Abstract
Background
Outcomes for pediatric cardiac surgery are commonly reported from international databases compiled from voluntary data submissions. Surgical outcomes for all children in a country or region are less commonly reported. We aimed to describe the bi‐national population‐based outcome for children undergoing cardiac surgery in Australia and New Zealand and determine whether the Risk Adjustment for Congenital Heart Surgery (RACHS) classification could be used to create a model that accurately predicts in‐hospital mortality in this population.
Methods and Results
The study was conducted in all children's hospitals performing cardiac surgery in Australia and New Zealand between January 2007 and December 2015. The performance of the original RACHS‐1 model was assessed and compared with an alternative RACHS‐ANZ (Australia and New Zealand) model, developed balancing discrimination with parsimonious variable selection. A total of 14 324 hospital admissions were analyzed. The overall hospital mortality was 2.3%, ranging from 0.5% for RACHS category 1 procedures, to 17.0% for RACHS category 5 or 6 procedures. The original RACHS‐1 model was poorly calibrated with death overpredicted (1161 deaths predicted, 289 deaths observed). The RACHS‐ANZ model had better performance in this population with excellent discrimination (Az‐ROC of 0.830) and acceptable Hosmer and Lemeshow goodness‐of‐fit (P=0.216).
Conclusions
The original RACHS‐1 model overpredicts mortality in children undergoing heart surgery in the current era. The RACHS‐ANZ model requires only 3 risk variables in addition to the RACHS procedure category, can be applied to a wider range of patients than RACHS‐1, and is suitable to use to monitor regional pediatric cardiac surgery outcomes.
Keywords: cardiac surgery, congenital heart disease, outcome and process assessment, pediatric, risk model
Subject Categories: Pediatrics, Risk Factors, Quality and Outcomes, Cardiovascular Surgery
Clinical Perspective
What Is New?
This population‐based study of a centralized system of providing pediatric cardiac services describes an overall hospital mortality for pediatric cardiac surgery of 2.3%.
The modified Risk Adjustment for Congenital Heart Surgery ‐ Australia and New Zealand (RACHS‐ANZ) prediction model reported in the study is calibrated to the standard of care in Australia and New Zealand in the current era.
Performance assessment of the model demonstrated excellent discrimination (Az‐ROC of 0.830) and acceptable goodness‐of‐fit.
What Are the Clinical Implications?
The infrastructure for surveillance of pediatric intensive care outcomes in Australia and New Zealand is well established.
This study shows that simply by adding surgical procedure codes to the data collected, the existing infrastructure developed for regional monitoring of intensive care outcomes could also provide a system for regional monitoring of pediatric cardiac surgery outcomes.
Introduction
Congenital heart disease is the most frequently occurring congenital anomaly at birth and a leading cause of infant death from birth defects.1 Many children with congenital heart disease require surgery, with some children needing multiple procedures during childhood. In order to monitor outcomes of hospitals performing pediatric cardiac surgery, methods of adjusting for procedural and patient risk are required. Three methods of scoring procedural complexity have been developed. The Aristotle Basic Complexity score2 and the Risk Adjustment for Congenital Heart Surgery (RACHS‐1) score3 were developed based on expert opinion while the Society of Thoracic Surgeons–European Association for Cardiothoracic Surgery (STS‐EACTS) Congenital Heart Surgery Mortality Score was developed more recently from empirical data contained in 2 large international databases.4 Procedure complexity categories, in combination with patient‐level risk factors, have been used to develop outcome prediction models for congenital heart surgery.3, 4
The Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database and the European Association for Cardiothoracic Surgery (EACTS) Congenital Heart Surgery Database enable participating cardiac surgical centers to benchmark risk‐adjusted outcomes with international standards. Notwithstanding the importance of international comparisons, there are additional benefits from regional comparison of health outcomes, for example in our context, comparing outcomes across Australia and New Zealand (ANZ). Regional analyses allow outcomes to be compared in settings where health systems and healthcare resources are similar, and where the social determinants of health in the population are also similar. Individual pediatric cardiac services in ANZ benchmark institutional outcomes using international databases, however, the infrastructure for regional comparisons does not currently exist.
Methods for benchmarking pediatric intensive care, such as the Paediatric Index of Mortality,5 estimate mortality risk at the time of admission to intensive care; however, to benchmark outcomes of surgical services where surgery, preoperative assessment, and postoperative care all potentially contribute to the outcome, it is important to use only preoperative risk factors and to assess outcome either at hospital discharge or a defined period such as 30 or 90 days after cardiac surgery.
