Abstract
Background
Cardiac catheterization is an important but costly component of health care for young patients with cardiac disease. Measurement of variation in their cost between hospitals and identification of the reasons for this variation may help reduce cost without compromising quality.
Methods and Results
Using data from Pediatric Health Information Systems Database from January 2007 to December 2015, the costs of 9 procedures were measured. Mixed‐effects multivariable models were used to generate case‐mix–adjusted estimates of each hospital's cost for each procedure and measure interhospital variation. Procedures (n=35 637) from 43 hospitals were studied. Median costs varied from $8249 (diagnostic catheterization after orthotopic heart transplantation) to $38 909 (transcatheter pulmonary valve replacement). There was marked variation in the cost of procedures between hospitals with 3.5‐ to 8.9‐fold differences in the case‐mix–adjusted cost between the most and least expensive hospitals. No significant correlation was found between hospitals’ procedure‐specific mortality rates and costs. Higher procedure volume was not associated with lower cost except for diagnostic procedures in heart transplant patients and pulmonary artery angioplasty. At the hospital level, the proportion of cases that were outliers (>95th percentile) was significantly associated with rank in terms of cost (Spearman's ρ ranging from 0.37 to 0.89, P<0.01).
Conclusions
Large‐magnitude hospital variation in cost was not explained by case‐mix or volume. Further research is necessary to determine the degree to which variation in cost is the result of differences in the efficiency of the delivery of healthcare services and the rate of catastrophic adverse outcomes and resultant protracted and expensive hospitalizations.
Keywords: congenital cardiac defect, congenital heart disease, cost, health services research, healthcare costs
Subject Categories: Congenital Heart Disease, Health Services
Clinical Perspective
What Is New?
The current study provides empirical measurements of the cost of pediatric/congenital cardiac catheterization laboratory procedures, demonstrating large‐scale differences in the cost of these procedures between hospitals that are not explained by case‐mix or procedural volume in most cases.
Hospitals with high costs for one procedure were more likely to also have high costs for other procedures.
Interhospital variation in cost is likely caused by both differences in the efficiency of the delivery of healthcare services and catastrophic adverse outcomes that result in protracted and expensive hospitalizations.
What Are the Clinical Implications?
Reducing cost without compromising clinical outcomes is an important goal, and the procedures with the greatest interhospital variation (patent ductus arteriosus, atrial septal defects, and transcatheter pulmonary valve replacement) are optimal candidates for cost reduction by optimizing clinical practice efficiency.
Simultaneously, across all procedures, the greatest potential savings are likely to be achieved by reducing the risk of outlier cases, as the most expensive 5% of cases are responsible for a disproportionate share of the cost for all procedures.
Preventing or reducing the risk of catastrophic adverse outcomes has the potential to lower costs while improving clinical outcomes.
Introduction
Congenital heart disease is the most common congenital anatomical defect, and the care of children with congenital heart disease incurs a disproportionately large proportion of pediatric healthcare spending relative to its prevalence, constituting 14% of the total inpatient hospital costs spent on children.1 Transcatheter interventions have proven to be effective therapies for a number of cardiac conditions, delivering excellent value (ie, lower cost and lower risk of adverse outcomes and morbidity) relative to traditional operative interventions.2, 3, 4, 5 As techniques for transcatheter interventions have proliferated, there has been a parallel increase in the utilization of pediatric/congenital cardiac catheterization laboratory (PCCL) procedures.6 In light of these trends, it is important to understand the cost impact of PCCL procedures in young patients, with the ultimate goals of ensuring high‐quality outcomes and delivering high‐value, economically efficient care to patients. Measuring the degree to which the cost of PCCL procedures varies between hospitals after case‐mix adjustment can demonstrate whether there is room for improvement in the efficiency of care delivered. Analogous studies in congenital cardiac surgery have demonstrated large‐magnitude variation in costs that were not explained by the incidence of adverse events.6, 7 Variation of this kind is evidence that hospitals can reduce the cost of these procedures without affecting the quality of care delivered.
Until recently, studying the variation in cost of PCCL procedures has not been possible because data sources with sufficient numbers of both patients and hospitals were not available. We addressed this problem by accessing data from the Pediatric Health Information Systems (PHIS) database, an administrative database of freestanding children's hospitals in the United States, to perform a multicenter retrospective observational study of PCCL costs and outcomes.
Methods
Data Source
The PHIS database is an administrative database that contains data from inpatient, emergency department, ambulatory surgery, and observation encounters from 48 not‐for‐profit, tertiary‐care, pediatric hospitals in the United States. This approach excludes catheterization procedures billed as outpatient procedures. The proportion of procedures billed this way at each hospital is not ascertainable but, as seen in previous studies, is not likely to differ substantially among hospitals.8, 9, 10 These hospitals are a subset of those hospitals affiliated with the Children's Hospital Association (CHA). Data quality and reliability are assured through a joint effort between CHA and participating hospitals. For the purposes of external benchmarking, participating hospitals provide discharge or encounter data including demographics, diagnoses, and procedures. The majority of these hospitals also submit resource utilization data (eg, pharmacy products, radiological studies, and laboratory studies) to PHIS. Data are deidentified at the time of data submission and subjected to a number of reliability and validity checks. The institutional review board of the Children's Hospital of Philadelphia has determined that all studies using data from PHIS do not constitute human subjects research in accordance with the Common Rule (45 CFR 46.102[f]). Data, analytic methods and study materials will not be made available to other researchers for the purposes of reproducing the results or replicating the procedures because such sharing is not consistent with the data use agreement between the study staff and CHA.
