Abstract
Background
Concerns exist about reliability of pressure‐wire‐guided coronary revascularization of non‐infarct‐related arteries (non‐IRA). We investigated whether physiological assessment of non‐IRA during the subacute phase of myocardial infarction might be flawed by microcirculatory dysfunction.
Methods and Results
We analyzed non‐IRA that underwent fractional flow reserve, coronary flow reserve, and the index of microcirculatory resistance assessment. Microcirculation and hyperemic response were evaluated in 49 acute myocardial infarction patients (59 non‐IRA) and compared with a matched control group of 46 stable angina (SA) patients (59 vessels). Time between acute myocardial infarction to physiological interrogation was 5.9±2.4 days. Fractional flow reserve was similar in both groups (0.79±0.11 in non‐IRA versus 0.80±0.13 in SA vessels, P=0.527). Lower coronary flow reserve values were found in non‐IRA compared with SA vessels (1.77 [1.25–2.76] versus 2.44 [1.63–4.00], P=0.018), primarily driven by an increased baseline flow in non‐IRA (rest mean transit time 0.58 [0.32–0.83] versus 0.65 s [0.39–1.20], P=0.045), whereas the hyperemic flow was similar (hyperemic mean transit time 0.26 [0.20–0.42] versus 0.26 s [0.18–0.35], P=0.873). No differences were found regarding index of microcirculatory resistance (15.6 [10.4–21.8] in non‐IRA versus 16.7 [11.6–23.6] U in SA vessels, P=0.559). During adenosine infusion, the hyperemic response was similar in both groups (non‐IRA versus SA vessels) in terms of the resistive reserve ratio (3.1±2.1 versus 3.7±2.2, P=0.118).
Conclusions
In the subacute phase of myocardial infarction, non‐IRA show an increased baseline flow that may cause abnormal coronary flow reserve despite preserved hyperemic flow. In non‐IRA, microcirculatory resistance and adenosine‐induced hyperemic response are similar to those found in SA patients. From a physiological perspective, these findings support the use of fractional flow reserve to interrogate non‐IRA during the subacute phase of myocardial infarction.
Keywords: coronary flow reserve, coronary microcirculation, fractional flow reserve, microcirculatory resistance, non‐infarct‐related arteries
Subject Categories: Angiography, Diagnostic Testing, Catheter-Based Coronary and Valvular Interventions, Revascularization
Short abstract
See Editorial Koh and Samady
Clinical Perspective
What Is New?
As compared with a matched control group of stable angina patients, we found in the subacute phase of myocardial infarction lower coronary flow reserve values in non‐infarct‐related arteries (non‐IRA), primarily driven by an increased resting flow, whereas hyperemic blood flow was similar.
However, fractional flow reserve, index of microcirculatory resistance, and the magnitude of the hyperemic response to adenosine were similar in non‐IRA and stable angina vessels.
What Are the Clinical Implications?
Concerns exist about reliability of pressure‐wire‐guided coronary revascularization of non‐IRA in patients with acute myocardial infarction and multivessel disease.
The pathophysiological framework of an acute myocardial infarction, including microcirculatory dysfunction or blunted hyperemic response, could transiently influence pressure‐wire‐based physiological assessment of non‐IRA.
From a physiological perspective, the findings of this study support the reliability of fractional flow reserve–based assessment of non‐IRA in the subacute phase of myocardial infarction.
Introduction
Pressure‐wire‐guided coronary revascularization can be safely applied in patients with stable angina (SA).1 In patients with acute myocardial infarction (AMI) with multivessel disease (MVD), fractional flow reserve (FFR)–based revascularization has been applied to non‐infarct‐related arteries (non‐IRA) as part of a complete revascularization strategy, compared with revascularization restricted to the culprit stenoses.2, 3 This information has generated great interest, as there is growing evidence supporting complete revascularization in AMI patients with MVD.4, 5
However, there is a paucity of data supporting the use of pressure‐wire‐guided coronary revascularization in the acute or subacute phase of AMI. Several pathophysiological factors could transiently influence pressure‐wire‐based physiological assessment of non‐IRA, including microcirculatory dysfunction, high left ventricular filling pressure, blunted hyperemic response to adenosine, and myocardial stunning extending also to remote myocardial territories. All this might contribute to underestimate the functional relevance of stenoses in non‐IRA, making physiology‐based deferral unsafe. In this regard, a recent pooled analysis of 2 large trials on physiology‐based revascularization identified an increased risk of cardiovascular events in patients presenting with acute coronary syndromes in whom percutaneous coronary intervention in nonculprit stenoses was deferred, compared with SA patients.6
As the use of pressure‐wires is progressively extending to patients with AMI and MVD, it is highly relevant to understand the underlying pathophysiological mechanisms potentially influencing the functional assessment of nonculprit stenoses. In this study, we sought to investigate the status of the microcirculation downstream non‐IRA in the subacute phase of an AMI and its impact on FFR values. In addition, we evaluate the magnitude of hyperemic response to adenosine in this clinical subset.
