Abstract
Background
The natural history and long‐term outcome in pediatric patients with idiopathic ventricular fibrillation (IVF) are poorly characterized. We sought to define the clinical characteristics and long‐term outcomes of a pediatric cohort with an initial diagnosis of IVF.
Methods and Results
Patients were included from an International Registry of IVF (consisting of 496 patients). Inclusion criteria were: (1) VF with no identifiable cause following comprehensive analysis for ischemic, electrical or structural heart disease and (2) age ≤16 years. These included 54 pediatric IVF cases (age 12.7±3.7 years, 59% male) among whom 28 (52%) had a previous history of syncope (median 2 syncopal episodes [interquartile range 1]). Thirty‐six (67%) had VF in situations associated with high adrenergic tone. During a median 109±12 months of follow‐up, 31 patients (57%) had recurrence of ventricular arrhythmias, mainly VF. Two patients developed phenotypic expression of an inherited arrhythmia syndrome during follow‐up (hypertrophic cardiomyopathy and long QT syndrome, respectively). A total of 15 patients had positive genetic testing for inherited arrhythmia syndromes. Ten patients (18%) experienced device‐related complications. Three patients (6%) died, 2 due to VF storm.
Conclusions
In pediatric patients with IVF, a minority develop a definite clinical phenotype during long‐term follow‐up. Recurrent VF is common in this patient group.
Keywords: idiopathic, defibrillator, ventricular fibrillation, complications, ventricular tachycardia, syncope
Subject Categories: Arrhythmias, Sudden Cardiac Death, Ventricular Fibrillation, Electrophysiology
Clinical Perspective
What Is New?
This is the first study to systematically evaluate long‐term outcomes among pediatric patients with an initial diagnosis of idiopathic ventricular fibrillation.
We demonstrate that among pediatric idiopathic ventricular fibrillation patients, arrhythmias commonly occur in the context of high adrenergic tone, and there is a high incidence of recurrent arrhythmic events.
Only a small minority of patients develop evidence of an underlying cardiac phenotype during long‐term follow‐up.
What Are the Clinical Implications?
Over the years, the risk of recurrent arrhythmic events continues to be elevated, and therefore, in addition to implantation of cardiac defibrillators, these patients should be followed up closely.
The incidence of complications related to implanted cardioverter/defibrillators in this pediatric population is relatively high, which may have important implications in terms of choice of device.
Introduction
The majority of cases of arrhythmic sudden cardiac death (SCD) in young patients are attributable to either inherited structural cardiac diseases or primary arrhythmic syndromes.1 In a subset of patients the cause of ventricular fibrillation remains undefined despite comprehensive diagnostic evaluation and is referred to as idiopathic ventricular fibrillation (VF). According to contemporary guidelines idiopathic VF is defined as resuscitated cardiac arrest that occurs in the absence of cardiac, metabolic, toxicological, and respiratory causes.2 Overall, idiopathic VF accounts for up to 8% of arrhythmic sudden cardiac death cases.3
There is a relative paucity of data on the natural history and long‐term outcome in patients of pediatric age who present with idiopathic VF (IVF). Available studies have largely focused on adult cohorts with IVF.4 The aim of the present study is to define the long‐term outcome in pediatric VF patients.
Methods
Data Collection
Patients were included from an International Registry of Idiopathic Ventricular Fibrillation (IVF) that consists of 496 IVF patients5 from 45 tertiary cardiac centers worldwide (collected between March 2004 and December 2015). The data are available on request from the authors. Criteria for inclusion in the registry were (1) sudden cardiac arrest with documented VF as the cause of arrest, (2) an absence of an underlying cause following comprehensive analysis for ischemic, electrical, infectious, or structural heart disease, and (3) age ≤16 years. The diagnostic criteria for IVF were based on the latest consensus guidelines.1
Patients were systematically assessed at the referring centers after the index VF episode (before being assigned a diagnosis of IVF). Data on past clinical symptoms, including previous syncopal episodes, were collected. Family history of SCD was defined as a sudden death that occurred before 55 years of age in the absence of any known underlying pathological conditions.