In this study, we used the existing infrastructure of the ANZPIC Registry (Australian and New Zealand Paediatric Intensive Care Registry) to investigate the feasibility of developing a method for regional surveillance of pediatric cardiac surgery outcome. Because the registry receives patient records from all institutions performing cardiac surgery in children in ANZ, the study also provided a unique opportunity to describe population‐based surgical outcomes. For surveillance of hospital performance, it is preferable to maximize the inclusion of cases managed by cardiac surgery programs in children's hospitals. The aim of the study was to develop and validate a modified and contemporary version of the RACHS model where surgery for acquired heart disease in children and congenital heart disease in adults could be included in addition to surgery for congenital heart disease in children.
Methods
The Stata code developed for the analysis may be obtained by contacting the first author at BrentM@adhb.govt.nz. Release of the data to researchers will be subject to the governance and confidentiality requirements of ANZICS CORE, as defined by the ANZICS CORE Data Access and Publication Policy. The policy and information request form are available at https://www.anzics.com.au/information-requests/.
Data Source
The ANZPIC Registry comprises records of all patients admitted to pediatric intensive care units (PICUs) in ANZ. Data were collected between January 1, 2007 and December 31, 2015. All 8 PICUs providing pre‐ and postoperative care to pediatric cardiac surgical patients in ANZ over this time period contributed data. Two neonatal intensive care units (NICUs) that routinely care for selected postoperative cardiac patients also contributed data between 2007 and 2010 inclusive.
Data Validation
The data validation processes of the registry have been described previously.6 An additional audit of cardiac surgical cases was performed in 2010. For each center, 5% of cases were randomly selected for audit with the random selection stratified by risk of death. A cardiologist from each site, blinded to the initial coding, reviewed the medical record and assigned a RACHS procedure category. The other variables in the RACHS‐1 model were also recoded by independent data collectors. Survival to hospital discharge was confirmed with the medical records department of each hospital and hospitals were asked to check whether there were additional intraoperative deaths of patients not admitted to PICU and therefore not registered.
Case Selection
Cardiac surgery was defined as surgery on the heart or intrathoracic great vessels. Children receiving extracorporeal life support were included if they underwent cardiac surgery during the hospital admission, while children receiving extracorporeal life support for indications other than support of cardiac surgery were not included. The initial data set contained all ICU admissions in which the patient had undergone a cardiac surgical procedure immediately before or during the stay in PICU or NICU. In the case of multiple PICU admissions during a single hospital admission, the information recorded for the admission during which the patient underwent the highest RACHS procedure category was used for analysis. Hospital admissions during which no cardiac surgical procedure had an applicable RACHS code were then excluded. As NICUs do not contribute to the ANZPIC Registry, staff from the 2 NICUs collected the information required for the study separately. The RACHS‐1 model was not validated in patients with acquired heart disease or in patients over 18 years of age. These patients were excluded from recalibration of the RACHS‐1 model and initial model development. Data from these excluded groups were subsequently reintroduced to each model under investigation and the effect of inclusion on discrimination and fit was assessed.
Model Development and Validation
Initially, the original RACHS‐1 model was applied to the entire data set to assess calibration and fit. The standardized mortality ratio was calculated by dividing the number of deaths recorded by the number of deaths predicted when applying the original RACHS‐1 model.
To build an alternative model, 33 patient variables were tested in combination with the RACHS procedure category in 93 logistic regression models using hospital mortality as the dependent variable. Continuous variables such as age, weight, and weight‐for‐age were added to a generalized additive model containing the RACHS procedure category and the plot of the smooth visually examined to determine whether transformation or division into categorical variables appeared appropriate. The risk variables can be found in Table S1 and logistic regression models in Table S2.
The fit of all models was compared using the Hosmer and Lemeshow goodness‐of‐fit (HL‐GOF). A χ2 test P>0.1 for the HL‐GOF test was used to indicate acceptable fit. Discrimination of the models was compared using the area under the receiver operator characteristic plot (Az‐ROC).7
For each model tested, the data were randomly divided into building and validation data sets in a 2:1 ratio. Out‐of‐sample performance was tested using 200 such random splits of the data. Fit statistics for each split were then computed, and the mean of the 200 HL‐GOF statistics and Az‐ROCs was recorded.