Methods
Study Design
We performed an observational, international, multicenter study involving patients with coronary artery disease who underwent comprehensive intracoronary physiology assessment with FFR, the index of microcirculatory resistance (IMR), and the coronary flow reserve (CFR). The status of the microcirculation downstream non‐IRA was evaluated with IMR and CFR and compared with that of target vessels of a cohort of SA patients selected by using a propensity score matching model, in order to avoid bias driven by baseline predictors of high IMR. Raw data from physiology studies were collected and independently analyzed at a central core laboratory. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
From an entire registry constituted by 4 tertiary centers (Hospital Clinico San Carlos, Madrid, Spain; Samsung Medical Center, Seoul, South Korea; Seoul National University Hospital, Seoul, South Korea; and VU Medical Center, Amsterdam, the Netherlands), we identified patients presenting with AMI (non–ST‐segment–elevation myocardial infarction [NSTEMI] and ST‐segment–elevation myocardial infarction [STEMI]) with MVD in whom revascularization of non‐IRA was guided by FFR at a staged procedure (after successful percutaneous intervention of the culprit vessel). The culprit vessel was selected based on the ECG, echocardiographic abnormalities, and angiographic appearance. Time between the onset of symptoms to physiological evaluation of the non‐IRA was collected. Exclusion criteria were unstable angina as the initial clinical presentation, vessels with subtotal stenoses or significant collaterals, and poor quality or no availability of the raw physiology studies. The study protocol was in accordance with the Helsinki declaration and participants gave written informed consent. Part of the population reported here belong to a series previously reported.7
Intracoronary Physiology Study
FFR, CFR, and IMR were obtained with a pressure‐temperature sensor fitted guidewire (Certus wire, St. Jude Medical, MN) as described elsewhere.8 Intracoronary nitrates were administered before physiology measurements. At baseline conditions, mean proximal aortic pressure and mean intracoronary pressure distal to the target stenoses (rest‐Pd) were measured. The mean transit time at rest (rest‐Tmn) was averaged after 3 bolus injections of saline at room temperature. Hyperemia was induced by infusion of adenosine (140 μg/kg per kg) through a femoral or antecubital vein during 2 minutes. At steady‐state hyperemia, mean proximal aortic pressure (hyperemic‐Pa), mean intracoronary pressure distal to the target stenoses (hyperemic‐Pd), and mean transit time (hyperemic‐Tmn) were measured. FFR was calculated as the ratio of hyperemic‐Pd to hyperemic‐Pa, IMR as the product between hyperemic‐Pd and hyperemic‐Tmn and corrected by the Yong's formula,9 and CFR was measured as the ratio of base‐Tmn to hyperemic‐Tmn.
Assessment of the Hyperemic Response
The hyperemic reactivity was evaluated by changes in the intracoronary distal pressure during adenosine infusion (named delta of Pd, from resting conditions to hyperemia), and the resistive reserve ratio (RRR). The RRR, an index aimed to evaluate the ability of the coronary microcirculation to achieve maximal hyperemia, was calculated as the ratio between baseline microcirculatory resistance (rest‐Tmn×rest‐Pd) and IMR, as described elsewhere10.
Statistical Analysis
Continuous variables are expressed as mean±SD or median with interquartile ranges, as appropriate. Categorical variables are presented as numbers and percentages. Demographic and clinical data were analyzed on a per‐patient basis. The remaining calculations were analyzed on a per‐vessel basis using the generalized estimating equation method to adjust intrasubject variability among vessels from the same patient. In the generalized estimating equation model, independence correlation structure fit for sandwich variable estimator was used. In the overall cohort, a propensity score model was applied to matching AMI patients with SA patients. The propensity model was adjusted by age, sex, previous MI, and target vessel to reduce differences in clinical predictors of high IMR.11 Participants were matched at the level of 1:1 vessel. The statistical analysis was performed with SPSS statistics, version 21 (IBM Corp., Armonk, NY), R, version 3.5.2 (R Foundation for Statistical Computing), and MedCalc software, version 17.6 (MedCalc Software bvba, Ostend, Belgium). A P<0.05 was considered statistically significant.
Results
Baseline Clinical and Lesion Characteristics
Out of our initial cohort of 357 patients (477 vessels) evaluated with FFR, IMR, and CFR, 56 patients presented with AMI with MVD, and 70 non‐IRA fulfilled the inclusion criteria. By applying the propensity score matching model, 59 non‐IRA from 49 AMI patients were matched with a control group of 59 target vessels from 46 SA patients (Figure 1). The propensity model efficiently avoided significant differences between the AMI group and SA group regarding age (62.3±9.9 versus 63.7±10.7 years, P=0.514), sex (male 90% versus 89%, P=0.916), previous MI (20% versus 20%, P=0.919), and target vessel (left anterior descending artery 45.8% versus 45.8%, P=1.00). Angiographic stenoses severity was similar between the matched groups (non‐IRA versus SA target vessels: % diameter stenosis 50.8±10.7 versus 50.8±14.4, P=0.994) (Figure 2A). Tables 1 and 2 show the clinical and coronary anatomy characteristics of AMI and SA matched groups.
Figure 1.
