Abstract
Background
The contemporary incidence of and reasons for early readmission after infective endocarditis (IE) are not well known. Therefore, we analyzed 30‐day readmission demographics after IE from the US Nationwide Readmission Database.
Methods and Results
We examined the 2010 to 2014 Nationwide Readmission Database to identify index admissions for a primary diagnosis of IE with survival at discharge. Incidence, reasons, and independent predictors of 30‐day unplanned readmissions were analyzed. In total, 11 217 patients (24.8%) were nonelectively readmitted within 30 days among the 45 214 index admissions discharged after IE. The most common causes of readmission were IE (20.5%), sepsis (8.7%), complications of device/graft (8.1%), and congestive heart failure (7.6%). In‐hospital mortality and the valvular surgery rates during the readmissions were 8.1% and 9.1%, respectively. Discharge to home or self‐care, undergoing valvular surgery, aged ≥60 years, and having private insurance were independently associated with lower rates of 30‐day readmission. Length of stay of ≥10 days, congestive heart failure, diabetes mellitus, renal failure, chronic pulmonary disease, peripheral artery disease, and depression were associated with higher risk. The total hospital costs of readmission were $48.7 million per year (median, $11 267; interquartile range, $6021–$25 073), which accounted for 38.6% of the total episodes of care (index+readmission).
Conclusions
Almost 1 in 4 patients was readmitted within 30 days of admission for IE. The most common reasons were IE, other infectious causes, and cardiac causes. A multidisciplinary approach to determine the surgical indications and close monitoring are necessary to improve outcomes and reduce complications in in‐hospital and postdischarge settings.
Keywords: epidemiology, infective endocarditis, readmission, surgery
Subject Categories: Infectious Endocarditis, Quality and Outcomes, Epidemiology
Clinical Perspective
What Is New?
This analysis of 45 214 admissions for a primary diagnosis of infective endocarditis with survival until discharge demonstrated that the rate of 30‐day unplanned readmissions was 24.8%, and the most common causes for readmission were infective endocarditis, other infectious causes, and cardiac causes.
Undergoing valvular surgery was independently associated with a lower risk of readmissions, whereas medical comorbidities were associated with a higher risk.
The proportion of patients undergoing valvular surgery, the in‐hospital mortality rates, and the costs during readmission were substantial.
What Are the Clinical Implications?
Thirty‐day readmission after infective endocarditis is common.
Some of the patients readmitted for repeated infective endocarditis may have relapsed or received suboptimal treatment, perhaps related to a short hospitalization period and/or inadequate assessment of surgical indications.
A multidisciplinary approach to evaluate surgical indications, adequate antibiotic administration duration, and close monitoring after discharge should be evaluated as methods to improve patient outcomes and reduce healthcare costs.
Introduction
Infective endocarditis (IE) is uncommon, but it is one of the most severe illnesses associated with substantial mortality and morbidity. The estimated incidence of IE in developed countries is 3 to 10 individuals per 100 000 person‐years.1, 2 The symptoms of IE are initially variable and clinically similar to those of other infectious diseases, which may contribute to the sudden occurrence of fatal complications, such as systemic embolization and heart failure.3, 4, 5 Previous studies have emphasized the importance of early diagnosis by echocardiography/microbiology, early initiation of high‐dosage antibiotics, and early surgical intervention, all of which are strongly associated with a reduced risk of in‐hospital mortality and morbidity.6, 7, 8 A substantially high rate of mortality was reported among patients undergoing treatment for IE, with the in‐hospital and 1‐year mortality being 15% to 20% and 18% to 40%, respectively.9, 10, 11 Despite its poor clinical outcomes, even postdischarge, there are surprisingly few multicenter cohort studies that have focused on readmission after IE. Rasmussen et al reported that the 1‐year readmission rate was 65% and that the mental/physical status and a higher comorbidity burden were associated with higher rates of 1‐year readmission.9 Given the rarity of IE, studies tend to have small sample sizes, limiting their ability to precisely determine early outcomes. Therefore, extensive studies in a multi‐institutional setting are required. It is vital to address the key factors and reasons associated with early readmission for patients and physicians to understand the expected outcomes immediately after recovery, eventually reducing the overall healthcare burden and improving patient outcome. To our knowledge, no study has systemically analyzed patient/hospital factors associated with unplanned 30‐day readmission from an epidemiological perspective using a nationally representative cohort. Furthermore, differences in standard practice between hospitals are reportedly associated with patient outcomes; thus, continued efforts to improve the management of IE are necessary.12 In this context, we examined the incidence, causes, and hospital costs of 30‐day readmission after IE using large real‐world data from the US Nationwide Readmission Database (NRD). We also analyzed the patient and hospital characteristics independently associated with 30‐day readmission.
Methods
The full data for this study are publicly available at the Healthcare Cost and Utilization Project,13 and the analysis method can be made available by request to the corresponding author.