A final model was selected after excluding models with a mean HL‐GOF χ2 test P≤0.1 and viewing the Az‐ROC in descending order. The RACHS‐ANZ model was selected by the authors, based on a compromise between model discrimination, ease and reproducibility of data collection, and parsimonious variable selection. The final coefficients for the selected model were then recalculated, including all data from the combined development and validation samples.8
Logistic regression and goodness‐of‐fit analyses were performed using Stata 13.1 for Windows. Generalized additive models and smoothed plots were generated using R 3.1.2. Two authors (B.M., A.S.) had full access to all the data in the study and take responsibility for its integrity and the data analysis. The human research ethics committee of the Children's Health Queensland, Brisbane, endorses the use of ANZPIC Registry data for research purposes. Consent was not required.
Results
Details of the cases included and excluded are shown in Figure 1. A total of 14 324 hospital admissions involving cardiac surgery were available for analysis. The overall mortality rate was 2.3%, varying between 0.5% in patients undergoing a RACHS category 1 procedure and 17.0% in patients undergoing a category 5 or 6 procedure (Table 1). A total of 539 patients could not be assigned a RACHS procedure code (Table 2). Patients undergoing procedures for acquired heart disease and patients aged >18 years constituted 4.7% and 0.7% of cases, respectively. The organization of surgical services for adults with congenital heart disease varies across Australian states and New Zealand. In this study, 99 of the 107 patients aged over 18 years who received surgery in a children's hospital were managed in Victoria or New Zealand. Between 2007 and 2010, 153 cases, representing 2.4% of cases during this period, received postoperative care in a NICU. Collection of data in the 2 NICUs was not continued after 2010 because of the small number of cases per annum and because the overall project aim was to develop a benchmarking system based on data that are routinely available via the ANZPIC Registry. Mortality rates associated with selected risk factors are shown in Table 3.
Figure 1.
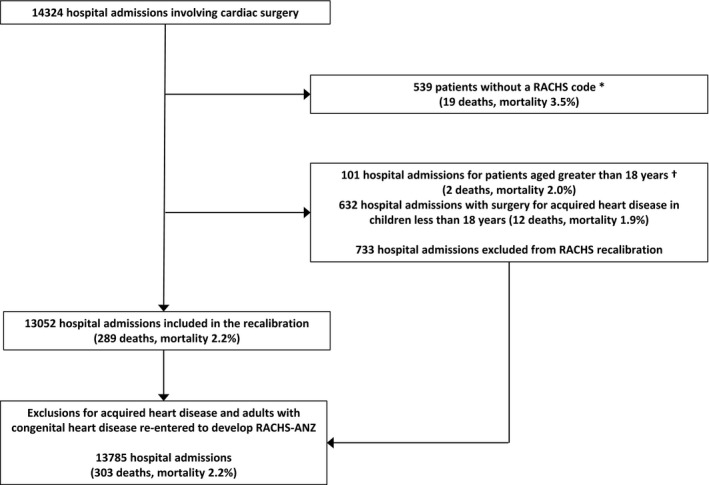
Flow diagram for cases analyzed over 9 years. *Includes 6 patients aged over 18 years and 36 patients receiving surgery for acquired heart disease; †Includes 7 patients aged over 18 years receiving surgery for acquired heart disease. RACHS‐ANZ indicates Risk Adjustment for Congenital Heart Surgery Score, Australia and New Zealand.
Table 1.
RACHS‐1 Risk Categories in All Patients and in Subgroups of Patients Aged >18 Years and Patients Who Received Surgery for Acquired Heart Disease
| RACHS‐1 Risk Category | All Cases | Surgery for Acquired Heart Disease | Age >18 Years | |||
|---|---|---|---|---|---|---|
| No. of Cases | No. of Deaths (%) | No. of Cases | No. of Deaths (%) | No. of Cases | No. of Deaths (%) | |
| 1 | 1758 | 8 (0.5) | 8 | 1 (12.5) | 13 | 0 |
| 2 | 5033 | 38 (0.8) | 64 | 1 (1.6) | 30 | 0 |
| 3 | 5207 | 104 (2.0) | 537 | 7 (1. 3) | 55 | 1 (1.8) |
| 4 | 1388 | 85 (6.1) | 28 | 3 (10.7) | 3 | 1 (33) |
| 5 | 23 | 6 (26.1) | 0 | … | 0 | … |
| 6 | 376 | 62 (16.5) | 2 | 0 | 0 | … |
| Total | 13 785 | 303 (2.2) | 639 | 12 (1.9) | 101 | 2 (2.0) |
RACHS indicates Risk Adjustment for Congenital Heart Surgery Score.
Table 2.