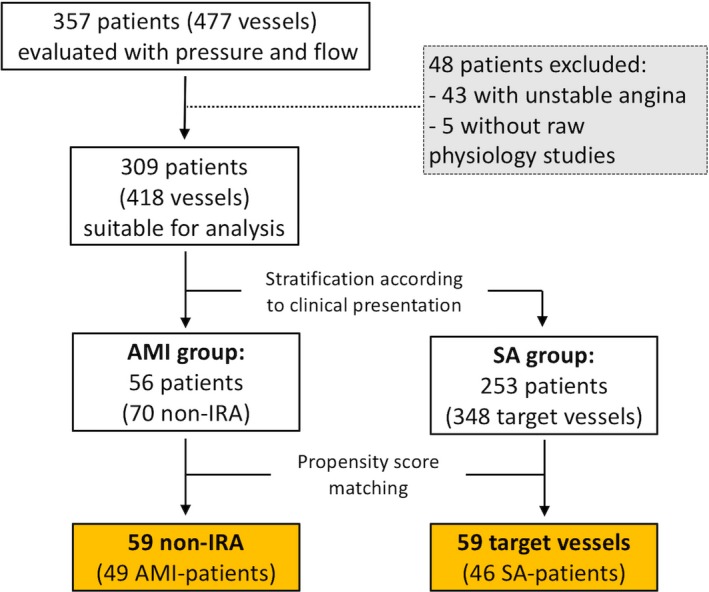
Study flowchart. AMI indicates acute myocardial infarction; non‐IRA, non‐infarct‐related arteries; SA, stable angina.
Figure 2.

Distribution of diameter stenoses and FFR values in non‐IRA and SA. Box‐and‐whisker plots show similar stenoses severity as judged by percent diameter stenosis (%DS) (A) and FFR (B) between non‐IRA and the matched‐control SA target vessels. The boxes show the median and first and third quartiles. Red dashed lines indicate the corresponding mean values. FFR indicates fractional flow reserve; non‐IRA, non‐infarct‐related arteries; SA, stable angina.
Table 1.
Baseline Clinical Characteristics
| Per‐Patient Analysis | AMI Group | SA Group | P Value |
|---|---|---|---|
| Age, y | 62.3±9.9 | 63.7±10.7 | 0.514 |
| Male | 44 (90) | 41 (89) | 0.916 |
| Cardiovascular risk factors | |||
| Hypertension | 30 (61) | 29 (63) | 0.856 |
| Diabetes mellitus | 14 (29) | 21 (46) | 0.053 |
| Dyslipidemia | 27 (55) | 27 (59) | 0.725 |
| Smoker | 23 (47) | 11 (24) | 0.020 |
| Obesity | 9 (18) | 3 (7) | 0.084 |
| Previous myocardial infarction | 10 (20) | 9 (20) | 0.919 |
| LVEF, % | 54.7±7.7 | 59.3±10.1 | 0.016 |
Values are mean±SD or n (%). AMI indicates acute myocardial infarction; LVEF, left ventricular ejection fraction; SA, stable angina.
Table 2.
Anatomic Characteristics of Non‐IRA and SA Target Vessels
| Per‐Vessel Analysis | Non‐IRA | SA‐Target Vessels | P Value |
|---|---|---|---|
| Target vessel | |||
| Left main | 1 (1.7) | 1 (1.7) | 1.0 |
| Left anterior descending | 27 (45.8) | 27 (45.8) | |
| Diagonal branch | 3 (5.1) | 3 (5.1) | |
| Left circumflex | 11 (18.6) | 11 (18.6) | |
| Obtuse marginal branch | 4 (6.8) | 4 (6.8) | |
| Right coronary artery | 13 (22) | 13 (22) | |
| 2D‐QCA parameters | |||
| Reference diameter, mm | 2.92±0.70 | 2.83±0.49 | 0.487 |
| MLD, mm | 1.2 (1.00–1.64) | 1.3 (0.91–1.67) | 0.460 |
| Diameter stenosis, % | 50.8±10.7 | 50.8±14.4 | 0.994 |
| Lesion length, mm | 9.4 (6.3–15.0) | 10.1 (6.2–14.6) | 0.514 |
Values are n (%), mean±SD, or median (interquartile range). MLD indicates minimum luminal diameter; non‐IRA, non‐infarct‐related arteries; SA, stable angina; 2D‐QCA, 2‐dimensional quantitative coronary angiography.
Trans‐Stenotic Pressure Gradient Indices
At baseline, no significant differences were found regarding trans‐stenotic pressure gradient between non‐IRA and SA target vessels: rest Pd/Pa 0.91 (0.86–0.95) versus 0.93 (0.88–0.97), P=0.567. During steady‐state hyperemia, the functional stenoses severity and the number of ischemic‐causing lesions were also similar between non‐IRA and SA‐target vessels: FFR 0.79±0.11 versus 0.80±0.13, P=0.527; number of vessels with FFR ≤0.80 28 (48%) versus 27 (46%), P=0.880, respectively (Figure 2B) (Table 3).
Table 3.
