Abstract
Background
Stroke survivors have high rates of mortality and recurrent stroke. Stroke patients are often unable to participate in decision making, highlighting the need for advance care planning (ACP) in poststroke care. We sought to better understand experiences and perceptions around stroke risk and ACP in our stroke clinic.
Methods and Results
Clinic patients completed the Planning After Stroke Survival survey assessing (1) advance directive (AD) documentation and ACP conversations, (2) factors associated with ADs and ACP, (3) perceptions of stroke risk, and (4) ACP needs. We used a physician survey and the electronic medical record to assess clinical and demographic information. We collected 219 surveys (78% response rate). Forty‐five percent reported having completed ADs, although the correlation between patient report and EMS documentation of ADs was low. Most patients (73%) had discussed ACP, and 58% desired additional conversation. Predictors of completing ADs included age (≥65 years; odds ratio, 4.8; 95% CI, 2.3–10.1), white race (odds ratio, 3.1; 95% CI, 1.2–7.8), milder poststroke disability (modified Rankin Scale score ≤1; odds ratio, 2.9; 95% CI, 1.3–6.4), having previously discussed ACP with a physician (odds ratio, 4.8; 95% CI, 2.0–11.7), and discussing risk of stroke recurrence (odds ratio, 2.2; 95% CI, 1.1–4.5).
Conclusions
Stroke survivors had low AD completion rates and desired more conversations about stroke risk and ACP. Completed ADs were inconsistently documented in the electronic medical record. These findings provide guidance to improve ACP in our stroke clinic and may provide a model for others interested in enhancing ACP and ultimately goal‐concordant care.
Keywords: advance care planning, advance directives, palliative care, stroke, stroke clinic
Subject Categories: Quality and Outcomes, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
Facing an elevated risk of recurrent stroke, future disability, and death, stroke survivors stand to benefit from advance care planning (ACP); however, this single‐center quality improvement project suggests that most stroke survivors do not have documented advance directives and desire additional information and discussion with their providers about ACP.
Factors associated with absence of ACP included younger age, nonwhite race, poor electronic medical record documentation, and increased stroke severity.
What Are the Clinical Implications?
This project suggests an important need for stroke‐specific ACP for survivors.
Areas for improvement could include (1) increasing the frequency and quality of conversations about recurrent stroke risk between patients and stroke providers; (2) increasing the frequency and accuracy of documentation of advance directives in the electronic medical record; and (3) improving access to ACP especially among the disabled and racial/ethnic minorities.
Recent advances in secondary stroke prevention and acute stroke treatment have moved stroke from the third‐ to the fifth‐leading cause of death in the United States.1 Even so, stroke survivors face high rates of subsequent poststroke illness and death, with 1‐ and 5‐year mortality estimates of 41% and 60%, respectively.2, 3 This excess mortality can be attributed in part to increased cardiovascular deaths, as well as increased rates of cancer, accidents, and suicides.4, 5 Nearly two thirds of stroke‐related deaths occur outside of the acute hospital setting.1 A high rate of stroke recurrence is another important contributor to poststroke death and disability: one quarter of the nearly 800 000 Americans with stroke each year are survivors of a previous stroke.6 History of transient ischemic attack reduces survival by 20% over 9 years, and even magnetic resonance imaging evidence of minor or subclinical stroke doubles the risk of overt stroke.7, 8 People who survive a stroke have a 2‐fold increased risk of developing dementia over those without stroke,9 and while 1 in 10 develops dementia after a first stroke, the incidence rises to 1 in 3 following a recurrent stroke.10
In addition to facing an elevated risk for future stroke and disability, many stroke survivors will be unable to communicate their values and treatment preferences in the acute setting. Stroke survivors are therefore a prime target for advance care planning (ACP), the process by which patients plan for, prepare, and communicate their personal values and goals for future medical care. ACP can give patients a voice when they cannot speak for themselves. The American Academy of Neurology recently identified discussions around patients’ goals of care and documentation of advance directives (ADs) as major quality improvement goals for patients hospitalized with neurologic illness.11 In addition, the American Heart Association/American Stroke Association 2014 scientific statement on palliative and end‐of‐life care in stroke makes explicit recommendations for patient‐ and family‐centered care and goals of care discussions.12