Cardiac Surgery Procedures in 539 Hospital Admissions Where a RACHS Procedure Code Could Not Be Assigned
| Procedure | Cases | Deaths |
|---|---|---|
| Pacemaker insertion or replacement | 191 | 2 |
| Heart transplant | 52 | 1 |
| PDA surgery ≤30 d | 43 | 1 |
| Pulmonary venous stenosis repair | 7 | 1 |
| Fontan conversion | 2 | 0 |
| Cardiac surgery other | 244 | 14 |
| Total | 539 | 19 |
PDA indicates patent ductus arteriosus.
Table 3.
Risk Factors for Mortality
| No. of Casesa | No. of Deathsb (%) | |
|---|---|---|
| RACHS procedure category 1 | 1758 | 8 (0.5) |
| RACHS procedure category 2 | 5033 | 38 (0.8) |
| RACHS procedure category 3 | 5207 | 104 (2.0) |
| RACHS procedure category 4 | 1388 | 85 (6.1) |
| RACHS procedure category 5 | 23 | 6 (26.1) |
| RACHS procedure category 6 | 376 | 62 (16.5) |
| Combination procedure | 3681 | 118 (3.2) |
| Major noncardiac structural anomaly | 939 | 45 (4.8) |
| ICU before surgery | 1742 | 142 (8.2) |
| Age ≤30 d | 2726 | 184 (6.8) |
| Age 31 d–1 y | 5025 | 80 (1.6) |
| Weight ≤2.5 kg | 383 | 46 (12.0) |
ICU indicates intensive care unit; RACHS, Risk Adjustment for Congenital Heart Surgery Score.
Inclusive of acquired heart disease and those aged over 18 years.
Deaths during the same hospital admission the procedure occurred.
Independent audit of 141 cases demonstrated agreement in RACHS category in 126 cases (89%), a higher code allocated by the auditor in 7 cases, and a lower category in 8 cases. The levels of agreement for prematurity, noncardiac structural anomaly, and combination procedure at the same operation were 97%, 91%, and 72%, respectively.
When applying the published RACHS‐1 model and original coefficients3 to the subset of patients for whom the original RACHS‐1 model was validated, 1161 deaths were predicted while 289 deaths were observed, giving a standardized mortality ratio of 0.25 (95% CI 0.20–0.30) and a HL‐GOF P<0.05. The within‐sample Az‐ROC was 0.815. Without altering the variables, the RACHS‐1 model was recalibrated and tested on development and validation data sets, respectively. For the recalibrated model, the mean P value for the out‐of‐sample HL‐GOF test was 0.250 and the mean Az‐ROC was 0.814.
Model Selection
Because of the paucity of cases in RACHS category 5, RACHS categories 5 and 6 were grouped together as a single category for all models tested. Various age and weight categories were tested. The use of transformed variables such as “age in days <2 years” and “weight <4.0 kg” in many of the models was because of analysis of the smoothed generalized additive model plots (Figure 2).
Figure 2.
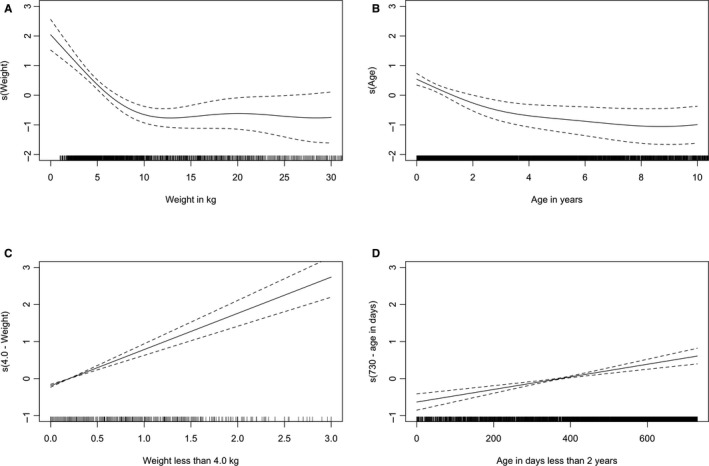
Four continuous variables plotted as smooths added to generalized additive models containing RACHS2‐4, RACHS5 or 6, and ICU before surgery. Dashed lines represent 2 standard errors above and below the estimate of the smooth. A, Weight in kg. B, Age in years. C, Weight transformed as weight <4.0 kg. D, Age transformed as days <2 years. ICU indicates intensive care unit; RACHS, Risk Adjustment for Congenital Heart Surgery Score.
