Abstract
Background
Several devices have been proposed to assess arterial stiffness in clinical daily use over the past few years, by estimating aortic pulse wave velocity (PWV) from a single measurement of brachial oscillometric blood pressure, using patented algorithms. It is uncertain if these systems are able to provide additional elements, beyond the contribution carried by age and blood pressure levels, in the definition of early vascular damage expressed by the stiffening of the arterial wall.
Methods and Results
The aim of our study was to compare the estimated algorithm‐based PWV values, provided by the Mobil‐O‐Graph system, with the standard noninvasive assessment of aortic PWV in patients with Marfan syndrome (ie, in subjects characterized by premature aortic stiffening and low blood pressure values). Aortic stiffness was simultaneously evaluated by carotid‐femoral PWV with a validated arterial tonometer and estimated with an arm cuff–based ambulatory blood pressure monitoring Mobil‐O‐Graph device on 103 patients with Marfan syndrome (50 men; mean±SD age, 38±15 years). Aortic PWV, estimated by the Mobil‐O‐Graph, was significantly (P<0.0001) lower (mean±SD, 6.1±1.3 m/s) than carotid‐femoral PWV provided by arterial tonometry (mean±SD, 8.8±3.1 m/s). The average of differences between PWV values provided by the 2 methods (±1.96×SD) was −2.7±5.7 m/s.
Conclusions
The Mobil‐O‐Graph provides PWV values related to an ideal subject for a given age and blood pressure, but it is not able to evaluate early vascular aging expressed by high PWV in the individual patient. This is well shown in patients with Marfan syndrome.
Keywords: arterial stiffness, early vascular aging, Marfan syndrome, Mobil‐O‐Graph, pulse wave velocity
Subject Categories: Diagnostic Testing, Vascular Disease, Blood Pressure, Quality and Outcomes
Clinical Perspective
What Is New?
-
The Mobil‐O‐Graph uses an algorithm essentially based on age and blood pressure values, providing aortic pulse wave velocity values related to an ideal subject for a given age and blood pressure.
In a cohort of patients with Marfan syndrome, the Mobil‐O‐Graph has proved not to be able to evaluate early vascular aging or precocious subclinical vascular damage expressed by high pulse wave velocity.
What Are the Clinical Implications?
The use of the Mobil‐O‐Graph and other devices applying similar algorithms strongly based on age and blood pressure values should be discouraged in clinical daily practice for the evaluation of early vascular aging by estimation of aortic pulse wave velocity.
Introduction
The evaluation of aortic pulse wave velocity (PWV) is a well‐established method for assessing aortic stiffness, which represents a predictor of cardiovascular mortality and morbidity, independently of the main known risk factors for cardiovascular disease.1 Currently, carotid‐femoral PWV (cf‐PWV) is widely accepted as a direct measurement of aortic stiffness and is recommended for this purpose.2
In recent years, other ways of estimating PWV have been proposed, to overcome the limitation of cf‐PWV, which requires carefully trained personnel. Assessment of aortic PWV was from a single measurement of oscillometric blood pressure (BP), using patented algorithms considering BP signal has been implemented in some devices. Moreover, the estimation of aortic PWV, calculated by an equation derived from the relationship of age and mean BP (ePWV), has demonstrated a predictive value in healthy subjects beyond traditional risk scores; thus, some might wonder about whether measuring cf‐PWV by tonometry should remain the recommended approach.3
The aim of our study was to compare the estimated algorithm‐based PWV, provided by the Mobil‐O‐Graph system, with the standard noninvasive measurement of cf‐PWV in Marfan syndrome (MFS) by tonometry. MFS is an autosomal dominant genetic disorder characterized by arterial stiffening attributable to altered synthesis of fibrillin‐1 protein. This protein plays an important role in connective and elastic tissue morphogenesis. The prognosis in MFS is mainly determined by the progressive aorta enlargement, potentially leading to aortic dissection and sudden death at a young age. The synthesis of abnormal fibrillin‐1 causes a change in the viscoelastic properties of the aorta, which involves an increase in the PWV. High values of PWV in patients with MFS are, therefore, related to the genetically induced structural alterations of the arterial wall. Thus, these alterations in arterial wall mechanical properties are related to the severity of genetic damage, regardless of age or BP values. Recent studies have shown that aortic diameter does not significantly correlate with mechanical and morphological tissue properties, and aneurismal diagnostic criteria based on aortic size do not provide sufficient indication of dissection or rupture potential.4 These studies seem to support the importance of early diagnosis of aortic stiffening in defining the risk of rupture of an aortic aneurysm in MFS. Early detection of aortic dissection risk could radically change the prognosis of patients with MFS. Previous studies showed increased aortic stiffness5, 6, 7, 8 and high values of PWV9, 10 in patients with MFS; these are independently associated with the aortic diameters at the sinuses of Valsalva and at the sinotubular junction (markers of aortic dissection risk), suggesting accelerated arterial aging. For these reasons, patients with MFS represent an ideal cohort to study the processes of early vascular aging.