Data Source and Study Population
Data were sourced from the 2010 to 2014 NRD, which contains essential patient and hospital information, for readmission analysis. Briefly, the NRD contains all‐payer hospital inpatient information and was developed by the Healthcare Costs and Utilization Project from the State Inpatient Database, representing 22 geographically dispersed states of the United States. The State Inpatient Database accounts for 51.2% of the total US population and 49.3% of all hospitalizations in the United States. Verified patient linkage numbers, “NRD_VisitLink,” were provided to track the discharges of the same patients within the states. Timing variables, “NRD_DaysToEvent,” were used to determine the number of days between each index discharge and subsequent readmission. The provided “weight” variables were used as a correction technique for analyzing statistics from the stratified database and accounting for national estimates. The NRD represented ≈15 and 35 million discharges for the unweighted and weighted volumes, respectively.13 Because the NRD is a commercial public database without any personally identifiable information, institutional review board approval and informed patient consent were waived for this study.
Patient and Hospital Characteristics
We included all index IE cases discharged with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes 4210, 4211, and 4219 between 2010 and 2014. As the ICD‐9‐CM codes show a high positive predictive value for the diagnosis of IE, on the basis of Duke's criteria, these codes could be used as extracting tools for studying outcomes in healthcare databases.3, 14, 15, 16 We excluded patients who were discharged in December of each year (from 2010 to 2014) as we could not determine 30‐day readmissions. Subsequently, we found 48 264 index admissions with a primary diagnosis of IE, after excluding those who (1) were aged <18 years; (2) had a length of stay of >180 days; (3) were transferred to a rehabilitation hospital; and (4) were nonstate residents. Among them, 3050 (in‐hospital mortality, 6.3%) patients died during index admissions. Eventually, the final study population size was 45 214 index admissions who survived to hospital discharge after IE. All unplanned readmissions (elective=0) within 30 days of index discharges were identified. If there were ≥2 readmissions after the index admission, only the first was included to analyze the factors associated with unplanned readmission among the discharged patients with IE (Figure). We identified patients’ comorbidities, in‐hospital outcomes, and in‐hospital procedures using ICD‐9‐CM codes, Elixhauser comorbidity software,17 and Clinical Classification Software,18 respectively. Clinical Classification Software was also used to identify the causes of readmission, summarizing the diagnosis codes in clinically meaningful categories. Exceptionally, among the causes of readmission, IE (codes 4210, 4211, and 4219) was separately categorized. According to a previous study,15 we identified causative pathogens of IE, including Staphylococcus, Streptococcus, gram‐negative bacteria, and fungi, using ICD‐9‐CM codes. The full list of codes used to identify these variables is provided in Table S1.
Figure 1.
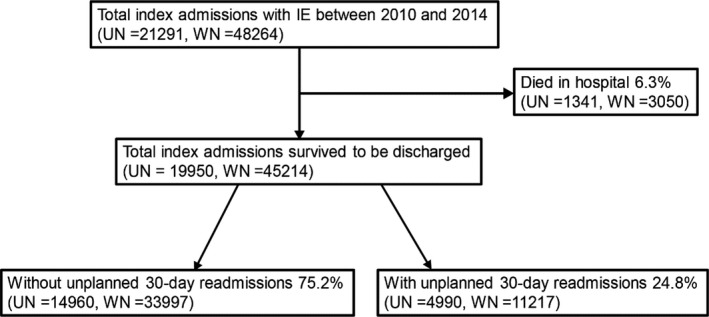
Flowchart of the study cohort. IE indicates infective endocarditis; UN, unweighted number; WN, weighted number (national estimates).
Statistical Analysis
All analyses were performed using R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). The Survey R package was used to analyze the weighted database.19 P<0.05 was considered to be statistically significant, and all null hypotheses were evaluated using 2‐sided tests. All analyses were performed and presented as weighted data representing the national estimates. The index IE cases were first identified. We calculated the proportion, causes, and costs of 30‐day readmissions. The costs of index IE cases were also determined. Subsequently, the baseline characteristics for the 30‐day readmissions were assessed. We used the χ2 test, Student's test, and Wilcoxon rank‐sum test for categorical, normally distributed continuous, and nonnormally distributed continuous variables, respectively. The normality of the continuous variables was determined by the Shapiro‐Wilk normality test. Categorical variables are expressed as percentages. Continuous variables are presented as mean±SD or median (interquartile range [IQR]), as appropriate. To identify independent predictors of 30‐day readmissions of IE, we performed multivariate logistic regression analysis using baseline characteristics. The multivariate logistic regression model was constructed using all patient and hospital variables because the cohort contained a large number of readmissions within 30 days. Odds ratios (ORs) and 95% CIs were used to report the results of the analyses.
The following unweighted data of patients with index IE were missing: (1) primary expected payer (62 [0.3%]), median household income for patient's zip code (359 [1.8%]), and disposition of the patient at discharge (23 [0.1%]). As the missing data were minimal (<5%), these variables were replaced with those of the dominant ones in each category. In addition, the unweighted hospital cost data were missing for 697 index admissions (3.5%) and 186 readmissions (3.7%). Cost analysis was performed on the basis of the data excluding these missing values.
Results
Baseline Patient and Hospital Characteristics
Among the 45 214 index admissions who survived to hospital discharge, 11 217 (24.8%) were readmitted within 30 days (Figure S1). When counting the multiple readmissions, we found that 1603 of 11 217 cases (14.3%) had ≥2 readmissions within 30 days.