When examining the RACHS categories without additional risk factors, the Az‐ROC was 0.765 for the group excluding patients aged over 18 years and those with acquired heart disease, and the mean HL‐GOF P value was 0.166. There was little difference in out‐of‐sample performance among the top‐performing models. The highest out‐of‐sample Az‐ROC when including all patients was 0.835, and when including only those patients for whom RACHS‐1 was originally validated was 0.834. The chosen RACHS‐ANZ model had an out‐of‐sample ROC of 0.830 (H‐L GOF P=0.216) for the entire data set and 0.830 (H‐L GOF P=0.226) for those in whom RACHS‐1 was applicable. The final RACHS‐ANZ model had the fewest variables of the top‐performing models (3 variables in addition to the RACHS category) and avoided use of the “combination procedure” and “major noncardiac structural anomaly” variables described in the RACHS‐1 model. Table 4 shows the results of logistic regression for the original model and for the final RACHS‐ANZ model. “Days <2 years of age” refers to days of age at admission to PICU, with no correction for gestation, with anyone 730 days old or older on admission to PICU being coded as zero. “Weight <4.0 kg” refers to the patient weight on admission to PICU (or in the case of immediate postoperative admissions, the presurgery weight), with anyone weighing 4.0 kg or more being coded as zero. “ICU before surgery” was coded as 1 (yes) if the patient was managed in ICU immediately before surgery or 0 (no) if the patient was admitted to ICU for recovery following surgery. An example of using the model to calculate the risk of death is provided in Appendix S2.
Table 4.
Recalibrated RACHS‐1 and RACHS‐ANZ Models Applied to the Population Used for the Original Version of RACHS‐1, and the Final RACHS‐ANZ Model Applied to the Population Inclusive of Children With Acquired Heart Disease and Adults With Congenital Heart Disease
| Recalibrated RACHS‐1a | RACHS‐ANZ—Limiteda | RACHS‐ANZ—Final Model | ||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | Coefficient (95% CI) | |
| RACHS 1 (reference) | 1.0 | 1.0 | 1.0 | ··· |
| RACHS 2 | 1.20 (0.53–2.74) | 1.38 (0.61–3.14) | 1.23 (0.57–2.67) | 0.2081 (−0.5666 to 0.9828) |
| RACHS 3 | 2.88 (1.31–6.34) | 3.09 (1.41–6.76) | 2.74 (1.32–5.69) | 1.0071 (0.2747–1.7395) |
| RACHS 4 | 5.51 (2.42–12.54) | 5.70 (2.54–12.76) | 5.09 (2.39–10.87) | 1.6280 (0.8704–2.3856) |
| RACHS 5 | 22.25 (6.41–77.16) | |||
| RACHS 6 | 16.14 (6.94–37.55) | |||
| RACHS 5 or 6 | 13.18 (5.76–30.20) | 11.23 (5.14–24.55) | 2.4189 (1.6371–3.2008) | |
| Neonate | 4.32 (2.84–6.56) | |||
| Infant | 2.07 (1.35–3.18) | |||
| Premature | 2.37 (1.72–3.28) | |||
| Combination procedure | 1.39 (1.08–1.80) | |||
| Major noncardiac structural anomaly | 2.46 (1.73–3.48) | |||
| ICU before surgeryb | 2.21 (1.68–2.89) | 2.29 (1.76–2.99) | 0.8294 (0.5648–1.0940) | |
| Weight <4.0 kg (per kg)c | 2.35 (1.89–2.92) | 2.34 (1.89–2.89) | 0.8482 (0.6352–1.0612) | |
| Days <2 y (per d)d | 1.0008 (1.0001–1.0014) | 1.0008 (0.0002–1.0014) | 0.000780 (0.000160–0.001399) | |
| Constant | −5.7416 (−6.4577 to −5.0256) | |||
ANZ indicates Australia and New Zealand; ICU, intensive care unit; OR, odds ratio; RACHS, Risk Adjustment for Congenital Heart Surgery Score.
Limited to those aged 18 years or less without acquired heart disease.
ICU before surgery: code as 1 if the patient was managed in ICU immediately before surgery or 0 if the patient was admitted to ICU for recovery following surgery.
Weight <4.0 kg=4.0—weight in kg. Code as zero if weight ≥4.0 kg.
Days <2 years=730—age in days. Code as zero if age ≥730 days.