When there is a lack of ACP, the responsibility of acute medical decision making typically falls on families and physicians. The families of patients with severe acute stroke have reported significant distress with surrogate medical decision making resulting from a need to make decisions under time pressure and having to make decisions that may result in death.13 The literature suggests that ACP may alleviate the confusion, anxiety and conflict that families experience when making surrogate decisions.14, 15, 16 Patients who have completed ACP are more likely to receive care that is congruent with their wishes,17 and this may be especially important for patients with stroke and their families, who have reported a dearth of preparation and discussion around what a future with severe disability might look like compared with the possibility of death.18
Despite the importance of ACP for stroke survivors, the documentation of patient preferences, surrogate decision makers, and ADs in the medical record are still relatively uncommon, even among critically ill stroke patients with a high risk of death,19 and most of the ADs available are not applicable for severe acute stroke.20 Barriers to ACP in the weeks following an acute stroke include a perceived lack of urgency for such conversations, patients’ lack of awareness of the severity of their illness, and a reluctance to engage in difficult conversations among healthcare providers.21 Nor should ACP conversations be limited to the acute setting; patient preferences are known to change over time, and goals‐of‐care discussions should be periodically revisited to assess for changes.12
Therefore, the aim of this quality improvement project was to explore the prevalence, experiences, and influencing factors around goals‐of‐care and ACP conversations among the stroke survivors who presented to our tertiary stroke clinic. We believe that a clearer understanding of these elements is the first step in our efforts to increase rates of ACP discussions and documentation, and ultimately to improve patient‐centered care and goal‐concordant health outcomes.
Methods
Study Population
From March 28 to July 28, 2017, all patients seen by one of our stroke physicians in the University of Washington (UW) stroke clinic, including new patients, posthospital follow‐up, and established clinic patients, were offered the opportunity to complete the Planning After Stroke Survival survey as part of a quality improvement project to assess factors related to ACP and recurrent stroke risk. This clinic is part of the comprehensive stroke center at Harborview Medical Center, which provides acute inpatient and ambulatory stroke care to patients across the Pacific Northwest. Patients are referred to this clinic for hospital follow‐up after discharge from the UW and other hospitals and by outpatient providers. Because our study enrolled all comers to our stroke clinic, we included patients with all types of stroke, transient ischemic attacks, cervical artery dissections without stroke, and stroke mimics. Because it was a quality improvement project, it was determined to be exempt from review by the UW Institutional Review Board.
Data Collection
The Planning After Stroke Survival survey assessed the following patient characteristics: demographics including age, race, and current living situation; experiences related to ACP including completion of an AD; prior conversations about ADs with a physician or family member; and level of interest in further ACP conversations and preferences regarding surrogate medical decision making. Those without ADs were asked if they were unfamiliar or “contemplating ADs.”22 We assessed how often they worried about having another stroke and their perceived risk of death, disability, and stroke recurrence. Surveys were voluntary and anonymous, and paper forms were collected in the clinic by medical staff. If patients were unable to complete the survey themselves, family members present for the clinic visit were asked to complete the survey on the patient's behalf. Non–English speakers had the opportunity to complete the survey with the assistance of an interpreter service.
Harborview Medical Center stroke clinic physicians, including 4 attending physicians and 4 vascular neurology fellows, were asked to complete a physician survey corresponding to each completed patient survey. Physicians provided clinical information regarding the acute stroke presentation, the patient's condition at the time of the clinic visit including modified Rankin Scale, and other relevant clinical data. Physician responses were completed immediately after the clinical encounter and were not made available to patients.