Methods
Because the participants in this study had a genetic disease, to minimize the possibility of unintentionally sharing information that can be used to reidentify private information, a subset of the data generated for this study is available from the corresponding author on reasonable request.
Study Population
Participants were recruited in a reference center for MFS (Marfan Clinic, Sacco Hospital, Milan, Italy), from January 2018 to May 2018. Only patients, aged ≥14 years, who strictly satisfied the revised Ghent criteria for the diagnosis of MFS11 were enrolled in the study. Patients underwent a clinical and dysmorphological evaluation, transthoracic echocardiography, and arterial tonometry on the same day. Genetic analysis was performed, when required by the Ghent criteria, at the Department of Molecular Genetics, Istituto Auxologico Italiano (Milan, Italy). Mutation screening, with the consent of the patient or of a guardian, was performed on genomic DNA extracted from peripheral blood cells. The entire coding region of the fibrillin‐1 gene (FBN1) was screened by direct sequencing. Polymerase chain reaction fragments were sequenced using the BigDye Terminator Kit (Applied Biosystems, Foster City, CA) and analyzed on the ABI Prism 3500 automated sequencer (Applied Biosystems).
The study was approved by the local Ethics Committee and performed according to ethical guidelines of the 1975 Declaration of Helsinki. All participants gave their written informed consent to the study procedures.
Estimation of Aortic Stiffness
All measurements were performed in the morning, in a quiet room at a stable temperature (20±2°C). Every patient was introduced to the investigators, familiarized with the procedure and devices, and laid supine for at least 10 minutes on a hospital bed. During this time, the investigators identified the carotid and femoral sites suitable for recording the arterial waveforms and marked them with a dermographic pen. Two operators took care of one patient at a time: one operator handled the cuff‐based devices (Mobil‐O‐Graph) on the left side of the patient, whereas the other operator dealt with the device measuring cf‐PWV (PulsePen) on the patient's right side, as recommended by the ARTERY Society guidelines.12 Measurements with PulsePen and Mobil‐O‐Graph devices started simultaneously, after entering patient data into the respective software. A second measurement was performed after 10 minutes from the first one, reversing the role of operators: the operator who in the first acquisition had performed the tonometry in the second dealt with the measurements provided by the Mobil‐O‐Graph, and vice versa. PWV values provided by the Mobil‐O‐Graph were downloaded into the computer and calculated off‐line and were, thus, not available during the test procedure.
The PulsePen tonometer (DiaTecne srl, Milan, Italy) was used as the reference standard method for assessing cf‐PWV, fully satisfying criteria fixed by ARTERY Society guidelines for validation of noninvasive hemodynamic measurement devices.12 The procedure has been described previously.13, 14 PulsePen is a pocket‐size, high‐fidelity tonometric sensor wirelessly connected to a laptop. PulsePen measures pulse wave transit time with the foot‐to‐foot method, identifying the wave foot by intersecting interpolating algorithm.14, 15 This procedure to define the foot of the pressure wave represents an evolution of the traditional intersecting tangent algorithm, allowing a more stable and precise identification of wave foot and showing a reduction in the variability of measurements.16 The software permits real‐time quality checks by the operator, providing a “quality index” during the recording of 10 cardiac cycles. The PulsePen software allows the acquisition of pulse wave signals only if the overlapping of pulse waves is >85%. The PulsePen is characterized by a 1‐kHz sampling rate. The PulsePen is marketed in 2 versions: the PulsePen‐ETT, offered with 2 tonometric probes; and the PulsePen‐ET, supplied with a single probe and an integrated electrocardiographic unit. In this study, cf‐PWV was measured with the latter system, by sequential recordings of the arterial pressure waveform at the right common carotid and right femoral artery. cf‐PWV was calculated as the distance between the sampling sites, divided by the time difference between the respective delays in the onset of femoral and carotid pulses with regard to the preceding R wave of an electrocardiographic recording. The distance traveled by the pulse waveform to the femoral artery site was estimated as 80% of the direct carotid‐to‐femoral distance, as recommended by a recent expert consensus document on the measurement of aortic stiffness in daily practice.17 Carotid‐to‐femoral distance was measured with a steel tape measure from the arterial marked points. BP was measured at the right brachial artery with a validated Omron‐705IT oscillometric digital BP monitoring device during each tonometric pulse wave recording.
