Abstract
Background
Women with non‐obstructive coronary artery disease have increased cardiovascular morbidity. The role of risk factors in this population has yet to be established. We aimed to study the predictive effect of triglycerides and the triglyceride/high‐density lipoprotein ratio on major adverse cardiovascular events (MACE) in patients with non‐obstructive coronary artery disease, and to explore the role of lipid lowering therapy in modifying this risk.
Methods and Results
This is a prospective cohort study enrolling patients with anginal symptoms referred to the cardiac catheterization laboratory for suspected ischemia, who were subsequently diagnosed with non‐obstructive coronary artery disease, defined as no stenosis >20% on angiography. All patients had baseline laboratory testing and were followed for 7.8±4.3 years for the development of major adverse cardiovascular events. We performed Cox proportional hazard testing to determine the effect of triglycerides on risk of major adverse cardiovascular events among men and women by baseline statin use. A total of 462 patients were included. Median age was 53 (Q1, Q3: 45, 62) years. In a Cox proportional hazard model stratified by statin use adjusting for confounders, among those not on baseline statins, triglycerides were independently predictive of major adverse cardiovascular events in women (per 50 mg/dL risk ratio: hazard ratio 1.25 [95% CI: 1.06, 1.47]; P=0.01). This was not true among men. The interaction between triglycerides and sex, and triglycerides and statin was statistically significant.
Conclusions
Triglyceride levels may play a key role in predicting cardiovascular‐specific risk in women, and statin use may be protective. Further investigation is necessary to better delineate the role of statin use in preventing cardiovascular risk.
Keywords: hyperlipidemia, outcome, prevention, triglycerides
Subject Categories: Coronary Artery Disease
Clinical Perspective
What Is New?
We present novel data suggesting a significant association between triglyceride levels, the triglyceride/high‐density lipoprotein ratio and major adverse cardiovascular events in women.
Additionally, we find that women with non‐obstructive coronary artery disease and elevated triglycerides who were on statins may be protected against future cardiovascular events.
What Are the Clinical Implications?
Further investigation is necessary in the form of larger randomized trials to assess the role of triglycerides in predicting cardiovascular risk in this population.
Statin use may be protective against future cardiovascular events and larger trials will help understand the role of statin therapy in women diagnosed with non‐obstructive coronary artery disease and elevated triglycerides.
Introduction
Coronary artery disease has been recognized as the number 1 cause of death in men and women, and hyperlipidemia as a modifiable risk factor.1 Metabolic changes associated with menopause and aging can contribute to dyslipidemia in women.2 Postmenopausal women may have higher triglyceride levels when compared with premenopausal women, and this may be associated with increased postmenopausal cardiovascular risk.3, 4 This is supported by data that have shown a greater increase in triglyceride levels with age in women versus men.5, 6 While the risk of hypertriglyceridemia has been well‐studied in secondary prevention, its role in patients with non‐obstructive coronary artery disease (NOCAD) and the effect of lipid lowering therapy has yet to be defined in this population.7, 8, 9
The lipid profile is a well‐recognized traditional risk factor that is associated with increased cardiovascular risk.10 The role of individual lipid subparticles, including high‐density lipoprotein (HDL), low‐density lipoprotein, and triglyceride levels is less well understood in patients with NOCAD.11, 12, 13 There are no set guidelines describing the need for lipid lowering therapy in this subset of patients either, and so further investigation is required to better understand the role of lipid levels and statin use in patients with NOCAD.
NOCAD is an increasingly recognized cause of ischemic symptoms, especially among women, and has been associated with a high prevalence of coronary endothelial dysfunction. This in turn is associated with significant cardiovascular morbidity and mortality.11, 12, 13, 14, 15, 16, 17 We aimed to define the role of triglycerides in predicting major adverse cardiovascular events (MACE) in patients with non‐obstructive coronary artery disease (NOCAD) and further stratify by sex and baseline statin use.