Physiology Characteristics of Non‐IRA and SA‐Vessels
| Per‐Vessel Analysis | Non‐IRA | SA‐Target Vessels | P Value |
|---|---|---|---|
| Mean rest Pa, mm Hg | 82 (75–93) | 93 (81–105) | 0.004 |
| Mean rest Pd, mm Hg | 74 (67–82) | 87 (72–97) | 0.004 |
| Rest Pd/Pa | 0.91 (0.86–0.95) | 0.93 (0.88–0.97) | 0.567 |
| Rest Tmn, s | 0.58 (0.32–0.83) | 0.65 (0.39–1.20) | 0.045 |
| Mean hyperemic Pa, mm Hg | 72 (60–80) | 83 (72–95) | 0.018 |
| Mean hyperemic Pd, mm Hg | 57 (43–67) | 70 (53–83) | 0.025 |
| Hyperemic Tmn, s | 0.26 (0.20–0.42) | 0.26 (0.18–0.35) | 0.873 |
| Delta‐Pa, mm Hg | −14 (−18: −4) | −11 (−16: −3) | 0.771 |
| Delta‐Pd, mm Hg | −16 (−23: −9) | −17 (−23: −11) | 0.984 |
| FFR | 0.79±0.11 | 0.80±0.13 | 0.527 |
| No. of vessels with FFR≤0.80 | 28 (48) | 27 (46) | 0.880 |
| IMR, U | 15.6 (10.4–21.8) | 16.7 (11.6–23.6) | 0.559 |
| No. of vessels with IMR≥25 | 10 (16.9) | 11 (18.6) | 0.809 |
| CFR | 1.77 (1.25–2.76) | 2.44 (1.63–4.00) | 0.018 |
| No. of vessels with CFR≤2.0 | 32 (54) | 21 (36) | 0.052 |
| RRR | 3.1±2.1 | 3.7±2.2 | 0.118 |
Values are median (interquartile range), mean±SD, or n (%). CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; non‐IRA: non‐infarct‐related arteries; Pa, mean aortic pressure; Pd, mean intracoronary distal pressure; RRR, resistive reserve ratio; SA, stable angina; Tmn, mean transit time.
Microcirculatory Resistance in the Non‐Infarcted Myocardium
The microcirculatory resistance in non‐IRA of AMI patients was similar to that of target vessels in SA patients (IMR 15.6 U [10.4–21.8] versus 16.7 U [11.6–23.6]; P=0.559) (Figure 3A). According to an IMR cutoff ≥25 U,12 no differences were found regarding percentage of high IMR values in non‐IRA and SA (10 [16.9%] versus 11 [18.6%] vessels, respectively; P=0.809) (Figure 3B). In addition, the 75th percentile of IMR was also similar between non‐IRA and SA vessels (21.8 versus 23.8 U, respectively; P=0.495).
Figure 3.

Comparison of IMR values between non‐IRA and SA. A, Plot figure shows a similar distribution of IMR values in non‐IRA and the matched‐control SA target vessels. Dashed lines represent the mean value for IMR. B, The number of vessels with IMR ≥25 was similar in non‐IRA and SA. IMR indicates index of microcirculatory resistance; non‐IRA, non‐infarct‐related arteries; SA, stable angina.
Coronary Flow Reserve in the Non‐IRA
A significant higher baseline coronary flow was found in non‐IRA compared with SA‐vessels: mean rest‐Tmn 0.58 sec (0.32–0.83) versus 0.65 sec (0.39–1.20), respectively; P=0.045. However, the hyperemic coronary flow was almost identical in non‐IRA and SA vessels: mean hyperemic‐Tmn 0.26 sec (0.20–0.42) versus 0.26 sec (0.18–0.35), respectively; P=0.873. As result, the CFR was significantly lower in non‐IRA compared with SA vessels (1.77 [1.25–2.76] versus 2.44 [1.63–4.00], respectively; P=0.018), and there was a higher number of vessels with depleted CFR in the non‐IRA group compared with the SA group (vessels with CFR ≤2: 32 [54%] versus 21 [36%], respectively; P=0.052) (Figure 4).
Figure 4.

Distribution of CFR values in non‐IRA and SA. A, CFR was significantly lower in non‐IRA as compared with that of the matched‐control group (SA target vessels). This difference can be explained when the components of CFR are analyzed separately: rest‐Tmn is significantly lower (higher flow) in non‐IRA (B), whereas hyperemic‐Tmn is very similar to that of SA target vessels (C). CFR indicates coronary flow reserve; Non‐IRA, non‐infarct‐related arteries; SA, stable angina; Tmn, mean transit time.
Correlation Between FFR, IMR, and CFR According to the Clinical Presentation
Figure 5 compares the correlation between FFR versus CFR and FFR versus IMR in non‐IRA and SA vessels. Whereas there was a modest but significant correlation between FFR and CFR in the SA group (r 0.356, P=0.006), no correlation was found in the non‐IRA (r 0.076, P=0.568). FFR and IMR did not correlate regardless of clinical presentation (r 0.062, P=0.641 in non‐IRA; r −0.126, P=0.340 in SA).
Figure 5.

Correlation between FFR, IMR, and CFR according to the clinical presentation. A significant correlation (modest) was found between CFR and FFR in the SA group (A), but not in the non‐IRA (B). IMR was not correlated with FFR regardless of clinical presentation (C, D). CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; non‐IRA, non‐infarct‐related arteries; SA, stable angina.
Assessment of the Hyperemic Reactivity to Adenosine
Among patients presenting with AMI, the mean time between the symptom's onset to physiological interrogation of non‐IRA was 5.9±2.4 days. During intravenous adenosine infusion, the hyperemic response as measured by delta intracoronary distal pressure (Δ Pd, mm Hg=hyperemic‐Pd−rest‐Pd) and the RRR (RRR=Baseline microcirculatory resistance/IMR) was similar between non‐IRA and SA groups (Δ Pd=−16 [−23: −9] versus −17 [−23: −11], P=0.984; RRR 3.1±2.1 versus 3.7±2.2, P=0.118, respectively) (Figure 6).
Figure 6.