The tested device evaluated in this study was the Mobil‐O‐Graph (I.E.M. GmbH, Stolberg, Germany),18 an automated oscillometric arm cuff–based ambulatory BP monitoring device. PWV values are derived from the inbuilt ARCSolver (Austrian Institute of Technology, Vienna, Austria) proprietary algorithm, which integrates age, central systolic blood pressure, and data derived from pulse wave analysis into a mathematical model.18, 19
The aortic PWV was also estimated using 2 equations derived by the Reference Values for Arterial Stiffness Collaboration3, 20:
e1PWV=9.5875−0.4025×Age+4.56×Age2/1000−2.621×Age2/1000×MAP/100+0.3176×Age×MAP/100−1.832×MAP/100, and e2PWV=4.62−0.13×Age+0.0018×Age2+0.0006×Age×MAP+0.0284×MAP,
where mean arterial pressure (MAP) was calculated as diastolic BP plus 40% of pulse pressure.21
Statistical Analysis
Data are reported as mean±SD or as absolute numbers and percentage, where appropriate. The average difference between 2 sets of PWV measurements was assessed with a 2‐tailed paired t test. A multivariate regression analysis was performed to evaluate the role of BP, heart rate, age, and age2 in affecting PWV for each device. The agreement between PWV measurements was analyzed according to the analysis described by Bland and Altman.22 In the first step, the data were plotted, and the line of identity was drawn to perform a visual inspection to gauge the degree of agreement between measurements. The regression line with coefficient of correlation was also reported. Second, the relative differences within each pair of measurements were plotted against the mean of the pair. The interoperator repeatability was assessed by considering the 2 measurements performed in each patient, and expressed as coefficient of repeatability (1.96×SD of differences between 2 measurements).22 As strongly recommended by Bland,23 the within‐subject coefficient of variation was calculated as the square root of the mean within‐subject variance ()/subject mean squared (), as follows: , where E[x] is the expected value of random variable x.
The number of enrolled patients followed the ARTERY Society recommendations for validation of noninvasive devices for assessing PWV.12 A sample size of at least 90 patients was recommended by these guidelines. Considering a 15% dropout rate, 103 patients were enrolled.
Results
PWV measurements were obtained successfully in all the enrolled patients, 50 men, aged 38±15 years. General characteristics of patients are shown in Table 1. Of patients with MFS, 77.7% were treated: 71.8% with angiotensin receptor–blocking agents and 45.6% with β blockers. This population with MFS was characterized by low BP values (mean±SD systolic BP, 117.8±13.8 mm Hg; mean±SD diastolic BP, 69.1±8.8mm Hg), without any increase of BP with age (Figure 1).
Table 1.
Basic Characteristics of the Population With MFS
| Variables | Total |
|---|---|
| Sex, men/women | 50:53 |
| Age, y | 38.2±14.9 |
| Height, cm | 179.2±11.2 |
| Weight, kg | 71.1±16.9 |
| Body mass index, kg/m2 | 22.0±4.1 |
| Body surface area, m² | 1.88±0.26 |
| Ghent diagnostic criteria | |
| Total score | 9.7±2.8 |
| Cardiovascular criterion | 81.6 |
| Ocular criterion | 50.5 |
| Family history | 72.8 |
| Fibrillin‐1 mutation | 72.8 |
| Systemic score ≥7 | 88.3 |
| General MFS features | |
| Replacement of the ascending aorta | 36.9 |
| Aortic valve spearing (David) | 27.2 |
| Aortic valve replacement (Bentall) | 9.7 |
| Wrist and thumb sign | 73.8 |
| Severe pectus excavatum | 28.2 |
| Pectus carinatum | 28.2 |
| Hind foot deformity | 1.0 |
| Pes planus | 66.0 |
| Spontaneous pneumothorax | 2.9 |
| Dural ectasia | 52.4 |
| Span ratio >1.05 | 69.9 |
| Scoliosis >20° | 69.9 |
| Reduced extension of elbows | 8.7 |
| Facial features | 63.1 |
| Myopia >3 diopters | 54.4 |
| Skin striae | 81.6 |
| Mitral valve prolapse | 83.5 |
| Treatment | |
| None | 22.3 |
| RAS antagonist | 71.8 |
| β Blocker | 45.6 |
Data are presented as mean±SD, or percentage. MFS indicates Marfan syndrome; RAS, renin‐angiotensin system.
Figure 1.