Study Population
We included children and adults undergoing one of 9 PCCL procedures: (1) device closure of patent ductus arteriosus (PDA), (2) device closure of ostium secundum atrial septal defect (ASD), (3) balloon valvuloplasty for pulmonic stenosis, (4) balloon valvuloplasty for aortic stenosis, (5) balloon or stent angioplasty of pulmonary arteries, (6) balloon or stent angioplasty of coarctation of the aorta, (7) diagnostic catheterization after orthotopic heart transplantation (OHT) with or without endomyocardial biopsy, (8) diagnostic catheterization for a patient with pulmonary hypertension (PH), and (9) transcatheter pulmonary valve replacement (TC‐PVR) between January 1, 2007, and September 30, 2015. The 7 interventional procedures are those that are used in the IMPACT (Improving Adult and Congenital Treatment) registry as benchmark procedures in the field of pediatric interventional cardiology.11, 12, 13 Diagnostic catheterizations in patients with PH and after OHT were chosen because these procedures are diagnostic and (within each group) would have consistent procedural complexity and risk, which we anticipated would result in similarly homogeneous costs. Differentiating between catheterizations with and without biopsy after OHT was not attempted. Given that the majority of cases after OHT include a biopsy, the costs most likely include the costs associated with biopsy. Participants were identified by International Classification of Diseases, Ninth Revision (ICD‐9) codes (Table S1). Codes for both diagnoses and procedures were used to improve the accuracy of cohort identification.8, 9 For PDA cases, ICD‐9 codes did not adequately differentiate between operative and transcatheter procedures, so Current Procedural Terminology supply codes identifying transcatheter closure were used to specifically identify transcatheter procedures (Table S1).
Cases from hospitals (1) that reported fewer than 25 total cardiac catheterization or total cardiac operative procedures per year over the study period or (2) that reported cardiac catheterization procedures and cardiac operations in <3 of the 9 years during the study period were excluded from analysis. This approach restricted the analysis to centers with stable reporting practices and adequate procedural volumes, as described previously.3, 4, 8, 9
Further participant‐level exclusions were applied to generate a homogeneous sample of procedures for analysis. To avoid including costs associated with lengthy hospitalization before catheterization, the study population was restricted to patients who underwent catheterization on the first 3 days of a hospitalization and who were >7 days of age at admission. Because intervention on critical valve stenosis in neonates represents a qualitatively different clinical scenario than in older children, only participants aged >30 days were included for aortic and pulmonary valvuloplasty.14 Balloon pulmonary valvuloplasty, ASD, PDA, balloon aortic valvuloplasty, and coarctation angioplasty cases were restricted to patients without other concomitant congenital heart disease. Procedures in which >1 PCCL procedure was coded were also excluded. However, it was noted during analyses that no TC‐PVR procedures also contained pulmonary artery angioplasty codes. Because these 2 procedures are almost always performed in tandem, we presumed that stent and/or balloon angioplasty of the main and branch pulmonary arteries were also performed during many if not all TC‐PVR cases.
Study Measures
Data were extracted from the PHIS database by direct query using ICD‐9 codes for diagnoses and procedures (Table S1). Patient‐level data included age, sex, race (white, black, Asian, other, or missing), insurance payer (private, Medicaid, other governmental insurance, or other), genetic syndromes, noncardiac congenital anomalies, and history of prematurity. Medical comorbidities were assigned using a coding system that has been described previously.15 Comorbidities in groups with <1% prevalence in the study population were grouped together in a single other medical condition category.
As described previously, several steps were undertaken to generate cost data that were comparable among hospitals and across the entire study period.2, 3, 4, 5 PHIS receives billing data directly from hospitals, including itemized charges, and converts these charges to costs using hospital‐ and department‐specific ratios of costs to charges. Details of hospital costs at the department level are not released by PHIS. Costs were also adjusted for regional wage‐price indexes to produce costs comparable between hospitals across the country. We further adjusted costs to account for inflation using the Consumer Price Index for medical care, as compiled by the US Bureau of Labor Statistics,16 in so doing adjusting pre‐2015 costs to year 2015 US dollars. PHIS includes data about inpatient and observation admissions but does not contain data about the fraction of procedures performed as outpatient procedures. Outpatient procedures represent a small proportion of the panel, but it should be noted that they will be associated with lower costs than other procedures.
Data Analysis
For analysis, the study population was divided into subgroups by the procedure performed. Baseline characteristics of each cohort were described using standard descriptive statistics. Continuous variables were expressed as median (range and interquartile range). Categorical variables were expressed as percentage (count). ANOVA, the Wilcoxon rank sum test, and χ2 tests were used to assess differences in distribution of baseline characteristics of the subgroups.
The first aim of the study was to characterize the distribution of costs of each procedure. Cost data are skewed, so costs were calculated for each subgroup and expressed as median, interquartile range, and range. To adjust for case mix, multivariable models for cost were calculated that included age, sex, race, insurance payer, genetic syndrome, and history of gastrointestinal, hematologic, neurological, pulmonary, or other condition as covariates. Prematurity was present in a significant proportion of PDA closure cases and not other case types; therefore, it was added as a covariate for the PDA models only. A random hospital intercept was added to account for clustering within hospitals. Generalized linear models were fit using a γ distribution (with a log link), which has demonstrated superior utility in simulation studies to address skewness in cost data.12 Models were calculated using robust variance estimation. These models have been used successfully in several previous studies of cost in congenital heart disease.3, 4, 10 A sensitivity analysis including hospital as a fixed effect was performed (rather than as a random effect) with similar results (data not shown). Using these models, conditional standardization was used to estimate the cost of a standard‐risk patient (aged 1–8 years, male, and white, with public insurance and no comorbidities) and 95% CIs at each hospital for each procedure. These standardized costs were compared, depicting the range of costs noted among hospitals in the study sample. To further characterize interhospital (intercluster) variation, intraclass correlation coefficients (ICCs) for cost between centers were calculated.1, 14 As a metric, ICCs are positive numbers between 0 and 1, with increasing numbers representing a greater degree of interhospital variation. By convention, ICCs ≥0.3 reflect substantial variation at the hospital level.17 ICCs provide a measure of the variation between centers across the entire range of costliness, providing data not captured in the ratio of most and least expensive hospitals.
Secondary analyses explored the reasons for hospital‐level variation in cost. First, we sought to determine if high cost was a consistent hospital characteristic across different procedures or if there were differences in the relative rankings of hospitals in terms of the cost of individual procedures. To do this, Spearman's rank correlation coefficients were calculated for hospital‐level adjusted costs between each of the study procedures, determining whether the most (and least) expensive hospitals were also the most expensive hospitals for other procedures. Next, the associations between hospital PCCL procedure volume and hospital cost of each procedure was evaluated. To do so, hospital catheterization laboratory volumes were divided into quartiles, as described previously (≤150, 150–300, 300–500, >500 cases/year),18 and these volume quartiles were added to previously calculated models. As a sensitivity analysis, an analogous analysis using hospital cardiothoracic surgical volume (as calculated previously7) was performed. A sensitivity analysis restricting analysis to hospitals with >10 of the procedure of interest was performed to confirm that outliers at small‐volume centers did not bias the observed results. Third, we sought to determine whether high‐cost centers delivered superior clinical outcomes, by calculating the Spearman's rank correlation coefficient for each hospital's procedure‐specific observed death rate and the case‐mix–adjusted cost of that procedure. Fourth, the degree to which costs were influenced by the minority of cases with extremely high cost was evaluated. To this end, the percentage of the total cost of all procedures performed due to the most expensive 5% of procedures was calculated. Then for each procedure, the Spearman's rank correlation coefficient between percentage of procedures at the hospital that were in the 5% of most expensive procedures and the adjusted median cost at that hospital was calculated.
