedentary | 37 (205) | Ref | 42 (219) | 0.293 | 12 (59) | <0.001 |
| Moderatea | 39 (215) | Ref | 34 (178) | 0.306 | 71 (355) | <0.001 |
| Vigorousb | 23 (127) | Ref | 24 (126) | <0.834 | 18 (89) | 0.334 |
| Urinary creatinine (mg/day), median (IQR) | ||||||
| Male | 1547 (1164–1951) | Ref | 1471 (1123–1775) | 0.051 | 1409 (1092–1787) | 0.004 |
| Female | 1209 (948–1522) | Ref | 1107 (881–1390) | 0.012 | 1103 (928–1307) | <0.001 |
| Household wealth categories, % (n) | ||||||
| Lowest | 14 (35) | Ref | 18 (44) | 0.016 | 29 (64) | 0.093 |
| Second | 14 (35) | Ref | 23 (55) | 0.294 | 23 (51) | 0.299 |
| Third | 18 (45) | Ref | 23 (55) | 0.540 | 19 (41) | 0.905 |
| Fourth | 23 (56) | Ref | 21 (51) | 0.803 | 16 (34) | 0.424 |
| Highest | 31 (75) | Ref | 15 (36) | 0.071 | 14 (30) | 0.073 |
| Added table salt with food | 59 (322) | Ref | 71 (370) | 66 (333) | ||
| Added table salt during cookingc % (n) | 100 (473) | Ref | 100 (497) | 1.000 | 100 (220) | 1.000 |
| Hours of sleep, % (n) | ||||||
| <6 h | 18 (96) | Ref | 24 (126) | 0.143 | 17 (86) | 0.856 |
| ≥6 to <9 h | 72 (395) | Ref | 61 (318) | 0.002 | 71 (357) | 0.762 |
| ≥9 h | 10 (56) | Ref | 15 (79) | 0.394 | 12 (60) | 0.731 |
| Alcohol consumption, % (n) | 4 (22) | Ref | 3 (15) | 0.873 | 4 (19) | 1.000 |
| Religion, % (n) | ||||||
| Hindu | 53 (289) | Ref | 55 (287) | 0.630 | 46 (233) | 0.112 |
| Muslim | 47 (258) | Ref | 45 (236) | 0.656 | 54 (270) | 0.108 |
| Self‐reported disease, % (n) | ||||||
| Hypertension | 18 (100) | Ref | 12 (61) | 0.310 | 15 (74) | 0.600 |
| Diabetes mellitus | 5 (29) | Ref | 4 (22) | 0.866 | 5 (23) | 1.000 |
| Chronic kidney disease | 2 (13) | Ref | 2 (11) | 1.000 | 2 (12) | 1.000 |
| Volume of 24‐h urine, median (IQR)d | 2224 (1655–2861) | Ref | 2030 (1515–2742) | 0.045 | 2026 (1323–2530) | <0.001 |
BMI indicates body mass index; EC, electrical conductivity; IQR, interquartile range; WHO, World Health Organization.
Work involves moderate‐intensity activity that causes small increases in breathing or heart rate such as brisk walking (or carrying light loads) for at least 10 minutes continuously.
Work involves vigorous‐intensity activity that causes large increases in breathing or heart rate (carrying or lifting heavy loads, digging or construction work) for at least 10 minutes continuously.
Data on use of salt during cooking were measured during the randomized‐controlled trial only. However. All households reported use of table salt during cooking, so this variable was not used for model adjustment.
We noticed participants 24‐hour volume changed across different visits or seasons. Median 24‐hour urine volume was highest (2224 mL) during December (visit 1 of the stepped‐wedge trial), and the lowest (1764) during April (visit 5 of the stepped‐wedge trial). Median 24‐hour urine volume was 2222 mL, 2176 mL, and 1994 during January (visit 2), February (visit 3), and March (visit 4) in stepped‐wedge trail.
We initially included all person‐visits in models, and then conducted separate restricted analyses among participants who were non‐hypertensive and non‐diabetic based on their self‐reported information. In sensitivity analyses, we included participants who reported no history of chronic kidney disease and person‐visits when urinary total protein was <300 mg/day.
To evaluate how water salinity may influence the risk of hypertension categories among the study population, we used multilevel multinomial logistic models with 3‐level random intercepts described above. We used the 2017 American Heart Association guidelines for hypertension categories—normal BP (SBP <120 mm Hg and DBP <80 mm Hg); elevated BP (SBP 120–129 and DBP <80); stage 1 (SBP 130–139 or DBP 80–89), and stage 2 (SBP ≥140 or DBP ≥90) hypertension.26 We also conducted propensity score‐matched analyses of person‐visits from the high and low water EC distribution. We calculated that we needed a sample size of 1344 in each group to detect a difference of 2 mm Hg SBP between person‐visits from low and high water EC distribution groups (standard deviation of SBP=18.5, power 80%, type 1 error 5%, 2‐sided). We initially selected 1344 person‐visits for those with stored water from the lowest EC distribution, and twice as many (1344×2=2688) person‐visits for those with stored water from the highest EC distribution. Then we matched the 1344 lowest EC person‐visits on listed covariates using nearest‐neighbor matching by Mahalanobis distance to select matched 1344 person‐visits (out of 2644 person‐visits) from the highest EC distribution. Finally, 1344 person‐visits from the lowest EC distribution and matched 1344 person‐visits from the highest EC distribution were used in propensity score‐matched analyses. In the propensity‐score matched subpopulation, we used similar multilevel linear models described above, but modeled salinity as a binary variable (high versus low EC).
To illustrate whether the shape of the associations between water salinity and BP is non‐linear or not, we used restricted cubic splines plots of water EC adjusted for covariates.