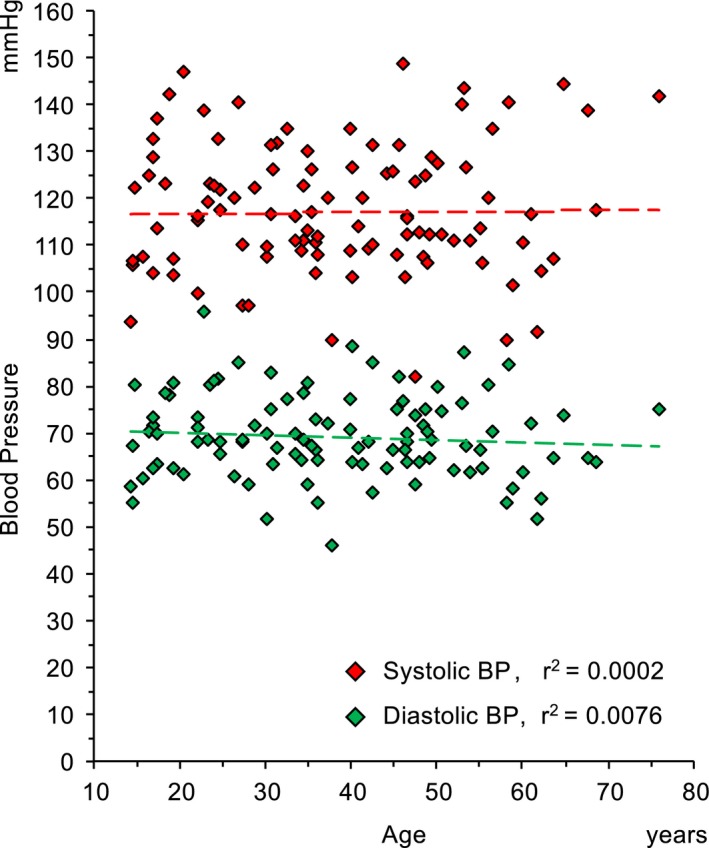
Distribution of systolic (red diamonds) and diastolic (green diamonds) blood pressure values according to age in patients with Marfan syndrome enrolled in the study.
As shown in Table 2, the interoperator coefficients of repeatability of PWV measurements, provided by PulsePen and Mobil‐O‐Graph, were 8.17% and 4.02%, respectively. Table 3 shows the change in heart rate and brachial BP values during the 2 measurements acquired by each device.
Table 2.
Repeatability Between Consecutive PWV Measurements
| Device | Difference, m/s | |d|, m/s | CV, % | CR, m/s |
|---|---|---|---|---|
| PulsePen | 0.05±1.08 | 0.78±0.74 | 8.17 | 2.12 |
| Mobil‐O‐Graph | 0.12±0.30 | 0.24±0.22 | 4.02 | 0.58 |
Difference indicates mean of differences±SD. CR indicates coefficient of repeatability (1.96×SD of differences); CV, coefficient of variation (square root of the mean); |d|, absolute mean of differences±SD; PWV, pulse wave velocity.
Table 3.
Change in Heart Rate and Brachial BP Values Between First and Second Measurement
| Variable | Mean | First Measurement | Second Measurement | P Value (First vs Second Measurement) |
|---|---|---|---|---|
| Omron 705IT (during PulsePen measurements) | ||||
| Systolic BP, mm Hg | 118.0±14.4 | 121.4±14.5 | 114.5±14.4 | <0.001 |
| Diastolic BP, mm Hg | 69.1±9.3 | 70.0±9.5 | 68.1±9.0 | 0.008 |
| Heart rate, bpm | 66.4±11.5 | 66.0±11.8 | 66.8±11.3 | 0.121 |
| Mobil‐O‐Graph | ||||
| Systolic BP, mm Hg | 116.7±12.9 | 118.7±12.8 | 114.7±13.1 | 0.628 |
| Diastolic BP, mm Hg | 72.1±9.0 | 73.2±9.0a | 71.0±8.9a | <0.001 |
| Heart rate, bpm | 66.1±11.9 | 66.3±12.0 | 66.0±11.8 | 0.075 |
Data are presented as mean±SD. BP indicates blood pressure; bpm, beats per minute.
P<0.05 between BP values provided by Omron 705IT and Mobil‐O‐Graph.
Aortic PWV, estimated by Mobil‐O‐Graph, was significantly lower than cf‐PWV provided by PulsePen (P<0.0001): PWV mean±SD=6.1±1.3 m/s versus 8.8±3.1 m/s, respectively (average of differences between PWV values provided by the 2 methods±1.96×SD=−2.7±5.6 m/s), with a greater underestimation for high values of PWV (Figure 2).
Figure 2.
