Abstract
Background
PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors effectively lower LDL (low‐density lipoprotein) cholesterol and have been shown to reduce cardiovascular outcomes in high‐risk patients. We used real‐world electronic health record data to characterize use of PCSK9 inhibitors, in addition to standard therapies, according to cardiovascular risk status.
Methods and Results
Data were obtained from 18 health systems with data marts within the National Patient‐Centered Clinical Research Network (PCORnet) using a common data model. Participating sites identified >17.5 million adults, of whom 3.6 million met study criteria. Patients were categorized into 3 groups: (1) dyslipidemia, (2) untreated LDL ≥130 mg/dL, and (3) coronary artery disease or coronary heart disease. Demographics, comorbidities, estimated 10‐year atherosclerotic cardiovascular disease risk, and lipid‐lowering pharmacotherapies were summarized for each group. Participants’ average age was 62 years, 50% were female, and 11% were black. LDL cholesterol ranged from 85 to 151 mg/dL. Among patients in groups 1 and 3, 54% received standard lipid‐lowering therapies and a PCSK9 inhibitor was prescribed in <1%. PCSK9 inhibitor prescribing was greatest for patients with coronary artery disease or coronary heart disease and, although prescribing increased during the study period, overall PCSK9 inhibitor prescribing was low.
Conclusions
We successfully used electronic health record data from 18 PCORnet data marts to identify >3.6 million patients meeting criteria for 3 patient groups. Approximately half of patients had been prescribed lipid‐lowering medication, but <1% were prescribed PCSK9 inhibitors. PCSK9 inhibitor prescribing increased over time for patients with coronary artery disease or coronary heart disease but not for those with dyslipidemia.
Keywords: coronary artery disease, lipids, PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, secondary prevention
Subject Categories: Cardiovascular Disease, Epidemiology, Secondary Prevention, Risk Factors
Clinical Perspective
What Is New?
We provide real‐world electronic health record data describing early use of the PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor class of medication.
Despite highly effective lipid‐lowering therapy, we document low usage.
What Are the Clinical Implications?
A substantial portion of patients with elevated atherosclerotic cardiovascular disease risk and elevated LDL (low‐density lipoprotein) cholesterol could benefit from additional lipid‐lowering therapy.
PCSK9 inhibitors, which effectively lower LDL cholesterol, had limited usage during this early surveillance period.
Additional surveillance and identification of ongoing barriers is important.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for ≈1 in every 3 deaths.1 An established risk factor for the development of CVD is elevated LDL (low‐density lipoprotein) cholesterol (LDL‐C), which is a common chronic condition in adults.1 Use of lipid‐lowering therapies, including statins and nonstatin therapies such as ezetimibe and PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors, reduces LDL‐C and lowers the risk of CVD outcomes.2, 3, 4 For every 1‐mmol/L reduction in LDL‐C from statins, there is a corresponding relative risk reduction in cardiovascular events of 12% and 22% after 1 and 4 years of treatment, respectively.5 Furthermore, aggressive lowering of LDL‐C beyond treatment guideline thresholds has been shown to provide even greater benefit in reduction of CVD outcomes.2, 6, 7 Despite these known benefits, a substantial proportion of patients at high risk for cardiovascular events, including patients with established CVD, receive suboptimal or no lipid‐lowering therapy.8, 9, 10, 11, 12
The 2013 American College of Cardiology/American Heart Association cholesterol guidelines recommend high‐intensity statins as first‐line therapy for patients with established CVD or high‐risk equivalents.13 Nonstatin lipid‐lowering therapies, including PCSK9 inhibitors, bile acid binding resins, and ezetimibe, may benefit patients who are intolerant to statins, who do not achieve optimal LDL‐C lowering following maximum tolerated statin therapy, or who have experienced ≥1 CVD event despite being treated with a statin. PCSK9 inhibitors are approved for use in participants with familial hypercholesterolemia or atherosclerotic CVD (ASCVD) who have not had adequate reduction in LDL‐C on a maximally tolerated dose of statin. The US Food and Drug Administration (FDA) has approved 2 PCSK9 inhibitors (alirocumab and evolocumab), both of which reduce LDL‐C levels 50% to 60% beyond that achieved from statin therapy alone,4, 7, 14, 15, 16, 17, 18 with sustained long‐term LDL‐C reduction over years of follow‐up without increased adverse effects from cumulative exposure.16 Randomized controlled trials of both PCSK9 inhibitors in combination with statins have demonstrated a reduced risk for cardiovascular events among patients with ASCVD or following acute coronary syndrome.7 In addition, some evidence for reduction of all‐cause death was observed for alirocumab,19 although no difference in all‐cause death was observed for evolocumab versus placebo.7 Furthermore, evidence from the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial indicates that evolocumab was equally effective in reducing cardiovascular events in ASCVD patients with baseline LDL‐C of <70 versus ≥70 mg/dL and among patients who were on maximal‐ versus submaximal‐potency statins.20
The objective of this National Patient‐Centered Clinical Research Network (PCORnet) project was to use real‐world electronic health record data to characterize the early use of PCSK9 inhibitors, in additional to standard lipid‐lowering therapies, according to cardiovascular risk status. We provide descriptions of the characteristics of the patient groups, the patients’ comorbid conditions, and the PCSK9 inhibitor prescribing trends over time.