The baseline patient and hospital characteristics at the index admissions are summarized in Table 1. The median age of the study population was 57 (IQR, 42–71) years, and 38.3% of them were women. The median length of stay was 10 (IQR, 6–17) days. The proportion of patients undergoing valvular surgery was 20.7%. In addition, 71.9% of patients (32 131) had ≥1 codes for causative pathogens and 3.9% (1778) had multiple codes. Streptococcus was the most common pathogen at 36.6%, followed by Staphylococcus (31.2%), gram‐negative bacteria (3.2%), and fungi (0.1%).
Table 1.
Baseline Patient and Hospital Characteristics of IE in the Index Hospitalization
| Characteristics | Total | 30‐d Readmission | P Value | |
|---|---|---|---|---|
| No | Yes | |||
| No. (weighted) | 45 214 | 33 997 | 11 217 | … |
| No. (unweighted) | 19 950 | 14 960 | 4990 | … |
| Age, median (IQR), y | 57 (42–71) | 57 (42–71) | 57 (41–71) | 0.78 |
| Aged ≥60 y, % | 45.7 | 45.8 | 45.5 | 0.8 |
| Aged <60 y, % | 54.3 | 54.2 | 54.5 | |
| LOS, median (IQR), d | 10 (6–17) | 9 (5–17) | 10 (6–18) | <0.001 |
| LOS ≥10 d, % | 50.4 | 49.3 | 53.8 | <0.001 |
| LOS <10 d, % | 49.6 | 50.7 | 46.2 | |
| Women, % | 38.3 | 37.7 | 40.1 | 0.01 |
| Weekend admission, % | 22.1 | 21.8 | 23.2 | 0.08 |
| Discharge location, % | ||||
| Routine (home or self‐care) | 30.8 | 32.9 | 24.7 | <0.001 |
| Home health care | 30.9 | 31.5 | 29.2 | |
| Transfer to SNF, ICF, or others | 29.1 | 27.7 | 33.3 | |
| Transfer to short‐term hospital | 4.7 | 4.9 | 4.3 | |
| Against medical advice | 4.4 | 3.0 | 8.5 | |
| Primary expected payer, % | ||||
| Medicare | 44.1 | 43.0 | 47.3 | <0.001 |
| Medicaid | 17.6 | 16.8 | 20.1 | |
| Self‐pay | 8.6 | 8.7 | 8.0 | |
| No charge | 1.2 | 1.2 | 1.4 | |
| Private | 24.8 | 26.4 | 19.9 | |
| Other | 3.8 | 3.9 | 3.4 | |
| Median household income, percentile, % | ||||
| 0–25th | 29.6 | 29 | 31.6 | 0.02 |
| 26th–50th | 25.3 | 25.3 | 25.3 | |
| 51st–75th | 22.8 | 23.1 | 22.0 | |
| 76th–100th | 22.2 | 22.6 | 21.2 | |
| Teaching status, % | ||||
| Teaching metropolitan | 59.2 | 58.9 | 60.0 | 0.28 |
| Nonteaching metropolitan | 33.9 | 34.1 | 33.6 | |
| Nonmetropolitan | 6.9 | 7.1 | 6.4 | |
| Bed size, % | ||||
| Small | 9.3 | 9.3 | 9.0 | 0.88 |
| Medium | 21.9 | 21.9 | 21.9 | |
| Large | 68.8 | 68.7 | 69.0 | |
| Bacteria, % | ||||
| Staphylococcus | 31.2 | 30.5 | 33.5 | <0.001 |
| Streptococcus | 36.6 | 37.7 | 33.2 | <0.001 |
| Gram‐negative bacteria | 3.2 | 3.0 | 3.7 | 0.04 |
| Fungi | 0.1 | 0.07 | 0.2 | 0.04 |
| Comorbidities, % | ||||
| Hypertension | 51.6 | 50.9 | 53.8 | 0.003 |
| Smoking history | 32.5 | 32.5 | 32.7 | 0.86 |
| Congestive heart failure | 30.2 | 28.5 | 35.5 | <0.001 |
| Diabetes mellitus | 24.2 | 23.1 | 27.5 | <0.001 |
| Renal failure | 22.7 | 20.9 | 28.1 | <0.001 |
| Atrial fibrillation | 19.5 | 19.2 | 20.3 | 0.18 |
| Chronic pulmonary disease | 18.2 | 17.3 | 20.9 | <0.001 |
| Coagulopathy | 16.1 | 15.7 | 17.4 | 0.02 |
| Peripheral artery disease | 13.1 | 12.6 | 14.6 | 0.002 |
| Depression | 11.2 | 10.7 | 12.7 | 0.002 |
| Obesity | 9.3 | 9.1 | 9.9 | 0.13 |
| Hypothyroidism | 8.9 | 8.8 | 9.2 | 0.46 |
| Prior stroke/TIA | 5.2 | 5.3 | 5.0 | 0.55 |
| Prior MI | 4.0 | 3.9 | 4.4 | 0.2 |
| In‐hospital outcomes, % | ||||
| Acute renal failure | 21.5 | 20.7 | 24.0 | <0.001 |
| Valvular surgery | 20.7 | 21.6 | 18.0 | <0.001 |
| Pulmonary embolism | 12.7 | 12.4 | 13.7 | 0.05 |
| Acute CI/TIA | 8.1 | 8.0 | 8.5 | 0.28 |
| Systemic embolism | 3.6 | 3.6 | 3.5 | 0.74 |
| Cardiogenic shock | 2.2 | 2.1 | 2.4 | 0.28 |
| Pacemaker/ICD implantation | 2.0 | 2.0 | 2.0 | 0.94 |
| Intracranial hemorrhage | 1.6 | 1.5 | 1.8 | 0.16 |
| Cardiac arrest | 0.8 | 0.8 | 1.0 | 0.46 |
CI indicates cerebral infarction; ICD, implantable cardioverter‐defibrillator; ICF, intermediate‐care facility; IE, infective endocarditis; IQR, interquartile range; LOS, length of stay; MI, myocardial infarction; SNF, skilled nursing facility; TIA, transient ischemic attack.
