Abstract
Background
Obtaining 24‐hour ambulatory blood pressure (BP) is recommended for the detection of masked or white‐coat hypertension. Our objective was to determine whether the magnitude of the difference between ambulatory and clinic BPs has prognostic implications.
Methods and Results
We included 610 participants of the AASK (African American Study of Kidney Disease and Hypertension) Cohort Study who had clinic and ambulatory BPs performed in close proximity in time. We used Cox models to determine the association between the absolute systolic BP (SBP) difference between clinic and awake ambulatory BPs (primary predictor) and death and end‐stage renal disease. Of 610 AASK Cohort Study participants, 200 (32.8%) died during a median follow‐up of 9.9 years; 178 (29.2%) developed end‐stage renal disease. There was a U‐shaped association between the clinic and ambulatory SBP difference with risk of death, but not end‐stage renal disease. A 5– to <10–mm Hg higher clinic versus awake SBP (white‐coat effect) was associated with a trend toward higher (adjusted) mortality risk (adjusted hazard ratio, 1.84; 95% CI, 0.94–3.56) compared with a 0– to <5–mm Hg clinic‐awake SBP difference (reference group). A ≥10–mm Hg clinic‐awake SBP difference was associated with even higher mortality risk (adjusted hazard ratio, 2.31; 95% CI, 1.27–4.22). A ≥−5–mm Hg clinic‐awake SBP difference was also associated with higher mortality (adjusted hazard ratio, 1.82; 95% CI, 1.05–3.15) compared with the reference group.
Conclusions
A U‐shaped association exists between the magnitude of the difference between clinic and ambulatory SBP and mortality. Higher clinic versus ambulatory BPs (as in white‐coat effect) may be associated with higher risk of death in black patients with chronic kidney disease.
Keywords: ambulatory blood pressure monitoring, chronic kidney disease, end‐stage renal disease, hypertension, mortality
Subject Categories: Hypertension, Nephrology and Kidney, Blood Pressure
Short abstract
See Editorial Parati et al
Clinical Perspective
What Is New?
In this study, we found that the magnitude of the difference between clinic and ambulatory blood pressure measurements taken in close time proximity was predictive of the risk of all‐cause mortality in a cohort of black patients with chronic kidney disease, but not with end‐stage renal disease.
What Are the Clinical Implications?
Our findings suggest that even if a patient with chronic kidney disease has “white‐coat” elevations in blood pressure, large differences between the clinic and ambulatory readings may be prognostic of worse outcomes, and these patients may need closer monitoring and appropriate cardiovascular risk modification.
Introduction
Several current guidelines recommend use of ambulatory blood pressure monitoring (ABPM) as the gold standard metric for the diagnosis and confirmation of hypertension in adults.1, 2, 3 This recommendation stems from a growing body of literature demonstrating that 24‐hour ambulatory blood pressures (ABPs) are more strongly associated with target organ damage compared with clinic blood pressures (BPs) in patients with chronic kidney disease (CKD).4, 5, 6, 7, 8
Most guidelines agree that it is normal for clinic BP to be up to 5 mm Hg higher than ABP in the general population, and standard definitions for the diagnosis of white‐coat and masked hypertension have been established.9, 10, 11, 12 Many studies have demonstrated a strong association between masked hypertension and risk of adverse cardiovascular and renal outcomes, but white‐coat hypertension has not been consistently associated with adverse outcomes.11, 13, 14, 15, 16, 17
Few studies have examined whether the magnitude of the absolute difference between clinic and ABPs among patients with CKD confers important prognostic information. In SPRINT (Systolic Blood Pressure Intervention Trial), large differences between clinic and ABPs were common, especially among patients with CKD randomized to the intensive treatment arm.18 These differences may be important as we intensify our approach to BP treatment in patients with CKD.12
Our objective was to examine whether the difference between clinic and ABP measurements has prognostic implications for risk of death and end‐stage renal disease (ESRD) among participants in the AASK (African American Study of Kidney Disease and Hypertension) Cohort Study, one of the largest observational studies of black patients with CKD. We hypothesized that large differences between clinic and ABP (either positive or negative) would be associated with higher risk of adverse outcomes among black patients with CKD.
Methods
AASK Cohort Study
We included participants from AASK Cohort Study, a National Institutes of Health–sponsored observational study that enrolled black patients with CKD who had not yet developed ESRD by the end of the AASK Cohort Study.19, 20, 21 The AASK Cohort Study began in April 2002 and ended in June 2007, and participants had clinic BPs and ABPM performed in close time proximity at study entry.22, 23, 24 At the start of the AASK Cohort Study, all participants were treated to a clinic BP target of <140/90 mm Hg based on results of the AASK Cohort Study;21, 24 the target was changed in 2004 to <130/80 mm Hg because of updated Joint National Committee guidelines.23, 25 Of AASK Cohort Study participants, 98% (674 of a total 691) had patient identifiers that facilitated ascertainment of ESRD and mortality outcomes. In our current analysis, we additionally excluded 64 participants who did not have ABPM data, deriving a final analytic cohort of 610 participants. Informed consent was obtained at all participating sites for participation in AASK Cohort Study and institutional review board approval for secondary data analysis was obtained at the University of California, San Francisco. Parent AASK Cohort Study data have been made publicly available at the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository.26
BP Measurements
Clinic BP
Clinic BPs were obtained by certified personnel using an aneroid device at the time of enrollment into the AASK Cohort Study.19, 22, 24 Three consecutive seated readings were obtained at each visit, and the average between the second and third seated readings was considered the clinic BP for the visit. The clinic BP taken from the initial AASK Cohort Study visit that was chronologically closest to ABPM performance was used to calculate the difference between clinic and ABPs. More than 96% of ABPMs were performed within 90 days of clinic BPs (median time difference=8 days).