Missing data were generally infrequent (<1% for most variables). However, there were >5% missing data for race. To mitigate bias, a separate categorical variable for missing race was generated. Otherwise, cases with missing data were excluded by case restriction. No imputation was used.
All data analysis was performed using Stata MP 15 (StataCorp). The threshold for statistical significance was P<0.05.
Results
Study Population
A total of 35 637 procedures in 20 730 participants from 43 hospitals were included in the study cohort (Figure 1). The study population had a median age of 4 years (interquartile range: 0–13 years) and was 53% male and 70% white (Table 1). Hospital mortality was <1% for all procedures other than diagnostic procedures in PH patients (1.6%) and PDA closure (1.8%). Median hospital length of stay was 1 day for all procedures (Table 2).
Figure 1.
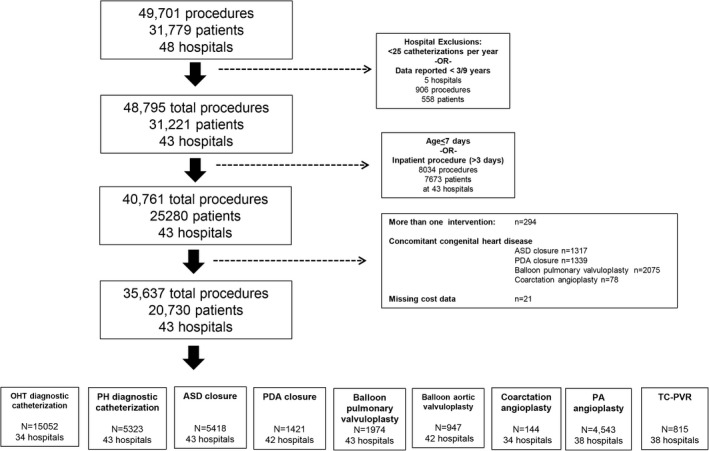
Study population. ASD indicates atrial septal defect; OHT, orthotopic heart transplantation; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Table 1.
Study Population
| Diagnostic Catheterization After OHT | Diagnostic Catheterization With PH | Device Closure of ASD | Transcatheter Closure of PDA | Balloon Pulmonary Valvuloplasty | Balloon Aortic Valvuloplasty | Coarctation Angioplasty | PA Angioplasty | TC‐PVR | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 15 053 | 5327 | 5424 | 1421 | 1980 | 949 | 144 | 4432 | 808 | |
| Hospitals, n | 34 | 43 | 43 | 42 | 43 | 42 | 34 | 38 | 38 | |
| Age, y | 11 (5–16) | 5 (1–13) | 6 (4–13) | 1 (0–3) | 1 (0–6) | 1 (0–10) | 1 (0–9) | 3 (1–8.5) | 15 (11–22) | <0.0001 |
| Age >18 y | 11 (1714) | 9.8 (521) | 8.7 (471) | 1.1 (15) | 6.2 (122) | 3.6 (34) | 4 (6) | 6.5 (294) | 34 (278) | |
| Female sex | 46 (6966) | 52 (2780) | 62 (3431) | 61 (881) | 49 (962) | 28 (265) | 43 (62) | 46 (2060) | 40 (320) | <0.001 |
| Race | <0.001 | |||||||||
| White | 70 (10 540) | 66 (3509) | 64 (3464) | 54 (763) | 68 (1337) | 78 (742) | 62 (89) | 66 (2940) | 70 (565) | |
| Black | 17 (2525) | 13 (669) | 8 (423) | 15 (206) | 9 (178) | 3 (31) | 19 (28) | 13 (578) | 10 (77) | |
| Asian | 2 (272) | 5 (259) | 4 (195) | 3 (49) | 3 (51) | 2 (16) | 2 (3) | 4 (175) | 4 (29) | |
| Other or >1 | 10 (1448) | 13 (713) | 18 (972) | 21 (304) | 16 (316) | 12 (117) | 13 (18) | 13 (564) | 11 (92) | |
| Not known/missing | 2 (268) | 3 (177) | 7 (370) | 7 (99) | 5 (98) | 5 (43) | 4 (6) | 4 (175) | 5 (42) | |
| Payer | <0.001 | |||||||||
| Private insurance | 42 (6324) | 43 (2267) | 47 (2569) | 39 (558) | 44 (871) | 49 (464) | 42 (61) | 43 (1924) | 49 (398) | |
| Medicaid | 38 (5690) | 36 (1934) | 34 (1821) | 45 (643) | 38 (755) | 31 (292) | 40 (57) | 36 (1607) | 29 (231) | |
| Other governmental insurance | 5 (799) | 6 (336) | 8 (418) | 8 (107) | 7 (137) | 6 (57) | 10 (14) | 6 (256) | 11 (86) | |
| Other source | 15 (2240) | 15 (2240) | 11 (616) | 8 (113) | 11 (217) | 14 (136) | 8 (12) | 15 (645) | 11 (90) | |
| Preprocedural conditions | ||||||||||
| Gastrointestinal | 6.2 (933) | 17.8 (947) | 1.5 (79) | 6.1 (86) | 2.0 (40) | 1.3 (12) | 6.9 (10) | 9.0 (399) | 4.0 (32) | <0.001 |
| Hematological | 8.2 (1240) | 5.6 (299) | 1.1 (57) | 1.8 (26) | 1.3 (26) | 1.8 (17) | 22.9 (33) | 7.2 (319) | 8.2 (66) | <0.001 |
| Neuromuscular | 2.4 (355) | 5.3 (282) | 1.9 (102) | 4.2 (60) | 0.7 (13) | 1.1 (10) | 4.2 (6) | 2.7 (120) | 2.5 (20) | <0.001 |
| Renal | 6.3 (945) | 3.4 (181) | 0.7 (36) | 2.0 (28) | 1.7 (33) | 1.9 (18) | 0.7 (1) | 2.2 (99) | 1.7 (14) | <0.001 |
| Respiratory | 1.5 (228) | 10.4 (556) | 0.7 (38) | 5.5 (78) | 0.7 (13) | 1.6 (18) | 5.6 (8) | 3.2 (146) | 1.4 (11) | <0.001 |
| Other noncardiac anatomical anomaly | 1.3 (192) | 5.5 (291) | 1.8 (99) | 2.1 (31) | 1.4 (28) | 1.2 (11) | 4.2 (6) | 2.5 (111) | 3.7 (30) | <0.001 |
| Genetic syndrome | 2 (249) | 18 (934) | 5 (284) | 8 (109) | 6 (116) | 3 (28) | 6 (9) | 10 (429) | 8 (64) | <0.001 |
| Prematurity | 0.0 (1) | 2.0 (108) | 0.1 (6) | 15.2 (216) | 2.0 (39) | 1.4 (13) | 0.7 (1) | 0.3 (11) | 0.1 (1) | <0.001 |
Values are expressed as median (interquartile range) or as % (n). ASD indicates atrial septal defect; OHT, orthotopic heart transplantation; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Table 2.