Methods
Because of specifications in the data‐use agreements that prohibit release of data mart–level data, it is not possible to release data generated from the data marts participating in this study.
Data Sources
Eighteen data marts from 7 clinical data research networks (CDRNs), including ADVANCE (Accelerating Data Value Across a National Community Health Center Network), LHSNet (Patient‐Centered Network of Learning Health Systems), Mid‐South, OneFlorida, PaTH, PORTAL (Patient Outcomes Research To Advance Learning), and REACHnet (Research Action for Health Network), participated in the study (Table S1). Data marts in PCORnet all followed the PCORnet Common Data Model (CDM) v3.1, which contains 15 data domains in relational schemas, including patient demographics, enrollment status, death status, cause of death, vital signs (eg, height, weight, and blood pressure), conditions (ie, diagnosed and self‐reported health conditions and diseases), encounters, diagnoses (ie, results of diagnostic process and medical coding within healthcare delivery), procedures, prescribing (ie, provider orders for medications), dispensing (ie, outpatient pharmacy dispensing; eg, filled prescriptions), and laboratory results. Data between January 1, 2015, and March 31, 2017, were included in the study. Each data mart received local institutional review board approval for exempt human subjects research before submitting data for inclusion in the study. No patients signed informed consent forms because approvals were granted for exempt status, as only aggregated deidentified data were provided by each data mart.
Study Cohort
We used the International Classification of Diseases, Ninth/Tenth Revisions, Clinical Modification (ICD‐9/10‐CM) or laboratory results coded with Logical Observation Identifiers Names and Codes (LOINC) to categorize patients into one of the following groups: (1) patients with dyslipidemia, (2) patients with LDL‐C ≥130 mg/dL who were not on any lipid‐lowering treatment, and (3) patients with coronary heart disease (CHD) or coronary artery disease (CAD) (Table S2). We considered only patients who were aged ≥18 years at the time of diagnosis. If a patient met the criteria for multiple groups, the patient was assigned to the highest risk group for which he or she satisfied criteria (CHD/CAD > LDL‐C ≥130 mg/dL who were not on any lipid‐lowering treatment > dyslipidemia). To validate the computable phenotypes created to place patients into 1 of the 3 groups, we performed a manual medical record review of 150 patients meeting criteria for the study, including 50 patients in each of the 3 patient groups. The queries used to formulate the cohorts can be accessed via GitHub (https://github.com/OneFLanalyst/PCSK9i.
Basic Demographics and Comorbid Conditions
Demographic information was obtained from the CDM's demographic and vital tables. Comorbid conditions were defined by ICD‐9/10‐CM codes (Table S3), and patients’ diagnoses were obtained from the diagnosis table in the CDM. The most recent valid height and weight measurements available between January 1, 2015, and March 31, 2017, were included in basic demographics and obtained from the vital signs table.
Risk Factors
CVD risk factors included estimated 10‐year ASCVD risk, smoking status, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (BP), LDL‐C, HDL (high‐density lipoprotein) cholesterol (HDL‐C), and triglycerides. Queries excluded invalid values based on prespecified range parameters.
The ASCVD risk score was calculated21, 22 for those in groups 1 and 2 when the required data were available: sex, age (20–79 years), race/ethnicity (black, white, and Hispanic), antihypertension medication status, diabetes mellitus, smoking status, total cholesterol, HDL‐C, and SBP.
Patients’ smoking status, BMI, and BP were obtained from the CDM vital table. If a patient had multiple vital records available, the most recent record was used for assessment. To identify current smokers, smoking, tobacco, and tobacco type were obtained from the vital table. The PCORnet CDM contains an original BMI field as well as height and weight fields. To determine the BMI, we used the most recent original BMI value available for the patient. If an original BMI value was not available, the same‐day height and weight were used to calculate the BMI. For height and weight, we used the most recent plausible values (ie, height ranging from 48 to 96 in and weight ranging from 50 to 1000 lb) available during the study period. BP measurements from ambulatory encounters were used to assess SBP and diastolic BP. SBP values between 70 and 250 mm Hg and diastolic BP values between 50 and 150 mm Hg were considered for analysis. LDL‐C, HDL‐C, and triglycerides were extracted based on either the LOINC codes or laboratory names from the lab result table in the CDM.
Medications
Medications were selected by RxNorm concept unique identifier or national drug code, depending on data available for each data mart. For this analysis, if the patient had a drug of interest (lipid‐lowering or other cardiovascular medication) prescribed or dispensed, the individual was counted as having had a record for that medication. The patient was counted once per drug for lipid‐lowering or class of cardiovascular medication.
To provide clarity for trends in PCSK9 inhibitor prescribing over time since FDA approval, we report the number of prescriptions for PCSK9 inhibitors and the rate of PCSK9 inhibitor prescription over time, along with 95% CIs. To do so, records were selected by prescription order date starting with July 1, 2015. Individuals were included in each 6‐month time period in which a record was available for the patient. The most recent record in each 6‐month period was selected per individual by drug. Of note, the final time period was 3 months (January–March 2017) because of data cutoff.