Ambulatory BP
ABPM was performed at the time of baseline enrollment into the AASK Cohort Study using a SpaceLabs 90207 device, with BPs taken every 30 minutes over a 24‐hour period.5, 13 Data from all ABP monitors were sent to the AASK Central Cardiovascular Core Laboratory and read centrally. Awake ABP readings were considered the mean of those BPs that were taken from 6 am to 12 am (midnight), as diaries were not available.5
Primary Predictor
We computed the difference between clinic BPs and awake ABP readings separately for systolic BP (SBP) and diastolic BP (DBP; henceforth termed clinic‐awake difference) as our primary predictor of interest. If clinic BP was higher than awake SBP readings by >5 mm Hg, this was considered a “white‐coat” effect (regardless of whether meeting criteria for hypertension). If clinic BP was lower than awake SBP readings, this was considered a “masked” effect (regardless of whether meeting criteria for hypertension). Most AASK cohort participants had a history of diagnosed hypertension and were receiving antihypertensive therapy.19, 21, 24 We were most interested in the difference between clinic and awake ABP readings because clinic BPs would be expected to differ more substantially from 24‐hour mean ABP because of its inclusion of nocturnal measurements. However, we also computed the difference between clinic and 24‐hour mean ABP readings separately (henceforth termed clinic‐ABP difference) as a secondary predictor.
Outcomes
To extend ascertainment of ESRD and vital status beyond the end of the AASK Cohort Study through to June 30, 2012, we performed linkage of AASK Cohort Study participants with the US Renal Data System and the Social Security Death Index.27, 28 All‐cause death was our primary outcome given the known benefit of BP control on mortality risk.29 ESRD was a secondary outcome, as the association between BP lowering and ESRD risk has traditionally been less robust.21, 30
Statistical Analysis
We used Cox proportional hazards models starting from the date of ABPM to determine the association between clinic‐awake (or clinic‐ABP) difference with death. We first performed unadjusted Cox models using cubic splines (with 3 evenly spaced knots at −21, 4.5, and 15 mm Hg for clinic‐ABP difference). We chose to begin our analysis with cubic splines because these models allowed for greater flexibility in modeling the association between BP and outcomes and can be used to detect nonlinear relationships. Then, we performed additional Cox models after categorizing the clinic‐awake and clinic‐ABP systolic and diastolic differences as ≥−5 mm Hg (ie, clinic BP is lower than awake or ABP by at least 5 mm Hg), −5 to <0 mm Hg, 0 to <5 mm Hg (reference), 5 to <10 mm Hg, and ≥10 mm Hg. The reference group was selected because of the general acceptance that clinic BPs can be up to 5 mm Hg higher than awake ABPs and still be considered normal.20 Although up to a 10–mm Hg difference is the normative difference between clinic and 24‐hour mean BPs (because of inclusion of nocturnal readings), we chose to use a 5–mm Hg difference as the reference group for both clinic‐awake and clinic‐ABP differences because at lower ranges of BPs (eg, SBP <120–130 mm Hg), the normative difference narrows to ≤5 mm Hg.29 Furthermore, recent data have suggested that black patients have higher nocturnal BPs, and the current normative standards (which were not developed in a racially diverse population) have been questioned.31
We examined the association between categories of the clinic‐awake or clinic‐ABP differences (using separate models for each predictor) with risk of death in unadjusted analyses, followed by a main adjusted model, including age, sex, estimated glomerular filtration rate (eGFR) by the CKD–Epidemiology Collaboration equation,32 heart disease (defined by a combination of self‐report, chart review, or baseline electrocardiogram reading), and proteinuria (measured at cohort entry) in model 1. In model 2, we added clinic SBP as a covariate to model 1. In model 3, we added either mean awake SBP or mean 24‐hour ambulatory SBP as a covariate to model 1, depending on whether the primary predictor is clinic‐awake SBP or clinic‐ambulatory SBP. Finally, because of the strong prognostic significance of sleep BPs in the literature compared with other ABPs,17 we also added sleep BPs in a sensitivity analysis in model 4. We categorized eGFR by CKD stages (eGFR ≥45, 30–<45, or <30 mL/min per 1.73 m2) and proteinuria (≥0.5 versus <0.5 g/g or missing) to handle nonnormality in both variables.
We repeated all analyses using DBP differences between clinic and ABPs. We also repeated all analyses using ESRD as our secondary outcome.