Outcomes of Study Procedures
| Diagnostic Catheterization After OHT | Diagnostic Catheterization With PH | Device Closure of ASD | Transcatheter Closure of PDA | Balloon Pulmonary Valvuloplasty | Balloon Aortic Valvuloplasty | Coarctation Angioplasty | PA Angioplasty | TC‐PVR | |
|---|---|---|---|---|---|---|---|---|---|
| In‐hospital mortality | 0.4 (59) | 1.6 (86) | 0.0 (0) | 1.8 (25) | 0.1 (2) | 0.4 (4) | 0.9 (38) | 0.7 (1) | 0.1 (1) |
| Length of stay, d | 1 (1–1) | 1 (1–3) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–1) |
| Observed cost (2015US$) | 8249 (6057–11 467) | 10 185 (6622–19 321) | 16 020 (12 247–21 215) | 12 793 (8696–26 792) | 10 969 (8147–14 834) | 13 791 (10 360–19 785) | 17 428 (11 809–29 039) | 16 288 (11 423–26 740) | 38 909 (23 417–58 703) |
| Standardized cost, 2015US$ (95% CI) | 10 829 (9142–12 827) | 21 423 (15 229–30 136) | 17 453 (15 310–19 895) |
Coil: 11 194 (8742–14 333) Device: 17 177 (13 226–22 308) |
15 840 (12 973–19 341) | 13 874 (11 909–16 163) |
Balloon: 18 148 (12 547–26 250) Stent: 23 230 (13 697–39 400) |
Balloon: 20 247 (16 700–24 546) Stent: 29 529 (23 282–37 453) |
43 640 (36 555–52 098) |
Values expressed as % (n) and median (interquartile range) except as noted. ASD indicates atrial septal defect; OHT, orthotopic heart transplantation; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Cost of PCCL Procedures
The median observed cost per case ranged from $8249 for a diagnostic procedure after OHT to $38 909 for TC‐PVR (Table 2). The cost of procedures generally increased in proportion to procedural technical complexity (Figure 2). The distribution of hospital‐level median observed costs for each procedure is depicted in Table S2.
Figure 2.
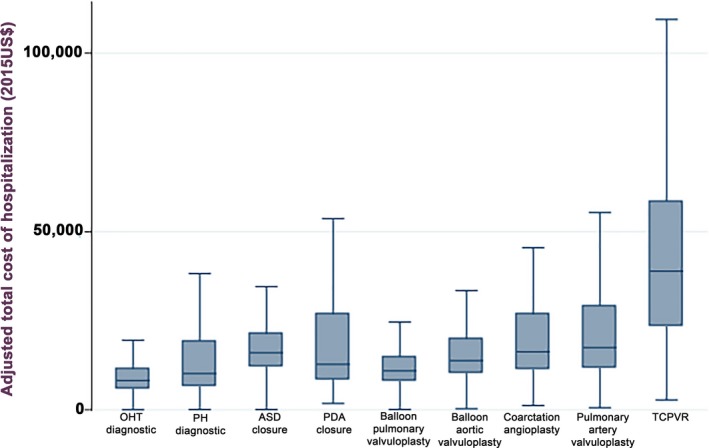
Cost of transcatheter procedures. Box and whisker plot of the observed costs of the 9 studied transcatheter procedures. Box and whiskers plot of study participants’ habitual exercise (expressed as hours/week) divided by diagnosis. The box plot demonstrates median, interquartile range, and outliers. Outliers >$100 000 are not shown. ASD indicates atrial septal defect; OHT, orthotopic heart transplant; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Mixed‐effects models for cost were calculated (Tables S3–S11) for each procedure, and case‐mix standardized costs were generated for each procedure (Table 2). These models were used to calculate postestimates of the cost of a standard case at each hospital (Table 3). There was significant variation in these estimates as well, with ratios between the most and least expensive hospitals ranging between 3.5 and 8.9. Although these measures indicate the range of cost differences between hospitals at the “extremes,” they do not capture the full degree of variation between all hospitals in the cohort—the variation over the entire distribution is better quantified by the ICC. The magnitude of interhospital variation in cost of the procedures was statistically significant but relatively small in magnitude for PH diagnostic procedures (ICC: 0.13). It was, however, much larger in magnitude for device closure of PDA (ICC: 0.36) and ASD (ICC: 0.43), as well as TC‐PVR (ICC: 0.46). Sensitivity analyses restricted analysis to hospitals with >10 procedures of interest were performed with no significant change in the results (data not shown).
Table 3.