Statistical Analysis
Continuous variables were summarized as means and standard deviations, and categorical variables were summarized as frequencies and percentages. The summary‐level data from each CDRN were aggregated within the 3 patient groups. For continuous variables, the overall averages were calculated based on the mean and sample size from each CDRN, and the overall standard deviations were calculated by taking the square root of (sum of variances×sample sizes/sum of sample sizes). For the categorical variables, the percentages were calculated as the sum of frequencies for all CDRNs divided by the group total. Lipid‐lowering medication use was low in some data marts and, therefore, reported as a threshold (eg, count of <11); exact counts were not available in some cases. Consequently, we imputed these thresholds to a count of 5 to estimate the sum of patients on a medication. This approach was applied to lipid‐lowering prescriptions only. All queries were developed and analyses performed in SAS v9.4 (SAS Institute).
Results
Among the 18 data marts that participated in this project, 17 520 612 individuals were included. Of those, 3 619 922 met criteria for categorization into 1 of the 3 risk groups of interest (Figure 1): (1) patients with dyslipidemia (n=2 224 395, 61%), (2) patients with LDL‐C ≥130 mg/dL and who were not on any lipid‐lowering treatment (n=452 625, 13%), and (3) patients with CHD or CAD (n=942 902, 26%). Results of the validation confirmed the computable phenotypes with positive predictive values from the medical record reviews of 86% for dyslipidemia, 98% for LDL‐C ≥130 mg/dL and not on lipid‐lowering treatment, and 84% for CHD or CAD.
Figure 1.
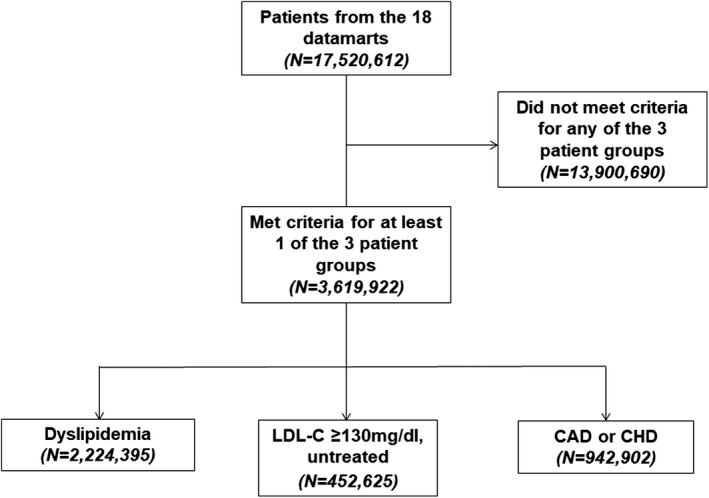
Flowchart of the number of patients meeting criteria for the 3 patient groups. CAD indicates coronary artery disease; CHD, coronary heart disease; LDL‐C, low‐density lipoprotein cholesterol.
Population Characteristics
Table 1 summarizes the demographics, comorbid risk conditions, cardiovascular risk factors, and estimated 10‐year ASCVD risk score according to patient group. The mean age of the population was 61.9 years (SD: 12.7), half of the patients were male, and the majority was of non‐Hispanic white ethnicity/race. Hypertension was the most common chronic cardiovascular condition, present in almost two thirds of the overall population and 78% of those with CAD or CHD. Diabetes mellitus was the next most prevalent comorbid risk condition, present in 29% overall. Not unexpectedly, patients with CAD or CHD had a higher prevalence of all comorbid conditions compared with the other patient groups. The average estimated 10‐year ASCVD risk score was 11.5% among those in group 1 and 6.1% among those in group 2. All individuals in group 2 had LDL‐C ≥130 mg/dL with no record of lipid‐lowering prescriptions in the data marts, including 26% with an estimated 10‐year ASCVD risk of ≥7.5%, and likely would benefit from a moderate‐ to high‐intensity statin. Average SBP and diastolic BP were 125±19 and 77±13 mm Hg, respectively, reflecting a population with fairly good BP control.
Table 1.