Methods
The current study was approved by the Mayo Clinic Institutional Review Board. All patients provided informed written consent. The authors were responsible for design and conduct of the study, study analyses, and drafting of the manuscript.
Design and Participants
We screened and enrolled consecutive patients presenting to the Mayo Clinic Cardiac Catheterization laboratory from January 1992 to August 2012 with signs and symptoms suggestive of ischemic cardiovascular disease including chest pain who were subsequently found to have non‐obstructive coronary disease (NOCAD) on invasive angiography. NOCAD was defined as no stenosis >20%. The data, analytic methods, and study materials are in this article and have been made available to other researchers for the purposes of reproducing the results or replicating the procedure.
Blood Measurements
Patients underwent baseline laboratory testing on enrollment. They were given detailed instructions to avoid alcohol, restrict exercise, and to not drink or eat anything except plain water for 12 hours before blood testing. Fasting venous blood samples were drawn with a vacutainer according to standard laboratory protocol. Samples were centrifuged, aliquoted, and transferred to the core laboratory for analysis. Analysis was performed within 12 hours of sample collection. Total cholesterol levels and triglycerides were measured using the enzymatic colorimetric assay, and serum high‐density lipoprotein‐cholesterol was measured using the homogeneous enzymatic colorimetric test. Low‐density lipoprotein was calculated according to standard procedure from these values. In addition, complete blood count with differential, electrolyte panel, and inflammatory markers were also collected.
Additionally, on enrollment, all patients completed a standardized validated questionnaire to assess baseline characteristics.18, 19, 20 Patients with a history of percutaneous coronary intervention, coronary artery bypass graft surgery, unstable angina pectoris, valvular heart disease, peripheral vascular disease, or known congestive heart failure were excluded from the study.
Subsequent Evaluation
All patients received a standardized questionnaire for assessment of their overall health several years after their initial coronary angiogram and evaluation. The standardized questionnaire was used to assess occurrence of major adverse cardiovascular events including stroke, rehospitalization, myocardial infarction, or death after a mean follow‐up of 7.8±4.3 years. Responses were verified by medical record review conducted by an investigator masked to the results of the standardized questionnaire.18, 19
Statistical Analysis
All data are displayed as median with the first and third quartiles listed in parenthesis. Demographic and baseline clinical data were compared using Student t test, ANOVA, and Wilcoxon tests for continuous data and Pearson Chi‐square test for categorical data. We used Cox proportional hazards models to assess the risk of MACE associated with dependent variables after adjusting for potential confounders. Non‐informative censoring was likely, and censoring was defined as time to receipt of survey form. Censoring for individual subjects was not related to the probability of an event occurring. Follow‐up was independent of clinical course and done through an independent questionnaire at a random time point. Additionally, hazard functions were proportional over time and the assumption of proportional hazards was met.
Results
Baseline Characteristics
A total of 519 patients were included in this study. Of these 54 patients were excluded because of lack of complete laboratory data. A total of 465 patients were included in the study. Median age of the population was 53 years. Hypertension was present in 41%, diabetes mellitus in 8%, and hyperlipidemia in 57%. Thirty‐seven percent of patients were on statins, and 47% took daily aspirin. There was a total of 316 men and 149 women included in the study. There was a total of 67 major adverse cardiovascular events (MACE), and Table 1 describes baseline characteristics by the occurrence of MACE. Table 2 describes baseline characteristics by sex.
Table 1.