Distribution of Case‐Mix–Adjusted Costs by Hospital
| Procedure | Minimum | 25% | Median | 75% | Maximum | Most:Least Expensive Hospitals | ICC |
|---|---|---|---|---|---|---|---|
| OHT diagnostic | 5070 | 6636 | 10 379 | 15 843 | 26 989 | 5.3 | 0.24 |
| PH diagnostic | 6640 | 15 125 | 23 783 | 30 136 | 59 629 | 8.9 | 0.13 |
| PDA closure | 7844 | 12 156 | 16 536 | 25 523 | 52 373 | 6.7 | 0.36 |
| ASD closure | 7643 | 14 092 | 16 865 | 21 275 | 53 454 | 7.0 | 0.43 |
| Balloon pulmonary valvuloplasty | 7092 | 11 682 | 14 523 | 20 061 | 33 610 | 4.7 | 0.24 |
| Balloon aortic valvuloplasty | 8099 | 11 343 | 13 144 | 16 952 | 28 032 | 3.5 | 0.21 |
| Coarctation angioplasty | 9510 | 20 717 | 22 587 | 25 506 | 39 100 | 4.1 | 0.17 |
| PA angioplasty | 9490 | 16 486 | 20 944 | 25 333 | 62 010 | 6.5 | 0.22 |
| TC‐PVR | 18 897 | 30 527 | 40 701 | 55 842 | 125 593 | 6.6 | 0.42 |
All costs expressed in 2015 US dollars. ASD indicates atrial septal defect; ICC, intraclass correlation coefficient; OHT, orthotopic heart transplantation; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Spearman's rank correlations were calculated for hospital‐specific adjusted cost for each pair of procedures (Table 4) to evaluate whether the most expensive hospitals for one procedure were also the most expensive for other PCCL procedures. Moderate or greater correlation (Spearman's ρ, range: 0.33–0.72; P<0.05) was seen between all interventional procedures except for PDA closure. For PDA closure, there was no significant correlation with cost of balloon aortic valvuloplasty, angioplasty of coarctation, and TC‐PVR, but there remained moderate (Spearman's ρ, range: 0.43–0.49; P<0.05) significant correlation with the cost of ASD closure, balloon pulmonary valvuloplasty, and pulmonary artery angioplasty. The cost of diagnostic studies for both OHT and PH patients were moderately correlated (Spearman's ρ=0.39, P=0.02) but were not consistent with interventional procedures.
Table 4.
Correlation of Hospital‐Level Adjusted Costs for Procedures
| Diagnostic OHT | Diagnostic PH | ASD Closure | PDA Closure | Balloon Pulmonary Valvuloplasty | Balloon Aortic Valvuloplasty | Coarctation Angioplasty | PA Angioplasty | TC‐PVR | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic OHT | … | 0.39 P=0.02 | 0.51 P=0.002 | 0.29 P=0.09 | 0.69 P<0.001 | 0.31 P=0.07 |
0.21 P=0.28 |
0.52 P=0.002 |
0.34 P=0.06 |
| Diagnostic PH | … | 0.43 P=0.004 | 0.25 P=0.10 | 0.34 P=0.02 | 0.14 P=0.39 |
−0.05 P=0.80 |
0.47 P=0.003 |
0.35 P=0.03 |
|
| ASD closure | … |
0.53 P=0.0003 |
0.62 P<0.0001 |
0.43 P=0.004 |
0.40 P=0.02 |
0.98 P<0.0001 |
0.70 P<0.0001 |
||
| PDA closure | … |
0.45 P=0.003 |
0.25 P=0.11 |
0.18 P=0.31 |
0.49 P=0.002 |
0.27 P=0.10 |
|||
| Balloon pulmonary valvuloplasty | … |
0.53 P=0.0003 |
0.49 P=0.003 |
0.61 P<0.0001 |
0.57 P=0.0002 |
||||
| Balloon aortic valvuloplasty | … |
0.40 P=0.02 |
0.38 P=0.02 |
0.34 P=0.04 |
|||||
| Coarctation angioplasty | … |
0.38 P=0.03 |
0.033 P=0.06 |
||||||
| PA angioplasty | … |
0.72 P<0.0001 |
|||||||
| TC‐PVR | … |
Table depicts ρ for Spearman's correlation of the ranks of hospital‐level adjusted costs between procedures. The P evaluates the null hypothesis that there is no correlation between ranks of cost between the 2 procedures. ASD indicates atrial septal defect; OHT, orthotopic heart transplantation; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Association Between Procedural Volume and Cost
For most procedures, there was not a significant association between PCCL volume quartile and the procedure's cost (Figure 3). For diagnostic catheterization in patients with OHT, undergoing catheterization at centers with 150 to 300 cases/year (cost ratio: 0.40; 95% CI, 0.35–0.47; P<0.001), 300 to 500 cases/year (cost ratio: 0.29; 95% CI, 0.21–0.41; P<0.001), or >500 cases/year (cost ratio: 0.17; 95% CI, 0.11–0.26; P<0.001) were associated with a lower total hospital cost than at a hospital with annual volume ≤150 cases/year. Undergoing a diagnostic catheterization with PH at a center with volume of 300 to 500 cases/year was associated with an increased cost (cost ratio: 1.81; 95% CI, 1.07–3.04; P=0.03) than at a hospital with annual volume ≤150 cases/year. Otherwise, PCCL procedural volume was not associated with costs. A sensitivity analysis was performed using hospital annual surgical volume (instead of catheterization volume) with similar results (data not shown).
Figure 3.
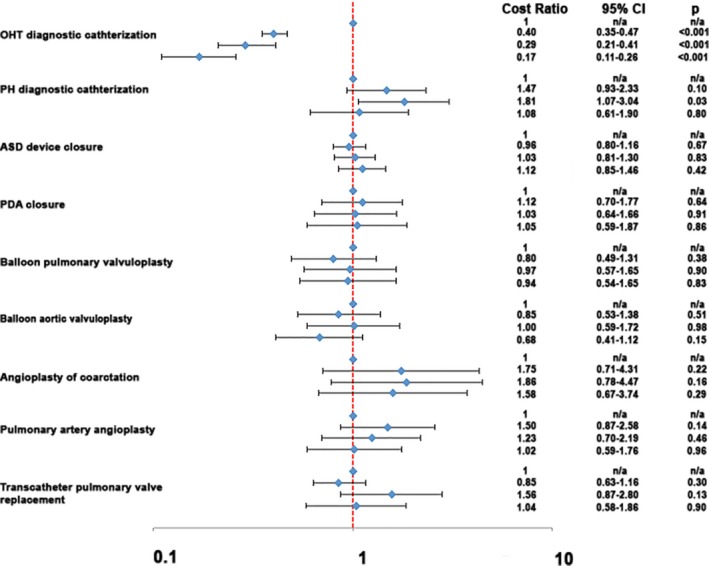
Association of hospital catheterization laboratory volume and cost. Forest plot depicts the results of a series of multivariable generalized linear models evaluating the association between hospital procedural volume and cost. Hospital catheterization volume is divided into 4 categories: ≤150 cases/year, 150 to 300 cases, 300 to 500, >500 cases (listed top to bottom for each procedure). For each procedure, the ratio of cost associated with higher procedure volume (relative to centers (≤150 cases/year) is marked with a blue diamond. The 95% CIs are depicted as brackets. A ratio of 1 is marked with a hashed orange line. Estimates to the left of this line reflect reduced cost with increasing volume, whereas estimates to the right reflect an association between increasing volume and higher cost. Results for a sensitivity analysis with hospital cardiothoracic surgical volume were similar but are not shown. ASD indicates atrial septal defect; n/a, not assessed; OHT, orthotopic heart transplant; PDA, patent ductus arteriosus; PH, pulmonary hypertension.