Exploring the mechanisms of water salinity and blood pressure associations
To explore the mechanisms by which water EC influences BP, we initially examined whether water EC was associated with daily urinary excretions of macro‐minerals such as Na+, K+, Ca2+, and Mg2+ using similar multilevel linear models and 3‐level random intercepts. We then assessed how SBP or DBP changes because of 1 SD unit increase in 24‐hour urinary Na+ (1 SD=74 mmol/day), K+ (1 SD=15 mmol/day), Ca2+ (1 SD=3 mmol/day), and Mg2+ (1 SD=3 mmol/day) excretions using separate multilevel linear models. We used 3 approaches of modeling for detecting the associations between each of the urine minerals and BP—(1) all person‐visits; (2) all person‐visits but adjusted for urinary creatinine; and (3) restricted analyses among person‐visits with complete 24‐hour urine collection based on creatinine index ≥0.7.27 Creatinine index was defined as the ratio of measured versus predicted daily urinary creatinine.27 Predicted daily urinary creatinine was calculated using the Kawasaki formula.28
Several variables were missing in the data set (EC [n=56, 0.9%]; BMI [n=85, 1.3%]; wealth index [n=34, 0.5%]; Na+ [n=97, 1.5%], K+ [n=97, 1.5%], urine creatinine [n=97, 1.5%], Ca2+ [n=405, 6%], Mg2+ [n=831, 13%]). We assumed data are missing not at random and applied multiple imputation (n=40 imputations) using chained equations conditional on the listed variables in the fully adjusted models. In sensitivity analyses, we also reported the associations of concurrent water EC categories with mean SBP and DBP using multilevel linear models in complete cases without imputing missing data. All results were considered statistically significant at the 5% level. We performed statistical analyses in Stata, version 15.0 and R, version 3.3.1.
Ethics
Informed written consent was obtained from all participants and household heads, and study protocols were approved by the Ethical Review Committee of International Centre for Diarrhoeal Disease Research, Bangladesh (PR‐15096).
Results
Study Participants and Characteristics
The median age and BMI of participants at enrollment were 40 (interquartile range: 31–54) years and 22 (interquartile range: 19–24) kg/m2 (Table 2). Most participants had normal weight (63%) as per WHO classification of BMI, were women (59%) and never smoked (52%).
Water Salinity and Blood Pressure
In all 6487 participant‐visits, 27% drank fresh water, 49% mild salinity, and 24% moderate‐salinity water. None of the water samples had high salinity (EC >10 mS/cm) based on the Food and Agricultural Organization classification. Spearman correlation coefficients suggests that participants whose drinking water EC was higher had lower SBP and DBP (Figure 3).
Figure 3.
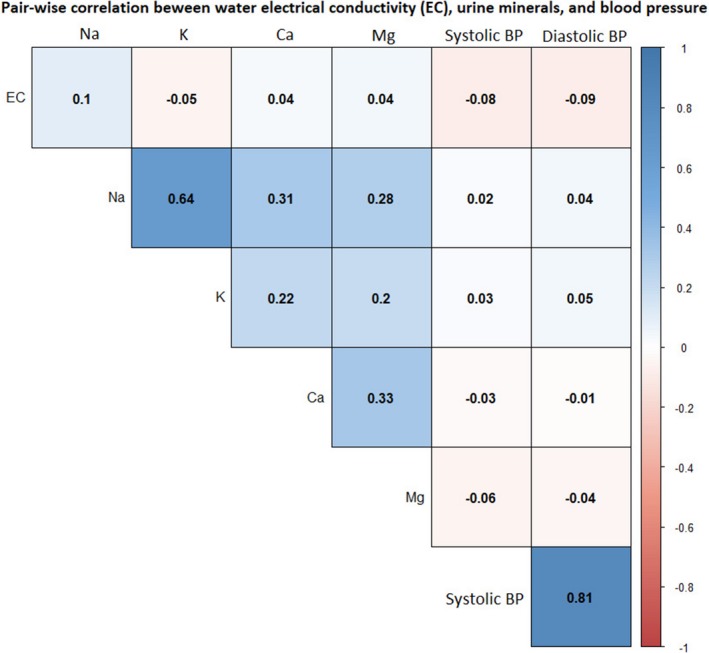
Pairwise correlation between households’ drinking water electrical conductivity, urinary minerals and systolic blood pressure in the pooled data. BP indicates blood pressure.
Compared with fresh water drinkers, mild‐salinity water drinkers had −1.55 (95% CI: −3.22–0.12) mm Hg SBP difference and −1.26 (95% CI: −2.21–−0.32) mm Hg DBP difference in the fully adjusted models (Table 3). Compared with fresh water drinkers, moderate‐saline water drinkers had −1.58 (95% CI: −3.13–−0.03) mm Hg SBP difference and −1.28 (95% CI: −2.10–−0.45) mm Hg DBP difference in the fully adjusted models (Table 3).
Table 3.
Association Between Household Drinking Water Salinity Categories and Household Members’ BP
| Outcomes | Drinking Water Electrical Conductivity Categories | |||
|---|---|---|---|---|
| Fresh Water (EC: 0 to <0.7 mS/cm) (β, 95% CI) | Mild‐Salinity Water (EC: 0.7 to <2 mS/cm) (β, 95% CI) | Moderate‐Salinity Water (EC: 2.0–10 mS/cm) (β, 95% CI) | ||
| Person‐visits of all participants | Systolic BP | |||
| Model 1a | Reference | −1.63 (−3.25–0.00) | −1.73 (−3.25–−0.20) | |
| Model 2b | Reference | −1.59 (−3.25–0.07) | −1.64 (−3.17–−0.12) | |
| Model 3c | Reference | −1.55 (−3.22–0.12) | −1.58 (−3.13–−0.03) | |
| Diastolic BP | ||||
| Model 1a | Reference | −1.33 (−2.22–−0.43) | −1.35 (−2.16–−0.55) | |
| Model 2b | Reference | −1.31 (−2.23–−0.38) | −1.32 (−2.13–−0.50) | |
| Model 3c | Reference | −1.26 (−2.21–−0.32) | −1.28 (−2.10–−0.45) | |
| Person‐visits of non‐hypertensive and non‐diabetic participants | Systolic BP | |||
| Model 1a | Reference | −1.43 (−2.78–−0.07) | −1.70 (−3.13–−0.26) | |
| Model 2b | Reference | −1.38 (−2.77–0.02) | −1.61 (−3.07–−0.16) | |
| Model 3c | Reference | −1.34 (−2.75–0.06) | −1.56 (−3.03–−0.08) | |
| Diastolic BP | ||||
| Model 1a | Reference | −1.12 (−1.98–−0.27) | −1.30 (−2.13–−0.48) | |
| Model 2b | Reference | −1.07 (−1.99–−0.17) | −1.25 (−2.10–−0.40) | |
| Model 3c | Reference | −1.04 (−1.97–−0.11) | −1.22 (−2.08–−0.36) | |
β refers to mean difference from the reference group. BP indicates blood pressure; EC, electrical conductivity.
Unadjusted model.