Baseline Characteristics by Occurrence of MACE
| Variable | MACE (n=67) | No MACE (n=452) | P Value |
|---|---|---|---|
| Age, y | 54 (45, 62) | 51 (42, 58) | 0.04 |
| Sex (female), n (%) | 38 (56.7) | 314 (68.1) | 0.07 |
| Hypertension, n (%) | 29 (43.3) | 181 (40.2) | 0.64 |
| Creatinine (mg/dL), median (Q1, Q3) | 1 (0.8, 1.2) | 1 (0.9, 1.1) | 0.73 |
| Type 2 diabetes mellitus, n (%) | 6 (9.0) | 37 (8.2) | 0.83 |
| Body mass index, kg/m2 | 28.8 (24.6, 32.9) | 27.8 (24.0, 31.9) | 0.46 |
| Statin use, n (%) | 28 (41.8) | 166 (36.7) | 0.43 |
| Estrogen use, n (%) | 15 (22.4) | 95 (21.0) | 0.80 |
| Total cholesterol, mg/dL | 190 (153, 247) | 189 (166, 220) | 0.32 |
| LDL, mg/dL | 119 (77, 146) | 107 (85, 134) | 0.15 |
| HDL, mg/dL | 50 (38, 61) | 52 (43, 63) | 0.23 |
| Triglycerides/HDL ratio, mg/dL | 2.5 (1.7, 3.6) | 2.2 (1.4, 4.1) | 0.52 |
| Triglycerides, mg/dL | 122 (90, 175) | 117 (81, 177) | 0.62 |
HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; MACE, major adverse cardiovascular events.
Table 2.
Baseline Characteristics by Sex
| Variable | Women (n=352) | Men (n=167) | P Value |
|---|---|---|---|
| Age, y | 51 (43, 60) | 54 (46, 62) | 0.01 |
| Hypertension, n (%) | 137 (38.9) | 73 (43.7) | 0.29 |
| Creatinine, mg/dL | 0.9 (0.8, 1) | 1.1 (1.0, 1.2) | 0.07 |
| Type 2 diabetes mellitus, n (%) | 22 (6.25) | 21 (12.5) | 0.01 |
| Body mass index, kg/m2 | 27.4 (23.6, 32.1) | 28.4 (25.0, 31.4) | 0.71 |
| Statin use, n (%) | 128 (36.4) | 66 (39.5) | 0.49 |
| Total cholesterol, mg/dL | 192 (168, 226) | 181 (155, 209) | 0.003 |
| LDL, mg/dL | 109 (88, 134) | 103 (81, 139) | 0.55 |
| HDL, mg/dL | 57 (48, 67) | 43 (35, 50) | 0.0001 |
| Triglycerides/HDL ratio, mg/dL | 1.9 (1.3, 3.3) | 3.4 (2.0, 5.1) | 0.0001 |
| Triglycerides, mg/dL | 113 (78, 231) | 143 (92, 195) | 0.004 |
HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein.
Major Adverse Cardiovascular Events
Patients were followed for the development of major adverse cardiovascular events (MACE), which was defined as any patient who developed a myocardial infarction, stroke, or cardiovascular death during the follow‐up period.
Triglycerides and Triglyceride/HDL Ratio
All patients
In all patients, including men and women, there was no significant difference in baseline triglyceride level between those who developed MACE and those who did not develop MACE (122 [Q1, Q3: 90, 175] mg/dL versus 117 [Q1, Q3: 81, 177] mg/dL; P=0.62). There was also no significant difference in triglyceride/HDL ratio in those who developed MACE when compared with those who did not (2.5 [Q1, Q3: 1.7, 3.6] versus 2.2 [Q1, Q3: 1.4, 4.1]; P=0.52). Triglyceride level and triglyceride/HDL ratio was not associated with MACE in a Cox proportional hazard model adjusting for confounders.
Men
There were a total of 29 MACE among men (Table 3). There was no significant difference in baseline triglyceride levels between men who developed MACE versus those that did not (118 [Q1, Q3: 81.3, 147.3] mg/dL versus 151 [Q1, Q3: 94, 205] mg/dL); P=0.10). There was also no significant difference in baseline triglyceride/HDL ratio between men who developed MACE versus those that did not (2.92 [Q1, Q3: 1.7, 4.1] versus 3.5 [Q1, Q3: 2.1, 5.5]; P=0.21).
Table 3.
Occurrence of MACE by Sex
| Men (n=167) | Women (n=352) | |
|---|---|---|
| Number of MACE | 29 (17.3%) | 38 (10.7%) |
| Hazard ratio | 1.42 (95% CI: 1.04, 1.93) | 0.81 (95% CI: 0.66, 1.02) |
MACE indicates major adverse cardiovascular events.