The clinical workup consisted of a baseline ECG, blood tests (including cardiac enzymes and electrolytes), toxicology screening, and cardiac imaging (echocardiography and/or cardiac magnetic resonance imaging). Ajmaline testing was performed in order to exclude a diagnosis of concealed Brugada syndrome. Isoproterenol or epinephrine testing and exercise stress testing (using a modified Bruce protocol adapted for pediatric patients) were performed to exclude long‐QT syndrome and catecholaminergic polymorphic ventricular tachycardia (VT). The aforementioned tests were performed at the investigating physician's discretion at the referring center. Signal‐averaged ECG and endomyocardial biopsies were also performed at the investigating physician's discretion. Time of implanted cardioverter/defibrillator (ICD) implantation and device programming were left to the decision of the referring physician (according to latest guidelines in order to reduce the possibility of inappropriate ICD shocks). The study was approved by the Institutional Review Board at CHU Bordeaux (inclusion in the IVF registry was approved by the institutional review boards at the respective institutions).
IVF Characteristics
The circumstances during the index VF event were categorized as (1) at rest, (2) daily activity, (3) sleep, (4) emotional stress, or (5) sport/strenuous activity. “At rest” was defined as SCD that occurred during no physical activity. “Daily activity” was defined as SCD that occurred during routine activities with very light physical activity. “Sleep” was defined as SCD occurring during bedtime or while asleep. Emotional stress was defined as intense emotion. Strenuous activity was defined as moderate to severe exercise (including in the setting of sport).
ECG Analysis
All ECGs (during the index hospitalization and at follow‐up) were reviewed by 2 independent electrophysiologists (K.T., V.K.). In case of disagreement, the ECG was reviewed by a third electrophysiologist (N.D.). ECGs were analyzed at a paper speed of 25 mm/s and an amplitude of 10 mm/mV. Digital calipers were used to perform measurements (EP Calipers version 1.8 [http://www.epstudiossoftware.com/], EP Studios, 2017). P‐wave duration and dispersion were calculated from the standard ECG during sinus rhythm where dispersion was defined as the difference between the minimum and maximum P‐wave duration. Early repolarization was defined according to the latest consensus guidelines.6 Abnormal QRS fragmentation was defined as either ≥4 spikes in 1 lead or >1 notches in the nadir of the R or S wave in 2 consecutive leads. Davignon criteria7 were used to exclude right and left ventricular hypertrophy. S waves were characterized in terms of amplitude and duration in lead I. T peak–T end interval was measured from the peak to the end of the T wave in the precordial leads.
Invasive Electrophysiology Study
Programmed ventricular stimulation was performed at the investigating physicians' discretion. A consistent stimulation protocol from the right ventricular apex and outflow tract was used (2 drive cycle lengths [600 and 400 ms] and 3 extrastimuli with a minimal coupling up to 180 ms [unless ventricular arrhythmia was induced or the ventricular effective refractory period was reached]). A sustained VT/VF episode, defined as an episode lasting >30 s or VT/VF requiring direct current cardioversion, was considered a positive electrophysiological study. Nonsustained episodes (<30 s) were classified as negative electrophysiological study.
Genetic Testing
When available, genetic testing for variants implicated in heritable cardiomyopathies and/or channelopathies (long QT syndrome, short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia) was performed per consensus guidelines. When a putative mutation was identified, cascade screening was performed in family members.2 The choice of genes targeted during genetic analysis was at the treating physician's discretion. Assignment of pathogenicity of identified variants was based on conventional guidelines.3 Repeat genetic testing with more extensive genetic panels was performed at the treating physician's discretion.
Follow‐Up
Patients were followed up with sequential ICD interrogations (at least every 4 months), ECG, and cardiac imaging (echocardiography). Appropriate and inappropriate therapies with an implantable defibrillator were documented. Genotype and phenotype reevaluations during follow‐up were performed at the investigating physician's discretion. Phenotypic reevaluation included repeat cardiac imaging, exercise stress testing, and pharmacological stress testing.
Statistical Analyses
Continuous variables were expressed as mean±standard deviation. Categorical variables were expressed as percentages. Qualitative variables were compared using the Fisher exact test, whereas the Mann‐Whitney test was used for continuous variables. Kaplan‐Meier analysis was also performed for arrhythmia‐free survival. A P<0.05 was considered to be statistically significant.