Adjusted for age, sex, and body mass index categories.
Additionally adjusted for physical activities and smoking status, household wealth, alcohol consumption, sleep hours, religion, and consumption of additional table salt with food.
In restricted analyses among non‐hypertensive and non‐diabetic participants, we found that compared with fresh water drinkers, mild‐salinity water drinkers had −1.34 (95% CI: −2.75–0.06) mm Hg mean SBP difference and −1.04 (95% CI: −1.97–−0.11) mm Hg DBP difference. In restricted analyses, compared with fresh water drinkers, moderate‐salinity water drinkers had −1.56 (95% CI: −3.03–−0.08) mm Hg mean SBP difference and −1.22 (95% CI: −2.08–−0.36) mm Hg DBP difference in the fully adjusted models (Table 3).
Compared with the fresh water drinkers, the fully adjusted odds ratio for the mild‐salinity water drinkers was 0.60 (95% CI: 0.43–0.84) for stage 1 hypertension and 0.56 (95% CI: 0.46–0.89) for stage 2 hypertension. Compared with the fresh water drinkers, the fully adjusted odds ratio for the moderate‐salinity water drinkers for stage 1 hypertension was 0.77 (95% CI: 0.51–1.17) and for stage 2 hypertension was 0.61 (95% CI: 0.35–1.09) (Table 4).
Table 4.
Odds Ratios of Having Elevated BP or Stage 1 or Stage 2 Hypertension, Relative to the Normal BP (SBP <120 mm Hg and DBP <80 mm Hg) Among Different Drinking Water Salinity Groups
| Water Salinity Categories | Elevated (SBP 120–129 and DBP <80) | Stage 1 Hypertension (SBP 130–139 or DBP 80–89) | Stage 2 Hypertension (SBP ≥140 or DBP ≥90) | |
|---|---|---|---|---|
| Model 2 | Fresh water (EC: <0.7 mS/cm) | Referent | Referent | Referent |
| Mild‐salinity water (EC: 0.7 to <2 mS/cm) | 0.88 (0.69–1.14) | 0.58 (0.42–0.81) | 0.54 (0.34–0.86) | |
| Moderate‐salinity water (EC: 2.0–10 mS/cm) | 0.91 (0.68–1.22) | 0.70 (0.47–1.04) | 0.59 (0.34–1.04) | |
| Model 3 | Fresh water (EC: <0.7 mS/cm) | Referent | Referent | Referent |
| Mild‐salinity water (EC: 0.7 to <2 mS/cm) | 0.92 (0.71–1.18) | 0.60 (0.43–0.84) | 0.56 (0.46–0.89) | |
| Moderate‐salinity water (EC: 2.0–10 mS/cm) | 0.96 (0.71–1.30) | 0.77 (0.51–1.17) | 0.61 (0.35–1.09) |
Model 1 is unadjusted (we did not report model 1 as it did not converge for the multilevel multinomial outcome); model 2: adjusted for age, sex, and body mass index; model 3: additionally, adjusted for physical activities and smoking status, household wealth, alcohol consumption, sleep hours, religion, and consumption of additional table salt with food. DBP indicates diastolic blood pressure; EC, electrical conductivity; SBP, systolic blood pressure.
In propensity score matching analyses, the matched high EC group had −1.64 (95% CI: −3.16–−0.12) mm Hg mean SBP difference and −1.54 (95% CI: −2.52–−0.58) mm Hg mean DBP difference in the fully adjusted models compared with the low EC group (Table 5). The water EC and BP restricted cubic spline plots suggest a non‐linear (Wald type test for non‐linearity, P<0.001 for SBP and <0.001 for DBP) and predominant negative association between drinking water EC and BP (Figure 4).
Table 5.
Propensity Score Matched Analyses for the Association of Low Versus High Water EC Distribution on BP
| Outcomes | Drinking Water Electrical Conductivity Categories | |
|---|---|---|
| Low Salinity (EC: <197.9 μS/cm) | Matched High Salinity (EC: >1803 μS/cm) | |
| Systolic BP (mean difference from the reference group) | ||
| Model 1a | Reference | −1.87 (−3.30–−0.44) |
| Model 2b | Reference | −1.74 (−3.24–−0.24) |
| Model 3c | Reference | −1.64 (−3.16–−0.12) |
| Diastolic BP (mean difference from the reference group) | ||
| Model 1a | Reference | −1.77 (−2.67–−0.87) |
| Model 2b | Reference | −1.65 (−2.60–−0.69) |
| Model 3c | Reference | −1.54 (−2.52–−0.58) |
BP indicates blood pressure; EC, electrical conductivity.
Unadjusted model.
Adjusted for age, sex, and body mass index categories.
Additionally adjusted for physical activities and smoking status, household wealth, alcohol consumption, sleep hours, religion, and consumption of additional table salt with food.
Figure 4.
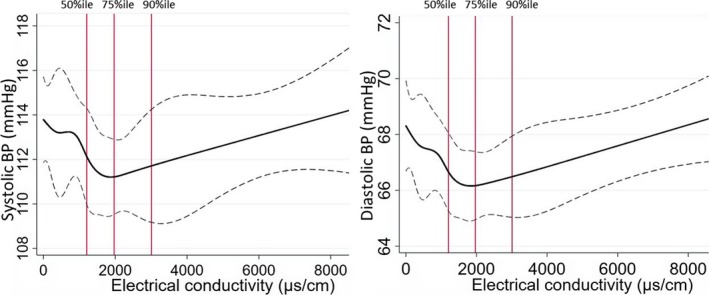
Restricted cubic spline plots (solid lines) and 95% CI (dashed lines) for the association between drinking water EC and blood pressure of the participants. Restricted cubic splines were plotted at EC cut points of 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentile. Distribution of EC data at 50% (median), 75%, and 90% illustrated as red vertical lines. BP indicates blood pressure; EC, electrical conductivity.
Water Salinity and Urinary Cations
Both mild‐ and moderate‐salinity water drinkers had higher urinary Na+, Ca2+, and Mg2+ excretion than the fresh water drinkers (Table 6). Compared with fresh water drinkers, mild‐salinity water drinkers had 4.8 (95% CI: −1.0–10.7) mmol/day higher mean urinary Na+, 1.3 (95% CI: 1.2–1.5) mmol/day higher mean urinary Ca2+, and 1.2 (95% CI: 1.1–1.4) mmol/day higher mean urinary Mg2+ in the fully adjusted models (Table 7). Moderate‐salinity water drinkers had 16.7 (95% CI: 11.3–22.0) mmol/day higher mean urinary Na+, 1.2 (95% CI: 1.1–1.4) mmol/day higher mean urinary Ca2+, and 1.2 (95% CI: 1.1–1.4) mmol/day higher mean urinary Mg2+ in the fully adjusted model than the fresh water drinkers (Table 7).