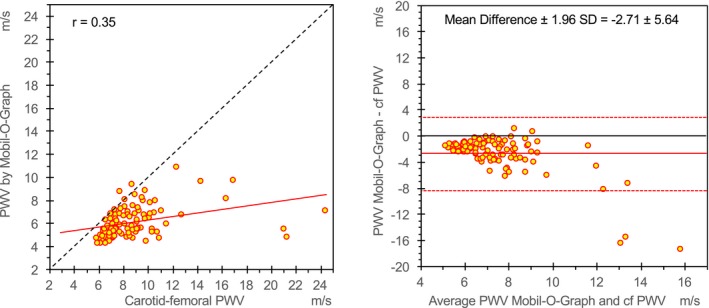
Relationship between aortic pulse wave velocity (PWV), estimated by the Mobil‐O‐Graph, and measured carotid‐femoral PWV (cf‐PWV). On the left, the scatterplot shows linear correlation between cf‐PWV measured by arterial tonometry (PulsePen device), the noninvasive reference method, and PWV estimated by the Mobil‐O‐Graph. A linear regression line (red solid line) and the identity line (black dashed line) are also shown. On the right, the Bland‐Altman plot shows differences observed between measurements to the average values. Red solid line shows the mean values of differences, and red dashed lines show ±1.96×SD of differences.
Figure 3 shows the distribution of PWV values related to age (left panel) and systolic BP (right panel). In both cases, a significant underestimation of PWV assessed by Mobil‐O‐Graph compared with cf‐PWV is evident (P<0.0001). cf‐PWV in patients with MFS was only weakly affected by age (r 2=0.21). On the contrary, aortic PWV, estimated by Mobil‐O‐Graph, was strongly related with age (r 2=0.86).
Figure 3.
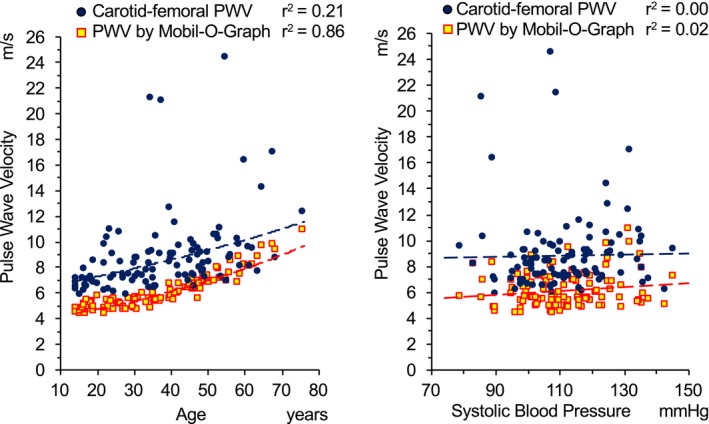
Distribution of aortic pulse wave velocity (PWV) values related to age (left panels) and systolic blood pressure (right panels) in different methodological approaches. Blue dots represent carotid‐femoral PWV values measured by PulsePen tonometer. Yellow squares with red border represent aortic PWV values estimated by the Mobil‐O‐Graph. Dashed lines show the relationship between age and PWV (exponential regression analysis) and between systolic blood pressure and PWV (linear regression analysis).
The values of e1PWV (mean±SD, 7.3±1.3 m/s) and e2PWV (mean±SD, 7.0±1.1 m/s) were both significantly lower than measured cf‐PWV (P<0.0001; average of differences between PWV values provided by the 2 methods±1.96×SD=−1.5±5.7 for e1PWV and −1.8±5.7 for e2PWV) (Figure 4), but significantly higher than PWV provided by Mobil‐O‐Graph (P<0.0001; average of differences between PWV values±1.96×SD=1.2±0.9 m/s for e1PWV and 0.9±0.9 m/s for e2PWV), and greatly affected by age (e1PWV, r 2=0.57; and e2PWV, r 2=0.68).
Figure 4.