Results
Patient Characteristics
Fifty‐four of the 496 patients in the IVF registry fulfilled inclusion criteria. Patient characteristics are summarized in Table 1. At the time of the index VF episode, the mean age was 12.7±3.7 years. Thirty‐two patients (59%) were male. Patients were predominantly of white descent (45 white [84%]; 5 Afro‐Caribbean [9%]; 4 Asian [7%]). Twenty‐eight patients (52%) had a prior history of syncope (median 2 syncopal episodes, interquartile range 1). Twenty‐four (85%) had effort‐induced syncope. The syncopal events occurred 54±10 days before the sudden cardiac arrest. Five patients (9%) had previously been diagnosed with epilepsy. None of the patients had a history of recreational drug use. Specific details of the circumstances of the index episode are included in Table 1. All 54 patients underwent ICD implantation.
Table 1.
Population Study
| Age, y | 12.7±3.7 |
| Male, n (%) | 32 (59%) |
| Ethnicity, n (%) | |
| White | 45 (84%) |
| Afro‐Caribbean | 5 (9%) |
| Asian | 4 (7%) |
| Circumstances of SCD, n (%) | |
| Sport/strenuous activity | 15 (28%) |
| Daily activity | 11 (20%) |
| Emotional stress | 11 (20%) |
| Rest | 12 (22%) |
| Sleep | 5 (10%) |
| Confirmation of IVF, n (%) | |
| Coronary angiogram | 24 (44%) |
| Imaging | |
| Echo | 54 (100%) |
| CMR | 38 (70%) |
| Treadmill test | 45 (83%) |
| Pharmacological tests, n (%) | |
| Isoproterenol | 38 (70%) |
| Ajmaline | 24 (44%) |
| Flecainide | 13 (24%) |
| Endomyocardial biopsy | 7 (13%) |
| Before the SCD event, n (%) | |
| Syncope | 28 (52%) |
| Epilepsy | 5 (9%) |
| Palpitations | 2 (4%) |
| Family history of SCD, n (%) | 15 (28%) |
| First degree | 10 (67%) |
| Second degree | 3 (20%) |
| Third degree | 2 (13%) |
CMR indicates cardiac magnetic resonance; Echo, echocardiogram; IVF, idiopathic ventricular fibrillation; SCD, sudden cardiac death.
Fifteen patients (28%) had a family history of SCD (10 [67%] a first‐degree relative; 3 [20%] a second‐degree relative; 2 [13%] a third‐degree relative). The mean age of sudden cardiac death in the relatives was 35.9 years (SD 19.7). In 1 case, sudden death occurred in 2 siblings.
Investigations
Imaging and Biopsy
Transthoracic echocardiography demonstrated a structurally normal heart in all patients (ejection fraction 61.5±6.2%). Cardiac magnetic resonance imaging was performed in 38 patients (70%) and confirmed the absence of structural heart disease. Coronary angiography was performed in 24 patients (44%) with no cases of anomalous coronary anatomy or significant stenosis identified. Seven patients (13%) underwent endomyocardial biopsy with negative results in all cases.
Exercise and Pharmacological Testing
Forty‐five patients (83%) underwent exercise stress testing, and 38 patients (70%) had an isoproterenol challenge. There were no QT interval abnormalities or sustained arrhythmias during these tests. In 1 patient the isoproterenol test induced multifocal ventricular ectopic beats. Ajmaline/flecainide challenge was performed in 37 patients (69%) and excluded Brugada syndrome.
ECG
None of the patients presented with left or right ventricular hypertrophy (based on the Davignon criteria). The early repolarization pattern was present in 31 patients (57.4%). Electrocardiographic characteristics are summarized in Table 2. The characteristics of the subset of patients with an early repolarization pattern are included in Table 3.
Table 2.