Compared with the patients who were not readmitted within 30 days, those readmitted were less likely to: (1) be routinely discharged (home or self‐care); (2) have undergone valvular surgery; (3) have private insurance; (4) and have streptococcal infections. On the other hand, the readmitted patients were more likely to: (1) have been hospitalized for ≥10 days; (2) be women; (3) have Medicare or Medicaid insurance; (4) be at the lowest quantile of median income; and (5) have staphylococcal infections. Hypertension, congestive heart failure, diabetes mellitus, renal failure, chronic pulmonary disease, coagulopathy, peripheral artery disease, depression, and acute renal failure were more prevalent in patients requiring 30‐day readmissions.
Independent Predictors of 30‐Day Readmissions
Table 2 summarizes the results of the multivariate logistic regression analysis of independent predictors for 30‐day readmission after IE. Discharge to home or self‐care (OR, 1.26; 95% CI, 1.14–1.40; P<0.001), undergoing valvular surgery (OR, 0.69; 95% CI, 0.61–0.78; P<0.001), aged ≥60 years (OR, 0.84; 95% CI, 0.75–0.94; P=0.003), and having private insurance (OR, 0.82; 95% CI, 0.72–0.93; P=0.002) were independently associated with lower rates of 30‐day readmission. A length of stay of ≥10 days (OR, 1.14; 95% CI, 1.04–1.26; P=0.005) and several comorbidities, including congestive heart failure (OR, 1.35; 95% CI, 1.23–1.47; P<0.001), diabetes mellitus (OR, 1.15; 95% CI, 1.04–1.27; P=0.008), renal failure (OR, 1.33; 95% CI, 1.21–1.47; P<0.001), chronic pulmonary disease (OR, 1.17; 95% CI, 1.06–1.29; P=0.002), peripheral artery disease (OR, 1.17; 95% CI, 1.04–1.33; P=0.01), and depression (OR, 1.22; 95% CI, 1.07–1.40; P=0.003), were independently associated with higher rates of 30‐day readmission. On the other hand, no causative pathogens of IE (Staphylococcus, Streptococcus, gram‐negative bacteria, and fungi) were independently associated with 30‐day readmission.
Table 2.
Independent Predictors of 30‐Day Readmission After IE via Multivariate Logistic Regression Analysis
| Predictors | OR | 95% CI | P Value |
|---|---|---|---|
| Aged ≥60 y | 0.84 | 0.75–0.94 | 0.003 |
| LOS ≥10 d | 1.14 | 1.04–1.26 | 0.005 |
| Women | 0.99 | 0.91–1.08 | 0.84 |
| Weekend admission | 1.02 | 0.93–1.12 | 0.67 |
| Discharge location | |||
| Routine (home or self‐care) | Reference | ||
| Home health care | 1.26 | 1.14–1.40 | <0.001 |
| Transfer to SNF, ICF, or others | 1.41 | 1.25–1.59 | <0.001 |
| Transfer to short‐term hospital | 1.12 | 0.94–1.34 | 0.2 |
| Against medical advice | 3.80 | 3.18–4.54 | <0.001 |
| Primary expected payer | |||
| Medicare | Reference | ||
| Medicaid | 1.07 | 0.94–1.22 | 0.31 |
| Self‐pay | 0.84 | 0.71–1.01 | 0.06 |
| No charge | 1.08 | 0.78–1.50 | 0.63 |
| Private | 0.82 | 0.72–0.93 | 0.002 |
| Other | 0.86 | 0.69–1.07 | 0.17 |
| Median household income, percentile | |||
| 0–25th | Reference | ||
| 26th–50th | 0.99 | 0.88–1.10 | 0.83 |
| 51st–75th | 0.98 | 0.88–1.09 | 0.68 |
| 76th–100th | 1.01 | 0.91–1.13 | 0.8 |
| Teaching status | |||
| Teaching metropolitan | Reference | ||
| Nonteaching metropolitan | 0.97 | 0.90–1.05 | 0.48 |
| Nonmetropolitan | 0.88 | 0.74–1.04 | 0.14 |
| Bed size | |||
| Small | Reference | ||
| Medium | 1.00 | 0.83–1.19 | 0.98 |
| Large | 1.02 | 0.86–1.20 | 0.84 |
| Bacteria | |||
| Staphylococcus | 0.95 | 0.86–1.05 | 0.3 |
| Streptococcus | 0.92 | 0.84–1.02 | 0.12 |
| Gram‐negative bacteria | 1.16 | 0.92–1.47 | 0.2 |
| Fungi | 2.12 | 0.91–4.94 | 0.08 |
| Comorbidities | |||
| Hypertension | 1.00 | 0.91–1.09 | 0.98 |
| Smoking history | 0.96 | 0.87–1.05 | 0.37 |
| Congestive heart failure | 1.35 | 1.23–1.47 | <0.001 |
| Diabetes mellitus | 1.15 | 1.04–1.27 | 0.008 |
| Renal failure | 1.33 | 1.21–1.47 | <0.001 |
| Atrial fibrillation | 0.99 | 0.89–1.10 | 0.85 |
| Chronic pulmonary disease | 1.17 | 1.06–1.29 | 0.002 |
| Coagulopathy | 1.11 | 1.00–1.24 | 0.05 |
| Peripheral artery disease | 1.17 | 1.04–1.33 | 0.01 |
| Depression | 1.22 | 1.07–1.40 | 0.003 |