Table 6.
Urinary Na+, K+, Ca2+, and Mg2+ Excretion by Drinking Water Salinity Categories
| Urinary Minerals | All Person‐Visits | Person‐Visits of Fresh Water Drinkers | Person‐Visits of Mild‐Salinity Water Drinkers | Person‐Visits of Moderate‐Salinity Water Drinkers |
|---|---|---|---|---|
| Urinary Na+ | ||||
| Mean (SD) | 165 (74) | 155 (73) | 166 (69) | 172 (83) |
| Median (IQR) | 154 (114–203) | 144 (108–191) | 158 (118–204) | 160 (112–218) |
| Urinary K+ | ||||
| Mean (SD) | 34 (15) | 34 (15) | 35 (15) | 33 (16) |
| Median (IQR) | 32 (24–42) | 32 (24–43) | 33 (24–42) | 30 (22–40) |
| Urinary Ca2+ | ||||
| Mean (SD) | 4 (3) | 3.2 (2.8) | 4.3 (3.1) | 3.4 (3.0) |
| Median (IQR) | 3 (1.6–5.1) | 2.5 (1.3–4.3) | 3.6 (2.1–5.7) | 2.6 (1.3–4.6) |
| Urinary Mg2+ | ||||
| Mean (SD) | 4 (3) | 3.3 (2.6) | 4.0 (2.8) | 4.0 (3.0) |
| Median (IQR) | 3.3 (2.1–4.8) | 2.8 (1.7–4.2) | 3.6 (2.4–5.0) | 3.5 (2.1–5.1) |
IQR indicates interquartile range.
Table 7.
Differences in Urinary Na+, K+, Ca2+ and Mg2+ Excretion Among Mild‐ and Moderate‐Salinity Water Drinkers Compared With Fresh Water Drinker When Adjusted for Different Level of Confounders
| Urinary Cations | Drinking Water Electrical Conductivity (EC) Categories | ||
|---|---|---|---|
| Fresh Water (EC: 0 to <0.7 mS/cm) | Mild‐Salinity Water (EC: 0.7 to <2 mS/cm) (β, 95% CI) | Moderate‐SalinityWater (EC: 2.0–10 mS/cm) (β, 95% CI) | |
| Urinary Na+ | |||
| Model 1a | Reference | 4.6 (−1.4–10.5) | 16.6 (11.3–21.9) |
| Model 2b | Reference | 5.0 (−0.8–10.8) | 16.9 (11.6–22.1) |
| Model 3c | Reference | 4.8 (−1.0–10.7) | 16.7 (11.5–22.0) |
| Urinary K+ | |||
| Model 1a | Reference | 0.6 (−1.4–2.7) | 0.0 (−2.00–2.00) |
| Model 2b | Reference | 0.7 (−1.4–2.7) | 0.1 (−1.9–2.0) |
| Model 3c | Reference | 0.8 (−1.2–2.8) | 0.2 (−1.8–2.1) |
| Urinary Ca2+ | |||
| Model 1a | Reference | 1.4 (1.2–1.5) | 1.2 (1.1–1.4) |
| Model 2b | Reference | 1.4 (1.2–1.5) | 1.2 (1.1–1.4) |
| Model 3c | Reference | 1.3 (1.2–1.5) | 1.2 (1.1–1.4) |
| Urinary Mg2+ | |||
| Model 1a | Reference | 1.2 (1.1–1.4) | 1.3 (1.1–1.4) |
| Model 2b | Reference | 1.2 (1.1–1.4) | 1.3 (1.1–1.4) |
| Model 3c | Reference | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) |
β refers to difference in mean urinary minerals between any water salinity and reference salinity group.
Unadjusted model.
Adjusted for age, sex, and body mass index categories.
Additionally adjusted for physical activities and smoking status, household wealth, alcohol consumption, sleep hours, religion, and consumption of additional table salt with food.
Urinary Cations and Blood Pressure
Higher urinary Na+ was associated with an increase in SBP, whereas higher urinary Ca2+ or urinary Mg2+ was associated with decreased SBP and DBP (Figure 5). A 74 mmol/day (1 SD) increase in urinary Na+ was associated with + 0.48 (95% CI: +0.14–+0.81) mm Hg higher mean SBP, and +0.00 (95% CI: −0.20–+0.20) mm Hg mean DBP difference in fully adjusted models. A 3 mmol/day (1 SD) increase in urinary Ca2+ was associated with −0.31 (95% CI: −0.01–−0.62) mm Hg lower mean SBP and −0.41 (95% CI: −0.16–−0.68) mm Hg lower mean DBP in fully adjusted models. A 3 mmol/day (1 SD) increase in urinary Mg2+ was associated with −0.7 (95% CI: −0.37–−0.97) mm Hg lower mean SBP and −0.3 (95% CI: −0.15–−0.51) mm Hg lower mean DBP in fully adjusted models (Figure 5). We found similar results in models additionally adjusted for urinary creatinine, or restricted among the complete 24‐hour urine collections (Figure 5).
Figure 5.
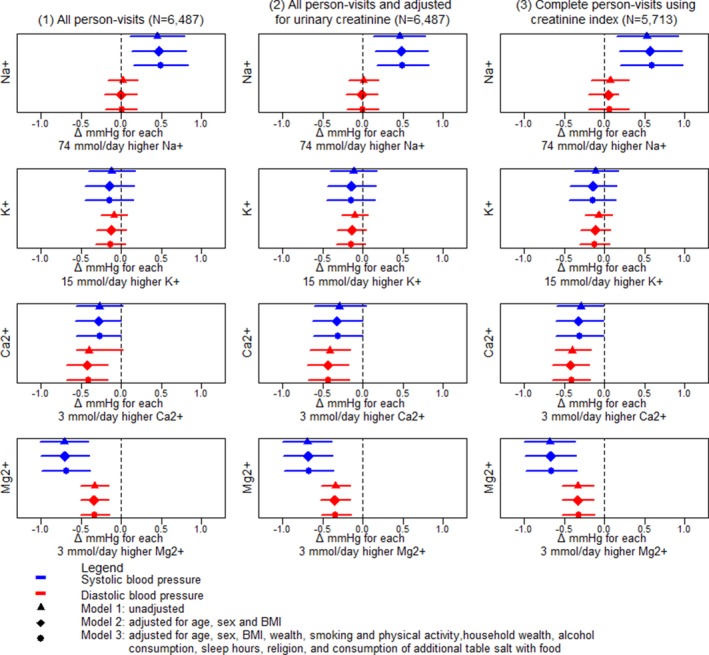
Association between 1 standard deviation higher urinary minerals and systolic and diastolic blood pressure considering (1) All person‐visits (2) All‐person‐visits and adjusting for urinary creatinine concentration, and (3) restricting the analyses among complete 24‐hour samples based on creatinine index. BMI indicates body mass index.