Patient Demographics, Comorbidities, Cardiovascular Risk Factors, and Non–Lipid‐Lowering Cardiovascular Medications by Patient Group
| Patient Characteristic | Group 1 (n=2 224 395) | Group 2 (n=452 625) | Group 3 (n=942 902) | Total (N=3 619 922) |
|---|---|---|---|---|
| Demographics | ||||
| Age (y), mean±SD | 60.5±13.9 | 52.1±13.8 | 70.0±12.2 | 61.9±12.7 |
| Male sex | 1 036 585 (46.6) | 179 850 (39.7) | 598 933 (63.5) | 1 815 368 (50.1) |
| BMI (kg/m2), mean±SD (n) | 30.8±7.1 (1 773 841) | 30.1±6.7 (438 692) | 29.7±6.8 (799 350) | 30.4±7.0 (3 011 883) |
| Race | ||||
| American Indian/Alaska Native | 20 858 (0.9) | 1910 (0.4) | 6698 (0.7) | 29 466 (0.8) |
| Asian | 61 636 (2.8) | 19 161 (4.2) | 13 781 (1.5) | 94 578 (2.6) |
| Native Hawaiian or other | 4424 (0.2) | 1301 (0.3) | 1147 (0.1) | 6872 (0.2) |
| Pacific Islander | ||||
| Black | 242 386 (10.9) | 65 267 (14.4) | 84 871 (9.0) | 392 524 (10.8) |
| White | 1 619 884 (72.8) | 322 992 (71.4) | 758 536 (80.4) | 2 701 412 (74.6) |
| Other/unknown | 275 194 (12.4) | 41 979 (9.3) | 77 853 (8.3) | 395 026 (10.9) |
| Ethnicity | ||||
| Hispanic or Latino | 251 263 (11.3) | 48 183 (10.6) | 50 935 (5.4) | 350 681 (9.7) |
| Not Hispanic or Latino | 1 562 845 (70.3) | 306 705 (67.8) | 753 048 (79.9) | 2 622 598 (72.4) |
| Other/unknown | 409 987 (18.4) | 97 729 (21.6) | 138 912 (14.7) | 646 628 (17.9) |
| Concomitant conditiona | ||||
| Obesity | 332 432 (14.9) | 49 916 (11.0) | 146 395 (15.5) | 528 743 (14.6) |
| Hypertension | 1 389 140 (62.5) | 142 960 (31.6) | 730 491 (77.5) | 2 262 591 (62.5) |
| Chronic kidney disease | 209 853 (9.4) | 13 363 (3.0) | 214 052 (22.7) | 437 268 (12.1) |
| PVD | 73 700 (3.3) | 4125 (0.9) | 123 749 (13.1) | 201 574 (5.6) |
| MI | 28 180 (1.3) | 1260 (0.3) | 250 939 (26.6) | 280 379 (7.7) |
| Ischemic stroke | 39 708 (1.8) | 1429 (0.3) | 36 477 (3.9) | 77 614 (2.1) |
| Hemorrhagic stroke | 6894 (0.3) | 482 (0.1) | 5 775 (0.6) | 13 151 (0.4) |
| TIA | 32 700 (1.5) | 1669 (0.4) | 27 830 (3.0) | 62 199 (1.7) |
| Diabetes mellitus | 671 859 (30.2) | 28 240 (6.2) | 356 244 (37.8) | 1 056 343 (29.2) |
| Cardiovascular risk factors | ||||
| Current smoker | 176 805 (8.7) | 46 615 (10.3) | 79 984 (9.0) | 303 404 (8.4) |
| SBP (mm Hg), mean±SD (n) | 125.5±18.4 (1 671 534) | 122.5±15.4 (434 611) | 125.0±20.5 (712 810) | 124.9±18.5 (2 818 955) |
| DBP (mm Hg), mean±SD (n) | 77.6±13.6 (1 660 714) | 77.7±10.5 (432 969) | 73.9±14.4 (701 935) | 76.7±13.4 (2 795 618) |
| LDL‐C (mg/dL), mean±SD (n) | 101.7±35.2 (919 031) | 150.7±19.1 (452 625) | 84.8±35.0 (362 646) | 110.7±31.7 (1 734 302) |
| HDL‐C (mg/dL), mean±SD (n) | 50.1±16.3 (861 299) | 54.0±15.7 (364 623) | 46.2±15.3 (315 549) | 50.2±15.9 (1 541 471) |
| Triglycerides (mg/dL), mean±SD (n) | 162.0±115.8 (725 416) | 140.1±76.3 (333 438) | 141.2±96.3 (268 058) | 152.3±103.3 (1 326 912) |
| 10‐year ASCVD risk score | ||||
| ASCVD risk estimate, % (n) | 11.5 (336 257) | 6.1 (211 111) | NA | 9.6 (547 368) |
| <5% | 120 011 (35.7) | 128 225 (60.7) | NA | 248 236 (45.4) |
| 5–7.5% | 41 927 (12.5) | 27 356 (13.0) | NA | 69 283 (12.7) |
| 7.5–10% | 34 326 (10.2) | 16 770 (7.9) | NA | 51 096 (9.3) |
| 10–20% | 81 445 (24.2) | 27 614 (13.1) | NA | 109 059 (19.9) |
| ≥20% | 58 548 (17.4) | 11 129 (5.3) | NA | 69 677 (12.7) |
| Non–lipid‐lowering cardiovascular medications | ||||
| ACEI | 573 027 (25.8) | 60 463 (13.4) | 298 114 (31.6) | 931 604 (25.7) |
| ARB | 267 835 (12.0) | 26 281 (5.8) | 142 935 (15.2) | 437 051 (12.1) |
| CCB | 392 416 (17.6) | 41 402 (9.1) | 255 718 (27.1) | 689 536 (19.0) |
| β‐Blocker | 485 336 (21.8) | 49 535 (10.9) | 515 828 (54.7) | 1 050 699 (29.0) |
| Thiazide diuretics | 435 815 (19.6) | 60 465 (13.4) | 150 109 (15.9) | 646 389 (17.9) |
| Aldosterone antagonists | 40 437 (1.8) | 5021 (1.1) | 54 380 (5.8) | 99 838 (2.8) |
Group 1 had dyslipidemia, group 2 had LDL‐C ≥130 mg/dL (untreated), and group 3 had coronary artery disease or coronary heart disease. Data are shown as n (%) except as noted. For variables with missing data, including BMI, cardiovascular risk factors, and the ASCVD risk score, the number of patients with available data is provided in parentheses. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CCB, calcium channel blocker; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; NA, not available; PVD, peripheral vascular disease; SBP, systolic blood pressure; TIA, transient ischemic stroke.