Electrocardiographic Characteristics
| Characteristics | Mean Value (SD) |
|---|---|
| Heart rate, bpm | 76.3 (21.2) |
| P wave duration, ms | 96.9 (17.2) |
| P wave dispersion, ms | 36.8 (17.6) |
| P wave amplitude, mV | 0.63 (3.66) |
| PR duration, ms | 159.4 (30.98) |
| QRS duration, ms | 82.9 (14.7) |
| IVS (Cornell), mm | 1.26 (0.79) |
| QTc (Bazett), ms | 416 (48.8) |
| S wave duration (I), ms | 30.3 (12.2) |
| S wave amplitude (I), mV | 0.23 (0.18) |
| T peak–T end, ms | 88.4 (22.2) |
| QRS fragmentation, N (%) | 25 (46.3%) |
| U wave, N (%) | 27 (50%) |
IVS indicates interventricular septum thickness (in mm); U wave, evidence of U wave on the 12‐lead ECG.
Table 3.
ECG Characteristics of Subset of Patients With Early Repolarization
| No ER (n=23) | ER (n=31) | P Value | |
|---|---|---|---|
| Female sex, N (%) | 9 (40.9%) | 11 (35.5%) | 0.63 |
| Age, y | 12.2 | 13.1 | 0.77 |
| HR (bpm) mean | 79 | 74.4 | 0.45 |
| P wave duration, ms | 97.8 | 96.6 | 0.78 |
| P wave amplitude, mV | 0.16 | 0.99 | 0.11 |
| P wave dispersion, ms | 38.4 | 36.06 | 0.58 |
| PR, ms | 152.5 | 164.6 | 0.18 |
| QRS duration, ms | 83.6 | 81.7 | 0.60 |
| QRS fragmentation, n (%) | 10 (45.5) | 14 (45.2) | 0.92 |
| IVS, mm | 10.9 | 13.7 | 0.22 |
| QTc, ms | 426.5 | 408.8 | 0.19 |
| S wave duration, ms | 26 | 17 | 0.82 |
| S wave amplitude, mV | 0.156 | 0.162 | 0.21 |
| U wave, N (%) | 12 (54.5) | 15 (50) | 0.66 |
| T Peak–T end, ms | 94.1 | 84.5 | 0.12 |
ER indicates early repolarization; IVS, interventricular septum thickness (in mm).
Invasive Electrophysiology Studies
An electrophysiological study was performed in 34 patients (63%). VF was induced in 3 patients (9%). There were no cases of inducible VT. Three patients (9%) had inducible atrial tachycardia.
Genetic Analysis
Forty‐five patients (83%) underwent genetic testing. A putative pathogenic mutation was identified in 15 of the 45 (33%) patients (RYR2 [n=7]; TRDN [n=2]; CALM1 [n=1]; CASQ2 [n=1]; SCN5A [n=1]; KCNH2 [n=1]; MYH7 [n=1]; SLC22A5 [n=1]). Two patients did not provide consent for disclosure of their genetic results. Based on the rapidly emerging data on genetic variation in normal populations, we reevaluated the prevalence of the reported genetic variants in the gnomAD database, which spans ≈123 000 exomes (http://gnomad.broadinstitute.org/). All variants were confirmed to be unique (Table 4).
Table 4.
Pathogenic Mutation Identified in 15 of 45 Patients
| Patient | Gene | Identified Variant | Homozygote/Heterozygote | MAF in gnomAD |
|---|---|---|---|---|
| 1 | RYR2 | R4608Q | Heterozygote | 0 |
| 5 | SLC22A5 | R471H | Heterozygote | 0 |
| 6 | CASQ2 | Deletion of Exon1 | Heterozygote | 0 |
| 17 | KCNH2 | A429P | Heterozygote | 0 |
| 18 | RYR2 | D4112N | Heterozygote | 0 |
| 19 | RYR2 | D4112N | Heterozygote | 0 |
| 22 | RYR2 | R4497C | Heterozygote | 0 |
| 29 | SCN5A | R1644H | Heterozygote | 0 |
| 37 | MYH7 | F48L | Heterozygote | 0 |
| 46 | CALM | N98S | Heterozygote | 0 |
| 47 | TRDN | c/53_56delACAG p.(Asp18AlafsTer14) | Compound heterozygote | 0 |
| 48 | TRDN | c.502G>T p.(Glu168Ter) | Compound heterozygote | 0 |
| 49 | RYR2 | c.7273T>C | Heterozygote | 0 |
| 50 | RYR2 | G3930R | Heterozygote | 0 |
| 51 | RYR2 | c.11648A>G | Heterozygote | 0 |
MAF indicates minor allele frequency.