| Obesity | 1.02 | 0.90–1.16 | 0.73 |
| Hypothyroidism | 0.99 | 0.86–1.14 | 0.88 |
| Prior stroke/TIA | 0.91 | 0.77–1.07 | 0.26 |
| Prior MI | 1.00 | 0.83–1.21 | 1 |
| In‐hospital outcomes | |||
| Acute renal failure | 1.07 | 0.97–1.18 | 0.17 |
| Valvular surgery | 0.69 | 0.61–0.78 | <0.001 |
| Pulmonary embolism | 1.07 | 0.94–1.21 | 0.3 |
| Acute CI/TIA | 1.07 | 0.93–1.24 | 0.36 |
| Systemic embolism | 0.93 | 0.73–1.19 | 0.58 |
| Cardiogenic shock | 1.15 | 0.88–1.50 | 0.32 |
| Pacemaker/ICD implantation | 1.05 | 0.79–1.40 | 0.74 |
| Intracranial hemorrhage | 1.17 | 0.87–1.56 | 0.3 |
| Cardiac arrest | 1.04 | 0.65–1.67 | 0.87 |
CI indicates cerebral infarction; ICD, implantable cardioverter‐defibrillator; ICF, intermediate‐care facility; IE, infective endocarditis; LOS, length of stay; MI, myocardial infarction; OR, odds ratio; SNF, skilled nursing facility; TIA, transient ischemic attack.
Causes and Costs of 30‐Day Readmissions
Among the 11 217 readmitted patients, the median time from index discharge to readmission was 10 (IQR, 4–18) days. In total, 899 patients (8.1%) died and 1025 patients (9.1%) underwent valvular surgery during their readmission. The most common causes of 30‐day readmission were IE, followed by sepsis, congestive heart failure, complications of device and graft, acute renal failure, and acute cerebrovascular disease; these were followed by valvular disease, cardiac arrhythmias, complications of surgical procedures, and pneumonia (Table 3). The median length of stay during readmission was 6 (IQR, 3–12) days. The median costs of index admissions with and without readmission were $20 323 (IQR, $11 701–$44 409) and $18 994 (IQR, $10 255–$44 930), respectively. The estimated total cost of readmission was $48.7 million (median, $11 267 [IQR, $6021–$25 073]), which accounted for 38.6% of the total episodes (index+first unplanned 30‐day readmission) (Figure S2).
Table 3.
Causes of 30‐Day Readmission After IE
| Causes | Total No. (Weighted) | Proportion, % |
|---|---|---|
| IE | 2298 | 20.5 |
| Sepsis | 975 | 8.7 |
| Complications of device and graft | 911 | 8.1 |
| Congestive heart failure | 857 | 7.6 |
| Acute renal failure | 398 | 3.5 |
| Acute cerebrovascular disease | 330 | 2.9 |
| Valvular disease | 325 | 2.9 |
| Cardiac arrhythmia | 305 | 2.7 |
| Complications of surgical procedures | 259 | 2.3 |
| Pneumonia | 257 | 2.3 |
| Anemia | 207 | 1.8 |
| Gastrointestinal tract hemorrhage | 202 | 1.8 |
| Respiratory tract failure | 187 | 1.7 |
| Nonspecific chest pain | 163 | 1.5 |
| Pericarditis and myocarditis | 138 | 1.2 |
| Fluid and electrolyte disorders | 136 | 1.2 |
| Hypertension with complications and secondary hypertension | 132 | 1.2 |
| Pleurisy/pneumothorax/pulmonary collapse | 130 | 1.2 |
| Diabetes mellitus with complications | 122 | 1.1 |
| Fever of unknown origin | 117 | 1.0 |
| Others | 2768 | 24.8 |
| Total | 11 217 | 100 |
IE indicates infective endocarditis.
Discussion
Thirty‐day readmission is an important metric used as an indicator for the assessment of the quality of hospital care and performance.20 For example, in the setting of heart failure, 30‐day readmission was significantly associated with subsequent mortality.21 In the present study, using data from the 2010 to 2014 NRD, the 30‐day readmission rate after IE was 24.8%, which was roughly comparable with the rates after primary diagnoses of congestive heart failure (25.7%), acute myocardial infarction (19.7%), and cardiac dysrhythmias (16.7%) reported in the 2014 NRD.13 A previous study analyzing patients with IE after discharge reported similar rates of readmission within 30 days at ≈25%, and the 90‐day and 1‐year rates were ≈40% and ≈65%, respectively.9 In our study as well as other studies, the readmissions after IE were clustered in the period immediately after discharge, and nearly 1 in 12 patients died during the readmission, which emphasizes the importance of early prevention and intervention to improve the short‐term outcome of this population.