Finally, we tested for prespecified interactions by adding interaction terms between the clinic‐awake SBP difference or clinic‐ABPM SBP difference with the following: (1) baseline clinic SBP; and separately (2) mean awake SBP (when clinic‐awake SBP difference is the primary predictor) or mean 24‐hour ambulatory SBP (when clinic‐ABPM SBP difference is the primary predictor). These analyses were designed to determine whether the magnitude of the clinic‐awake or clinic‐ABP differences and their associations with death differed depending on the level of achieved SBP.
STATA14 was used for statistical analyses. For all analyses, P<0.05 was considered statistically significant.
Results
Characteristics of the 610 AASK Cohort Study participants are shown in Tables 1 and 2. Overall mean age was 60.5 years, 38% of the cohort were women, and mean eGFR was 39 mL/min per 1.73 m2. Mean clinic SBP was 134±20 mm Hg, and mean clinic DBP was 80±12 mm Hg, at cohort entry. In general, more women had white‐coat effect and fewer women had masked effect, but there were no differences in age, prior BP target randomization arm, baseline eGFR, or proteinuria between different categories of the clinic‐awake difference (Table 1). Heart disease was more prevalent among those with masked effect compared with those with white‐coat effect. The distribution of the clinic, 24‐hour ABP, and ABP awake BPs is shown in Table 2. Approximately 24% of patients had white‐coat effect and 64% had masked effect using the difference between clinic and awake SBP.
Table 1.
Characteristics of AASK Cohort Study Participants Included for Analysis by Categories of Difference Between Clinic and Awake SBP
| Characteristics | Clinic BP–Mean Awake ABP, mm Hg | ||||
|---|---|---|---|---|---|
| White‐Coat Effect | Reference | Masked Effect | |||
| ≥10 | 5 to <10 | 0 to <5 | −5 to <0 | ≥−5 | |
| (N=93) | (N=56) | (N=70) | (N=97) | (N=294) | |
| Age, y | 60.4±9.3 | 61.2±8.4 | 59.5±10.9 | 58.5±9.7 | 60.1±10.6 |
| Female sex | 43 (46.2) | 33 (58.9) | 29 (41.4) | 36 (37.1) | 92 (31.3) |
| Heart disease | 35 (35) | 34 (61) | 56 (58) | 65 (70) | 197 (67) |
| eGFR, mL/min per 1.73 m2 | 39.9±16.5 | 36.3±15.0 | 35.9±15.2 | 42.7±16.3 | 39.2±15.2 |
| Proteinuria, median (IQR), g/ga | 0.05 (0.02–0.1) | 0.09 (0.04–0.34) | 0.07 (0.03–0.26) | 0.05 (0.03–0.29) | 0.06 (0.03–0.32) |
| Randomized to strict BP control during the AASK Cohort Study | 49 (52.7) | 30 (53.6) | 30 (42.9) | 48 (49.5) | 147 (50.0) |
Data are given as mean±SD or number (percentage), unless otherwise indicated. AASK indicates African American Study of Kidney Disease and Hypertension; ABP, ambulatory BP; BP, blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SBP, systolic BP.
Missing in N=81.
Table 2.
Mean BP Among Each of the Categories of the Difference Between Clinic and Awake SBP
| BP Variable | Clinic BP–Mean Awake ABP, mm Hg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White‐Coat Effect | Reference | Masked Effect | ||||||||
| ≥10 | 5 to <10 | 0 to <5 | −5 to <0 | ≥−5 | ||||||
| (N=93) | (N=56) | (N=70) | (N=97) | (N=294) | ||||||
| SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | |
| Mean clinic BP at visit closest to ABPM performance, mm Hg | 152.0±21.8 | 92.1±12.9 | 143.2±17.4 | 86.6±9.6 | 140.0±16.3 | 83.3±9.8 | 131.4±15.9 | 77.8±8.9 | 125.4±16.2 | 74.3±10.8 |
| Mean 24‐h ABPM, mm Hg | 131.2±17.0 | 77.9±11.0 | 134.7±17.7 | 77.5±11.2 | 136.7±16.6 | 81.7±10.2 | 133.0±15.6 | 80.8±10.1 | 140.2±16.8 | 82.7±10.2 |
| Mean awake ABPM, mm Hg | 132.1±16.9 | 79.3±11.2 | 135.9±17.6 | 79.1±11.4 | 137.6±16.2 | 83.0±10.4 | 134.0±15.7 | 82.2±10.2 | 140.9±16.6 | 83.9±10.1 |
ABP indicates ambulatory BP; ABPM, ABP monitoring; BP, blood pressure; DBP, diastolic BP; SBP, systolic BP.