Pharmacotherapy
Twenty patients (37%) were discharged following the index VF with no drug therapy. Eighteen (33%) were commenced on a selective β‐blocker. One patient was discharged with quinidine.
Outcomes
Arrhythmia Recurrences
Patients were followed up for a median time of 109±12 months after the index VF episode. Thirty‐one patients (57%), experienced a recurrence of ventricular arrhythmias (23 [74%] had only VF; 8 [26%] had only sustained VT). Four patients (13%) experienced both VT and VF episodes. The patients with recurrences experienced a median of 2 appropriate shocks (interquartile range 5.5). The first recurrence of ventricular arrhythmia occurred after a mean of 6.4 years after the index VF episode (Kaplan‐Meier curve, Figure 1).
Figure 1.
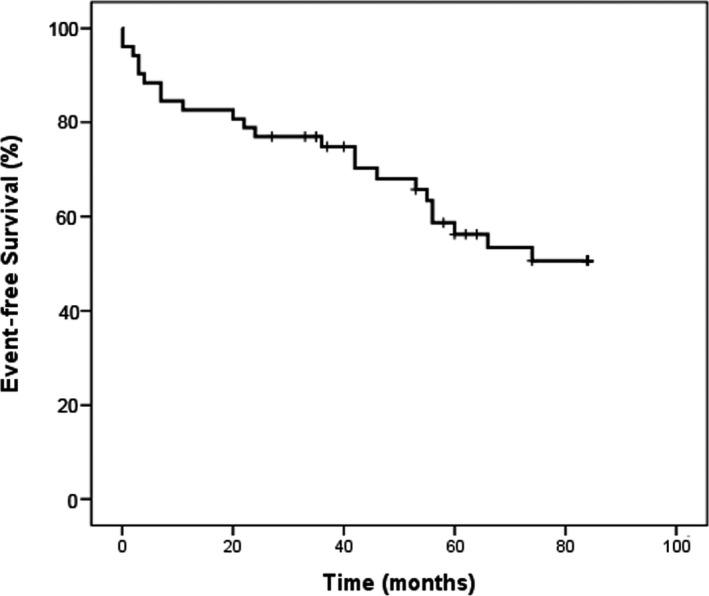
Kaplan–Meier curve demonstrating freedom from ventricular arrhythmias.
In 12 of 31 patients (39%), recurrences of ventricular arrhythmia occurred in the context of high adrenergic tone (exercise or intense stress/emotion), and the remaining recurrences occurred at rest. The arrhythmia recurrences according to clinical characteristics and specific genotypes are represented in Tables S1 and S2. One patient developed paroxysmal atrial fibrillation. The characteristics of individuals with VT/VF‐related ICD shocks are included in Table 5. Nine (33%) patients with VF recurrences were drug‐naive. Among the 36 patients in whom the index VF episode occurred in the context of high adrenergic tone, 15 (42%) had recurrent ventricular arrhythmias. In the remaining 18 patients (nonadrenergic group), 12 (67%) experienced a recurrence of VF. Recurrence of arrhythmia occurred in 4 patients treated with selective β‐blockers and in 1 patient treated with mexiletine. Three patients (6%) died during follow‐up. Two died due to an arrhythmic storm. One patient remained in a neurovegetative state following the arrest until death.
Table 5.
Recurrences of Ventricular Arrhythmias
| Sex | Past Syncope | Family History | EP Study | Genetic | Death | |
|---|---|---|---|---|---|---|
| VF group (n=23) | 16 male | 12/23 (52%) | 8/23 (35%) |
Performed in 3/12 Inducible (13%) |
18/23 (78%) 1 CALM+ 2 Triadin + 4 RyR2+ |
2 (9%) |
| VT group (n=8) | 6 male | 5/8 (62.5%) | 5/8 (62%) |
Performed in 6/8 (75%) No inducible ventricular tachycardia |
Performed in 7/8 (88%) RyR2+ (2) and Triadin+ (2) |
1 (14%) |
EP indicates electrophysiological; VF, ventricular fibrillation; VT, ventricular tachycardia.