Relevant clinical information was abstracted from patient medical charts, including admission National Institutes of Health Stroke Scale (NIHSS) score, medical comorbidities, and documentation of palliative care consultation during the initial hospitalization. Documentation of ADs in our electronic medical record (EMR) on admission and discharge were also recorded and compared with patient self‐report of AD status in the clinic.
Study data were collected and managed using the Research Electronic Data Capture tools hosted at UW. Research Electronic Data Capture is a secure, web‐based application designed to support data capture for research and quality improvement studies.23 The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical Analysis
Descriptive statistics including mean, SD, median, and interquartile range were calculated for baseline characteristics of participants. We chose to use patient report of having completed ADs as our primary outcome rather than ADs identified in the EMR for 2 reasons: (1) several studies have shown poor documentation of AD and ACP discussions in EMRs24, 25, 26, 27; and (2) this project was aimed at patient's experiences with ACP, and therefore their own account of having completed AD was deemed more relevant. Secondary outcomes included whether a patient had participated in ACP or discussed recurrent stroke risk with a physician or family member and how often they worried about stroke recurrence. We used chi‐squared and Fisher's exact tests to look for differences in the distribution of categorical patient characteristics and outcomes of interest. Student's t test was used to test differences for continuous variables (eg, age). We used multivariate logistic regression to estimate the odds ratios of patient characteristics (age, race, having discussed ADs or ACP, NIHSS on admission, current living situation, sex, or being seen as a new versus follow‐up clinic patient) that were associated with each outcome (AD status, having previously discussed ACP with a family member or a physician, attitude toward surrogate decision makers, frequency of worrying about stroke recurrence).
All data analysis was performed using STATA software.28
Results
During the survey period, 280 patients were seen in our stroke clinic. We collected 219 pairs of patient and physician surveys corresponding with a 78% response rate. Among returned patient surveys, response rates for individual questions ranged from 86% (n=188) to 100% (n=218). Most patients (91%) completed the surveys themselves; in the remaining 9%, a family member completed the survey on the patient's behalf.
Patient Characteristics
Outpatient follow‐up occurred at a median of 5 months after the stroke (interquartile range, 3–12 months). The mean age of patients was 60 years, and 46% of participants were women (Table 1). Patients tended to have mild strokes with a median NIHSS of 4 on initial presentation, and mild poststroke disability with a median modified Rankin Scale score of 1 (interquartile range, 0–2) at the time of the clinic visit. Over half of respondents (n=127; 63%) were new to the clinic, and all others were returning after a previous visit. One hundred twenty (58%) had been admitted to either of the 2 UW‐affiliated tertiary care hospitals for their initial stroke care; all others (n=99; 42%) were referred from other hospitals or outpatient providers. Medical records were abstracted for 206 patients (94%) but missing for 13 of the patients who were referred from outside facilities (Table 2).
Table 1.
Baseline Characteristics of Survey Respondents
| Variable | Patient Respondents, n (%) |
|---|---|
| Age, y (median, IQR), n=219 | 61 (50–70) |
| >65 y | 130 (59) |
| <65 y | 89 (41) |
| Female sex, n=219 | 101 (46) |
| Race, n=216 | |
| White | 162 (75) |
| Black | 23 (11) |
| Asian | 22 (10) |
| Other | 9 (4) |
| Current housing situation, n=217 | |
| Home alone | 43 (20) |
| Home with family members | 162 (75) |
| Skilled nursing facility | 10 (5) |
| Other | 2 (1) |
| Time since last stroke—months (IQR), n=208 | 5 (3–11) |
| New to clinic, n=208 | 127 (61) |
| Stroke type, n=212 | |
| Ischemic | 144 (68) |
| Intraparenchymal hemorrhage | 30 (14) |
| Nonstroke (TIA, dissection, etc) | 38 (18) |
| mRS, n=197 | |
| 0–1 | 128 (65) |
| 2 | 69 (35) |
| Previously discussed ACP with a physician, n=212 | |
| Yes | 155 (73) |
| No | 57 (27) |
| Discussed the risk of stroke recurrence with a physician, n=206 | |
| Yes | 84 (41) |
| No | 86 (42) |
| Don't know | 36 (18) |
ACP indicates advance care planning; IQR, interquartile range; mRS, modified Rankin Scale; TIA, transient ischemic attack.