Discussion
The data set used in this study is unique in that it represents all pediatric cardiac surgery and some selected representation of adult congenital cardiac surgery undertaken in the population of 2 countries during a 9‐year period. The centralization of cardiac surgery services in ANZ results in pediatric cardiac surgery being undertaken at 1 institution in New Zealand, and in 4 Australian states. In New South Wales, surgery is undertaken in 2 hospitals that are now part of the same governance system (The Sydney Children's Hospitals Network). In Queensland there is a single pediatric cardiac service; however, the service was relocated to a different hospital twice during the study period. Pediatric cardiac surgery is not performed in 4 Australian states and territories. With all pediatric cases included, the study overcomes potential selection bias inherent in reports from voluntary registries or databases: when data submission is voluntary, the case‐mix and outcomes of contributing institutions may differ from those of institutions that do not contribute.
If the original RACHS‐1 model is applied to the data set, the standardized mortality ratio is 0.25 (0.20–0.30). That is, 75% of the children predicted to die in this study population, using standards developed in 1996 from 32 institutions in the United States, survived. Although RACHS‐1 has been validated in a number of settings, most reports do not contain model coefficients to allow comparison of risk‐adjusted outcomes for the entire surgical population; however, mortality rates within each RACHS‐1 category have improved considerably since 1996.9, 10, 11, 12 The exact explanation for this is unknown, although the tendency for mortality prediction models to increasingly overpredict death over time has been noted previously.12, 13, 14, 15, 16, 17 It is possible that incremental gain has been achieved through advances in surgery, anesthesia, perfusion, and intensive care via the use of newer equipment, medications, and techniques. Both the recalibrated RACHS‐1 model and the RACHS‐ANZ model set a higher standard for benchmarking of cardiac surgical mortality.
During development of the modified prediction model, we examined alternative variable selection for patient specific factors, but we did not alter the system of categorizing procedures. The RACHS‐ANZ model includes age as a continuous predictor up to 2 years of age, rather than the age categories from the original RACHS‐1 model.3 Of note, the age used in this analysis differs from RACHS‐1 and STS‐EACTS because it uses the age at admission to PICU, rather than age at operation, which was not available in the ANZPIC data set. It is likely that neonates with congenital heart disease that became clinically unstable during the first weeks of life were admitted to PICU for stabilization, although operative repair or palliation may not have occurred until the child was in a different RACHS‐1 or STS‐EACTS age category. Similarly the RACHS‐ANZ model includes weight as a continuous predictor up to 4.0 kg rather than the category used in the harmonized RACHS‐1 method (500–2500 g).18 With age and weight defined as continuous variables, rather than by categories, the effect of performing surgery near a defined cut point is reduced. Our analysis suggests that the risk of surgery in very young and low weight children reduces gradually over the first 2 years and as weight increases to 4 kg, rather than by stepwise reductions at specific ages or weights.
The RACHS‐1 model includes the variable “combination procedure” defined as “2 or more distinct cardiac surgical procedures performed simultaneously.” The final model does not include “combination procedure,” because of the poor level of agreement between the coding of the data collectors and independent auditors. There was also variation between hospitals in the coding of the variable “major noncardiac structural anomaly.” Dropping these variables resulted in only a small reduction in discrimination, while inclusion of physiological data for patients admitted to PICU before surgery or substituting weight‐for‐age z‐score for weight resulted in only minor improvements in discrimination. Therefore, these variables were not included in the final model. Patients receiving intensive care immediately before surgery had an increased risk of mortality. Preoperative patient factors that indicate a requirement for intensive care, such as shock or the need for mechanical ventilation, have previously been associated with increased mortality risk in congenital heart surgery.19
Recent international efforts to standardize congenital heart disease nomenclature and risk assessment are commendable for many reasons. The publication of a validated STS‐EACTS mortality prediction model, developed from pooled data from databases in Europe and the United States, represents a landmark achievement in this field. A revised RACHS‐1 model, described as a harmonization of 2 similar methods developed to benchmark congenital heart surgery in the United States, has recently been published.18 Similar to our study, this report allowed population‐based estimates of outcome as the database captured ≈95% of pediatric hospital admissions in the United States.
One reason in favor of using RACHS‐1 is that the simpler procedure categorization may result in more accurate data. In our study the discrimination of the procedure category alone, without patient‐specific covariates, was 0.77, only marginally lower than that reported for the STS‐EACTS score without covariates (0.79).4 With the addition of patient covariates, the discrimination of our model (0.83) was similar to that reported for an equivalent STS‐EACTS model (0.82) and similar to that reported for the original and updated versions of the RACHS‐1 models (0.81 and 0.82).3, 18 A direct comparison of the RACHS and STS‐EACTS systems for categorizing surgical procedures was beyond the scope of this study and could be investigated in the future.