Device‐Related Complications
Ten patients (18%) experienced device‐related complications (ventricular lead fracture [n=5]; lead insulation defect [n=1]; further device procedures due to lead recall [n=2]; late lead dislodgement [n=1]; device infection [n=1]). Eleven patients (20%) experienced inappropriate shocks (sinus tachycardia [n=1]; atrial arrhythmias [n=3]; noise on the ventricular channel [n=3], lead fracture [n=3]; sensing lead insulation defect [n=1]). The mean time from implant to complication was 41±14 months; the mean number of inappropriate shocks was 1.9±1 (Table 6).
Table 6.
ICD Characteristics and Complications Encountered During the Follow‐Up
| ICD characteristics | |
| Endocardial implant, n (%) | 47 (87%) |
| Single‐chamber ICD | 45 (96%) |
| Dual‐chamber ICD | 2 (4%) |
| Epicardial implant | 4 (7%) |
| Subcutaneous ICD | 3 (6%) |
| ICD complications | |
| Inappropriate shocks, n (%) | 11 (20%) |
| Sinus tachycardia | 1 (9%) |
| Atrial arrhythmias | 3 (27%) |
| Lead fracture | 3 (27%) |
| Noise on the V channel | 3 (27%) |
| Lead insulation defect | 1 (9%) |
| Device‐related complications, n (%) | 10 (18%) |
| Lead fracture | 5 (50%) |
| Lead recall | 2 (20%) |
| Pocket infection | 1 (10%) |
| Sensing lead insulation defect | 1 (10%) |
| Lead dislodgement | 1 (10%) |
ICD indicates implanted cardioverter/defibrillator.
Repeat Testing
Patients underwent systematic phenotype reevaluation 78±11 months after the index VF episode: all patients underwent repeat ECGs and transthoracic echocardiography. Forty‐five percent had a repeat cardiac magnetic resonance; 90% had a repeat stress test (with adult exercise testing protocols); 62% had a repeat isoproterenol challenge. Repeat genetic sequencing with an extended genetic panel was performed in 54% of the population at the discretion of the treating physician. One patient (2%) developed phenotypic evidence of hypertrophic cardiomyopathy during follow‐up. Of note, that patient had previously been identified as a MYH7 variant carrier (patient 37, Table 4). None of the patients who underwent repeat exercise stress testing or isoproterenol challenge had exercise‐induced arrhythmias. Repeat genetic testing did not reveal additional variants. None of the long QT gene carriers had a prolonged QT interval at the time of the index event; 1 of the patients (patient 29 in Table 4) had evidence of QT prolongation during follow‐up (SCN5A mutation carrier; QT interval 495 ms). The results of phenotypic reevaluation are included in Figure 2.
Figure 2.
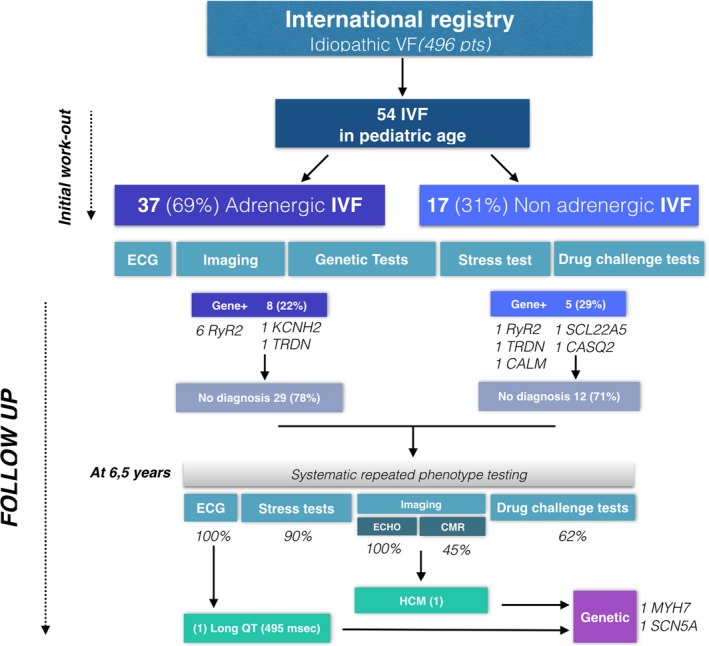
Summary of phenotypic evaluation following index episode and at follow‐up (78±11 months). CMR indicates cardiac magnetic resonance; ECHO, echocardiogram; HCM, hypertrophic cardiomyopathy; IVF, idiopathic ventricular fibrillation; VF, ventricular fibrillation.