We determined that the most common cause of readmission for IE within 30 days was a primary diagnosis of IE, followed by sepsis, congestive heart failure, complications from a device/graft, acute renal failure, acute cerebrovascular disease, valvular disease, and cardiac dysrhythmias. Similarly, Rasmussen et al reported that primary diagnoses of IE, congestive heart failure, atrial fibrillation, and ischemic stroke were leading causes of readmission over the 1‐year period.9 This evidence emphasizes the importance of managing risk factors for infectious and cardiac disease, even after discharge. Furthermore, IE was the most frequent cause of nonelective readmission, regardless of the postdischarge period, in both our own study and the study by Rasmussen et al.9 Generally, because an antibiotic treatment period of ≥4 weeks is considered appropriate, IE recurring within 10 weeks, from 10 weeks to 6 months or less, and >6 months from the start of treatment at the index admission is defined as suboptimal treatment, relapse, and recurrence, respectively.22, 23 Previous long‐term studies reported rates of relapse or recurrence of ≈5% to 15%.9, 22, 23 On the other hand, readmission for IE within 30 days occurred after ≈5% of all index admissions (20.5% of 24.8% readmitted cases) in this study. Because the observation period was different from previous studies, it was difficult to simply compare the rates between them. Considering the short‐term observation period in this study, most of the patients readmitted for IE were presumed to have relapsed or received suboptimal treatment, possibly caused by a short hospitalization (median, 10 days) or an inadequate initial assessment for surgical indications. Furthermore, the diagnosis of readmission was based on the ICD‐9‐CM codes, which might include a certain likelihood of misdiagnosis. Nevertheless, these findings underscored the severe inadequacies in the initial care of patients with IE in real‐world practices and strongly suggested that considering more intensive treatments, such as prolonged antibiotic therapy and close monitoring, even after discharge is necessary to prevent adverse outcomes in the high‐risk populations.
To our knowledge, this study is the first to identify several independent predictors of 30‐day readmission after surviving IE discharge, extending the results of previous studies examining the association between mortality and clinical variables. We found that undergoing valvular surgery was strongly associated with a lower risk of 30‐day readmission. The readmissions involved a substantial rate of mortality, and valvular surgery was performed in 1 of 11 readmitted patients in our study. Again, this suggests that a certain proportion of patients were discharged after inadequate assessment for surgical indications and received antibiotic treatment for an insufficient period. Moreover, surgery would not be an option in high‐risk patients, even in those with a surgical indication,24 which causes inevitable selection bias in this context. Chu et al reported that ≈24% of patients with a surgical indication for IE did not undergo surgery, with the common reasons being a poor prognosis regardless of treatment, hemodynamic instability, death before surgery, stroke, and sepsis.25 A previous study reported that surgical intervention was independently associated with lower 6‐month mortality, even after adjustment for operative propensity and risk.26 Collectively, these facts suggested that careful decision making about surgical indications by a multidisciplinary team, including echocardiologists, cardiac surgeons, neurologists, and infectious disease specialists, is mandatory for improving outcomes as well as reducing readmissions and healthcare costs after discharge.
Routine discharge (home or self‐care) was independently associated with a lower rate of readmission in this study. Routine discharge may represent good cerebral performance and low frailty in patients, indicating that patients with more severe illness were more likely to be readmitted after IE.
Several comorbidities were independent predictors of 30‐day readmission after IE in the multivariate model (namely, congestive heart failure [P<0.001], diabetes mellitus [P=0.008], renal failure [P<0.001], chronic pulmonary disease [P=0.002], peripheral artery disease [P=0.01], and depression [P=0.003]), all of which were associated with higher risk. These results were in line with those of previous studies that demonstrated that a higher burden of comorbidity was an independent predictor of long‐term mortality9, 25, 26 and readmission.9 With regard to the aforementioned factors, congestive heart failure has been reported to be associated with mortality as it reflects the overall severity of IE, including the presence of severe valvular dysfunction, other comorbid cardiovascular disease, or an unstable hemodynamic status.27 Previous studies have shown that elevated serum creatinine is associated with increased mortality as it also reflects systemic issues, such as renal artery embolism and antibiotic toxicity.28 Existing chronic kidney disease might serve as a risk factor for the development of acute renal failure and heart failure. The association of altered mental health with 6‐month mortality and readmissions has also been reported previously, and this may reflect poor cerebral perfusion, metabolic instability, and physical frailty.9, 29 It is vital to develop effective interventions that can improve the patients’ mental health to reduce these adverse outcomes. Taken together, these findings suggest that carefully recognizing these common comorbidities may be valuable in predicting adverse events and managing this high‐risk population.