The diagnostic codes used to define concomitant conditions are provided in Table S3.
Average LDL‐C in group 2 was 151±19 mg/dL, whereas those in groups 1 and 3 had LDL‐C of 102±35 and 85±35 mg/dL, respectively, on average. With regard to cardioprotective and BP‐lowering medications, as expected, patients in group 3 had the most prevalent use overall.
Lipid‐Lowering Medications
Lipid‐lowering medications (prescriptions or dispensings) were present for 1 688 960 (47%) of the overall population. Use of any lipid‐lowering medication occurred in 1 134 598 (51.0%) patients in group 1 and 554 362 (58.8%) patients in group 3 (Table 2). By definition, no lipid‐lowering medications were used among patients in group 2. Use of any PCSK9 inhibitor was most prevalent among patients in group 3 (n=1952, 0.21%). Use of a PCSK9 inhibitor among those in group 1 (n=362, 0.02%) was less common. Because some lipid‐lowering medication use was low and reported by some data marts as below threshold (n<11), and thus exact counts were not available, we imputed those below‐threshold medications to a count of 5. The imputed data are available in Table 3.
Table 2.
Frequencies of Prescribed or Dispensed Lipid‐Lowering Medications by Patient Group
| Group 1 (n=2 224 395) | Group 3 (n=942 902) | Total (N=3 167 297) | |
|---|---|---|---|
| Any lipid‐lowering prescription | 1 134 598 (51.0) | 554 362 (58.8) | 1 688 960 (46.7) |
| Statins | |||
| Lovastatin | 52 402 (2.4) | 12 892 (1.4) | 65 294 (1.8) |
| Simvastatin | 356 943 (16.0) | 122 236 (13.0) | 479 179 (13.2) |
| Atorvastatin | 565 766 (25.4) | 346 529 (36.8) | 9 122 295 (25.1) |
| Pravastatin | 169 738 (7.6) | 74 989 (8.0) | 244 727 (6.7) |
| Rosuvastatin | 74 553 (3.4) | 69 442 (7.4) | 143 995 (4.0) |
| Pitavastatina | 2138 (0.1) | 1990 (0.2) | 4128 (0.1) |
| Fluvastatina | 1034 (0.05) | 594 (0.1) | 1628 (0.04) |
| Nonstatin therapies, n (%) | |||
| Ezetimibea | 25 522 (1.1) | 27 818 (3.0) | 53 340 (1.5) |
| Fenofibric acida | 6611 (0.3) | 4442 (0.5) | 11 053 (0.3) |
| Colesevelama | 4390 (0.2) | 2692 (0.3) | 7082 (0.2) |
| Cholestyramine | 9747 (0.4) | 5268 (0.6) | 15 015 (0.4) |
| Colestipola | 3203 (0.1) | 1578 (0.2) | 4781 (0.1) |
| PCSK9 inhibitors | |||
| Any PCSK9 inhibitor | 362 (0.02) | 1952 (0.21) | 2314 (0.06) |
| Evolocumaba | 222 (0.01) | 1226 (0.13) | 1448 (0.04) |
| Alirocumaba | 140 (0.01) | 726 (0.08) | 866 (0.02) |
Group 1 had dyslipidemia and group 3 had coronary artery disease or coronary heart disease. Data are shown as n (%). Patients may have been prescribed >1 lipid lowering medication during the study (due to switching or adding of medications). All prescriptions per person were captured and are reflected in the table. PCSK9 indicates proprotein convertase subtilisin/kexin type 9.
Category contained below‐threshold values (<11 patients) among ≥1 of the participating data marts. Thus, the count in the table is lower than the actual count.
Table 3.