Risk of All‐Cause Mortality
During a median follow‐up of 9.9 years, 200 (of 610) participants died. A U‐shaped association was found between the magnitude of the clinic‐awake SBP difference (either positive or negative) and the risk of death (Figure—Panel A). Results of the association when the magnitude of the clinic‐awake difference was examined categorically are shown in Table 3. Compared with the reference group (clinic‐awake SBP difference of 0–<5 mm Hg), participants with a clinic SBP that was 5 to <10 mm Hg higher than awake SBP had at least a 1.84 times greater risk of death (model 1), although this finding did not achieve statistical significance. Those with a >10–mm Hg higher clinic SBP than awake SBP had a 2.31 times higher risk of death (Table 3). There was no difference in the risk of death among study participants with a clinic‐awake SBP difference of −5 to 0 mm Hg compared with the reference group in model 1 (hazard ratio, 1.15 [95% CI, 0.59–2.24]). However, if the clinic‐awake SBP difference was ≥−5 mm Hg, the adjusted risk of death was statistically significantly higher than that for the reference group (hazard ratio, 1.82 [95% CI, 1.05–3.15]). Additional adjustment accounting for demographics, kidney function, heart disease, proteinuria, as well as clinic, awake, or sleep SBP did not substantially alter findings (models 2–4, Table 3).
Figure 1.
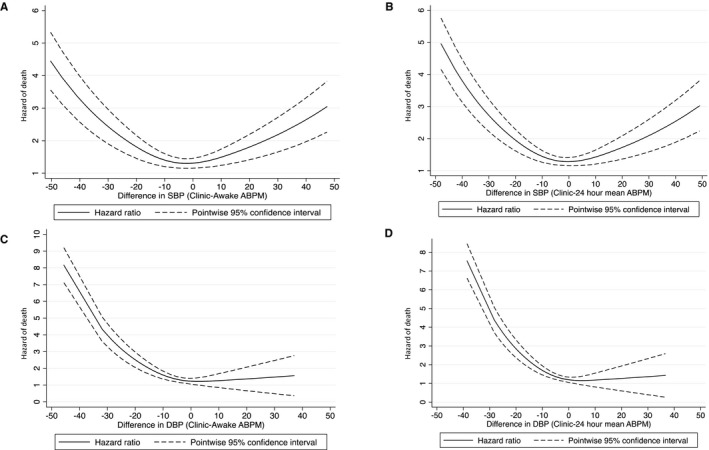
Association between clinic‐awake or clinic–ambulatory blood pressure (ABP) and risk of death in unadjusted models. Risk of death based on difference between clinic and ABP monitoring (ABPM) systolic blood pressure (SBP; 24 hour and awake). A, U‐shaped association between the magnitude of the clinic‐awake ABPM SBP difference and the risk of death. B, U‐shaped association between the magnitude of the clinic–24‐hour mean ABPM SBP difference and the risk of death. C and D, Clinic‐ABPM diastolic blood pressure (DBP) not associated with risk of death.
Table 3.
Difference Between Clinic and Mean Awake ABP and Long‐Term Risk of Death
| Systolic BP, mm Hg | Fully Adjusted Models, Hazard Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| (Clinic–Awake Ambulatory BP) | N | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 |
| “White‐coat effect” | ||||||
| ≥10 | 93 | 2.30 (1.27–4.18) | 2.31 (1.27–4.22) | 2.10 (1.14–3.86) | 2.62 (1.43–4.81) | 2.53 (1.38–4.62) |
| 5 to <10 | 56 | 2.07 (1.07–4.02) | 1.84 (0.94–3.56) | 1.85 (0.95–3.61) | 1.98 (1.01–3.87) | 1.96 (1.01–3.84) |
| 0 to <5 | 70 | Reference | Reference | Reference | Reference | Reference |
| “Masked effect” | ||||||
| −5 to <0 | 97 | 1.04 (0.54–2.02) | 1.15 (0.59–2.24) | 1.25 (0.64–2.43) | 1.22 (0.62–2.37) | 1.21 (0.62–2.36) |
| ≥−5 | 294 | 1.82 (1.06–3.13) | 1.82 (1.05–3.15) | 2.14 (1.22–3.75) | 1.79 (1.03–3.09) | 1.76 (1.02–3.05) |
Model 1: All fully adjusted models are adjusted for age, sex, heart disease, estimated glomerular filtration rate at cohort entry, and proteinuria. Model 2: Adjusted model+clinic systolic BP. Model 3: Adjusted model+either mean awake ambulatory systolic BP (Table 3) or mean 24‐hour ambulatory systolic BP (Table 4), depending on whether the difference is between clinic‐awake systolic BP or clinic–ABP monitoring systolic BP. Model 4: Adjusted model+mean 24‐hour asleep systolic BP. ABP indicates ambulatory BP; BP, blood pressure.
Similar results were found when examining the association between clinic‐systolic ABP difference and the risk of death. We found a U‐shaped association between the magnitude of the clinic‐ambulatory SBP difference (either positive or negative) and the risk of death (Figure—Panel B). A higher risk of death was also noted among individuals with categories of clinic‐ambulatory SBP difference of >5 mm Hg or ≥−5 mm Hg in unadjusted analysis (Table 4); with further adjustment in model 1, a ≥10– or ≥−5–mm Hg difference in clinic ambulatory SBP was statistically significantly associated with risk of death (Table 4). Additional adjustments for clinic SBP, 24‐hour mean, or sleep SBP did not substantially alter findings (models 2–4).
Table 4.