Hyperemic response to adenosine. A, In the non‐IRA, adenosine caused a drop of intracoronary distal pressure (delta Pd, from base to hyperemia) similar to that observed in the control matched group of SA‐vessels. Red dashed lines represent the estimated mean drop (delta) (Table 3). B, distribution of the RRR values in both groups. In this Box‐and‐whisker plots figure, the boxes show the median and first and third quartiles; * represents an outlier. Hyp indicates hyperemic; non‐IRA, non‐infarct‐related arteries; Pd, distal pressure; RRR, resistive reserve ratio; SA, stable angina.
Physiology Parameters in Non‐IRA for STEMI and NSTEMI Patients
Among the overall AMI cohort, 30 patients (37 non‐IRA) presented with STEMI and 26 patients (33 non‐IRA) with NSTEMI. Functional severity in non‐IRA was similar between STEMI and NSTEMI patients (FFR 0.79±0.09 versus 0.79±0.12, P=0.626), as well as the number of non‐IRA with ischemic lesions (FFR ≤0.80: 16 [43%] versus 15 [45%], respectively; P=0.848) (Table 4). The microcirculatory resistance and CFR in non‐IRA were similar regardless of the type of AMI (STEMI versus NSTEMI: IMR 15.2 [10.2–18.9] versus 17.2 [11.2–24.8] U, P=0.110; CFR 1.79 [1.43–2.73] versus 1.61 [1.12–2.94], P=0.990) (Figure 7).
Table 4.
Comparison of Clinical, Angiographic Characteristics and Physiology Parameters in the Non‐IRA According to the Type of AMI
| STEMI Group | NSTEMI Group | P Value | |
|---|---|---|---|
| Troponin‐I | 68.0 (38.3–97.7) | 3.1 (1.3–4.8) | <0.01 |
| Target vessel | |||
| Left main | 1 (2.7) | 1 (3.0) | 0.36 |
| Left anterior descending | 17 (45.9) | 11 (33.3) | |
| Diagonal branch | 3 (8.1) | 2 (6.1) | |
| Left circumflex | 5 (13.5) | 9 (27.3) | |
| Obtuse marginal branch | 5 (13.5) | 3 (9.1) | |
| Right coronary artery | 6 (16.2) | 7 (21.2) | |
| Rest Pd/Pa | 0.91 (0.87–0.95) | 0.90 (0.84–0.94) | 0.267 |
| FFR | 0.79±0.09 | 0.79±0.12 | 0.626 |
| Vessels with FFR≤0.80 | 16 (43) | 15 (45) | 0.848 |
| IMR, U | 15.2 (10.2–18.9) | 17.2 (11.2–24.8) | 0.110 |
| CFR | 1.79 (1.43–2.73) | 1.61 (1.12–2.94) | 0.990 |
| Vessels with CFR≤2.0 | 19 (51) | 20 (61) | 0.479 |
Values are n (%), median (IQR), or mean±SD. AMI indicates acute myocardial infarction; CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; non‐IRA, non‐infarct‐related arteries; NSTEMI, non–ST‐segment–elevation myocardial infarction; Pa, mean aortic pressure, Pd, mean intracoronary distal pressure; STEMI, ST‐segment–elevation myocardial infarction.
Figure 7.

Distribution of FFR, IMR, and CFR values in non‐IRA according to the type of acute myocardial infarction. In the non‐IRA, distribution of FFR, IMR, and CFR values was similar regardless of the type of acute myocardial infarction. Dashed lines represent mean values. CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Discussion
The findings made in this study have implications for the use and interpretation of both pressure‐ and flow‐based physiological indices in the subacute phase of AMI. On the one hand, we found that resting coronary flow in non‐IRA is significantly higher than in SA patients. This potentially explains why CFR is decreased in non‐IRA, and why it should be used with caution to draw conclusions on the status of the coronary microcirculation downstream non‐IRA after AMI. The presence of a higher resting flow also casts doubts as to the accuracy of nonhyperemic resting indices of stenosis severity in non‐IRA. On the other hand, the absence of differences in the hyperemic flow and microcirculatory resistance between non‐IRA and SA vessels provide mechanistic support to the validity of hyperemic indices, such as FFR, in decision making during the subacute phase of AMI.
The occurrence of MVD in patients with AMI is frequent and entails a worse prognosis.13, 14 Recently, FFR‐based revascularization of non‐IRA has been shown in trials to improve prognosis compared with a culprit‐only percutaneous coronary intervention strategy in patients with AMI,2 and to reduce repeat revascularization rates compared with an angiography‐based strategy.3 However, a comparable safety of FFR as a decision‐making tool in AMI patients to that widely documented in SA is missing, and data supporting the use of FFR and new resting pressure–based indices like iFR (instantaneous wave‐free ratio) is scarce in this clinical subset. In addition, given the differences in the physiological framework of SA and AMI (which could cause transient variations in FFR values), conclusions on safety cannot be transferred from one to the other clinical scenarios.15 The concerns about equivocal decision‐making with FFR and iFR in the context of AMI stems from theoretical reasons and clinical evidence. Infarct‐related extravascular compression (caused by intramyocardial hemorrhage and edema) and intravascular obstruction (caused by endothelial cell swelling and embolized particulate) increase microcirculatory resistance, limiting myocardial flow.16 It is not well known whether such pathophysiological changes extend also to remote myocardial territories affecting functional assessment of non‐IRA during the acute or subacute stage of MI. In addition, a recent study based on large randomized trials suggests an excess of events at 1‐year follow‐up in patients with AMI in whom revascularization deferral of non‐IRA was based on FFR or iFR, compared with SA patients.6
In this regard, major pending questions include whether revascularization of non‐IRA should be performed during the acute or subacute stages of an AMI, and whether physiological guidance of non‐IRA results in any advantage over angiography‐based revascularization. Our research is of interest in the discussion of these topics.