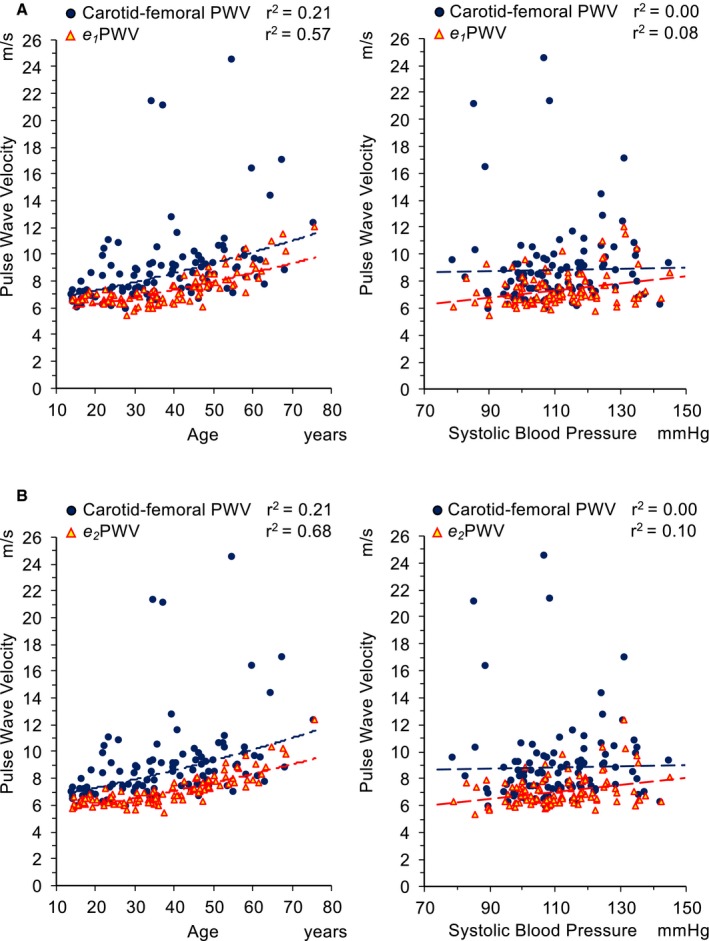
Distribution of aortic pulse wave velocity (PWV) values related to age (left panels) and systolic blood pressure (right panels) in different methodological approaches. Blue dots represent carotid‐femoral PWV values measured by PulsePen tonometer. Yellow triangles with red border represent aortic PWV values estimated (e1 PWV in A and e2 PWV in B) using equations derived by the Reference Values for Arterial Stiffness Collaboration.20 Dashed lines show the relationship between age and PWV (exponential regression analysis) and between systolic blood pressure and PWV (linear regression analysis).
Determinant factors of PWV values, estimated by PulsePen and Mobil‐O‐Graph, were investigated by multivariate analysis (Table 4), considering as independent variables heart rate, systolic blood pressure, diastolic blood pressure, age, and age2. This multivariate analysis showed that in this population with MFS, cf‐PWV was significantly affected by heart rate. The coefficient of correlation of this model was 18.4%. cf‐PWV was not affected by age and blood pressure. On the other hand, Mobil‐O‐Graph was strongly affected by age, age2, and systolic blood pressure, with a minor contribution of diastolic blood pressure. The coefficient of correlation of this model was 98.6%.
Table 4.
Multiple Regression Analysis With PWV Estimated by PulsePen and Mobil‐O‐Graph as Dependent Variable
| Dependent Variable | Independent Variables | Regression Coefficient | SE | Lower 95% CL | Upper 95% CL | Standardized Coefficient | P Value |
|---|---|---|---|---|---|---|---|
| PWV by PulsePen (r 2 model=0.184) | Intercept | 2.5447 | 2.3257 | −2.0421 | 7.1316 | 0.2752 | |
| Age | 0.0775 | 0.0662 | −0.0531 | 0.2080 | 0.3904 | 0.2432 | |
| Age2 | 0.0000 | 0.0008 | −0.0016 | 0.0016 | −0.0013 | 0.9968 | |
| Systolic BP | 0.0259 | 0.0164 | −0.0065 | 0.0583 | 0.1297 | 0.1159 | |
| Diastolic BP | −0.0352 | 0.0274 | −0.0891 | 0.0188 | −0.1105 | 0.2000 | |
| Heart rate | 0.0393 | 0.0177 | 0.0043 | 0.0743 | 0.1530 | 0.0279 | |
| PWV by Mobil‐O‐Graph (r 2 model=0.986) | Intercept | 1.3687 | 0.1447 | 1.0833 | 1.6541 | <0.0001 | |
| Age | −0.0304 | 0.0040 | −0.0383 | −0.0226 | −0.3379 | <0.0001 | |
| Age2 | 0.0014 | 0.0000 | 0.0013 | 0.0015 | 1.2574 | <0.0001 | |
| Systolic BP | 0.0339 | 0.0013 | 0.0315 | 0.0364 | 0.3287 | <0.0001 | |
| Diastolic BP | −0.0064 | 0.0018 | −0.0100 | −0.0028 | −0.0438 | 0.0006 | |
| Heart rate | 0.0011 | 0.0010 | −0.0010 | 0.0031 | 0.0094 | 0.3034 |
BP indicates blood pressure; CL, confidence limits; PWV, pulse wave velocity; r 2, coefficient of determination; SE, standard error.
A multivariate regression analysis considering only age2 and systolic BP showed a coefficient of determination of PWV estimated by Mobil‐O‐Graph of 98% (Figure 5), according to the formula:
Figure 5.