Table 2.
Patient Characteristics From Abstraction of the Electronic Medical Record
| Variable | Patient Respondents, n (%) |
|---|---|
| Discharge destination after stroke hospitalization, n=189 | |
| Home | 112 (59) |
| Skilled nursing facility | 19 (10) |
| Inpatient rehabilitation | 53 (28) |
| Other | 5 (3) |
| NIHSS on admission, n=79 | |
| Median NIHSS (IQR) | 4 (1–7) |
| Received tPA or thrombectomy, n=219 | 22 (10) |
IQR indicates interquartile range; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
Most patients had suffered an ischemic stroke (n=145/213; 68%), followed by intraparenchymal hemorrhage in 30 (14%) and transient ischemic attack in 20 (9%). Thirteen patients (6%) had cerebrovascular disease without stroke, including cerebral venous sinus thrombosis (n=2), or cervical artery dissection (n=10; 5%). The remaining 5 patients had no clinically definite stroke and were diagnosed with other vascular disease (fibromuscular dysplasia and symptomatic subclavian artery stenosis; n=1 each) or were considered stroke mimics (seizure, magnetic resonance imaging–negative hemisensory complaints and transient global amnesia; n=1 each). The most common discharge destination was home (n=111/189; 59%) followed by inpatient rehab (n=53; 28%) and a skilled nursing facility (n=19; 10%). Thirty‐day readmission to a UW hospital after the initial stroke occurred in 15 patients (8%).
Completion of Advance Directives
Almost half of survey respondents (n=94/209; 45%) reported having completed AD (Table 3). An additional 20% of patients were not sure if they had completed ADs. Among the 73 (35%) who reported not having ADs, most were either unfamiliar with the concept or were “contemplating ADs.” There was no difference in patient report of having completed ADs when evaluated by NIHSS on admission, current living situation, sex, or being seen as a new versus follow‐up clinic patient. Independent predictors of reporting completed ADs included older age, white race, low level of poststroke disability based on a modified Rankin Scale score of 0 to 1 on follow‐up, having previously discussed ACP with a physician, and having discussed the risk of stroke recurrence with a physician (Figure).
Table 3.
Planning After Stroke Survival Survey Responses (n=219, Actual Response Rate to Individual Questions Varied From 94% to 97%)
| Variable | Patient Respondents, n (%) |
|---|---|
| Do you currently have an advance directive? n=209 | |
| Yes | 94 (45) |
| No | 73 (35) |
| Don't know | 42 (20) |
| If you do not have an advance directive, why not? n=73 | |
| No response | 17 (30) |
| I am not familiar with advance directives | 23 (41) |
| I choose not to complete an advance directive | 5 (9) |
| I plan to, but have not yet completed it | 17 (30) |
| Other | 11 (20) |
| Have you ever discussed with a doctor, in a face‐to‐face discussion, the kind of medical care you would want if you were too sick to speak for yourself? n=212 | |
| Yes | 155 (73) |
| Have you ever discussed with a family member or friend the kind of medical care you would want if you were too sick to speak for yourself? n=214 | |
| Yes | 171 (80) |
| Would you like to have a discussion of this type with your stroke doctor? n=211 | |
| Yes | 123 (58) |
| No | 53 (25) |
| I don't know | 35 (17) |
| Have you ever discussed with a doctor your risk of having another stroke? n=206 | |
| Yes | 84 (41) |
| No | 86 (42) |
| I don't know | 36 (18) |
| How often do you worry about having another stroke? n=207 | |
| Every day | 10 (5) |
| More than half of days | 3 (1) |
| Several days a week | 124 (60) |
| Not at all | 70 (34) |
Figure 1.