Difference Between Clinic and Mean 24‐Hour ABP and Long‐Term Risk of Death
| Systolic BP, mm Hg | Fully Adjusted Models, Hazard Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| (Clinic–Ambulatory BP) | N | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 |
| ≥10 | 104 | 2.34 (1.29–4.22) | 2.18 (1.20–3.95) | 1.95 (1.07–3.56) | 2.47 (1.35–4.49) | 2.36 (1.30–4.28) |
| 5 to <10 | 58 | 1.99 (1.02–3.89) | 1.59 (0.81–3.13) | 1.59 (0.81–3.13) | 1.72 (0.87–3.38) | 1.71 (0.87–3.37) |
| 0 to <5 | 77 | Reference | Reference | Reference | Reference | Reference |
| −5 to <0 | 91 | 1.31 (0.68–2.53) | 1.38 (0.72–2.67) | 1.50 (0.78–2.91) | 1.45 (0.75–2.81) | 1.34 (0.70–2.60) |
| ≥−5 | 280 | 2.07 (1.20–3.56) | 1.85 (1.07–3.21) | 2.20 (1.25–3.87) | 1.80 (1.04–3.11) | 1.69 (0.97–2.93) |
Model 1: All fully adjusted models are adjusted for age, sex, heart disease, estimated glomerular filtration rate at cohort entry, and proteinuria. Model 2: Adjusted model+clinic systolic BP. Model 3: Adjusted model+either mean awake ambulatory systolic BP (Table 3) or mean 24‐hour ambulatory systolic BP (Table 4), depending on whether the difference is between clinic‐awake systolic BP or clinic–ABP monitoring systolic BP. Model 4: Adjusted model+mean 24‐hour asleep systolic BP. ABP indicates ambulatory BP; BP, blood pressure.
We tested for but did not find significant interactions between categories of the clinic‐awake or clinic‐ABP difference with baseline clinic SBP, mean awake SBP, or 24‐hour SBP (all P>0.05).
The relationship between DBP and outcomes of interest differed from that observed for SBP. Specifically, presence of clinic DBP higher than ambulatory DBP (either awake or mean 24‐hour DBP in a white‐coat effect) was not associated with risk of death in our spline‐based analyses (Figure–Panels C and D). However, clinic DBP lower than ambulatory DBP (masked effect) appeared to have a continuous and graded association with mortality risk that was similar to that observed for SBP. When the clinic‐awake or clinic‐ABPM DBP differences were categorized, a difference ≥−5 mm Hg was statistically significantly associated with mortality risk in adjusted analyses (data not shown).
Risk of ESRD
A total of 178 participants developed ESRD during follow‐up. The continuous relationship between the magnitude of the clinic‐awake and clinic‐ABP difference with risk of ESRD is shown in Figure S1A and S1B for SBPs and Figure S1C and S1D for DBPs. There was no statistically significant association between the magnitude of the clinic‐awake or clinic‐ABP systolic or diastolic difference and risk of ESRD in unadjusted analyses (Tables S1 and S2). In adjusted analyses, extremes of differences (>10 or ≥−5 mm Hg) in clinic‐awake or clinic‐ambulatory DBP were associated with a higher risk of ESRD in adjusted analyses, and further adjustment for ABPM parameters did not substantially alter results (data not shown).
Discussion
In the population with CKD, ≈10% to 30% of patients are reported to have white‐coat hypertension, and ≈30% to 60% of patients with CKD have masked hypertension.4, 8, 13, 33, 34 However, neither masked nor white‐coat hypertension can be detected without out‐of‐office BP measurements, and 24‐hour ABPM remains the preferred standard measure of out‐of‐office BPs.10, 35 Although many observational studies have demonstrated the prognostic significance of ABPM parameters that support the routine ascertainment of ABPs, most of these studies have either examined the prognostic significance of individual parameters (eg, nocturnal SBP) as continuous variables or categorized clinic BPs as white‐coat, masked, or confirmed hypertension for study.5, 7, 8, 13, 36
In this study, we found that, compared with a clinic‐awake or clinic‐ABP difference of 0 to <5 mm Hg (considered a normal difference), there was a continuous, U‐shaped relationship between positive or negative absolute differences in clinic‐awake or clinic‐ABP SBP measurements with mortality risk. Specifically, when a white‐coat effect was present (clinic SBP higher than ambulatory SBP by ≥5 mm Hg), the clinic‐awake and clinic‐ABP differences were strongly associated with mortality risk in a graded manner. When a masked effect was present (clinic SBP lower than ambulatory SBP), the clinic‐awake and clinic‐ABP differences were only associated with mortality risk if this absolute difference was ≥5 mm Hg. These findings were not attenuated after adjustment for clinic SBP, ambulatory SBP, sleep SBP, or proteinuria, suggesting that the absolute difference between clinic and ABP provides independent prognostic information beyond achieved SBP. The magnitude of the clinic‐awake or clinic‐ABPM SBP difference was not associated with risk of ESRD.