Frequencies of Prescribed or Dispensed Lipid‐Lowering Medications by Patient Group, After Imputation of Below‐Threshold Counts
| Group 1 (n=2 224 395) | Group 3 (n=942 902) | Total (N=3 167 297) | |
|---|---|---|---|
| Any lipid‐lowering prescription | 1 134 598 (51.0) | 554 362 (58.8) | 1 688 960 (46.7) |
| Statins | |||
| Lovastatin | 52 402 (2.4) | 12 892 (1.4) | 65 294 (2.1) |
| Simvastatin | 356 944 (16.0) | 122 236 (13.0) | 479 180 (15.1) |
| Atorvastatin | 565 765 (25.4) | 346 532 (36.8) | 912 297 (28.8) |
| Pravastatin | 169 739 (7.6) | 74 989 (8.0) | 244 728 (7.7) |
| Rosuvastatin | 74 555 (3.4) | 69 442 (7.4) | 143 997 (4.5) |
| Pitavastatina | 2143 (0.1) | 2000 (0.2) | 4143 (0.1) |
| Fluvastatina | 1039 (0.05) | 619 (0.1) | 1658 (0.05) |
| Nonstatin therapies | |||
| Ezetimibea | 25 522 (1.1) | 27 818 (3.0) | 53 340 (1.7) |
| Fenofibric acida | 6616 (0.3) | 4462 (0.5) | 11 078 (0.3) |
| Colesevelama | 4395 (0.2) | 2707 (0.3) | 7102 (0.2) |
| Cholestyramine | 9747 (0.4) | 5268 (0.6) | 15 015 (0.5) |
| Colestipola | 3208 (0.1) | 1583 (0.2) | 4791 (0.2) |
| PCSK9 inhibitors | |||
| Any PSCK9 inhibitor | 437 (0.02) | 2002 (0.21) | 2439 (0.08) |
| Evolocumaba | 257 (0.01) | 1251 (0.13) | 1508 (0.05) |
| Alirocumaba | 180 (0.01) | 751 (0.08) | 931 (0.03) |
Group 1 had dyslipidemia and group 3 had coronary artery disease or coronary heart disease. Data are shown as n (%). Any below‐threshold values (<11 patients for a category from a data mart) were assigned a value of 5 to generate an imputed count of the medication.PCSK9 indicates proprotein convertase subtilisin/kexin type 9.
Category contained below‐threshold values (<11 patients) among ≥1 of the participating data marts. Thus, the count in the table is lower than the actual count.
Rates of PCSK9 inhibitor prescribing over time are shown in Figure 2. Among patients in group 3 (CAD or CHD), PCSK9 inhibitor prescribing increased substantially over time, whereas prescribing among those with dyslipidemia remained low and stable over time.
Figure 2.
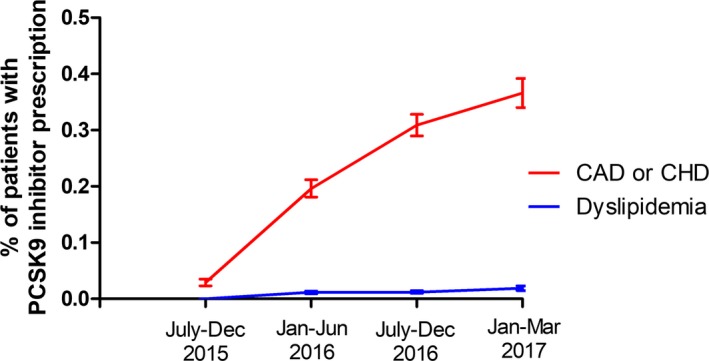
Trends in PCSK9 inhibitor prescriptions, July 2015 to March 2017. PCSK9 inhibitor prescription rates with 95% CIs during 6‐month intervals except the final reported time period (January–March 2017), which represents only 3 months. CAD indicates coronary artery disease; CHD, coronary heart disease; PCSK9, proprotein convertase subtilisin/kexin type 9.
Discussion
This large, multicenter project included 18 data marts from 7 Patient‐Centered Outcomes Research Institute (PCORI) CDRNs, capturing >17.5 million patients in the United States. Of these 17.5 million patients, >3.5 million met the criteria for 3 patient groups, including (1) patients with dyslipidemia, (2) patients with LDL‐C ≥130 mg/dL and who were not on lipid‐lowering therapy, and (3) patients with CAD or CHD. Approximately half of the patients had been prescribed or dispensed lipid‐lowering medications, and the most common medications were statins. With regard to PCSK9 inhibitor use specifically, we identified low use overall, although use increased over time for patients with CAD or CHD.
The data described in this article, obtained from electronic health record data marts across the United States, are consistent with previously published studies regarding low PCSK9 inhibitor use.23, 24 Many factors may contribute to the low rates of prescribing for PCSK9 inhibitors, including cost, recent approval, initial lack of outcomes data, prior authorization requirements, and lack of insurance approval. The high out‐of‐pocket cost for copays affects access to PCSK9 inhibitors, despite Medicare and other third‐party payer coverage for PCSK9 inhibitors.25 Despite the evidence of the effectiveness of PCSK9 inhibitors, their use is limited by the high cost of treatment, which is estimated to be $14 000 per year.26 As such, most cost‐effectiveness studies have concluded that at the current price, PCSK9 inhibitors are not cost‐effective at the commonly accepted threshold of $100 000 per quality‐adjusted life‐year gained.26, 27 Furthermore, physicians may have lower LDL‐C thresholds for prescribing PCSK9 inhibitors than the thresholds at which payers will approve the prescription.28 Payer denials are also commonly attributed to inadequate or missing documentation, patients not currently being treated with maximally tolerated statins, and other formulary restrictions.28 It is estimated that nearly 2 in 3 prescriptions for PCSK9 inhibitors are rejected, and during 6 months of follow‐up of 3472 patients prescribed PCSK9 inhibitors, patients who were rejected experienced higher rates of cardiovascular events compared with those approved for a PCSK9 inhibitor (7.3 versus 6.3 per 100 person‐years).29 Consequently, a large number of cardiovascular events may be preventable with improved access to PCSK9 inhibitors and improved adherence to evidence‐based treatment, including PCSK9 inhibitors when indicated, among patients who are at increased risk of subsequent cardiovascular events.