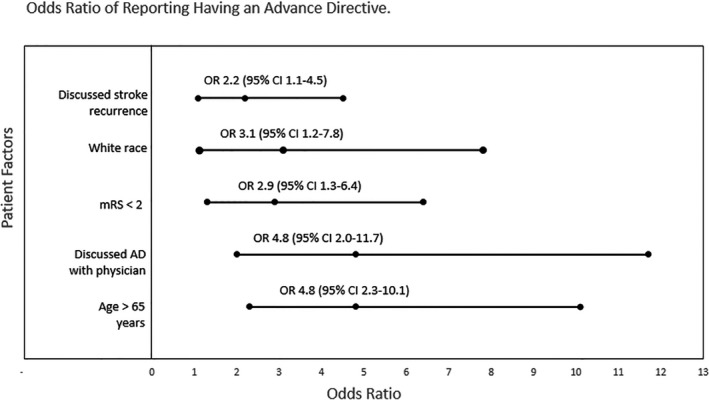
Forest plot showing odds ratios of patient characteristics independently associated with reporting having an advance directive at the time of clinic visit. ADs indicates advanced directives; mRS, modified Rankin Scale.
EMR documentation of AD at the time of clinic follow‐up was identified in 39% of all stroke clinic patients seen during the study period (n=288), but the EMR contained information inconsistent with many patients’ self‐reported status. Our EMR contained AD documentation for only 36 (46%) of the patients who reported having ADs. Of 72 patients who stated that they did not have ADs, 26 (36%) were found to have documented ADs in our EMR.
Patient Experience With ACP
Many patients (n=155; 73%) reported having previously discussed ACP with a physician, and over half (n=123; 58%) were interested in having additional ACP conversations with their stroke doctor. One quarter stated that they did not wish to discuss ACP with their stroke doctor, over half of whom (28/53; 53%) had already completed ADs.
Patients aged 65 years or older were more likely to have discussed ACP with a doctor compared with patients younger than 65 (83% versus 66%; P=0.006). We found no difference by race in having discussed ACP with a physician (73% in both whites and nonwhites; P=0.99). Nonwhites were significantly less likely, however, to have discussed ACP with their family members (66% versus 85%; P=0.003). Neither severity of NIHSS on admission nor modified Rankin Scale score at follow‐up were associated with having previously discussed ACP with a physician. Older patients were also no more likely to have discussed risk of stroke recurrence with a physician (40% of those 65 years or older versus 43% in those younger than 65; P=0.65).
Risk and Worry About Future Stroke, Death, or Disability
Two thirds of stroke survivors reported worrying several days a week or more about having another stroke. Patients who were seen for a follow‐up visit worried less about stroke recurrence compared with patients seen in the stroke clinic for the first time: almost half (49%) of follow‐up patients reported worrying “not at all” compared with 25% of new patients (P=0.001). Time from stroke (in months) was not predictive of the frequency of worrying. Older age was not associated with worry about stroke recurrence (7% in patients 65 years or older versus 5% in those younger than 65; P=0.45).
Medical Decision Making
Among patients who completed the survey on their own, most would accept medical decisions to be made either by their physician (146/184; 79%) and/or a family member (166/186; 89%) if they were not able to participate in acute decision making themselves. Only 25 (14%) did not wish their doctor to be involved in medical decision making, and 11 (6%) did not wish their family to be involved. There was no statistical difference in these factors when the analysis was based on race, age, or time since last stroke. Those who did not want a family member to make medical decisions on their behalf were no more likely to have completed ADs (odds ratio, 1.17; 95% CI, 0.4–3.8; P=0.8).