A few prior studies have examined the implications of the magnitude of the difference between clinic and ABP measurements in the context of variations in the response of this difference to antihypertensive therapy, and changes to these differences have been observed as aging occurs in populations without CKD.36, 37, 38 Our study results extend those from prior observations to examine the association of those differences with mortality and ESRD, arguably outcomes of greatest interest to patients. Consistent with prior studies that have shown that masked hypertension is associated with risk of adverse outcomes,39, 40 we found that masked clinic‐awake or clinic‐ABPM SBP differences of ≥5 mm Hg were associated with a higher risk of death, regardless of whether absolute SBP met the definition for hypertension. It is possible that these findings may be driven by the well‐performed protocol‐driven AASK clinic BP readings, which have important prognostic value and are likely lower than non–protocol‐driven clinic BP readings.
We also found that clinic SBP readings that represented a white‐coat effect were strongly associated with the risk of death. A few studies of patients in the general population have suggested that presence of white‐coat hypertension is associated with higher‐risk cardiovascular outcomes and may be a precursor to the development of sustained hypertension.40, 41, 42, 43, 44, 45 Our findings are consistent with recent studies that also demonstrated that white‐coat hypertension was not benign and was associated with cardiovascular risk during long‐term follow‐up.17, 46 Prior studies have noted that the prevalence of white‐coat hypertension is more common among women in the general population,47, 48 whereas masked hypertension may be more common in men.49 Our findings are consistent with those of the literature in the general population, and in the setting of a new diagnosis of hypertension, confirmation of the diagnosis using ABPM, especially in black women, could help prevent overtreatment.
Having a large difference between clinic and ABPs may also reflect heightened variability or “volatility” of BP measurements or increased levels of sympathetic overactivity, which may be associated with long‐term cardiovascular risk. However, it is also possible that these findings are specific to the black population with CKD, as little is known about racial differences in the prognostic value of ABP in the population with CKD. On the basis of our findings, a clinic‐ABP difference of >5 mm Hg, suggestive of white‐coat phenomena, should not be completely ignored as benign among black patients with CKD. Thus, we believe that even in the presence of white‐coat hypertension, patients with CKD who exhibit a large difference between clinic and ABPs may need closer follow‐up and intensive cardiovascular risk reduction, as these patients are at elevated risk for death during long‐term follow‐up. In contrast, those patients with smaller magnitudes of difference (eg, <5 mm Hg) between ambulatory and clinic BPs may be at lower risk for adverse cardiovascular events.
We did not find the difference between clinic‐ABP or clinic‐awake SBP or DBP to be consistently associated with the risk of ESRD. Prior studies of patients with CKD have also not found ABP measurements to be consistently associated with renal outcomes, especially after accounting for other risk factors for CKD progression.7 Our results are overall consistent with the null effects of several adult BP‐lowering trials on ESRD outcomes.21, 23, 29, 30
The strengths of this study include the use of a well‐characterized cohort and availability of well‐performed research‐grade clinic BPs and ABPs. Limitations include the focus on a black population with hypertension‐attributed CKD, so our results may not generalize to all patients with CKD. Also, the number of participants with white‐coat effect was smaller, and this warrants replication with a larger sample size with a higher number of events. The AASK Cohort Study was also unique in the long‐term relationship between participants and study coordinators who obtained BP readings, which may have reduced white‐coat effects during clinic measurements.50 We also do not have causes of death and, therefore, are unable to examine the risk of cardiovascular‐related mortality. Finally, although repeated ABPMs were performed every 2 years during the duration of the AASK Cohort Study, the number of participants with ABPM declined precipitously after the baseline ABPM; and those who died would not have had a subsequent ABPM (which may lead to survivor bias), so we have chosen not to include analysis of subsequent ABPs in this study.
In conclusion, the magnitude of the difference between clinic and ambulatory SBP measurements has prognostic significance and should be considered during BP assessments among patients with CKD. Our results suggest that the presence of a white‐coat effect may be associated with higher risk of death, even when clinic or ambulatory SBPs are controlled. We believe that it is important to invest in more widespread implementation of ABPM and efforts to obtain consistent, high‐quality, clinic BP measurements to accurately collect data that may inform antihypertensive therapy in patients with CKD. Further studies are needed to confirm these findings in more racially diverse populations and to examine whether therapeutic changes to alter clinic‐ABP differences may lead to improved outcomes.
Sources of Funding
This research was supported by the National Institutes of Health grants K23 HL131023 (Ku), R01DK104130 (Tuot), and K23DK100468 and R03DK111881 (Hsu). The AASK (African American Study of Kidney Disease and Hypertension) Cohort Study was conducted by AASK Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosures
None.
Supporting information
Table S1. Difference Between Clinic and Mean Awake Ambulatory Blood Pressure and Risk of ESRD
Table S2. Difference Between Clinic and Mean Ambulatory Blood Pressure and Risk of ESRD
Figure S1. Risk of ESRD based on difference between office and ABPM systolic and diastolic (24 hour and awake).