The final RACHS‐ANZ model has 3 additional patient covariates that are easy to define and collect. The performance of the model was unchanged when patient groups not previously included in RACHS‐1 were introduced to the model (children in whom surgery was for acquired heart disease or adults in whom surgery was for congenital heart disease). The aim was to maximize cases included for benchmarking of hospital mortality.
This study has a number of limitations. The sample size for the data quality audit was small relative to size of the study; the audit indicated only 90% agreement in coding of some variables; however, there was not a systematic bias in the inconsistencies. Reports of this information are unusual and could be seen as a strength of the study. Exclusion of 3.8% of patients in whom a RACHS‐1 category could not be assigned is a limitation. The results justify inclusion of adults with congenital heart disease in the analysis of the overall population of patients receiving cardiac surgery in children's hospitals. However, because of the small number of adult patients in the study, the model should not be considered a robust predictor of mortality in the population of adult patients with congenital heart disease.
We developed a method for assessing the risk‐adjusted outcome of cardiac surgery in children's hospitals in ANZ. There are clear benefits from international benchmarking achieved by participation in large international databases. Additional complementary information is obtained by benchmarking at a regional level, because background variation from a number of sources is reduced. Compared with the diverse range of healthcare systems contributing to international databases, there is greater homogeneity in the systems for delivering complex pediatric specialty care in ANZ. The centralized system of care and full participation reduces potential selection bias, while a local registry further aids consistency by providing standardized training of data collectors and methods for maintaining data quality.
Conclusion
The original RACHS‐1 model overpredicts mortality in children undergoing congenital heart surgery in the current era. RACHS‐ANZ uses the minimum number of variables needed to achieve acceptable model performance, and out‐of‐sample validation demonstrated acceptable fit and excellent discrimination. The new model can be applied to a wider range of patients than RACHS‐1, including adults undergoing surgery for congenital heart disease and children having surgery for acquired heart disease. Many regions already collect the data needed to monitor PICU performance; collecting just 1 extra variable, the cardiac surgery procedure code, adds the ability to monitor regional pediatric cardiac surgery outcomes using RACHS‐ANZ.
Author Contributions
Slater was responsible for project inception, design, and format of data collection variables, supervision, and final edit. Alexander coordinated the project data collection, assisted in data validation steps, as well as assisted in the literature search and editing of the manuscript. McSharry wrote software for data validation and patient audits, audited NZ data, performed the statistical analysis, assisted in the coordination of all centers, produced all tables and graphs, and wrote the manuscript draft. Shann, Winlaw, and Gentles provided opinions as to how to analyze data and edited the manuscript.
Sources of Funding
The ANZPIC Registry is supported by the Australian and New Zealand Intensive Care Society, the Ministry of Health (New Zealand), and State and Territory Health Departments through the Australian Health Ministers’ Advisory Council.
Disclosures
None.
Supporting information
Appendix S1. Australian and New Zealand Intensive Care Society Paediatric Study Group Management Committee Members.
Data S1. Sample calculation of the predicted risk of death using the RACHS‐ANZ model.
Table S1. Risk Variables Tested During the Development of an Alternative Logistic Regression Model
Table S2. Alternative Logistic Regression Models Tested
Acknowledgments
The authors would like to express their sincerest thanks to all those involved in the maintenance of the ANZPIC Registry, as well as those involved in the maintenance and overseeing of the various cardiac surgical databases, who allowed us access to their data. In particular we would like to thank Shelley Tregea and Julieta Woosley for their hard work keeping the ANZPIC Registry data as accurate as possible, and all those contributing to the ongoing maintenance of the ANZPIC Registry.
(J Am Heart Assoc. 2019;8:e011390 DOI: 10.1161/JAHA.118.011390.)