Association Between Clinical Outcomes and Cost
To determine whether higher cost care was associated with improved outcomes, the correlation between the observed mortality risk and hospital cost was calculated (Table S12). For diagnostic catheterizations after OHT, there was a significant association between the hospital risk of mortality and case‐mix–adjusted cost (Spearman's ρ=0.59, P=0.00002); however, there was no significant association for other procedures.
Association of Frequency of Outlier Cases and Costs
To assess the degree to which outliers influenced the cost of procedures, the percentage of each hospital's total cost of procedures that were attributable to the most expensive 5% of procedures was calculated (Table 5), revealing a range between 15% and 56%. Procedures with the largest percentage of total costs due to outliers were diagnostic procedures in patients after OHT (48%) or in patients with PH (56%) along with PDA closure procedures (49%), whereas those with the lowest ratio were ASD closure (15%) and TC‐PVR (20%). Outliers for valvuloplasty and angioplasty procedures accounted for an intermediate proportion (32–37%) of total costs. At the hospital level, there was a significant correlation between the proportion of outlier cases and the hospital's case‐mix–adjusted cost per procedure (Spearman's ρ, range: 0.37–0.86; P<0.05) for all procedures (Table 6).
Table 5.
Contributions of Outliers to Total Cost
| Sum of the 5% Most Costly Procedures (2015US$) | Sum of the Remaining 95% of Procedures (2015US$) | Percentage of Total Costs Due to the Most Expensive 5% of Cases (%) | |
|---|---|---|---|
| OHT diagnostic | 112 131 998 | 121 556 871 | 48 |
| PH diagnostic | 110 250 807 | 85 536 726 | 56 |
| ASD closure | 14 335 447 | 82 578 400 | 15 |
| PDA closure | 46 622 700 | 48 981 112 | 49 |
| Balloon pulmonary valvuloplasty | 10 965 043 | 21 681 340 | 34 |
| Balloon aortic valvuloplasty | 7 958 414 | 13 335 911 | 37 |
| Coarctation angioplasty | 1 251 951 | 2 678 445 | 32 |
| PA angioplasty | 54 400 020 | 91 371 234 | 37 |
| TC‐PVR | 7 515 444 | 30 765 391 | 20 |
ASD indicates atrial septal defect; OHT, orthotopic heart transplantation; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Table 6.
Rank Correlation of the Adjusted Cost per Hospital and Percentage of Cases at That Hospital That Were in the Most Expensive 5% of Procedures
| Spearman's ρ | P Value | |
|---|---|---|
| OHT diagnostic | 0.76 | <0.0001 |
| PH diagnostic | 0.81 | <0.0001 |
| ASD closure | 0.86 | <0.0001 |
| PDA closure | 0.49 | 0.0009 |
| Balloon pulmonary valvuloplasty | 0.53 | 0.0003 |
| Balloon aortic valvuloplasty | 0.37 | 0.02 |
| Coarctation angioplasty | 0.66 | <0.0001 |
| PA angioplasty | 0.47 | 0.003 |
| TC‐PVR | 0.48 | 0.003 |
ASD indicates atrial septal defect; OHT, orthotopic heart transplant; PA, pulmonary artery; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TC‐PVR, transcatheter pulmonary valve replacement.
Discussion
This multicenter observational study demonstrated large‐magnitude, between‐hospital variation in the costs of a panel of PCCL procedures, even after adjustment for measurable confounders. Hospitals with high costs for one PCCL intervention were also likely to have high cost for other procedures. Interhospital variation in cost was not explained by differences in the overall death rate between hospitals or by differences in procedural volume.
This study is the first, to our knowledge, to evaluate interhospital cost variation of PCCL procedures. The presence of large‐magnitude variation not explained by case mix highlights the possibility of achieving cost savings for these procedures and that this can be accomplished without compromising quality of care.
Congenital heart defects are relatively rare, but they incur a disproportionately large amount of healthcare costs in children.1 The majority of these costs are associated with hospitalizations for open‐heart surgeries,19 but PCCL procedures are also an important contributor to the overall costs. Previous studies demonstrated large‐magnitude variation in the cost of panels of congenital heart operations among US children's hospitals.6, 7 Some but not all of the interhospital variation seen in these studies is attributable to the risk of adverse events, and the rest is attributable to differences in routine care.6, 7 To our knowledge, no studies to date have researched how costs of PCCL procedures vary between centers. The current study demonstrated similar large‐magnitude variation among hospitals in costs. Interestingly, in studies of congenital heart surgery, the largest range of costs in their panels of operations were in low‐complexity operations.6 In contrast, interhospital variation in cost of PCCL procedures was of similar magnitude across the range of technical complexity. This result may be because catheterization procedures have uniform and very short postprocedure lengths of stay, and that aspect was an important determinant of cost in congenital heart surgeries.
An important consideration in studying the economic impact of medical care is to determine whether existing higher costs are associated with improved quality, because higher costs may be warranted if they are associated with reduced risk of adverse outcomes. In the current study, however, no correlation was seen between hospital average costs and mortality rate, in contrast to findings in congenital heart surgery, for which the lowest cost hospitals generally had superior risk‐adjusted outcomes.20 To our knowledge, no evidence shows that higher cost care is associated with improved outcomes in any aspect of pediatric/congenital cardiac care. If anything, in this study, analyses of high‐cost outlier cases suggested that these outlier cases, which are perhaps attributable to adverse events and consequent intensive resource utilization, are a major driver of hospitals’ overall costs for PCCL procedures. Interventions that identify high‐risk patients or patient groups and those that potentially prevent the progression of minor adverse events to catastrophic downstream consequences may reduce total costs and improve outcomes.