Coronary Flow in Non‐IRA
We found significantly lower CFR values in non‐IRA as compared with the control matched SA vessels. However, when the components of the flow ratio were analyzed separately, we found that the impairment of CFR in non‐IRA was not caused by a decrease in maximal flow, but was driven by an increase in resting flow in non‐IRA (Table 3 and Figure 4). Our observations on this aspect are supported by previous research.
Bax et al evaluated the evolutionary changes of CFR and microcirculatory resistance in both IRA and non‐IRA in 73 patients with an anterior STEMI through intracoronary Doppler technique.17 The CFR, measured immediately after reperfusion, increased significantly at 1‐week and 6‐month follow‐up in both groups. They stated that low CFR in remote regions during AMI is probably the consequence of a disturbed autoregulation. Van Herck also found an increase in baseline flow in non‐IRA, which was proportional to myocardial infarction size assessed with magnetic resonance imaging.18 More recently, de Waard et al reported, based on intracoronary Doppler flow velocity measurements, a significantly lower CFR and increased baseline flow in non‐IRA in patients with AMI, compared with SA patients.19 Of note, these authors also documented a decrease in maximal flow in non‐IRA, but such difference might be because their control group did not include flow‐limiting stenosis.
In other words, the decrease in CFR in non‐IRA denotes a different physiological framework than in SA patients, but should not be interpreted in the sense that myocardial blood supply is decreased nor that the subtended microcirculation is impaired in non‐IRA. A higher resting coronary flow in AMI patients may be the result of neurohumoral compensatory mechanisms triggered by the acute myocardial damage.20 Yet, the increase in resting flow in AMI patients may have implications for functional stenosis assessment based on resting translesional pressure ratios, such as iFR or Pd/Pa. The findings of our study may explain the overestimation of stenosis severity of iFR in AMI patients reported in the i‐STEMI trial. In that study, the classification agreement between acute and follow‐up iFR measurements obtained in non‐IRA was moderate (78%).21 Of note, classification agreement was influenced by time interval from acute to follow‐up iFR. The authors acknowledged that the main benefit of iFR in the assessment of non‐IRA during the acute stage of AMI would be ruling out functional stenosis significance.
Microcirculatory Resistance in Non‐IRA
We also investigated the microcirculation in non‐IRA from a resistive perspective. An increase in microcirculatory resistance in the context of AMI may result from intramyocardial hemorrhage, edema, microvascular obstruction, or extravascular compression,22 limiting maximal coronary flow. In this regard, mirroring similar hyperemic Tmn, the microcirculatory resistance in our study was similar in non‐IRA and the matched SA vessels in terms of IMR, the 75th percentile of IMR values, and the number of vessels with high IMR (Table 3 and Figure 3). In our study population, the angiographic and functional severity of non‐IRA stenoses was intermediate and similar to that of the control matched vessels (Figure 2). This is in concordance with the clinical practice guidelines in which the use of pressure wires is recommended to guide revascularization of intermediate coronary stenoses1. Our results support the findings reported by previous studies that used a different methodology. Ntalianis et al investigated the reliability of FFR in non‐IRA during the acute phase of a myocardial infarction and 1 month later in 75 STEMI patients and 26 NSTEMI patients.23 Interestingly, they found that FFR and IMR values did not change during the acute phase and follow‐up. However, whether 1 month is enough time for resolution of potential transient microcirculatory dysfunction affecting remote myocardial territories was not addressed by such study; additionally, the number of vessels evaluated with IMR was very low (14 patients). A recent animal experiment supports the concept that local high microcirculatory resistance increases the local FFR value but does not affect IMR or FFR in remote coronary vessels.24 Lee et al investigated in swine whether local microcirculatory damage also extends to a distant myocardial territory and affects FFR values in non‐IRA. They injected microspheres into the left anterior descending artery and artificially created an epicardial stenosis with angioplasty balloons. They found that FFR values proportionally increase to the local microcirculatory damage measured by IMR in the infarcted vessel, whereas IMR and FFR values in non‐IRA remained similar. Although such study was carried out in animals and used artificial methods, its results support our findings in real patients in whom we did not find differences between non‐IRA and the control matched SA‐vessels in terms of IMR and FFR values.
Hyperemic Response to Adenosine in Non‐IRA
The physiological framework of FFR makes mandatory the achievement of maximal myocardial hyperemia. It remains unclear whether myocardial damage associated with AMI may impair maximal hyperemia in remote, noninfarcted territories. To investigate this phenomenon, we quantified the hyperemic response in non‐IRA of AMI patients and the control group of SA vessels by assessing the modification of intracoronary pressure caused by adenosine, as well as the changes in microcirculatory resistance.10 Overall, we found that the hyperemic reactivity to adenosine in non‐IRA during the subacute phase of a myocardial infarction was not blunted, compared with patients with SA. The shift in intracoronary pressure (distal to the interrogated stenosis) caused by adenosine infusion was of similar magnitude in AMI and SA patients (Table 3) and, likewise, the variation in the microcirculatory resistance caused by adenosine, as determined by the RRR, was of similar magnitude between both groups (Figure 6).