Previous studies have reported that Staphylococcus was one of the most common causative agents of IE,30 and the cause was associated with higher in‐hospital and late mortality attributed to high resistance to antibiotics compared with other agents.24, 25 Patients with IE with Staphylococcus often present with antibiotic resistance and difficult‐to‐treat pathologic conditions, and a significant prognostic benefit of early surgery was observed among them.7 In contrast, no causative agents, including Staphylococcus (P=0.3), Streptococcus (P=0.12), gram‐negative bacteria (P=0.2), and fungi (P=0.08), could predict 30‐day readmission after IE in our study. This result was consistent with that of a previous study reporting that Staphylococcus was not associated with repeated episodes of IE in an adjusted model.23 Collectively, we speculated that the patients who survived to discharge after IE were at a similar risk, regardless of their microbiological cause.
Finally, our study is the first to demonstrate that readmissions after surviving IE discharge result in a significant economic burden, with a total cost of $48.7 million per year, which accounted for ≈38.4% of the total episodes of care. This fact emphasizes the importance of early and close interventions to prevent readmission in these patients, particularly among those with several risk factors for readmission. In the future, the effect of more targeted and individualized follow‐up interventions compared with that of normal interventions should be established.
Limitations
Our study has several limitations. First, as with similar studies based on retrospective administrative data, the effect of unmeasured confounding factors could not be fully excluded. Second, data on the surgical indications for IE were not available, such as information on vegetation size, severity of valvular regurgitation, cardiac function, and timing/severity of an embolic event. Third, we could not validate the precise diagnosis of IE and describe its details in a clinically logical way (eg, active endocarditis, valve lesions resulting from treated endocarditis, and other). However, other previous studies had also used ICD‐9‐CM codes as a substitute for chart‐based diagnosis of IE.14, 15 Fourth, as readmissions could not be tracked across different states, the absolute number of readmissions might be underestimated. Fifth, the causative pathogens were not identified from blood culture results, but multiple codes for pathogens were only found in 3.9% of patients. We noted that a similar approach was used in a previous study.15 Despite these limitations, we believe that the NRD provides valuable and robust information in the context of the quality of care and cost immediately after IE.
Conclusions
Discharge after IE showed a high rate of 30‐day readmission, with the main associated causes being IE, other infectious causes, and heart failure. In‐hospital mortality, the proportion of patients undergoing valvular surgery, and the costs accumulated during the readmissions were substantial. Undergoing valvular surgery, as well as other patient‐ and hospital‐related factors, was associated with readmissions. Careful consideration of surgical indications by a multidisciplinary team and recognition of these predictors of intervention in high‐risk patients are critical for reducing healthcare costs and improving patient outcomes.
Disclosures
None.
Supporting information
Table S1. International Classification of Diseases, Ninth Edition, Clinical Modification, Elixhauser Comorbidity Software, and Clinical Classification Software Were Used to Identify Patient/Hospital Characteristics
Figure S1. Kaplan‐Meier curve of 30‐day readmissions after infective endocarditis.
Figure S2. Estimated mean costs of index admissions and 30‐day readmissions after infective endocarditis.
(J Am Heart Assoc. 2019;8:e011598 DOI: 10.1161/JAHA.118.011598.)
References
- 1. Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population‐based profile of 1536 patients in Australia. Eur Heart J. 2010;31:1890–1897. [DOI] [PubMed] [Google Scholar]
- 2. Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York State, 1998‐2013. JAMA. 2017;317:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heiro M, Helenius H, Hurme S, Savunen T, Metsärinne K, Engblom E, Nikoskelainen J, Kotilainen P. Long‐term outcome of infective endocarditis: a study on patients surviving over one year after the initial episode treated in a Finnish teaching hospital during 25 years. BMC Infect Dis. 2008;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Durack DT, Lukes AS, Bright DK; Duke Endocarditis Service . New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200–209. [DOI] [PubMed] [Google Scholar]
- 5. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. [DOI] [PubMed] [Google Scholar]
- 6. Steckelberg JM, Murphy JG, Ballard D, Bailey K, Tajik AJ, Taliercio CP, Giuliani ER, Wilson WR. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med. 1991;114:635–640. [DOI] [PubMed] [Google Scholar]
- 7. Lalani T, Cabell CH, Benjamin DK, Lasca O, Naber C, Fowler VG Jr, Corey GR, Chu VH, Fenely M, Pachirat O, Tan RS, Watkin R, Ionac A, Moreno A, Mestres CA, Casabé J, Chipigina N, Eisen DP, Spelman D, Delahaye F, Peterson G, Olaison L, Wang A; International Collaboration on Endocarditis‐Prospective Cohort Study (ICE‐PCS) Investigators . Analysis of the impact of early surgery on in‐hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment‐selection bias. Circulation. 2010;121:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang DH, Kim YJ, Kim SH, Sun BJ, Kim DH, Yun SC, Song JM, Choo SJ, Chung CH, Song JK, Lee JW, Sohn DW. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–2473. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen TB, Zwisler AD, Thygesen LC, Bundgaard H, Moons P, Berg SK. High readmission rates and mental distress after infective endocarditis: results from the national population‐based CopenHeart IE survey. Int J Cardiol. 2017;235:133–140. [DOI] [PubMed] [Google Scholar]
- 10. Hill EE, Herijgers P, Herregods MC, Peetermans WE. Evolving trends in infective endocarditis. Clin Microbiol Infect. 2006;12:5–12. [DOI] [PubMed] [Google Scholar]
- 11. Slipczuk L, Codolosa JN, Davila CD, Romero‐Corral A, Yun J, Pressman GS, Figueredo VM. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8:e82665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drye EE, Normand SL, Wang Y, Ross JS, Schreiner GC, Han L, Rapp M, Krumholz HM. Comparison of hospital risk‐standardized mortality rates calculated by using in‐hospital and 30‐day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Agency for Healthcare Research and Quality . Introduction to the HCUP Nationwide Readmissions Database (NRD) 2014. https://www.hcup-us.ahrq.gov/db/nation/nrd/NRD_Introduction_2014.jsp. Accessed September 1, 2018
- 14. Shih CJ, Chu H, Chao PW, Lee YJ, Kuo SC, Li SY, Tarng DC, Yang CY, Yang WC, Ou SM, Chen YT. Long‐term clinical outcome of major adverse cardiac events in survivors of infective endocarditis: a nationwide population‐based study. Circulation. 2014;130:1684–1691. [DOI] [PubMed] [Google Scholar]
- 15. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070–2076. [DOI] [PubMed] [Google Scholar]
- 16. Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. [DOI] [PubMed] [Google Scholar]
- 17. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in‐hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 18. US Agency for Healthcare Research and Quality . Clinical Classifications Software (CCS) for ICD‐9‐CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed September 1, 2018.