Factors affecting aortic pulse wave velocity (PWV) estimated by Mobil‐O‐Graph. Aortic PWV, estimated by the Mobil‐O‐Graph, is strongly associated (r 2=0.98) with age2 and brachial systolic blood pressure (SBP).
Discussion
Currently, the Mobil‐O‐Graph is considered an attractive approach to estimate aortic PWV, performing easy and operator‐independent measurements. The ARCSolver algorithm inbuilt in the Mobil‐O‐Graph was developed from invasive aortic PWV recordings in a large population of patients undergoing cardiac catheterization. According to the statements by the developers of the system, the ARCSolver algorithm estimates aortic PWV with a regression based on pulse waveform characteristics, age, and systolic BP.18, 19 Validation studies showed a good agreement between PWV provided by Mobil‐O‐Graph and aortic PWV invasively assessed.18, 19 Our study was designed to check if an algorithm essentially based on age and BP, which gives a reliable estimate of aortic PWV in the general population, is also able to identify conditions of early vascular aging.
A population of patients with a diagnosis of MFS was involved in our study. MFS is characterized by abnormal fibrillin‐1 synthesis, which causes degradation of the elastin fibers in the arterial wall,4 higher interfibrillar spaces, and decreased elastin fiber concentration.24, 25 The result of this process is increased aortic stiffness.4, 9, 10, 26
The histological characteristics and the alterations in viscoelastic properties of the large arteries observed in young patients with MFS are similar to the alterations usually found in elderly individuals,4 thus delineating in all respects a condition of early vascular aging. The main purpose of our study is, therefore, not only to study whether the Mobil‐O‐Graph is able to evaluate vascular damage in patients with MFS, but rather to verify if the Mobil‐O‐Graph is able to identify a condition of early vascular aging.
In recent studies,9, 10 our research group provided clear evidence that aortic stiffness evaluated as cf‐PWV is significantly increased in patients with MFS, suggesting accelerated arterial aging.4, 27 cf‐PWV emerged as an independent predictor of aortic diameter at the sinuses of Valsalva and at the sinotubular junction, which are considered at present the most reliable markers of risk of aortic dissection in MFS.28 Other rigorous studies, conducted with arterial tonometry,29 echocardiography,30, 31 or magnetic resonance imaging,7, 8, 26, 32 highlighted that the evaluation of aortic viscoelastic properties, and particularly of aortic PWV, could have a relevant clinical role in the estimation of aortic dissection risk in MFS.
The cohort enrolled in this study included several young adults (52% aged 18–45 years), with BP values, on average, lower compared with the general population values. The reason for low BP values is not the syndrome itself, which is characterized by normal blood pressure,33 but the use of antihypertensive medications as β blockers and angiotensin II type 1 (AT1) receptor antagonists, which are usually administered to patients for prevention of aortic complications to the maximal dosage allowed.34, 35 The values of systolic and diastolic blood pressures, which were found in our patients, are in line with those of patients with MFS enrolled in recent pharmacological trials.34, 35 The use of antihypertensive medications is likely the reason for the lack of relationship between BP values and age, observed in our population with MFS.
Our study shows that, in our cohort with MFS, Mobil‐O‐Graph delivered significantly lower PWV values than cf‐PWV measurement and showed only a weak relationship with cf‐PWV provided by PulsePen tonometer, as shown by the Bland‐Altman plot and analysis. cf‐PWV was measured by skilled operators; the interoperator short‐term repeatability was good and in line with previous studies performed by the same operators.16, 36 Thus, the possibility of an operator‐induced systematic error is unlikely.
In a general population, cf‐PWV is significantly affected by age and BP values.20, 37 In particular, aortic PWV increases exponentially with advancing age,38, 39 and this increase is more pronounced in the presence of high BP values.20 In our population with MFS, the multivariate analysis showed only a weak influence of age on cf‐PWV (relative r 2=14%), without any action of BP and heart rate. On the other hand, the algorithm used by the Mobil‐O‐Graph was essentially based on age2 and systolic BP (r 2=0.98), whereas the third component declared by the developers of the algorithm (ie, the pulse waveform characteristics) seems to impact weakly on the PWV provided by the ARCSolver algorithm. However, it cannot be excluded that the component derived from the analysis of pulse waveform is, in turn, strongly affected by age and/or the systolic blood pressure.