Sensitivity Analyses
Whenever we restricted the analyses to participants who did not report a history of chronic kidney disease and for person‐visits when urinary total protein was <300 mg/day, we found that mild‐salinity water drinkers had −1.32 (95% CI: −2.82–0.17) mm Hg SBP difference and −1.40 (95% CI: −2.25–−0.55) mm Hg DBP difference in the fully adjusted models compared with the fresh water drinkers (Table 8). Moderate saline water drinkers had −1.40 (95% CI: −3.14–0.34) mm Hg SBP difference and −1.29 (95% CI: −2.24–−0.33) mm Hg DBP difference in the fully adjusted models compared with the fresh water drinkers (Table 8).
Table 8.
Sensitivity Analyses of Association Between Drinking Water Salinity Categories and Participants BP When Analyses was Restricted Among Participants With No Chronic Disease and Whose Urinary Protein was <300 mg/day
| Outcomes | Drinking Water Electrical Conductivity (EC) Categories | |||
|---|---|---|---|---|
| Fresh Water (EC: 0 to <0.7 mS/cm) (β, 95% CI) | Mild‐Salinity Water (EC: 0.7 to <2 mS/cm) (β, 95% CI) | Moderate‐Salinity Water (EC: 2.0–10 mS/cm) (β, 95% CI) | ||
| No chronic disease and urinary protein <300 mg/d | Systolic BP | |||
| Model 1a | Reference | −1.44 (−2.81–−0.08) | −1.59 (−3.23–0.05) | |
| Model 2b | Reference | −1.39 (−2.86–0.08) | −1.49 (−3.17–0.18) | |
| Model 3c | Reference | −1.32 (−2.82–0.17) | −1.40 (−3.14–0.34) | |
| Diastolic BP | ||||
| Model 1a | Reference | −1.45 (−2.20–−0.70) | −1.37 (−2.29–−0.45) | |
| Model 2b | Reference | −1.46 (−2.28–−0.64) | −1.33 (−2.27–−0.40) | |
| Model 3c | Reference | −1.40 (−2.25–−0.55) | −1.29 (−2.24–−0.33) | |
| Non‐hypertensive, non‐diabetic, no chronic kidney disease, and urinary protein <300 mg/d | Systolic BP | |||
| Model 1a | Reference | −1.35 (−2.51–−0.20) | −1.63 (−3.24–−0.02) | |
| Model 2b | Reference | −1.28 (−2.57–−0.00) | −1.54 (−3.24–0.16) | |
| Model 3c | Reference | −1.21 (−2.51–0.09) | −1.44 (−3.19–0.31) | |
| Diastolic BP | ||||
| Model 1a | Reference | −1.33 (−2.07–−0.60) | −1.29 (−2.32–−0.25) | |
| Model 2b | Reference | −1.31 (−2.09–−0.55) | −1.26 (−2.31–−0.21) | |
| Model 3c | Reference | −1.26 (−2.07–−0.46) | −1.20 (−2.282–−0.12) | |
β refers to mean difference from the reference group. BP indicates blood pressure.
Unadjusted model.
Adjusted for age, sex, and body mass index categories.
Additionally adjusted for physical activities and smoking status, household wealth, alcohol consumption, sleep hours, religion, and consumption of additional table salt with food.
In complete case analyses without missing data imputation, mild‐salinity water drinkers had −1.54 (95% CI: −3.32–0.23) mm Hg SBP difference and −1.30 (95% CI: −2.31–−0.30) mm Hg DBP difference in the fully adjusted models compared with the fresh water drinkers (Table 9). Moderate‐saline water drinkers had −1.36 (95% CI: −3.06–0.32) mm Hg SBP difference and −1.19 (95% CI: −2.07–−0.32) mm Hg DBP difference in the fully adjusted models compared with the fresh‐water drinkers (Table 9).
Table 9.
Sensitivity Analyses of Association Between Drinking Water Salinity Categories and Participants BP Without Missing Data Imputation
| Outcomes | Drinking Water Electrical Conductivity Categories | |||
|---|---|---|---|---|
| Fresh Water (EC: 0 to <0.7 mS/cm) (β, 95% CI) | Mild‐Salinity Water (EC: 0.7 to <2 mS/cm) (β, 95% CI) | Moderate‐Salinity Water (EC: 2.0–10 mS/cm) (β, 95% CI) | ||
| Person‐visits of all participants | Systolic BP | |||
| Model 1a | Reference | −1.62 (−3.27–0.02) | −1.52 (−3.13–−0.08) | |
| Model 2b | Reference | −1.59 (−3.34–0.16) | −1.47 (−3.10–0.16) | |
| Model 3c | Reference | −1.54 (−3.32–0.23) | −1.36 (−3.06–0.32) | |
| Diastolic BP | ||||
| Model 1a | Reference | −1.33 (−2.24–−0.42) | −1.24 (−2.24–−0.42) | |
| Model 2b | Reference | −1.35 (−2.33–−0.37) | −1.24 (−2.10–−0.39) | |
| Model 3c | Reference | −1.30 (−2.31–−0.30) | −1.19 (−2.07–−0.32) | |
| Person‐visits of non‐hypertensive and non‐diabetic participants | Systolic BP | |||
| Model 1a | Reference | −1.45 (−2.81–−0.09) | −1.56 (−3.04–−0.08) | |
| Model 2b | Reference | −1.39 (−2.83–0.04) | −1.50 (−3.00–0.00) | |
| Model 3c | Reference | −1.34 (−2.79–0.11) | −1.41 (−2.96–0.13) | |
| Diastolic BP | ||||
| Model 1a | Reference | −1.14 (−2.00–−0.28) | −1.21 (−2.04–−0.38) | |
| Model 2b | Reference | −1.14 (−2.04–−0.24) | −1.21 (−2.05–−0.36) | |
| Model 3c | Reference | −1.09 (−2.01–−0.17) | −1.15 (−2.02–−0.30) | |
β refers to mean difference from the reference group. BP indicates blood pressure.