In a Cox proportional hazard model stratified by statin use, among those not on baseline statins, after adjusting for age, lipid lowering drug use, hypertension, bone mass index, and sex, triglycerides were not independently predictive of MACE in men (per 50 mg/dL risk ratio: hazard ratio 0.76 [95% CI: 0.50, 1.14]; P=0.18). Among those on statins, after adjusting for the same confounders, triglycerides were also not independently predictive of MACE in men (per 50 mg/dL risk ratio: hazard ratio 0.71 [95% CI: 0.42, 1.02]; P=0.07].
Women
Among women, there were a total of 38 MACE over the follow‐up period of 7.8±4.3 years (Table 3). Baseline triglyceride levels were significantly higher in patients who developed MACE when compared with those that did not (123 [Q1, Q3: 100, 191] mg/dL versus 109 [Q1, Q3: 73 164.5] mg/dL; P=0.02) (Figure 1). Triglyceride/HDL ratio was also significantly higher in those who developed MACE when compared with those that did not (2.3 [Q1, Q3: 1.7, 3.6] versus 1.9 [Q1, Q3: 1.2, 3.1]; P=0.02) (Figure 2).
Figure 1.
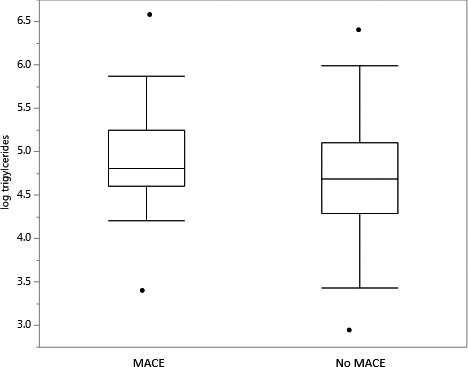
Triglycerides and major adverse cardiovascular events. This figure depicts log triglycerides in women who develop MACE and women who do not. MACE indicates major adverse cardiovascular events.
Figure 2.
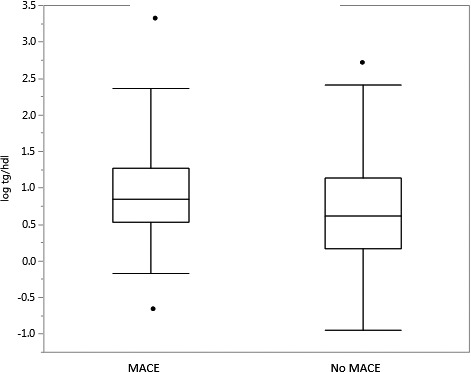
Triglyceride/high‐density lipoprotein ratio and major adverse cardiovascular events in women. This figure depicts log triglycerides/high‐density lipoprotein ratio in women who develop MACE and women who do not. MACE indicates major adverse cardiovascular events.
In a Cox proportional hazard model stratified by statin use, among those not on baseline statins, after adjusting for age, lipid lowering drug use, hypertension, body mass index, and sex, triglyceride levels were independently predictive of MACE in women (per 50 mg/dL risk ratio: hazard ratio 1.25 [95% CI: 1.06, 1.47]; P=0.01). Among those taking statins after adjusting for the same confounders, triglycerides was not predictive of MACE (per 50 mg dL risk ratio 0.85 [95% CI: 0.53, 1.22]; P=0.47). In a separate Cox model, the interaction between sex and triglycerides and statin use and triglycerides were both significant (P=0.004 and P=0.0048, respectively).
Discussion
The current study has 3 main findings. First, triglycerides may be independently associated with MACE in women with NOCAD not on lipid lowering therapy. Second, this association is not seen among men. Lastly, baseline use of lipid lowering therapy and sex both modify the effect of triglycerides on MACE. These findings suggest that hypertriglyceridemia may be associated with increased MACE in women presenting with suspected ischemia and normal coronary arteries and that statins may have a protective effect.