Acknowledgments
We are grateful to the study participants, research staff, and investigators of the AASK (African American Study of Kidney Disease and Hypertension) Cohort Study. The data from the AASK Cohort Study reported herein were supplied, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repositories. This article was not prepared in collaboration with investigators of the AASK Cohort Study and does not necessarily reflect the opinions or views of the AASK Cohort Study, the NIDDK Central Repositories, or NIDDK grants MD000182, UL1TR000124, and P30AG021684. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The interpretation and reporting of the data presented herein are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
(J Am Heart Assoc. 2019;8:e011013 DOI: 10.1161/JAHA.118.011013.)
References
- 1. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the TASK Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 2. Parati G, Stergiou G, O'Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 3. Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192–204. [DOI] [PubMed] [Google Scholar]
- 4. Bangash F, Agarwal R. Masked hypertension and white‐coat hypertension in chronic kidney disease: a meta‐analysis. Clin J Am Soc Nephrol. 2009;4:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, Faulkner ML, Hiremath L, Jamerson KA, Lea JP, Lipkowitz MS, Pogue VA, Rostand SG, Smogorzewski MJ, Wright JT, Greene T, Gassman J, Wang X, Phillips RA. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7:1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, De Nicola L. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171:1090–1098. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2016;27:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 10. ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 13. Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–27. [DOI] [PubMed] [Google Scholar]
- 14. Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O'Brien E, Ohkubo T, Padfield P, Palatini P, Pickering TG, Redon J, Revera M, Ruilope LM, Shennan A, Staessen JA, Tisler A, Waeber B, Zanchetti A, Mancia G. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–785. [DOI] [PubMed] [Google Scholar]
- 15. Minutolo R, Borrelli S, Scigliano R, Bellizzi V, Chiodini P, Cianciaruso B, Nappi F, Zamboli P, Conte G, De Nicola L. Prevalence and clinical correlates of white coat hypertension in chronic kidney disease. Nephrol Dial Transplant. 2007;22:2217–2223. [DOI] [PubMed] [Google Scholar]
- 16. Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, Nappi F, Stanzione G, Conte G, De Nicola L. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. Am J Kidney Dis. 2014;64:744–752. [DOI] [PubMed] [Google Scholar]
- 17. Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz‐Hurtado G, Segura J, Rodriguez‐Artalejo F, Williams B. Relationship between clinic and ambulatory blood‐pressure measurements and mortality. N Engl J Med. 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 18. Drawz PE, Pajewski NM, Bates JT, Bello NA, Cushman WC, Dwyer JP, Fine LJ, Goff DC Jr, Haley WE, Krousel‐Wood M, McWilliams A, Rifkin DE, Slinin Y, Taylor A, Townsend R, Wall B, Wright JT, Rahman M. Effect of intensive versus standard clinic‐based hypertension management on ambulatory blood pressure: results from the SPRINT (Systolic Blood Pressure Intervention Trial) ambulatory blood pressure study. Hypertension. 2017;69:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER III, Norris K, O'Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley‐Brown D, Tisher CC, Toto RD, Wright JT Jr, Xu S. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. [DOI] [PubMed] [Google Scholar]
- 20. Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, Randall OS, Wang SR. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol. 2003;14:S154–S165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas‐Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 22. Appel LJ, Middleton J, Miller ER III, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14:S166–S172. [DOI] [PubMed] [Google Scholar]
- 23. Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X. Intensive blood‐pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sika M, Lewis J, Douglas J, Erlinger T, Dowie D, Lipkowitz M, Lash J, Cornish‐Zirker D, Peterson G, Toto R, Kusek J, Appel L, Kendrick C, Gassman J. Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) clinical trial and cohort study. Am J Kidney Dis. 2007;50:78–89, 89.e71. [DOI] [PubMed] [Google Scholar]
- 25. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 26. National Institute of Diabetes and Digestive and Kidney Diseases. African American Study of Kidney Disease and Hypertension (AASK). NIDDK Central Repository . https://repository.niddk.nih.gov/home/. Accessed May 24, 2018.