Unadjusted model.
Adjusted for age, sex, and body mass index categories.
Additionally adjusted for physical activities and smoking status, household wealth, alcohol consumption, sleep hours, religion, and consumption of additional table salt with food.
Discussion
Our analyses suggest that in seawater intrusion affected southwest coastal Bangladesh, drinking mild‐salinity water was associated with lower BP. We also found drinking mild‐salinity water was associated with lower risks of stage 1 and stage 2 hypertension among the study population.
We suspect that the effects of drinking mild‐ and moderate‐salinity water on BP may be attributable to high Ca2+ and Mg2+ present in saline water. Similar to other study findings conducted in southwest coastal Bangladesh,9, 12, 13, 29 we found that drinking mild‐ and moderate‐salinity water was associated with higher urinary Na+, and higher urinary Na+ was associated with higher SBP. We additionally found that drinking mild‐ and moderate‐salinity water EC was associated with higher urinary Ca2+ and Mg2+, and both urinary minerals were associated with lower SBP and DBP. We hypothesize that the BP‐lowering effects of Ca2+ and Mg2+ counteracted the harmful effects of Na+, reflected by the overall inverse association between drinking mild‐ and moderate‐salinity water EC and BP. Similarly, BP‐lowering effects of drinking water rich in Ca2+ and Mg2+ have been observed across many regions of the world.30, 31 Drinking water rich in Ca2+ and Mg2+ was associated with reduced cardiovascular and cerebrovascular mortality.32, 33
These findings may be generalizable to other seawater intrusion‐affected coastal regions. The predominant cations in seawater are Na+, Ca2+, and Mg2+.34 These minerals have been reported in high concentrations in groundwater of seawater intrusion affected coastal regions across the world including deltas,35 arid or semi‐arid regions,5 peninsula,36 and islands.37 A hydro‐geological survey in Bangladesh suggests that groundwater hardness—a measure of Ca and Mg salts—is the highest in seawater intrusion‐affected southwest Bangladesh,38 where the groundwater is of the Na‐Ca‐Mg‐HCO3‐Cl type. When communities in seawater intrusion‐affected areas drink Na+, Ca2+, and Mg2+ rich water, their intakes of these minerals increase, evident in our study as high urinary Na+, Ca2+, and Mg2+ concentrations. People in Bangladesh have lower intake of Ca and Mg through their regular diet,39 therefore, drinking water can be an important source of these minerals. In settings where communities have higher dietary intake of Ca and Mg, intake of these minerals though drinking water may be less beneficial.
Experimental studies suggest that Ca2+ and Mg2+ can counterbalance the effect of Na+ on BP.15 Entry of Na+ across the cell membrane of vascular smooth muscle precedes smooth muscle contraction that increases vascular tone and BP.40 In contrast, Ca2+ and Mg2+ decrease BP by stabilizing the cell membrane of the vascular smooth muscle by binding to the plasma membrane,41, 42 which in turn interferes with the ionic conductance that diminishes vascular tone.43 Ca2+ and Mg2+ concentrations below physiological levels destabilizes the cell membrane, causing greater Na+ entry across the cell membrane and attenuates smooth muscle contraction.44 Increased dietary intake of Ca2+ and Mg2+ also facilitates urinary excretion of Na+ by a variety of mechanisms including increased release of atrial natriuretic peptide, reduced sympathetic outflow and interference with Na+ re‐absorption by kidneys.45, 46
Our analyses have several key limitations. First, we were unable to measure the concentrations of individual minerals in water because of high costs. This precludes the understanding of exact mineral exposure through high EC water. We also lack bioavailability data for minerals from drinking water, however, studies support high bioavailability of Ca and Mg from drinking water.47 We also did not collect mineral intake data of the participants through diet, which precludes our understanding of what percentage of urinary mineral concentrations were coming from food or drinking water. Although 24‐hour urine collection is the ideal method for urinary mineral measurements,27 it may be biased by over‐ or under‐collection of urine samples.27 We attempted to minimize bias by analyzing data from participants with complete 24‐hour urine collection based on the urinary creatinine index.23 Several studies have reported Na+ induces calciuria or Ca2+ excretion through urine.48 Therefore, high urinary Ca2+ among study participants could be partially because of the influence of Na+ on kidneys in addition to Ca2+ intake through high EC water. Whenever we restricted the analyses excluding the self‐reported chronic kidney participants and those with >300 mg/day urinary total protein, the findings were slightly attenuated. We only had a few self‐reported chronic kidney participants, but we were unable to measure renal function of the participants using serum creatinine or estimated glomerular filtration rate as we did not collect blood samples of the participants. We had few high‐salinity water drinkers thereby limiting insight on the shape of the EC and BP dose response curve, however, this may reflect community behavior as many people report that high EC water has a disagreeable taste. Moderate‐salinity water drinkers had higher urinary Na+ than the mild‐salinity water drinkers but no differences were observed for urinary Mg2+. High‐salinity water drinkers may have hypertension due to increased Na intake, but we could not assess this. BP has a diurnal variation and participants whose BP was measured in the morning may had higher BP than participants whose BP was measured around noon or afternoon.49 We did not collect the exact time of BP measurement and thereby were unable to control for it, which likely introduced measurement error for BP.
The nuanced effects of drinking water salinity on blood pressure in Bangladesh are consistent with other observations. Blood Mg concentration was lower and mortality after hospitalization was higher in areas served by desalinated water in Israel compared with areas served by non‐desalinated water.50 Populations exposed to desalinated water had higher risks for ischemic heart disease.51 Those that have low‐salinity drinking water (eg, rainwater, desalinated water, reverse osmosis water) should explore adding calcium and magnesium to their water sources to reduce the risks of blood pressure and cardiovascular diseases.52 Similarly, adding calcium and magnesium to drinking water may be a useful strategy for reducing the population burden of hypertension when drinking water sources have low levels of these minerals. Ensuring optimum concentrations of Ca2+ and Mg2+ in drinking water may be an important public health and nutritional intervention to ensure fulfillment of daily requirements of these essential macro‐minerals since evidence suggests that globally concentrations of these minerals are decreasing in the diet.53, 54
Sources of Funding
This research was funded by Wellcome Trust, UK, Our Planet, Our Health Award (Grant # 106871/Z/15/Z).