Several studies have reported increased risk of coronary artery disease with increased fasting and/or non‐fasting triglyceride levels in postmenopausal women.5, 21 There is a growing body of evidence suggesting the role of triglycerides in predicting cardiovascular risk, especially among women.22, 23, 24,24, 25, 26, 27 Menopausal status and aging may play a key role in linking increased triglyceride levels to increased cardiovascular risk in this population.2, 3, 4 The majority of women enrolled in this study are postmenopausal, and thus their risk may differ from premenopausal women and men further explaining the effect we report. As women age and estrogen levels decrease, this may contribute to increased triglyceride levels and increased cardiovascular risk. A study by Lindquist and colleagues suggested that there may be an age‐specificity in association between lipids and cardiovascular risk in women, with triglyceride levels playing a greater role in older postmenopausal women.28 Bittner and colleagues also reported that the triglyceride/HDL ratio may predict all‐cause mortality in women.22 Our findings extend these previous studies. We report this association in women, but not among men. Moreover, we have found that statin use and sex both modify the effect of triglycerides on MACE, suggesting a sex‐specific association between triglycerides and MACE. Our data also support a potential protective role for statin therapy in these patients.
Initially, patients with severe hypertriglyceridemia were thought to develop atherosclerosis. Recent data suggest that patients with severe hypertriglyceridemia have triglyceride lipoproteins too large to enter the arterial intima, thus not leading to atherosclerosis, while patients with moderate triglyceride elevations, have an increased risk of atherosclerosis because lipoproteins are small enough to enter into the arterial wall.29, 30 In the current study, the median triglyceride levels in both groups are not severely elevated, yet these patients appear to have an elevated risk of MACE when compared with women with normal triglyceride levels. This suggests that even a small elevation in triglycerides may be associated with increased risk of MACE, and perhaps that these women should be managed more aggressively with both risk stratification and preventive medicine. These studies provide a plausible mechanism by which even moderately elevated triglyceride levels may play a key role in increasing cardiovascular risk. We do not see this relationship in men, and we find that sex modifies the effect of trigylcerides on MACE further supporting that triglyceride elevation may have a sex‐specific effect.
The triglyceride to HDL ratio has also been cited as a marker of poor cardiovascular outcomes in patients.31 This ratio has also been associated with worse cardiovascular outcomes in patients with chronic kidney, ischemic stroke and cardiovascular disease.27, 32, 33, 34, 35 Gaziano and colleagues found that the triglyceride to HDL ratio predicted a 16‐fold increase in myocardial infarction in patients with no prior history of coronary artery disease.36 In a report from the WISE (Women's Ischemia Syndrome Evaluation) study, the triglyceride/HDL ratio was found to be a powerful independent predictor of all‐cause mortality and cardiovascular events in a similar population of women with suspected ischemia, but no obstructive plaque on angiography.22 This study did not report the effect of statin use nor did it explore an association of the triglyceride/HDL ratio and MACE in men. Our study extends the findings of Bittner and colleagues, and highlights the role of both triglycerides and triglyceride/HDL ratio in predicting MACE while also reporting the sex‐specific difference in effect, and the protective role of baseline statin therapy in modifying risk.
We also found that statins may exert a protective effect in these women with NOCAD. This is supported by previous data by Chow and colleagues suggesting plaque attenuation secondary to statin use even in those with NOCAD and minimal plaque. Authors used coronary computed tomographic angiography to visualize non‐obstructive plaque and found that patients with NOCAD had an incremental increase in mortality per additional segment of non‐obstructive plaque, and baseline statin use was associated with reduced mortality.37 Our findings are consistent with this study underscoring the potential importance of statin therapy in patients without critical CAD (coronary artery disease) on coronary angiography.