Another potential source of cost variation is difference in the costs associated with routine care. Efforts focused on improving the efficiency of care (eg, reducing unnecessary laboratory, radiographic, and cardiac testing in these patients; streamlining periprocedural care; standardizing PCCL practice) could reduce costs without compromising excellent clinical outcomes. The current study identified PDA closure, ASD closure, and TC‐PVR as procedures with the greatest degree of interhospital variation; therefore, they would be ideal targets for interventions to reduce unnecessary practice variation and unnecessary medical goods and services. Previous studies have demonstrated significant practice variation in the referral patterns and practices for several of these procedures,4, 10, 14, 20 suggesting that modifiable aspects of practice exist that could reduce cost without compromising quality of care. The choice of the site for postprocedure recovery is a potentially important variable but was beyond the scope of this study. Another important determinant of several PCCL procedures is the cost of devices.2, 3 One way that hospitals have reduced costs significantly in bundled‐care demonstration experiments for joint replacement is that they have engaged in competitive bidding for orthopedic implants and other medical equipment, with significant reduction in the prices of these devices.22 However, the relatively smaller number of suppliers with approved devices for PCCL procedures in the United States may make competitive bidding less effective in this context.
Surprisingly, hospital volume was not influential on costs for the majority of PCCL procedures. Across medicine, procedural volume repeatedly has been associated with improved clinical outcomes, and this has been shown in both congenital heart surgery23, 24, 25, 26, 27 and PCCL procedures.8, 9 For diagnostic catheterizations after OHT, increased procedural volume was associated with reduced cost, but with no significant association between volume and cost for the rest of the panel of PCCL procedures. This finding underscores the importance of evaluating the efficiency of healthcare delivery across the entire range of PCCL programs.
The current study has several additional limitations. It is a retrospective observational study that utilizes an administrative data set. To maximize the validity of our findings, we attempted to stringently identify the procedures included in the cohort (varying the methods by the procedure in question) and to limit those included to representative cases. We acknowledge that some clinically relevant data are not available in an administrative database, and this may result in unmeasured confounding. Also, as noted, data from outpatient catheterizations are not included in PHIS and therefore are not analyzed in this study. Many PCCL procedures are billed as observation or inpatient procedures, but some proportion (depending on hospital and insurer policies) are likely billed as outpatient procedures. These excluded outpatient procedures are more likely to be lower cost, so the current estimate of median cost is potentially inflated; however, because the proportion of outpatient procedures is likely not differential among hospitals, this exclusion should not affect the quantification of interhospital variation. Third, the cost data used were extrapolated from billing records using cost‐to‐charge ratios. This technique is widely used but is an indirect measure of resource utilization. Fourth, the costs measured are for the entire hospitalization, and for cases with a prolonged postprocedural hospitalization, some costs not strictly associated with the procedure would included in these totals. We acknowledge that we cannot differentiate costs associated with the procedure and postprocedural care in the current study. In addition, although our analysis attempted to determine whether there was any association between cost of care and quality of outcome, it is not possible in this study to determine the optimal level of spending for individual cases. Finally, the focus of this study is on cost as a measure of resource utilization, viewed from the perspective of the healthcare system as a whole rather than the payer perspective. Addressing charges and the relative reimbursement paid to hospitals, as has been done in previous studies,28 is beyond the scope of the current study.
Conclusion
There is significant between‐hospital variation in the cost of transcatheter procedures that is not explained by case mix. No evidence shows that higher cost resulted in better quality of care or better outcomes. Variability in cost is likely due to both differences in the efficiency of the delivery of healthcare services and the incidence rate of catastrophic adverse outcomes from PCCL procedures that result in protracted and expensive hospitalizations.
Sources of Funding
This research was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (K23 HL130420‐01). This project was supported in part by the Cardiac Center Clinical Research Core at the Children's Hospital of Philadelphia. The funding agencies had no role in the planning or execution of the study, nor did they edit the article as presented. The article represents the opinions of the authors alone.
Disclosures
Dr O'Byrne has received modest speaking fees from Gore Medical. The remaining authors have no disclosures to report.
Supporting information
Table S1. Procedures and International Classification of Diseases, Ninth Revision Codes
Table S2. Distribution of Hospital‐Level Median Observed Costs
Table S3. Mixed‐Effects Models for the Cost of Diagnostic Catheterization Following Orthotopic Heart Transplantation (n=14 304)
Table S4. Mixed Effects Models for the Cost of Diagnostic Catheterization for Pulmonary Hypertension (n=5024)
Table S5. Mixed Effects Models for the Cost of Transcatheter ASD Closure (n=5238)
Table S6. Mixed Effects Models for the Cost of Transcatheter PDA Closure (n=1284)
Table S7. Mixed Effects Models for the Cost of Balloon Pulmonary Valvuloplasty (n=1922)
Table S8. Mixed Effects Models for the Cost of Balloon Aortic Valvuloplasty (n=918)
Table S9. Mixed Effects Models for the Cost of Coarctation Angioplasty (n=141)
Table S10. Mixed Effects Models for the Cost of Angioplasty of Pulmonary Arteries (n=4273)
Table S11. Mixed Effects Models for the Cost of Transcatheter Pulmonary Valve Replacement (n=808)
Table S12. Rank Correlation of Hospital Procedure‐Specific Mortality Rate and Adjusted Cost
(J Am Heart Assoc. 2019;8:e011543 DOI: 10.1161/JAHA.118.011543.)