Disclosures
None.
Acknowledgments
We acknowledge with gratitude the commitment of Wellcome Trust, UK to its research efforts. We are grateful to United Nations Children's Fund colleagues Dr Boluwaji Onabolu and Ms Nargis Akter for their support during site selection and study implementation. We are grateful to the study participants for their support and cooperation. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. We are grateful to Dhaka University colleagues for their assistance conducting the study.
(J Am Heart Assoc. 2019;8:e012007 DOI: 10.1161/JAHA.119.012007.)
References
- 1. Small C, Nicholls RJ. A global analysis of human settlement in coastal zones. J Coast Res. 2003;19:584–599. [Google Scholar]
- 2. Van Weert F, Van der Gun J, Reckman J. Global overview of saline groundwater occurrence and genesis. International Groundwater Resources Assessment Centre 2009. Available at: https://www.un-igrac.org/resource/global-overview-saline-groundwater-occurrence-and-genesis-0. Accessed January 1, 2019.
- 3. Cooper HH, Kohout FA, Henry HR, Glover RE. Sea water in coastal aquifers. Department of the Interior, US Geological Survey Water‐Supply Paper 1613‐C 1964. Available at: https://pubs.usgs.gov/wsp/1613c/report.pdf. Accessed March 18, 2019.
- 4. Taylor RG, Scanlon B, Döll P, Rodell M, Van Beek R, Wada Y, Longuevergne L, Leblanc M, Famiglietti JS, Edmunds M. Ground water and climate change. Nat Clim Chang. 2013;3:322–329. [Google Scholar]
- 5. Alfarrah N, Walraevens K. Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi‐arid regions. Water. 2018;10:143. [Google Scholar]
- 6. Lam Y, Surkan PJ, Winch PJ, Nizame FA. Freshwater access in high salinity regions: impacts and adaptation insights from the Ganges River Delta. J Glob Health Rep. 2018;2:e2018007. [Google Scholar]
- 7. Khan AE, Ireson A, Kovats S, Mojumder SK, Khusru A, Rahman A, Vineis P. Drinking water salinity and maternal health in coastal Bangladesh: implications of climate change. Environ Health Perspect. 2011;119:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talukder MRR, Rutherford S, Phung D, Islam MZ, Chu C. The effect of drinking water salinity on blood pressure in young adults of coastal Bangladesh. Environ Pollut. 2016;214:248–254. [DOI] [PubMed] [Google Scholar]
- 9. Khan AE, Scheelbeek PFD, Shilpi AB, Chan Q, Mojumder SK, Rahman A, Haines A, Vineis P. Salinity in drinking water and the risk of (pre) eclampsia and gestational hypertension in coastal Bangladesh: a case‐control study. PLoS One. 2014;9:e108715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhoades J. Salinity: electrical conductivity and total dissolved solids. Methods of soil analysis part 3—chemical methods. Madison, Wisconsin, USA. Soil Sci Soc Am J. 1996;Series 5.3:417–435. [Google Scholar]
- 11. Hem JD. Study and interpretation of the chemical characteristics of natural water. Department of the Interior, US Geological Survey Water‐Supply Paper 2254; 1985. Available at: https://pubs.usgs.gov/wsp/wsp2254/pdf/wsp2254a.pdf. Accessed March 18, 2019.
- 12. Al Nahian M, Ahmed A, Lázár AN, Hutton CW, Salehin M, Streatfield PK. Drinking water salinity associated health crisis in coastal Bangladesh. Elementa Science of the Anthropocene. 2018. DOI: 10.1525/elementa.143. [DOI] [Google Scholar]
- 13. Scheelbeek PF, Chowdhury MA, Haines A, Alam DS, Hoque MA, Butler AP, Khan AE, Mojumder SK, Blangiardo MA, Elliott P. Drinking water salinity and raised blood pressure: evidence from a cohort study in coastal Bangladesh. Environ Health Perspect. 2017;125:057007 DOI: 10.1289/ehp659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure: meta‐analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–1632. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of magnesium supplementation on blood pressure: a meta‐analysis of randomized double‐blind placebo‐controlled trials. Hypertension. 2016;68:324–333. [DOI] [PubMed] [Google Scholar]
- 16. Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: a meta‐analysis of randomized clinical trials. Ann Intern Med. 1996;124:825–831. [DOI] [PubMed] [Google Scholar]
- 17. Naser AM, Unicomb L, Doza S, Ahmed KM, Rahman M, Uddin MN, Quraishi SB, Selim S, Shamsudduha M, Burgess W. Stepped‐wedge cluster‐randomised controlled trial to assess the cardiovascular health effects of a managed aquifer recharge initiative to reduce drinking water salinity in southwest coastal Bangladesh: study design and rationale. BMJ Open. 2017;7:e015205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Global physical activity questionnaire (GPAQ) analysis guide. 2012. Available at: https://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf?ua=1. Accessed March 18, 2019.