To our knowledge, the current study is the first to assess the association of triglycerides, the triglyceride/HDL ratio, and the role of sex and statin use in predicting outcomes in men and women with suspected ischemia. Our findings suggest that women who develop MACE have significantly higher triglycerides and triglyceride:HDL ratio when compared with those that do not develop MACE. This relationship is not noted in the men in our population. Our findings also highlight the potential importance of statin use in managing patients with NOCAD, even in those with borderline elevation in triglyceride levels.38, 39, 40 Thus, triglycerides may be an important predictor of events in this population of women with suspected ischemia. Few studies have explored risk factor reduction in patients with NOCAD. Our cohort is unique in that it represents a group of men and women with suspected ischemia in whom coronary angiography has not shown obstructive disease.
This study has several strengths. First, this is a prospective cohort of patients with substantial follow up of >6 to 7 years. Moreover, the event rate in the analysis is about 10%, allowing for appropriate analysis. The robust data collected by study coordinators over a long follow‐up time allowed for detailed analysis of risk factors in this population of patients. Moreover, the inclusion of men in our study is an important strength as it allows a deeper understanding of sex‐specific risk factors in patients with premature atherosclerosis and non‐obstructive coronary artery disease.
There are also some key limitations that must be considered when interpreting the results. These findings are based upon cross‐sectional data on initial presentation. Data on outcomes were obtained via a combination of questionnaires and chart review, potentially limiting validity. While baseline use of statins is known, the previous duration of therapy, compliance, and lifestyle habits is unknown. Additionally, another important limitation to consider is that subsequent initiation or cessation of statin use may also impact outcomes and this information was not collected on the follow‐up questionnaire. However, we have adjusted for traditional cardiovascular risk factors in the analysis to account for this. These limitations highlight the need for treatment intervention studies in this population of patients to further understand the role of triglycerides and statin use in predicting outcomes in postmenopausal women with NOCAD.
The current study highlights the importance of further investigation to better understand the role of traditional and non‐traditional risk factors, and traditional medical therapies in management of NOCAD. There are no evidence‐based guidelines driving treatment of NOCAD because of significant knowledge gaps.41 Moreover, few have studied men with NOCAD, who also may have adverse events. An in depth understanding of risk factors in both population is important. While numerous large randomized trials guide treatment of obstructive CAD and have made a considerable impact in this field, there is little known about the role of risk factor modification and medication therapy in this population of patients who have yet to develop obstructive CAD or ischemia. Moreover, sex‐specific differences and the need for different treatments in patients with NOCAD have not been measured with long‐term data from large cohorts and call for additional research to understand both mechanistically the role of triglycerides in increasing risk of CAD among women, but also the protective effect of statins.41
Conclusions
The management of patients with NOCAD continues to be challenging, despite its significant associated cardiovascular morbidity and mortality. Our findings suggest that using the triglyceride levels and the triglyceride/HDL ratio in women may help predict MACE in this population, and that statin use may have a role in reducing cardiovascular events in this population. We do not note such an association among men and report a sex‐specific effect of triglycerides on the occurrence of adverse cardiovascular events. Further investigation in the form of both mechanistic studies and large randomized controlled trials are required to further define this relationship and delineate clear therapies for women with non‐obstructive coronary artery disease.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e009442 DOI: 10.1161/JAHA.118.009442.)