Discussion
Stroke survivors face a heightened risk for recurrent stroke, disability, and death and therefore stand to benefit from both ACP and having documented ADs. In this cross‐sectional observation of stroke survivors presenting to a tertiary care stroke clinic, three quarters had discussed ACP with a physician and/or a member of their family, but most expressed a desire for additional ACP discussion with their physician. Predictors of having AD in our clinic, including older age and being white, were consistent with prior studies. Documentation in our EMR did not correlate with patients’ self‐reported AD status, lacking electronic documentation of many patients’ completed ADs. This quality improvement project identified opportunities to better document ADs in the EMR, reduce the age and race gap for completing ADs, and improve our conversations about ACP with our stroke survivors.
Next steps include:
Developing a standardized method to screen our stroke clinic patients for current AD status and properly documenting this in the EMR. This can be routinely performed by medical assistants along with medication reconciliation.
Developing and evaluating a tool to help clinicians educate stroke survivors about the importance of ACP and inviting them to have this conversation. This may include clinician education around sensitive discussions of individualized risk for stroke recurrence and other long‐term effects as well as exploring patient values around future dementia and disability.
Our study relied on stroke neurologists; however, a collaborative approach to ACP involving primary care providers, geriatricians, social workers, nurses, and palliative care physicians would provide a more comprehensive approach.
Finally, the results of all ACP discussion and documentation should be readily available in the EMR to both inpatient and outpatient care providers.
Efforts to integrate ACP into the EMR in a standardized fashion have already begun at some institutions, and will likely play a significant role in the care of high‐risk patient populations such as ours.29, 30, 31 Electronic tools may be useful to educate or remind patients about ACP, as well as to facilitate documentation. The lack of correlation between patients’ self‐reported AD status and that documented in our outpatient EMR in our study is consistent with previous studies suggesting that documentation in the EMR, when it does exist, is often out of date, inaccurate, or inaccessible.25, 26, 27 This poor documentation is worrisome, as the EMR is a primary source of information for providers in the acute setting if patients cannot participate in decision making and family members are not immediately available. The problem is compounded by the fact that many institutions use distinct EMR systems for inpatient and outpatient care, and documentation may not be readily shared between the 2 systems.
This quality improvement study has several limitations. First, our study relied on patient self‐report, which is inherently susceptible to recall bias. Patients may have experienced confusion with the terms advance directive and advance care planning as used in the survey. Prior studies have reported low familiarity with these terms in the general public.32 However, the surveys were completed in the clinic, and medical assistants as well as physicians were available to provide clarification. Second, there was limited racial diversity among respondents, three quarters of whom identified as white. This proportion is consistent with the composition of the local population within the catchment area of the medical centers and may not be a reflection of disparate healthcare access. Third, most respondents had mild strokes and mild poststroke deficits. Only 10 respondents required care at a skilled nursing facility at the time of follow‐up. Therefore, we did not study the population with the most severe strokes, a group that may disproportionately lack access to stroke clinic follow‐up care and may have the greatest need for AD conversations while simultaneously being less likely to have completed ADs. This represents a problem that needs to be addressed, possibly through in‐home or nursing home visits or by telemedicine.
We expect that many patients will have discussed poststroke ACP with a primary care physician or other provider before their stroke clinic visit. This is especially likely given the 5 months’ median interval between initial hospitalization and follow‐up in our clinic. We did not assess the frequency of ACP discussions with a primary care or other healthcare provider before stroke clinic follow‐up. As a national quality measure, all patients should have received stroke education, including education around stroke risk, during their initial hospitalization. However, stroke patients typically present at their worst, and in‐hospital goals‐of‐care conversations are often held with surrogates, making it likely that patients would not recall previous conversations.33 We take these factors to reinforce the importance of repeating ACP discussions on a recurring basis, including in the clinic setting.20 It is worth noting that there are now Current Procedural Terminology billing codes available for prolonged service (eg, 99354) or for ACP (eg, 99497 or 99498), which allow for physicians to be remunerated for time spent discussing these issues with their patients.