References
- 1. Keren R, Luan X, Localio R, Hall M, McLeod L, Dai D, Srivastava R; Pediatric Research in Inpatient Settings (PRIS) Network . Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166:1155–1164. [DOI] [PubMed] [Google Scholar]
- 2. O'Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J. 2015;169:727–735.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative pulmonary valve replacement (from the Pediatric Health Information Systems Database). Am J Cardiol. 2016;117:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Byrne ML, Glatz AC, Mercer‐Rosa L, Gillespie MJ, Dori Y, Goldmuntz E, Kawut S, Rome JJ. Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. Am J Cardiol. 2015;115:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, Kogon B, Oster ME. Transcatheter versus surgical closure of atrial septal defects in children: a value comparison. JACC Cardiovasc Interv. 2016;9:79–86. [DOI] [PubMed] [Google Scholar]
- 6. Pasquali SK, Sun JL, d'Almada P, Jaquiss RDB, Lodge AJ, Miller N, Kemper AR, Lannon CM, Li JS. Center variation in hospital costs for patients undergoing congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2011;4:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasquali SK, Jacobs ML, He X, Shah SS, Peterson ED, Hall M, Gaynor JW, Hill KD, Mayer JE, Jacobs JP, Li JS. Variation in congenital heart surgery costs across hospitals. Pediatrics. 2014;133:e553–e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Byrne ML, Glatz AC, Shinohara RT, Jayaram N, Gillespie MJ, Dori Y, Rome JJ, Kawut S. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. Am Heart J. 2015;169:823–832.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Byrne ML, Glatz AC, Hanna BD, Shinohara RT, Gillespie MJ, Dori Y, Rome JJ, Kawut SM. Predictors of catastrophic adverse outcomes in children with pulmonary hypertension undergoing cardiac catheterization: a multi‐institutional analysis from the Pediatric Health Information Systems Database. J Am Coll Cardiol. 2015;66:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Byrne ML, Shinohara RT, Grant EK, Kanter JP, Gillespie MJ, Dori Y, Rome JJ, Glatz AC. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: an observational study using data from the Pediatric Health Information Systems database. Am Heart J. 2017;192:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vincent RN, Moore J, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Ringel R, Rome J, Martin GR. Procedural characteristics and adverse events in diagnostic and interventional catheterisations in paediatric and adult CHD: initial report from the IMPACT Registry. Cardiol Young. 2016;26:70–78. [DOI] [PubMed] [Google Scholar]
- 12. Martin GR, Beekman RH, Ing FF, Jenkins KJ, McKay CR, Moore JW, Ringel RE, Rome JJ, Ruiz CE, Vincent RN. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:20–25. [DOI] [PubMed] [Google Scholar]
- 13. Moore JW, Vincent RN, Beekman RH, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Li Y, Ringel R, Rome J, Martin GR; NCDR IMPACT Steering Committee . Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2014;64:2439–2451. [DOI] [PubMed] [Google Scholar]
- 14. Glatz AC, Kennedy KF, Rome JJ, O'Byrne ML. Variations in practice patterns and consistency with published guidelines for balloon aortic and pulmonary valvuloplasty. JACC Cardiovasc Interv. 2018;11:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:1–5. [DOI] [PubMed] [Google Scholar]
- 16. United States Department of Labor Bureau of Labor Statistics. Consumer Price Index, Medical Care. Available at: www.bls.gov/data.
- 17. Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies. Am J Epidemiol. 1999;149:876–883. [DOI] [PubMed] [Google Scholar]
- 18. Jayaram N, Spertus JA, O'Byrne ML, Chan PS, Kennedy KF, Bergersen L, Glatz AC. Relationship between hospital procedure volume and complications following congenital cardiac catheterization: a report from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J. 2017;183:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinto NM, Waitzman N, Nelson R, Minich LL, Krikov S, Botto LD. Early childhood inpatient costs of critical congenital heart disease. J Pediatr. 2018;203:371–379.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasquali SK, Jacobs JP, Bove EL, Gaynor JW, He X, Gaies MG, Hirsch‐Romano JC, Mayer JE, Peterson ED, Pinto NM, Shah SS, Hall M, Jacobs ML. Quality‐cost relationship in congenital heart surgery. Ann Thorac Surg. 2015;100:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Byrne ML, Kennedy KF, Rome JJ, Glatz AC. Variation in practice patterns in device closure of atrial septal defects and patent ductus arteriosus: an analysis of data from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J. 2018;196:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navathe AS, Troxel AB, Liao JM, Nan N, Zhu J, Zhong W, Emanuel EJ. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177:214–219. [DOI] [PubMed] [Google Scholar]
- 23. Checchia PA, McCollegan J, Daher N, Kolovos N, Levy F, Markovitz B. The effect of surgical case volume on outcome after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;129:754–759. [DOI] [PubMed] [Google Scholar]
- 24. Bazzani LG, Marcin JP. Case volume and mortality in pediatric cardiac surgery patients in California, 1998–2003. Circulation. 2007;115:2652–2659. [DOI] [PubMed] [Google Scholar]
- 25. Hirsch JC, Gurney JG, Donohue JE, Gebremariam A, Bove EL, Ohye RG. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pediatr Cardiol. 2008;29:713–717. [DOI] [PubMed] [Google Scholar]
- 26. Welke KF, O'Brien SM, Peterson ED, Ungerleider RM, Jacobs ML, Jacobs JP. The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. J Thorac Cardiovasc Surg. 2009;137:1133–1140. [DOI] [PubMed] [Google Scholar]
- 27. Pasquali SK, Li JS, Burstein DS, Sheng S, O'Brien SM, Jacobs ML, Jaquiss RDB, Peterson ED, Gaynor JW, Jacobs JP. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics. 2012;129:e370–e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bergersen L, Brennan A, Gauvreau K, Connor J, Almodovar M, Dinardo J, David S , Triedman J, Banka P, Emani S, Mayer JE. A method to account for variation in congenital heart surgery charges. Ann Thorac Surg. 2015;99:939–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Procedures and International Classification of Diseases, Ninth Revision Codes
Table S2. Distribution of Hospital‐Level Median Observed Costs
Table S3. Mixed‐Effects Models for the Cost of Diagnostic Catheterization Following Orthotopic Heart Transplantation (n=14 304)
Table S4. Mixed Effects Models for the Cost of Diagnostic Catheterization for Pulmonary Hypertension (n=5024)
Table S5. Mixed Effects Models for the Cost of Transcatheter ASD Closure (n=5238)
Table S6. Mixed Effects Models for the Cost of Transcatheter PDA Closure (n=1284)
Table S7. Mixed Effects Models for the Cost of Balloon Pulmonary Valvuloplasty (n=1922)
Table S8. Mixed Effects Models for the Cost of Balloon Aortic Valvuloplasty (n=918)
Table S9. Mixed Effects Models for the Cost of Coarctation Angioplasty (n=141)
Table S10. Mixed Effects Models for the Cost of Angioplasty of Pulmonary Arteries (n=4273)
Table S11. Mixed Effects Models for the Cost of Transcatheter Pulmonary Valve Replacement (n=808)
Table S12. Rank Correlation of Hospital Procedure‐Specific Mortality Rate and Adjusted Cost