Discussion
The main findings of the present study are as follows: (1) the majority of SCD events in pediatric IVF patients occur in the context of high adrenergic tone; (2) close to a fifth of pediatric IVF patients have variants in genes implicated in catecholaminergic polymorphic ventricular tachycardia; (3) pediatric IVF patients have a high risk of recurrent arrhythmic events during long‐term follow‐up; (4) only a small minority of patients develop evidence of an underlying cardiac phenotype during long‐term follow‐up. (5) The incidence of ICD‐related complications in this pediatric population is relatively high.
The majority of reports on IVF to date have focused on adult populations. There is a relative paucity of data on IVF in pediatric populations. In a large cohort of adult IVF patients, Visser and colleagues reported that, during long‐term follow‐up, a specific diagnosis was identified in up to a fifth of patients4 of whom almost 50% were found to have an underlying cardiomyopathy. In contrast to these findings, during a similar follow‐up period specific diagnoses were identified in only 4% of our pediatric cohort. It is important to emphasize, however, that because our cohort was of pediatric age at the index VF event, the initial evaluation was less comprehensive than that routinely performed in adult cohorts. Further, although reevaluation of our cohort during follow‐up did not reveal a specific underlying diagnosis, the strategies for reevaluation were variable. Our findings may, however, suggest that the underlying arrhythmogenic substrate in pediatric patients is distinct from that of adult IVF patients and may have a low diagnostic yield.
A potential pathogenic genetic variant was identified in a third of the patients in our cohort. In turn, variants in RYR2 were identified in close to half of the genotype‐positive patients. Consistent with our finding, Lahrouchi et al8 reported that in patients with sudden arrhythmic death syndrome, RYR2 variants were the most commonly identified pathogenic/likely pathogenic variants following postmortem genetic testing (43% of genotype‐positive cases). In the study by Visser and colleagues,4 on the other hand, the overall yield for genetic testing was lower at 15%, with only a quarter of genotype‐positive patients harboring catecholaminergic polymorphic ventricular tachycardia gene variants. Of note, patients included in our study (as in that of Lahrouchi et al) were significantly younger than the patients included by Visser et al. It is important to note, however, that a significant proportion of apparently pathogenic RYR2 variants may not be disease causing.9 Furthermore, although the yield of genetic test may increase with broad multiphenotype genetic testing, this approach may be associated with a higher incidence of variants of uncertain significance.10
Further, in the study of Visser and colleagues,4 a third of adult IVF patients experienced recurrent arrhythmic events. We observed a higher frequency of recurrent arrhythmic events in our pediatric IVF cohort, with close to half of cases experiencing recurrences. Importantly, despite an overall higher arrhythmic burden, the recurrent arrhythmic events in our cohort occurred at a later stage as compared with those reported in the adult population. These findings suggest that, in pediatric IVF patients, the disease process is associated with a more malignant course, with ongoing arrhythmic risk extending over a more prolonged period of time.
The choice of ICD device is an important consideration in pediatric populations. In recent years increasing evidence has emerged supporting the use of subcutaneous ICD as an alternative to transvenous systems.11 Close to a fifth of patients in our cohort experienced device‐related complications, which predominantly consisted of lead‐related issues. These findings may support the use of subcutaneous ICD in pediatric populations.
In addition to a family history of SCD, ECG features such as Tpeak‐Tend >100 ms with U waves and right bundle branch block were associated with more arrhythmia recurrences in our cohort. Our findings are in line with similar results in the adult population,12 and these ECG markers could potentially modulate arrhythmic risk stratification. However, based on the limited size of our cohort, these results should be interpreted with caution.