In conclusion, stroke survivors in our outpatient stroke clinic have a high burden of unmet ACP needs. Best practices on how to optimally address the gap between the conversations patients want to have about ACP and the actual conversations occurring in the stroke clinic have not yet been elucidated. Furthermore, to be of use to clinicians in emergency settings, the results of these discussions must be readily available in the EMR. Research is needed to identify the most effective ways in which stroke physicians can address the risk and anxiety that stroke survivors face in a sensitive and timely manner and to show that such efforts will relieve anxiety, enhance goal‐concordant care, and improve quality of life for stroke survivors and their families.
Sources of Funding
Funding was generously provided by a research grant from the University of Washington Housestaff Association.
Disclosures
Dr Creutzfeldt receives support from the NIH (K23NS099421). The remaining authors have no disclosures to report.
Acknowledgments
Special thanks are due to the clinic staff, especially Tracy Ezell, Dakota Johnson, Crystal Kelly, Monica Ancheta, Suzanne Olson, and Aaron Guzik, who generously assisted in administering and collecting surveys in the clinic. We also thank the stroke clinic physicians who participated in this quality improvement study by completing assessments of patients’ stroke type and stroke risk. Additional thanks go to Dr Hillary Lum, Adreanne Brungardt, and Dr J. Randall Curtis for reviewing the manuscript and providing valuable feedback.
(J Am Heart Assoc. 2019;8:e011317 DOI: 10.1161/JAHA.118.011317.)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Richey M, Rodriquez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Munter P; American Heart Association Statistics Committee and Stroke Statistics Subcomittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bronnum‐Hansen H, Davidsen M, Thorvaldsen P. Long‐term survival and causes of death after stroke. Stroke. 2001;32:2131–2136. [DOI] [PubMed] [Google Scholar]
- 3. Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief. 2016;267:1–8. [PubMed] [Google Scholar]
- 4. Burns JD, Rabinstein AA, Roger VL, Stead LG, Christianson TJ, Killian JM, Brown RD Jr. Incidence and predictors of myocardial infarction after transient ischemic attack: a population‐based study. Stroke. 2011;42:935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–646. [DOI] [PubMed] [Google Scholar]
- 6. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HG, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriquez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 7. Gattellari M, Goumas C, Garden F, Worthington JM. Relative survival after transient ischaemic attack: results from the Program of Research Informing Stroke Management (PRISM) study. Stroke. 2012;43:79–85. [DOI] [PubMed] [Google Scholar]
- 8. Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, Kamel H. Silent brain infarction and risk of future stroke: a systematic review and meta‐analysis. Stroke. 2016;47:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. [DOI] [PubMed] [Google Scholar]
- 10. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre‐stroke and post‐stroke dementia: a systematic review and meta‐analysis. Lancet Neurol. 2009;8:1006–1018. [DOI] [PubMed] [Google Scholar]
- 11. Josephson SA, Ferro J, Cohen A, Webb A, Lee E, Vespa PM. Quality improvement in neurology: inpatient and emergency care quality measure set: executive summary. Neurology. 2017;89:730–735. [DOI] [PubMed] [Google Scholar]
- 12. Holloway RG, Arnold RM, Creutzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, Rabinstein AA, Saposnik G, Sheth KN, Zahuranec DB, Zipfel GJ, Zorowitz RD; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Clinical Cardiology . Palliative and end‐of‐life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1887–1916. [DOI] [PubMed] [Google Scholar]
- 13. de Boer ME, Depla M, Wojtkowiak J, Visser MC, Widdershoven GA, Francke AL, Hertogh CM. Life‐and‐death decision‐making in the acute phase after a severe stroke: interviews with relatives. Palliat Med. 2015;29:451–457. [DOI] [PubMed] [Google Scholar]