- 19. Ostchega Y, Zhang G, Sorlie P, Hughes JP, Reed‐Gillette DS, Nwankwo T, Yoon S. Blood pressure randomized methodology study comparing automatic oscillometric and mercury sphygmomanometer devices: National Health and Nutrition Examination Survey, 2009–2010. Natl Health Stat Report. 2012;1–15. Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.248.4291&rep=rep1&type=pdf. Accessed March 18, 2019. [PubMed] [Google Scholar]
- 20. World Health Organization . Affordable Technology: Blood Pressure Measuring Devices for Low Resource Settings. Geneva, Switzerland: World Health Organization; 2005. Available at: https://apps.who.int/iris/bitstream/handle/10665/43115/9241592648.pdf?sequence=1&isAllowed=y. Accessed March 18, 2019. [Google Scholar]
- 21. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 22. Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort RT; Group PS . Albuminuria assessed from first‐morning‐void urine samples versus 24‐hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol. 2008;168:897–905. [DOI] [PubMed] [Google Scholar]
- 23. Edwards O, Bayliss R, Millen S. Urinary creatinine excretion as an index of the completeness of 24‐hour urine collections. Lancet. 1969;294:1165–1166. [DOI] [PubMed] [Google Scholar]
- 24. Albert V, Subramanian A, Rangarajan K, Pandey RM. Agreement of two different laboratory methods used to measure electrolytes. J Lab Physicians. 2011;3:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rhoades JD, Kandiah A, Mashali AM. The Use of Saline Waters for Crop Production. Chapter 2—Saline Waters as Resources. Rome, Italy: Food and Agricultural Organization of the United Nations; 1992. Available at: http://www.fao.org/3/a-t0667e.pdf. Accessed March 18, 2019. [Google Scholar]
- 26. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 27. John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJ, Patel SM. Accuracy and usefulness of select methods for assessing complete collection of 24‐hour urine: a systematic review. J Clin Hypertens (Greenwich). 2016;18:456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawasaki T, Uezono K, Itoh K, Ueno M. Prediction of 24‐hour urinary creatinine excretion from age, body weight and height of an individual and its application. Nihon Koshu Eisei Zasshi. 1991;38:567–574. [PubMed] [Google Scholar]
- 29. Scheelbeek PF, Khan AE, Mojumder S, Elliott P, Vineis P. Drinking water sodium and elevated blood pressure of healthy pregnant women in salinity‐affected coastal areas novelty and significance. Hypertension. 2016;68:464–470. [DOI] [PubMed] [Google Scholar]
- 30. Sauvant M‐P, Pepin D. Drinking water and cardiovascular disease. Food Chem Toxicol. 2002;40:1311–1325. [DOI] [PubMed] [Google Scholar]
- 31. Monarca S, Donato F, Zerbini I, Calderon RL, Craun GF. Review of epidemiological studies on drinking water hardness and cardiovascular diseases. Eur J Cardiovasc Prev Rehabil. 2006;13:495–506. [DOI] [PubMed] [Google Scholar]
- 32. O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. [DOI] [PubMed] [Google Scholar]
- 33. Sengupta P. Potential health impacts of hard water. Int J Prev Med. 2013;4:866–875. [PMC free article] [PubMed] [Google Scholar]
- 34. Nani M, Zura S, Majid F, Jaafar A, Mahdzir A, Musa M. Potential health benefits of deep sea water: a review. Evid Based Complement Alternat Med. 2016;2016:6520475 DOI: 10.1155/2016/6520475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoque MA, Butler AP. Medical hydrogeology of Asian deltas: status of groundwater toxicants and nutrients, and implications for human health. Int J Environ Res Public Health. 2015;13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park Y, Lee J‐Y, Kim J‐H, Song S‐H. National scale evaluation of groundwater chemistry in Korea coastal aquifers: evidences of seawater intrusion. Environ Earth Sci. 2012;66:707–718. [Google Scholar]
- 37. Chandrajith R, Chaturangani D, Abeykoon S, Barth JA, van Geldern R, Edirisinghe E, Dissanayake CB. Quantification of groundwater–seawater interaction in a coastal sandy aquifer system: a study from Panama, Sri Lanka. Environ Earth Sci. 2014;72:867–877. [Google Scholar]
- 38. Kinniburgh D, Smedley P. Arsenic contamination of groundwater in Bangladesh: summary. BGS Technical Report WC/00/19. 2001. Available at: https://www.bgs.ac.uk/downloads/start.cfm?id=2221. Accessed March 18, 2019.
- 39. Bromage S, Ahmed T, Fawzi WW. Calcium deficiency in Bangladesh: burden and proposed solutions for the first 1000 days. Food Nutr Bull. 2016;37:475–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hall JE. Guyton and Hall Textbook of Medical Physiology E‐Book. Philadelphia, PA, USA: Elsevier Health Sciences; 2015. [Google Scholar]
- 41. Bohr DF. Vascular smooth muscle: dual effect of calcium. Science. 1963;139:597–599. [DOI] [PubMed] [Google Scholar]
- 42. Wu C‐C, Bohr DF. Mechanisms of calcium relaxation of vascular smooth muscle. Am J Physiol Heart Circ Physiol. 1991;261:H1411–H1416. [DOI] [PubMed] [Google Scholar]
- 43. Laurant P, Touyz RM. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J Hypertens. 2000;18:1177–1191. [DOI] [PubMed] [Google Scholar]
- 44. Palant CE, Stern N, Meyer A, Tuck ML, Lee DB, Yanagawa N. Modulation of aortic smooth muscle cell membrane potential by extracellular calcium. Hypertension. 1989;14:549–555. [DOI] [PubMed] [Google Scholar]
- 45. Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens (Greenwich). 2008;10:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hatton DC, Yue Q, McCarron DA. Mechanisms of calcium's effects on blood pressure. Semin Nephrol. 1995;15:593–602. [PubMed] [Google Scholar]
- 47. World Health Organization . Calcium and magnesium in drinking‐water: public health significance. 2009. Available at: https://www.who.int/water_sanitation_health/publications/publication_9789241563550/en/. Accessed March 18, 2019.
- 48. Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006;25:271S–276S. [DOI] [PubMed] [Google Scholar]
- 49. White WB. Importance of blood pressure control over a 24‐hour period. J Manag Care Pharm. 2007;13:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shlezinger M, Amitai Y, Goldenberg I, Shechter M. Desalinated seawater supply and all‐cause mortality in hospitalized acute myocardial infarction patients from the Acute Coronary Syndrome Israeli Survey 2002–2013. Int J Cardiol. 2016;220:544–550. [DOI] [PubMed] [Google Scholar]
- 51. Shlezinger M, Amitai Y, Akriv A, Gabay H, Shechter M, Leventer‐Roberts M. Association between exposure to desalinated sea water and ischemic heart disease, diabetes mellitus and colorectal cancer; A population‐based study in Israel. Environ Res. 2018;166:620–627. [DOI] [PubMed] [Google Scholar]
- 52. Naser AM, Martorell R, Narayan KV, Clasen TF. First do no harm: the need to explore potential adverse health implications of drinking rainwater. Environ Sci Technol. 2017;51:5865–5866. [DOI] [PubMed] [Google Scholar]
- 53. Beal T, Massiot E, Arsenault JE, Smith MR, Hijmans RJ. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS One. 2017;12:e0175554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosanoff A. Changing crop magnesium concentrations: impact on human health. Plant Soil. 2013;368:139–153. [Google Scholar]