- 19. Lumley T. Analysis of complex survey samples. https://cran.r-project.org/web/packages/survey/survey.pdf. Accessed September 1, 2018.
- 20. Medicare.gov . 30‐Day unplanned readmission and death measures. https://www.medicare.gov/hospitalcompare/Data/30-day-measures.html. Accessed September 1, 2018.
- 21. Arundel C, Lam PH, Khosla R, Blackman MR, Fonarow GC, Morgan C, Zeng Q, Fletcher RD, Butler J, Wu WC, Deedwania P, Love TE, White M, Aronow WS, Anker SD, Allman RM, Ahmed A. Association of 30‐day all‐cause readmission with long‐term outcomes in hospitalized older Medicare beneficiaries with heart failure. Am J Med. 2016;129:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mansur AJ, Dal Bo CM, Fukushima JT, Issa VS, Grinberg M, Pomerantzeff PM. Relapses, recurrences, valve replacements, and mortality during the long‐term follow‐up after infective endocarditis. Am Heart J. 2001;141:78–86. [DOI] [PubMed] [Google Scholar]
- 23. Alagna L, Park LP, Nicholson BP, Keiger AJ, Strahilevitz J, Morris A, Wray D, Gordon D, Delahaye F, Edathodu J, Miró JM, Fernández‐Hidalgo N, Nacinovich FM, Shahid R, Woods CW, Joyce MJ, Sexton DJ, Chu VH. Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis ‐ Prospective Cohort Study. Clin Microbiol Infect. 2014;20:566–575. [DOI] [PubMed] [Google Scholar]
- 24. Habib G, Tribouilloy C, Thuny F, Giorgi R, Brahim A, Amazouz M, Remadi JP, Nadji G, Casalta JP, Coviaux F, Avierinos JF, Lescure X, Riberi A, Weiller PJ, Metras D, Raoult D. Prosthetic valve endocarditis: who needs surgery? A multicentre study of 104 cases. Heart. 2005;91:954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chu VH, Park LP, Athan E, Delahaye F, Freiberger T, Lamas C, Miro JM, Mudrick DW, Strahilevitz J, Tribouilloy C, Durante‐Mangoni E, Pericas JM, Fernández‐Hidalgo N, Nacinovich F, Rizk H, Krajinovic V, Giannitsioti E, Hurley JP, Hannan MM, Wang A; International Collaboration on Endocarditis (ICE) Investigators . Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation. 2015;131:131–140. [DOI] [PubMed] [Google Scholar]
- 26. Park LP, Chu VH, Peterson G, Skoutelis A, Lejko‐Zupa T, Bouza E, Tattevin P, Habib G, Tan R, Gonzalez J, Altclas J, Edathodu J, Fortes CQ, Siciliano RF, Pachirat O, Kanj S, Wang A; International Collaboration on Endocarditis (ICE) Investigators . Validated risk score for predicting 6‐month mortality in infective endocarditis. J Am Heart Assoc. 2016;5:e003016 DOI: 10.1161/JAHA.115.003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis‐Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallace SM, Walton BI, Kharbanda RK, Hardy R, Wilson AP, Swanton RH. Mortality from infective endocarditis: clinical predictors of outcome. Heart. 2002;88:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasbun R, Vikram HR, Barakat LA, Buenconsejo J, Quagliarello VJ. Complicated left‐sided native valve endocarditis in adults: risk classification for mortality. JAMA. 2003;289:1933–1940. [DOI] [PubMed] [Google Scholar]
- 30. Selton‐Suty C, Celard M, Le Moing V, Doco‐Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B; AEPEI Study Group . Preeminence of Staphylococcus aureus in infective endocarditis: a 1‐year population‐based survey. Clin Infect Dis. 2012;54:1230–1239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Ninth Edition, Clinical Modification, Elixhauser Comorbidity Software, and Clinical Classification Software Were Used to Identify Patient/Hospital Characteristics
Figure S1. Kaplan‐Meier curve of 30‐day readmissions after infective endocarditis.
Figure S2. Estimated mean costs of index admissions and 30‐day readmissions after infective endocarditis.