Limitations
Patients were recruited from a large number of centers over a prolonged period of time (more than a decade) in multiple countries. Therefore, the workup of patients following the VF episode and ICD programming was not standardized. It must be emphasized that guidelines for evaluation of these patients evolved over the time period of the study; therefore, there was some variation in the investigation of patients following episodes of aborted sudden death. During follow‐up, close to half of the population did not undergo comprehensive genetic testing. Medications were prescribed at the physician's discretion, and we acknowledge that is also a potential confounding factor. Among patients who did not have a cardiac magnetic image performed (30%), we acknowledge that a diagnosis of myocarditis could have been missed. Genetic testing was performed in the absence of a clear phenotype and on a discretionary basis. Where performed, genetic testing involved a candidate‐gene approach that targeted the common genes implicated in cardiac channelopathies and cardiomyopathies.
Conclusion
Our findings suggest that both the clinical course and underlying arrhythmogenic substrate in pediatric patients with IVF are distinct from those previously reported for adult IVF cohorts.
Sources of Funding
The study was supported by Equipex MUSIC ANR‐11‐EQPX‐0030 and IHU LIRYC ANR‐10‐IAHU‐04.
Disclosures
Drs Haïssaguerre, Hocini, Jaïs, and Sacher have received lecture fees from Biosense Webster and Abbott. Drs Denis, Derval, Jaïs, and Sacher received speaking honoraria and consulting fees from Boston Scientific. The remaining authors have no disclosures to report.
Supporting information
Table S1. Characteristics of Patients With RyR2 and Triadic Mutations
Table S2. Clinical Characteristics of Genotype‐Positive Patients
(J Am Heart Assoc. 2019;8:e011172 DOI: 10.1161/JAHA.118.011172.)
References
- 1. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N; ESC Scientific Document Group . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Wilde AA, Horie M, Cho Y. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 3. Myerburg RJ, Kessler KM, Zaman L, Conde CA. Survivors of prehospital cardiac arrest. JAMA. 1982;247:1485–1490. [PubMed] [Google Scholar]
- 4. Visser M, van der Heijden JF, van der Smagt JJ, Doevendans PA. Long‐term outcome of patients initially diagnosed with idiopathic ventricular fibrillation: a descriptive study. Circ Arrhythm Electrophysiol. 2016;9:e004258. [DOI] [PubMed] [Google Scholar]
- 5. Haïssaguerre M, Derval N, Sacher F, Jesel L. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 6. Macfarlane PW, Antzelevitch C, Haissaguerre M, Huikuri HV. The early repolarization pattern: a consensus paper. J Am Coll Cardiol. 2015;66:470–477. [DOI] [PubMed] [Google Scholar]
- 7. Davignon A, Rautaharju P, Boisselle E, Soumis F. Normal ECG standards for infants and children. Pediatr Cardiol. 1980;1:123–131. [Google Scholar]
- 8. Lahrouchi N, Raju H, Lodder EM, Papatheodorou E. Utility of post‐mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am Coll Cardiol. 2017;69:2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapplinger JD, Pundi KN, Larson NB, Callis TE, Tester DJ, Bikker H, Wilde AAM, Ackerman MJ. Yield of the RYR2 genetic test in suspected catecholaminergic polymorphic ventricular tachycardia and implications for test interpretation. Circ Genom Precis Med. 2018;11:e001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mellor G, Laksman ZWM, Tadros R, Roberts JD. Genetic testing in the evaluation of unexplained cardiac arrest: from the CASPER (Cardiac Arrest Survivors With Preserved Ejection Fraction Registry). Circ Cardiovasc Genet. 2017;11:e001686. [DOI] [PubMed] [Google Scholar]
- 11. Burke MC, Gold MR, Knight BP, Barr CS. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2‐year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol. 2015;65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 12. Panikkath R, Reinier K, Uy‐Evanado A, Teodorescu C. Prolonged Tpeak‐to‐Tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Patients With RyR2 and Triadic Mutations
Table S2. Clinical Characteristics of Genotype‐Positive Patients