- 27. Ku E, Gassman J, Appel LJ, Smogorzewski M, Sarnak MJ, Glidden DV, Bakris G, Gutierrez OM, Hebert LA, Ix JH, Lea J, Lipkowitz MS, Norris K, Ploth D, Pogue VA, Rostand SG, Siew ED, Sika M, Tisher CC, Toto R, Wright JT Jr, Wyatt C, Hsu CY. BP control and long‐term risk of ESRD and mortality. J Am Soc Nephrol. 2016;28:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ku E, Lipkowitz MS, Appel LJ, Parsa A, Gassman J, Glidden DV, Smogorzewski M, Hsu CY. Strict blood pressure control associates with decreased mortality risk by APOL1 genotype. Kidney Int. 2017;91:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modiligfiligcation of Diet in Renal Disease Study Group . The effects of dietary protein restriction and blood‐pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. [DOI] [PubMed] [Google Scholar]
- 31. Ravenell J, Shimbo D, Booth JN III, Sarpong DF, Agyemang C, Beatty Moody DL, Abdalla M, Spruill TM, Shallcross AJ, Bress AP, Muntner P, Ogedegbe G. Thresholds for ambulatory blood pressure among African Americans in the Jackson Heart Study. Circulation. 2017;135:2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, Ruilope LM. Differences between office and 24‐hour blood pressure control in hypertensive patients with CKD: a 5,693‐patient cross‐sectional analysis from Spain. Am J Kidney Dis. 2013;62:285–294. [DOI] [PubMed] [Google Scholar]
- 34. Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol. 2006;26:503–510. [DOI] [PubMed] [Google Scholar]
- 35. Becker GJ, Wheeler DC, De Zeeuw D, Fujita T, Furth SL, Holdaas H, Mendis S, Oparil S, Perkovic V, Saad Rodrigues CI, Sarnak MJ, Schernthaner G, Tomson CRV, Zoccali C. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int, 2012;2 Suppl.:337–414. [Google Scholar]
- 36. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Zampi I, Gattobigio R, Sacchi N, Porcellati C. White coat hypertension and white coat effect: similarities and differences. Am J Hypertens. 1995;8:790–798. [DOI] [PubMed] [Google Scholar]
- 37. Banegas JR, Messerli FH, Waeber B, Rodriguez‐Artalejo F, de la Sierra A, Segura J, Roca‐Cusachs A, Aranda P, Ruilope LM. Discrepancies between office and ambulatory blood pressure: clinical implications. Am J Med. 2009;122:1136–1141. [DOI] [PubMed] [Google Scholar]
- 38. Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Segura J, Oliveras A, Martell N, Garcia‐Puig J, Williams B. Clinic versus daytime ambulatory blood pressure difference in hypertensive patients: the impact of age and clinic blood pressure. Hypertension. 2017;69:211–219. [DOI] [PubMed] [Google Scholar]
- 39. Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, Huan Y, Keane MG, Kusek JW, Makos GK, Miller ER III, Soliman EZ, Steigerwalt SP, Taliercio JJ, Townsend RR, Weir MR, Wright JT Jr, Xie D, Rahman M. Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;11:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fukuhara M, Arima H, Ninomiya T, Hata J, Hirakawa Y, Doi Y, Yonemoto K, Mukai N, Nagata M, Ikeda F, Matsumura K, Kitazono T, Kiyohara Y. White‐coat and masked hypertension are associated with carotid atherosclerosis in a general population: the Hisayama study. Stroke. 2013;44:1512–1517. [DOI] [PubMed] [Google Scholar]
- 41. Mancia G, Bombelli M, Cuspidi C, Facchetti R, Grassi G. Cardiovascular risk associated with white‐coat hypertension: pro side of the argument. Hypertension. 2017;70:668–675. [DOI] [PubMed] [Google Scholar]
- 42. Cerasola G, Cottone S, Nardi E, D'Ignoto G, Volpe V, Mule G, Carollo C. White‐coat hypertension and cardiovascular risk. J Cardiovasc Risk. 1995;2:545–549. [PubMed] [Google Scholar]
- 43. Glen SK, Elliott HL, Curzio JL, Lees KR, Reid JL. White‐coat hypertension as a cause of cardiovascular dysfunction. Lancet. 1996;348:654–657. [DOI] [PubMed] [Google Scholar]
- 44. Muldoon MF, Nazzaro P, Sutton‐Tyrrell K, Manuck SB. White‐coat hypertension and carotid artery atherosclerosis: a matching study. Arch Intern Med. 2000;160:1507–1512. [DOI] [PubMed] [Google Scholar]
- 45. Muscholl MW, Hense HW, Brockel U, Doring A, Riegger GA, Schunkert H. Changes in left ventricular structure and function in patients with white coat hypertension: cross sectional survey. BMJ. 1998;317:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kushiro T, Kario K, Saito I, Teramukai S, Sato Y, Okuda Y, Shimada K. Increased cardiovascular risk of treated white coat and masked hypertension in patients with diabetes and chronic kidney disease: the HONEST Study. Hypertens Res. 2017;40:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Myers MG, Reeves RA. White coat effect in treated hypertensive patients: sex differences. J Hum Hypertens. 1995;9:729–733. [PubMed] [Google Scholar]
- 48. Abir‐Khalil S, Zaimi S, Tazi MA, Bendahmane S, Bensaoud O, Benomar M. Prevalence and predictors of white‐coat hypertension in a large database of ambulatory blood pressure monitoring. East Mediterr Health J. 2009;15:400–407. [PubMed] [Google Scholar]
- 49. Hwang ES, Choi KJ, Kang DH, Nam GB, Jang JS, Jeong YH, Lee CH, Lee JY, Park HK, Park CH. Prevalence, predictive factor, and clinical significance of white‐coat hypertension and masked hypertension in Korean hypertensive patients. Korean J Intern Med. 2007;22:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cobos B, Haskard‐Zolnierek K, Howard K. White coat hypertension: improving the patient‐health care practitioner relationship. Psychol Res Behav Manag. 2015;8:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Difference Between Clinic and Mean Awake Ambulatory Blood Pressure and Risk of ESRD
Table S2. Difference Between Clinic and Mean Ambulatory Blood Pressure and Risk of ESRD
Figure S1. Risk of ESRD based on difference between office and ABPM systolic and diastolic (24 hour and awake).