References
- 1. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd‐Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. [DOI] [PubMed] [Google Scholar]
- 2. Kolovou GD, Bilianou HG. Influence of aging and menopause on lipids and lipoproteins in women. Angiology. 2008;59:54S–57S. [DOI] [PubMed] [Google Scholar]
- 3. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009;169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. [DOI] [PubMed] [Google Scholar]
- 5. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high‐density lipoprotein cholesterol level: a meta‐analysis of population‐based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 6. Criqui MH, Heiss G, Cohn R, Cowan LD, Suchindran CM, Bangdiwala S, Kritchevsky S, Jacobs DR Jr, O'Grady HK, Davis CE. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328:1220–1225. [DOI] [PubMed] [Google Scholar]
- 7. Austin MA. Epidemiology of hypertriglyceridemia and cardiovascular disease. Am J Cardiol. 1999;83:13F–16F. [DOI] [PubMed] [Google Scholar]
- 8. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Ther. 2016;23:e862–e870. [DOI] [PubMed] [Google Scholar]
- 9. Kim KH, Kim CH, Jeong MH, Ahn Y, Kim YJ, Cho MC, Kim W, Kim JJ; Other Korea Acute Myocardial Infarction Registry Investigators . Differential benefit of statin in secondary prevention of acute myocardial infarction according to the level of triglyceride and high density lipoprotein cholesterol. Korean Circ J. 2016;46:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller M, Seidler A, Kwiterovich PO, Pearson TA. Long‐term predictors of subsequent cardiovascular events with coronary artery disease and ‘desirable’ levels of plasma total cholesterol. Circulation. 1992;86:1165–1170. [DOI] [PubMed] [Google Scholar]
- 11. Pepine CJ, Ferdinand KC, Shaw LJ, Light‐McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN; ACC CVD in Women Committee . Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reyes BJ, Hallak O, Elhabyan AK, Lucas BD Jr, Kasem H. Angina with “normal” coronary arteries. JAMA. 2005;293:2468–2469; author reply 2469. [DOI] [PubMed] [Google Scholar]
- 14. Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293:477–484. [DOI] [PubMed] [Google Scholar]
- 15. Chilappa K, Aronow WS, Rajdev A, Ahn C, Kalapatapu K, Pucillo AL, Monsen CE. Mortality at long‐term follow‐up of patients with no, nonobstructive, and revascularized 1‐, 2‐, and 3‐vessel obstructive coronary artery disease. Med Sci Monit. 2010;16:RA120–RA123. [PubMed] [Google Scholar]
- 16. Beltrame JF. Assessing patients with myocardial infarction and nonobstructed coronary arteries (MINOCA). J Intern Med. 2013;273:182–185. [DOI] [PubMed] [Google Scholar]
- 17. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 18. Prasad M, McBane R, Reriani M, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with increased risk of venous thromboembolism. Thromb Res. 2016;139:17–21. [DOI] [PubMed] [Google Scholar]
- 19. Prasad M, Reriani M, Khosla S, Gossl M, Lennon R, Gulati R, Prasad A, Lerman LO, Lerman A. Coronary microvascular endothelial dysfunction is an independent predictor of development of osteoporosis in postmenopausal women. Vasc Health Risk Manag. 2014;10:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reriani M, Flammer AJ, Li J, Prasad M, Rihal C, Prasad A, Lennon R, Lerman LO, Lerman A. Microvascular endothelial dysfunction predicts the development of erectile dysfunction in men with coronary atherosclerosis without critical stenoses. Coron Artery Dis. 2014;25:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. [DOI] [PubMed] [Google Scholar]
- 22. Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, Bairey‐Merz CN, Sopko G. The triglyceride/high‐density lipoprotein cholesterol ratio predicts all‐cause mortality in women with suspected myocardial ischemia: a report from the Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2009;157:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Botta RM, Migliore MG, Sorrentino M, Pagano G. Triglyceride and cholesterol levels during pregnancy in type I, type II and gestational diabetic women. Boll Soc Ital Biol Sper. 1984;60:2055–2059. [PubMed] [Google Scholar]
- 24. Mazza A, Tikhonoff V, Schiavon L, Casiglia E. Triglycerides + high‐density‐lipoprotein‐cholesterol dyslipidaemia, a coronary risk factor in elderly women: the CArdiovascular STudy in the ELderly. Intern Med J. 2005;35:604–610. [DOI] [PubMed] [Google Scholar]