References
- 1. van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJM. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;8:50–60. [DOI] [PubMed] [Google Scholar]
- 2. Lacour‐Gayet F, Clarke D, Jacobs J, Comas J, Daebritz S, Daenen W, Gaynor W, Hamilton L, Jacobs M, Maruszsewski B, Pozzi M, Spray T, Stellin G, Tchervenkov C, Mavroudis C. The Aristotle score: a complexity‐adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25:911–924. [DOI] [PubMed] [Google Scholar]
- 3. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus‐based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. [DOI] [PubMed] [Google Scholar]
- 4. O'Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour‐Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, Edwards FH. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. [DOI] [PubMed] [Google Scholar]
- 5. Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, Slater A. Paediatric Index of Mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med. 2013;14:673–681. [DOI] [PubMed] [Google Scholar]
- 6. Straney L, Clements A, Alexander J, Slater A. Quantifying variation of paediatric length of stay among intensive care units in Australia and New Zealand. Qual Saf Health Care. 2010;19:e5. [DOI] [PubMed] [Google Scholar]
- 7. Zweig MH, Campbell G. Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 8. Normand ST, Glickman ME, Sharma RGVRK, McNeil BJ. Using admission characteristics to predict short‐term mortality from myocardial infarction in elderly patients: results from the cooperative cardiovascular project. JAMA. 1996;275:1322. [PubMed] [Google Scholar]
- 9. Al‐Radi OO, Harrell FE, Caldarone CA, McCrindle BW, Jacobs JP, Williams MG, Van Arsdell GS, Williams WG. Case complexity scores in congenital heart surgery: a comparative study of the Aristotle Basic Complexity score and the Risk Adjustment in Congenital Heart Surgery (RACHS‐1) system. J Thorac Cardiovasc Surg. 2007;133:865–875. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs JP, Jacobs ML, Lacour‐Gayet FG, Jenkins KJ, Gauvreau K, Bacha E, Maruszewski B, Clarke DR, Tchervenkonv CI, Gaynor JW, Spray TL, Stellin G, O'Brien SM, Elliott MJ, Mavroudis C. Stratification of complexity improves the utility and accuracy of outcomes analysis in a multi‐institutional congenital heart surgery database: application of the Risk Adjustment in Congenital Heart Surgery (RACHS‐1) and Aristotle Systems in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database. Pediatr Cardiol. 2009;30:1117–1130. [DOI] [PubMed] [Google Scholar]
- 11. Miyata H, Murakami A, Tomotaki A, Takaoka T, Konuma T, Matsumura G, Sano S, Takamoto S. Predictors of 90‐day mortality after congenital heart surgery: the first report of risk models from a Japanese database. J Thorac Cardiovasc Surg. 2014;148:2201–2206. [DOI] [PubMed] [Google Scholar]
- 12. Tsang VT, Brown KL, Synnergren MJ, Kang N, de Leval MR, Gallivan S, Utley M. Monitoring risk‐adjusted outcomes in congenital heart surgery: does the appropriateness of a risk model change with time? Ann Thorac Surg. 2009;87:584–587. [DOI] [PubMed] [Google Scholar]
- 13. Slater A, Shann F. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5:447–454. [DOI] [PubMed] [Google Scholar]
- 14. Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. [DOI] [PubMed] [Google Scholar]
- 15. Au WK, Sun MP, Lam KT, Cheng LC, Chiu SW, Das SR. Mortality prediction in adult cardiac surgery patients: comparison of two risk stratification models. Hong Kong Med J. 2007;13:293–297. [PubMed] [Google Scholar]
- 16. Ivanov J, Weisel RD, David TE, Naylor CD. Fifteen‐year trends in risk severity and operative mortality in elderly patients undergoing coronary artery bypass graft surgery. Circulation. 1998;97:673–680. [DOI] [PubMed] [Google Scholar]
- 17. Mann SL, Marshall MR, Holt A, Woodford B, Williams AB. Illness severity scoring for Intensive Care at Middlemore Hospital, New Zealand: past and future. N Z Med J. 2010;123:47–65. [PubMed] [Google Scholar]
- 18. Jenkins KJ, Koch Kupiec J, Owens PL, Romano PS, Geppert JJ, Gauvreau K. Development and validation of an Agency for Healthcare Research and Quality indicator for mortality after congenital heart surgery harmonized with Risk Adjustment for Congenital Heart Surgery (RACHS‐1) methodology. J Am Heart Assoc. 2016;5:e003028 DOI: 10.1161/JAHA.115.003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobs JP, O'Brien SM, Pasquali SK, Kim S, Gaynor JW, Tchervenkov CI, Karamlou T, Welke KF, Lacour‐Gayet F, Mavroudis C, Mayer JE, Jonas RA, Edwards FH, Grover FL, Shahian DM, Jacobs ML. The importance of patient‐specific preoperative factors: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2014;98:1653–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Australian and New Zealand Intensive Care Society Paediatric Study Group Management Committee Members.
Data S1. Sample calculation of the predicted risk of death using the RACHS‐ANZ model.
Table S1. Risk Variables Tested During the Development of an Alternative Logistic Regression Model
Table S2. Alternative Logistic Regression Models Tested