Limitations and Strengths
Some limitations of our study deserve mention. First, only aggregate data were available from each data mart, and this did not allow for specific clarity of some data elements; for example, the linkage of laboratory data with prescription data was not possible. Second, because of the aggregated nature of the data, we were unable to fully understand data missingness. Third, because some missingness of data was required to calculate ASCVD risk, we were not able to further stratify our analysis by 10‐year estimated ASCVD risk category within each patient group. Fourth, we did not retrieve information on niacin use, which may have limited our ability to fully characterize nonstatin lipid‐lowering therapy use in the patient groups. Fifth, we did not have information on insurance status or insurance type to explore whether the lipid‐lowering prescribing patterns differed by insurance type. Sixth, we did not determine the proportion of each patient group that met eligibility criteria for PCSK9 inhibitors. Finally, because the PCSK9 inhibitors are relatively new and not widely prescribed, data marts frequently produced below‐threshold values, so we were unable to determine the actual count of patients on PCSK9 inhibitors. Nevertheless, our study has many strengths, including the large number of patients captured (>17.5 million) among 18 data marts. Including data from data marts that are geographically diverse allows for inclusion of patients receiving care from multiple distinct healthcare systems that span various care models, practice settings, demographics, and practice patterns. In addition, the large sample size as a result of pooling data from multiple data marts offers a distinct opportunity to study rare events and, as in our case, use of rarely prescribed medications to generate real‐world evidence that is more generalizable than single‐center study data would be. Indeed, the results of our study may be more generalizable than single‐center studies because of the geographic diversity of the 18 data marts, which include a variety of practice settings across the United States and variability in the underlying patient populations.
Conclusion
Among >3.6 million patients meeting the criteria for 3 patient groups in 18 PCORI data marts, approximately half had been prescribed lipid‐lowering medication, and the most common medications were statins. Overall, <1% of patients were prescribed PCSK9 inhibitors. Between July 2015 and March 2017, no apparent differences were observed in the number of patients prescribed PCSK9 inhibitors over time for those with dyslipidemia; however, increased PCSK9 inhibitor use was observed over time for patients with CAD or CHD.
Sources of Funding
This study was funded by the Patient‐Centered Outcomes Research Institute (PCORI) Award (CDRN‐1501‐26692 contract amendment). The statements presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its board of governors, or its methodology committee.
Disclosures
Cooper‐DeHoff received research funding from OneFlorida Clinical Data Research Network funded by the Patient‐Centered Outcomes Research Institute (no. CDRN‐1501‐26692 and RI‐CRG‐1807‐0003) at the level of modest. Linton receives research funding from the National Institutes of Health (P01 HL116263) at the level of significant and by Merck, ISIS, Genzyme, Sanofi, and Regeneron and is a consultant or on advisory board for Merck, Retrophin, Amgen, and RegenXBio at the level of modest. The remaining authors have no disclosures to report.
Supporting information
Table S1. List of Participating Clinical Data Research Networks and Data Marts
Table S2. Definition of the 3 Patient Groups, Ranked Lowest to Highest Risk
Table S3. Diagnostic Codes to Identify Comorbid Conditions
Acknowledgments
We would like to acknowledge all of the clinical data research network data mart analysts who provided data for this study.
(J Am Heart Assoc. 2019;8:e011246 DOI: 10.1161/JAHA.118.011246)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 4. Navarese E, Kołodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB Sr, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta‐analysis. Ann Intern Med. 2015;163:40–51. [DOI] [PubMed] [Google Scholar]
- 5. Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Boren J, Farnier M, Ference BA, Gielen S, Graham I, Grobbee DE, Hovingh GK, Luscher TF, Piepoli MF, Ray KK, Stroes ES, Wiklund O, Windecker S, Zamorano JL, Pinto F, Tokgozoglu L, Bax JJ, Catapano AL. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J. 2018;39:1131–1143. [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 7. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 8. Bittner V, Deng L, Rosenson RS, Taylor B, Glasser SP, Kent ST, Farkouh ME, Muntner P. Trends in the use of nonstatin lipid‐lowering therapy among patients with coronary heart disease: a retrospective cohort study in the Medicare population 2007 to 2011. J Am Coll Cardiol. 2015;66:1864–1872. [DOI] [PubMed] [Google Scholar]