Microcirculation Downstream Non‐IRA in STEMI and NSTEMI Patients
We also evaluated the microcirculation in non‐IRA according to the type of AMI. This is because the extension of myocardial damage, usually larger in STEMI than in non‐STEMI, might be associated with the degree of remote microcirculatory impairment. In this study, despite a larger myocardial damage in STEMI than in NSTEMI patients (troponin I [units] 68.0 versus 3.1, P<0.01), the distribution of IMR and CFR values in non‐IRA was similar between both AMI groups (Figure 7). In agreement with these findings, the physiological relevance of non‐culprit stenoses in non‐IRA was similar between STEMI and NSTEMI patients (Table 4). This supports that our findings can be applied to non‐IRA in patients with either type of myocardial infarction.
Study Limitations
Because physiological assessment was performed only in non‐IRA, we cannot determine the association between the extension of local myocardial damage in terms of IMR in the culprit vessel and the status of the remote microcirculation. A second limitation is that physiological assessment was not repeated at follow‐up; therefore we cannot determine the evolutionary changes in the microcirculation subtended to non‐IRA over time. Another limitation is that the influence of the medication received by AMI patients in the results of our study was not assessed. Other relevant hemodynamic parameters that can potentially influence pressure and flow intracoronary measurements within the context of an AMI, such as the left ventricle filling pressure, were not systematically determined. In addition, our study is limited to assessing the coronary physiological implications of AMI to support or not the use of invasive pressure‐wire measurements in guiding revascularization of non‐IRA. We compared the microcirculation subtended to non‐IRA with that of a matched control group of patients with SA. However, it is important to bear in mind that AMI patients have a clinically increased risk at long term compared with SA patients, and that these differences cannot be explained only on the basis of FFR (or iFR) alone. In this regard, a limitation of our study is that the impact of our findings on long‐term cardiovascular outcomes was not evaluated.
Conclusions
In the subacute phase of myocardial infarction, non‐IRA show an increased baseline flow that may cause abnormal CFR despite preserved hyperemic flow. In non‐IRA, microcirculatory resistance and adenosine‐induced hyperemic response are similar to those found in SA patients. From a physiological perspective, these findings support the use of FFR to interrogate non‐IRA during the subacute phase of myocardial infarction.
Disclosures
Dr Joo Myung Lee received a Research Grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. Dr Bon‐Kwon Koo received an Institutional Research Grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. The remaining authors have no disclosures to report.
(J Am Heart Assoc.2019;8:e011534 DOI: 10.1161/JAHA.118.011534.)
References
- 1. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 2. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted H‐H, Villadsen AB, Aarøe J, Jensen SE, Raungaard B, Køber L; DANAMI‐3—PRIMULTI Investigators . Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3—PRIMULTI): an open‐label, randomised controlled trial. Lancet Lond Engl. 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 3. Smits PC, Abdel‐Wahab M, Neumann F‐J, Boxma‐de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E; Compare‐Acute Investigators . Fractional flow reserve‐guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. [DOI] [PubMed] [Google Scholar]
- 4. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG; PRAMI Investigators . Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. [DOI] [PubMed] [Google Scholar]
- 5. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Escaned J, Ryan N, Mejía‐Rentería H, Cook CM, Dehbi H‐M, Alegria‐Barrero E, Alghamdi A, Al‐Lamee R, Altman J, Ambrosia A, Baptista SB, Bertilsson M, Bhindi R, Birgander M, Bojara W, Brugaletta S, Buller C, Calais F, Silva PC, Carlsson J, Christiansen EH, Danielewicz M, Di Mario C, Doh J‐H, Erglis A, Erlinge D, Gerber RT, Going O, Gudmundsdottir I, Härle T, Hauer D, Hellig F, Indolfi C, Jakobsen L, Janssens L, Jensen J, Jeremias A, Kåregren A, Karlsson A‐C, Kharbanda RK, Khashaba A, Kikuta Y, Krackhardt F, Koo B‐K, Koul S, Laine M, Lehman SJ, Lindroos P, Malik IS, Maeng M, Matsuo H, Meuwissen M, Nam C‐W, Niccoli G, Nijjer SS, Olsson H, Olsson S‐E, Omerovic E, Panayi G, Petraco R, Piek JJ, Ribichini F, Samady H, Samuels B, Sandhall L, Sapontis J, Sen S, Seto AH, Sezer M, Sharp ASP, Shin E‐S, Singh J, Takashima H, Talwar S, Tanaka N, Tang K, Van Belle E, van Royen N, Varenhorst C, Vinhas H, Vrints CJ, Walters D, Yokoi H, Fröbert O, Patel MR, Serruys P, Davies JE, Götberg M. Safety of the deferral of coronary revascularization on the basis of instantaneous wave‐free ratio and fractional flow reserve measurements in stable coronary artery disease and acute coronary syndromes. JACC Cardiovasc Interv. 2018;11:1437–1449. [DOI] [PubMed] [Google Scholar]