- 14. Sudore RL, Lum HD, You JJ, Hanson LC, Meier DE, Pantilat SZ, Matlock DD, Rietjens JAC, Korfage IJ, Ritchie CS, Kitner JS, Teno JM, Thomas J, McMahan RD, Heyland DK. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53:821–832.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dy S. Ensuring documentation of patients’ preferences for life‐sustaining treatment: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. (Evidence Reports/Technology Assessments, No. 211. Chapter 30. Available at: http://www.ncbi.nlm.nih.gov/books/NBK133401/. Accessed December 15, 2017. [Google Scholar]
- 17. Brinkman‐Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end‐of‐life care: a systematic review. Palliat Med. 2014;28:1000–1025. [DOI] [PubMed] [Google Scholar]
- 18. Kendall M, Cowey E, Mead G, Barber M, McAlpine C, Stott DJ, Boyd K, Murray SA. Outcomes, experiences and palliative care in major stroke: a multicentre, mixed‐method, longitudinal study. CMAJ. 2018;190:E238–E246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson MT, Vickrey BG, Holloway RG, Chong K, Williams LS, Brook RH, Leng M, Parikh P, Zingmond DS. The lack of documentation of preferences in a cohort of adults who died after ischemic stroke. Neurology. 2016;86:2056–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alonso A, Dorr D, Szabo K. Critical appraisal of advance directives given by patients with fatal acute stroke: an observational cohort study. BMC Med Ethics. 2017;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green T, Gandhi S, Kleissen T, Simon J, Raffin‐Bouchal S, Ryckborst K. Advance care planning in stroke: influence of time on engagement in the process. Patient Prefer Adherence. 2014;8:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fried TR, Redding CA, Robbins ML, Paiva A, O'Leary JR, Iannone L. Stages of change for the component behaviors of advance care planning. J Am Geriatr Soc. 2010;58:2329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curtis JR, Sathitratanacheewin S, Starks H, Lee RY, Kross EK, Downey L, Sibley J, Lober W, Loggers ET, Fausto JA, Lindvall C, Engelberg RA. Using electronic health records for quality measurement and accountability in care of the seriously ill: opportunities and challenges. J Palliat Med. 2018;21:S52–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lakin JR, Isaacs E, Sullivan E, Harris HA, McMahan RD, Sudore RL. Emergency physicians’ experience with advance care planning documentation in the electronic medical record: useful, needed, and elusive. J Palliat Med. 2016;19:632–638. [DOI] [PubMed] [Google Scholar]
- 26. Platts‐Mills TF, Richmond NL, LeFebvre EM, Mangipudi SA, Hollowell AG, Travers D, Biese K, Hanson LC, Volandes AE. Availability of advance care planning documentation for older emergency department patients: a cross‐sectional study. J Palliat Med. 2017;20:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grudzen CR, Buonocore P, Steinberg J, Ortiz JM, Richardson LD. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647–651. [DOI] [PubMed] [Google Scholar]
- 28. StataCorp . Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 29. Green MJ, Levi BH. The era of “e”: the use of new technologies in advance care planning. Nurs Outlook. 2012;60:376–383.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durbin CR, Fish AF, Bachman JA, Smith KV. Systematic review of educational interventions for improving advance directive completion. J Nurs Scholarsh. 2010;42:234–241. [DOI] [PubMed] [Google Scholar]
- 31. Bose‐Brill S, Kretovics M, Ballenger T, Modan G, Lai A, Belanger L, Koesters S, Pressler‐Vydra T, Holloman C, Wills C. Testing of a tethered personal health record framework for early end‐of‐life discussions. Am J Manag Care. 2016;22:e258–e263. [PubMed] [Google Scholar]
- 32. Foundation CH . Final Chapter: Californians’ Attitudes and Experiences With Death and Dying. Oakland, CA: California HealthCare Foundation; 2012. Available at: www.chcf.org. Accessed December 10, 2017. [Google Scholar]
- 33. Kessels RP. Patients’ memory for medical information. J R Soc Med. 2003;96:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]