- 9. Johansen ME, Green LA, Sen A, Kircher S, Richardson CR. Cardiovascular risk and statin use in the United States. Ann Fam Med. 2014;12:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makam RC, Erskine N, McManus DD, Lessard D, Gore JM, Yarzebski J, Goldberg RJ. Decade‐long trends (2001 to 2011) in the use of evidence‐based medical therapies at the time of hospital discharge for patients surviving acute myocardial infarction. Am J Cardiol. 2016;118:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Punekar RS, Fox KM, Richhariya A, Fisher MD, Cziraky M, Gandra SR, Toth PP. Burden of first and recurrent cardiovascular events among patients with hyperlipidemia. Clin Cardiol. 2015;38:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verma A, Visintainer P, Elarabi M, Wartak S, Rothberg MB. Overtreatment and undertreatment of hyperlipidemia in the outpatient setting. South Med J. 2012;105:329–333. [DOI] [PubMed] [Google Scholar]
- 13. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 14. AlHajri L, AlHadhrami A, AlMheiri S, AlMutawa Y, AlHashimi Z. The efficacy of evolocumab in the management of hyperlipidemia: a systematic review. Ther Adv Cardiovasc Dis. 2017;11:155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catapano AL, Lee LV, Louie MJ, Thompson D, Bergeron J, Krempf M. Efficacy of alirocumab according to background statin type and dose: pooled analysis of 8 ODYSSEY Phase 3 clinical trials. Sci Rep. 2017;7:45788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Kassahun H, Ruzza A, Ma Y, Somaratne R, Raal FJ. Long‐term low‐density lipoprotein cholesterol‐lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open‐label OSLER‐1 extension study. JAMA Cardiol. 2017;2:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCullough PA, Ballantyne CM, Sanganalmath SK, Langslet G, Baum SJ, Shah PK, Koren A, Mandel J, Davidson MH. Efficacy and safety of alirocumab in high‐risk patients with clinical atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia (from 5 placebo‐controlled ODYSSEY trials). Am J Cardiol. 2018;121:940–948. [DOI] [PubMed] [Google Scholar]
- 18. Toth PP, Worthy G, Gandra SR, Sattar N, Bray S, Cheng LI, Bridges I, Worth GM, Dent R, Forbes CA, Deshpande S, Ross J, Kleijnen J, Stroes ESG. Systematic review and network meta‐analysis on the efficacy of evolocumab and other therapies for the management of lipid levels in hyperlipidemia. J Am Heart Assoc. 2017;6:e005367 DOI: 10.1161/JAHA.116.005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz GG, Szarek M, Bhatt DL, Bittner V, Diaz R, Edelberg J, Goodman SG, Hanotin C, Harrington R, Jukema JW, Lecorps G, Moryusef A, Pordy R, Roe M, White HD, Zeiher A, Steg PG. The ODYSSEY OUTCOMES Trial: topline results. Alirocumab in patients after acute coronary syndrome. American College of Cardiology ‐ 67th Scientific Sessions ‐ March 10, 2018. Available at: https://accscientificsession.acc.org/~/media/ScientificSessions/ACC18/PDFs/Sanofi-stream/Session-401-ODYSSEY-slides.pdf. Accessed July 26, 2018.
- 20. Giugliano RP, Keech A, Murphy SA, Huber K, Tokgozoglu SL, Lewis BS, Ferreira J, Pineda AL, Somaratne R, Sever PS, Pedersen TR, Sabatine MS. Clinical efficacy and safety of evolocumab in high‐risk patients receiving a statin: secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal‐potency statin in a randomized clinical trial. JAMA Cardiol. 2017;2:1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd‐Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qureshi WT, Kaplan RC, Swett K, Burke G, Daviglus M, Jung M, Talavera GA, Chirinos DA, Reina SA, Davis S, Rodriguez CJ. American College of Cardiology/American Heart Association (ACC/AHA) Class I Guidelines for the Treatment of Cholesterol to Reduce Atherosclerotic Cardiovascular Risk: Implications for US Hispanics/Latinos Based on Findings From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). J Am Heart Assoc. 2017;6:e005045 DOI: 10.1161/JAHA.116.005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knowles JW, Howard WB, Karayan L, Baum SJ, Wilemon KA, Ballantyne CM, Myers KD. Access to nonstatin lipid‐lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135:2204–2206. [DOI] [PubMed] [Google Scholar]
- 24. Hess GP, Natarajan P, Faridi KF, Fievitz A, Valsdottir L, Yeh RW. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017;136:2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kazi DS, Lu CY, Lin GA, DeJong C, Dudley RA, Chen R, Tseng CW. Nationwide coverage and cost‐sharing for PCSK9 inhibitors among Medicare Part D plans. JAMA Cardiol. 2017;2:1164–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins‐Domingo K. Cost‐effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–753. [DOI] [PubMed] [Google Scholar]
- 27. Annemans L, Packard CJ, Briggs A, Ray KK. ‘Highest risk‐highest benefit’ strategy: a pragmatic, cost‐effective approach to targeting use of PCSK9 inhibitor therapies. Eur Heart J. 2018;39:2546–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen JD, Cziraky MJ, Jacobson TA, Maki KC, Karalis DG. Barriers to PCSK9 inhibitor prescriptions for patients with high cardiovascular risk: results of a healthcare provider survey conducted by the National Lipid Association. J Clin Lipidol. 2017;11:891–900. [DOI] [PubMed] [Google Scholar]
- 29. Baum SJ, Chen C‐C, Rane P, Patel J, Maya J, Harrison D, Yurgin N, Wade R, Desai N. Cardiovascular risk in patients denied access to PCSK9i therapy. J Am Coll Cardiol. 2018;71:A1760. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of Participating Clinical Data Research Networks and Data Marts
Table S2. Definition of the 3 Patient Groups, Ranked Lowest to Highest Risk
Table S3. Diagnostic Codes to Identify Comorbid Conditions