- 25. LaRosa JC. Triglycerides and coronary risk in women and the elderly. Arch Intern Med. 1997;157:961–968. [PubMed] [Google Scholar]
- 26. Dotevall A, Johansson S, Wilhelmsen L, Rosengren A. Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women. A prospective 18‐year follow‐up of the BEDA study. Diabet Med. 2004;21:615–622. [DOI] [PubMed] [Google Scholar]
- 27. Wu H, Xiong L, Xu Q, Wu J, Huang R, Guo Q, Mao H, Yu X, Yang X. Higher serum triglyceride to high‐density lipoprotein cholesterol ratio was associated with increased cardiovascular mortality in female patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2015;25:749–755. [DOI] [PubMed] [Google Scholar]
- 28. Lindquist P, Bengtsson C, Lissner L, Bjorkelund C. Cholesterol and triglyceride concentration as risk factors for myocardial infarction and death in women, with special reference to influence of age. J Intern Med. 2002;251:484–489. [DOI] [PubMed] [Google Scholar]
- 29. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 30. Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima‐inner media. Arterioscler Thromb Vasc Biol. 1995;15:534–542. [DOI] [PubMed] [Google Scholar]
- 31. Unal HU, Basaran Y, Gezer M. The triglyceride to high‐density lipoprotein cholesterol ratio is a useful marker to predict unfavorable cardiovascular outcomes, but other confounding factors should be considered. J Clin Hypertens (Greenwich). 2016;18:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sonmez A, Yilmaz MI, Saglam M, Unal HU, Gok M, Cetinkaya H, Karaman M, Haymana C, Eyileten T, Oguz Y, Vural A, Rizzo M, Toth PP. The role of plasma triglyceride/high‐density lipoprotein cholesterol ratio to predict cardiovascular outcomes in chronic kidney disease. Lipids Health Dis. 2015;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng QW, Wang H, Sun CZ, Xing FL, Zhang HQ, Zuo L, Gu ZT, Yan FL. Triglyceride to high‐density lipoprotein cholesterol ratio predicts worse outcomes after acute ischaemic stroke. Eur J Neurol. 2017;24:283–291. [DOI] [PubMed] [Google Scholar]
- 34. Chen HY, Tsai WC, Chiu YL, Hsu SP, Pai MF, Yang JY, Peng YS. Triglyceride to high‐density lipoprotein cholesterol ratio predicts cardiovascular outcomes in prevalent dialysis patients. Medicine (Baltimore). 2015;94:e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salazar MR, Carbajal HA, Espeche WG, Aizpurua M, Marillet AG, Leiva Sisnieguez CE, Leiva Sisnieguez BC, Stavile RN, March CE, Reaven GM. Use of the triglyceride/high‐density lipoprotein cholesterol ratio to identify cardiometabolic risk: impact of obesity? J Investig Med. 2017;65:323–327. [DOI] [PubMed] [Google Scholar]
- 36. Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high‐density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. [DOI] [PubMed] [Google Scholar]
- 37. Chow BJ, Small G, Yam Y, Chen L, McPherson R, Achenbach S, Al‐Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann PA, Kim YJ, Leipsic J, LaBounty T, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK; CONFIRM Investigators . Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: an InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol. 2015;35:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikdahl E, Hisdal J, Rollefstad S, Olsen IC, Kvien TK, Pedersen TR, Semb AG. Rosuvastatin improves endothelial function in patients with inflammatory joint diseases, longitudinal associations with atherosclerosis and arteriosclerosis: results from the RORA‐AS statin intervention study. Arthritis Res Ther. 2015;17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strey CH, Young JM, Lainchbury JH, Frampton CM, Nicholls MG, Richards AM, Scott RS. Short‐term statin treatment improves endothelial function and neurohormonal imbalance in normocholesterolaemic patients with non‐ischaemic heart failure. Heart. 2006;92:1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adel A, Abdel‐Salam Z, Nammas W. Low‐dose statin therapy improves endothelial function in type 2 diabetic patients with normal serum total cholesterol: a randomized placebo‐controlled study. J Clin Hypertens (Greenwich). 2010;12:820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paul TK, Sivanesan K, Schulman‐Marcus J. Sex differences in nonobstructive coronary artery disease: recent insights and substantial knowledge gaps. Trends Cardiovasc Med. 2017;27:173–179. [DOI] [PubMed] [Google Scholar]