- 7. Mejía‐Rentería H, Lee JM, Lauri F, van der Hoeven NW, de Waard GA, Macaya F, Pérez‐Vizcayno MJ, Gonzalo N, Jiménez‐Quevedo P, Nombela‐Franco L, Salinas P, Núñez‐Gil I, Del Trigo M, Goto S, Lee HJ, Liontou C, Fernández‐Ortiz A, Macaya C, van Royen N, Koo B‐K, Escaned J. Influence of microcirculatory dysfunction on angiography‐based functional assessment of coronary stenoses. JACC Cardiovasc Interv. 2018;11:741–753. [DOI] [PubMed] [Google Scholar]
- 8. Ng MKC, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. [DOI] [PubMed] [Google Scholar]
- 9. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A, Ng MK. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. [DOI] [PubMed] [Google Scholar]
- 10. Layland J, Carrick D, McEntegart M, Ahmed N, Payne A, McClure J, Sood A, McGeoch R, MacIsaac A, Whitbourn R, Wilson A, Oldroyd K, Berry C. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non‐ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:231–236. [DOI] [PubMed] [Google Scholar]
- 11. Lee JM, Layland J, Jung J‐H, Lee H‐J, Echavarria‐Pinto M, Watkins S, Yong AS, Doh J‐H, Nam C‐W, Shin E‐S, Koo B‐K, Ng MK, Escaned J, Fearon WF, Oldroyd KG. Integrated physiologic assessment of ischemic heart disease in real‐world practice using index of microcirculatory resistance and fractional flow reserve: insights from the International Index of Microcirculatory Resistance Registry. Circ Cardiovasc Interv. 2015;8:e002857. [DOI] [PubMed] [Google Scholar]
- 12. Liu A, Wijesurendra RS, Liu JM, Forfar JC, Channon KM, Jerosch‐Herold M, Piechnik SK, Neubauer S, Kharbanda RK, Ferreira VM. Diagnosis of microvascular angina using cardiac magnetic resonance. J Am Coll Cardiol. 2018;71:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Park D‐W, Clare RM, Schulte PJ, Pieper KS, Shaw LK, Califf RM, Ohman EM, Van de Werf F, Hirji S, Harrington RA, Armstrong PW, Granger CB, Jeong M‐H, Patel MR. Extent, location, and clinical significance of non‐infarct‐related coronary artery disease among patients with ST‐elevation myocardial infarction. JAMA. 2014;312:2019–2027. [DOI] [PubMed] [Google Scholar]
- 14. Dziewierz A, Siudak Z, Rakowski T, Zasada W, Dubiel JS, Dudek D. Impact of multivessel coronary artery disease and noninfarct‐related artery revascularization on outcome of patients with ST‐elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER Registry). Am J Cardiol. 2010;106:342–347. [DOI] [PubMed] [Google Scholar]
- 15. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 16. Mejía‐Rentería H, van der Hoeven N, van de Hoef TP, Heemelaar J, Ryan N, Lerman A, van Royen N, Escaned J. Targeting the dominant mechanism of coronary microvascular dysfunction with intracoronary physiology tests. Int J Cardiovasc Imaging. 2017;33:1041–1059. [DOI] [PubMed] [Google Scholar]
- 17. Bax M, de Winter RJ, Koch KT, Schotborgh CE, Tijssen JGP, Piek JJ. Time course of microvascular resistance of the infarct and noninfarct coronary artery following an anterior wall acute myocardial infarction. Am J Cardiol. 2006;97:1131–1136. [DOI] [PubMed] [Google Scholar]
- 18. Van Herck PL, Paelinck BP, Haine SE, Claeys MJ, Miljoen H, Bosmans JM, Parizel PM, Vrints CJ. Impaired coronary flow reserve after a recent myocardial infarction: correlation with infarct size and extent of microvascular obstruction. Int J Cardiol. 2013;167:351–356. [DOI] [PubMed] [Google Scholar]
- 19. de Waard GA, Hollander MR, Teunissen PFA, Jansen MF, Eerenberg ES, Beek AM, Marques KM, van de Ven PM, Garrelds IM, Danser AHJ, Duncker DJ, van Royen N. Changes in coronary blood flow after acute myocardial infarction: insights from a patient study and an experimental porcine model. JACC Cardiovasc Interv. 2016;9:602–613. [DOI] [PubMed] [Google Scholar]
- 20. Schäfer U, Kurz T, Jain D, Hartmann F, Dendorfer A, Tölg R, Raasch W, Dominiak P, Katus H, Richardt G. Impaired coronary flow and left ventricular dysfunction after mechanical recanalization in acute myocardial infarction: role of neurohumoral activation? Basic Res Cardiol. 2002;97:399–408. [DOI] [PubMed] [Google Scholar]
- 21. Thim T, Götberg M, Fröbert O, Nijveldt R, van Royen N, Baptista SB, Koul S, Kellerth T, Bøtker HE, Terkelsen CJ, Christiansen EH, Jakobsen L, Kristensen SD, Maeng M. Nonculprit stenosis evaluation using instantaneous wave‐free ratio in patients with ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2017;10:2528–2535. [DOI] [PubMed] [Google Scholar]
- 22. McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010;3:715–722. [DOI] [PubMed] [Google Scholar]
- 23. Ntalianis A, Sels J‐W, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR, Wijns W, Pijls NHJ, De Bruyne B. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274–1281. [DOI] [PubMed] [Google Scholar]
- 24. Lee JM, Kim HK, Lim KS, Park J‐K, Choi KH, Park J, Hwang D, Rhee T‐M, Yang JH, Shin E‐S, Nam C‐W, Doh J‐H, Hahn J‐Y, Koo B‐K, Jeong MH. Influence of local myocardial damage on index of microcirculatory resistance and fractional flow reserve in target and nontarget vascular territories in a porcine microvascular injury model. JACC Cardiovasc Interv. 2018;11:717–724. [DOI] [PubMed] [Google Scholar]