The PWV resulting from the Mobil‐O‐Graph can fit well with the real aortic PWV in the general population and can provide a relevant estimate of cardiovascular risk, relying on classic risk factors (age and blood pressure). On the other hand, this approach may not be able to provide additional prognostic information beyond that already supplied by these risk factors. Aortic PWV is currently considered an independent predictor of coronary heart disease and stroke, and its usefulness is mainly to reclassify the cardiovascular risk beyond traditional risk factors.1 This main point of strength of PWV measurement may, thus, be lost when using the Mobil‐O‐Graph to assess arterial stiffness in the single patient.
Similarly, the estimation of PWV by equations derived by PWV reference values (e1PWV and e2PWV) yielded to a significant underestimation of aortic stiffness in patients with MFS. The Mobil‐O‐Graph and other systems using an algorithm‐based approach to assess arterial stiffness cannot provide estimates of PWV values reflecting other factors beyond those included in the algorithm (ie, age and BP levels). The PWV values provided by the Mobil‐O‐Graph are related to an ideal subject for a given age and systolic BP, but may not be able to evaluate subclinical vascular damage expressed by high aortic stiffness in the individual patient, as shown in this population of patients with MFS.
The results of this study question the ability of algorithm‐based systems, such as the Mobil‐O‐Graph, to provide an accurate evaluation of early vascular aging. Other factors beyond age and changes in BP levels might well play a role in this process. Indeed, a BP‐based algorithm for evaluation of PWV could also lead to misleading results when exploring PWV in conditions in which BP changes significantly, as in response to pharmacological treatment, diet, and physical activity or after exposure to environmental factors. In these cases, PWV variations obtained through an algorithm considering BP values and age reflect changes in BP levels rather than real variations in arterial distensibility.
The main limitation of this study was the use of a noninvasive approach (cf‐PWV) as a reference standard method for the assessment of aortic PWV. However, angiography is not a diagnostic method usually recommended in patients with MFS; thus, the use of invasive methods for this study was not ethically allowed, and the noninvasive recommended method for the assessment of aortic stiffness is currently considered cf‐PWV.2, 40
Conclusions
The results of our study, even based on a small and rare population, seem to suggest that an algorithm‐based system, such as the Mobil‐O‐Graph, could not be an adequate method to assess arterial stiffness. This is more evident in peculiar clinical conditions in which other factors beyond age and changes in BP levels might play a role, providing misleading clinical information in the single patient. These results should be confirmed by further studies conducted in different populations.
Disclosures
P. Salvi reported receiving consulting fees from DiaTecne srl, manufacturer of systems for assessing the arterial stiffness. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e011440 DOI: 10.1161/JAHA.118.011440.)
References
- 1. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greve SV, Blicher MK, Kruger R, Sehestedt T, Gram‐Kampmann E, Rasmussen S, Vishram JK, Boutouyrie P, Laurent S, Olsen MH. Estimated carotid‐femoral pulse wave velocity has similar predictive value as measured carotid‐femoral pulse wave velocity. J Hypertens. 2016;34:1279–1289. [DOI] [PubMed] [Google Scholar]
- 4. Sulejmani F, Pokutta‐Paskaleva A, Ziganshin B, Leshnower B, Iannucci G, Elefteriades J, Sun W. Biomechanical properties of the thoracic aorta in Marfan patients. Ann Cardiothorac Surg. 2017;6:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groenink M, Rozendaal L, Naeff MS, Hennekam RC, Hart AA, van der Wall EE, Mulder BJ. Marfan syndrome in children and adolescents: predictive and prognostic value of aortic root growth for screening for aortic complications. Heart. 1998;80:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vitarelli A, Conde Y, Cimino E, D'Angeli I, D'Orazio S, Stellato S, Padella V, Caranci F. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol. 2006;97:571–577. [DOI] [PubMed] [Google Scholar]
- 7. Nollen GJ, Groenink M, Tijssen JG, Van Der Wall EE, Mulder BJ. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J. 2004;25:1146–1152. [DOI] [PubMed] [Google Scholar]
- 8. Kroner ES, Scholte AJ, de Koning PJ, van den Boogaard PJ, Kroft LJ, van der Geest RJ, Hilhorst‐Hofstee Y, Lamb HJ, Siebelink HM, Mulder BJ, Groenink M, Radonic T, van der Wall EE, de Roos A, Reiber JH, Westenberg JJ. MRI‐assessed regional pulse wave velocity for predicting absence of regional aorta luminal growth in Marfan syndrome. Int J Cardiol. 2013;167:2977–2982. [DOI] [PubMed] [Google Scholar]
- 9. Salvi P, Grillo A, Marelli S, Gao L, Salvi L, Viecca M, Di Blasio AM, Carretta R, Pini A, Parati G. Aortic dilatation in Marfan syndrome: role of arterial stiffness and fibrillin‐1 variants. J Hypertens. 2018;36:77–84. [DOI] [PubMed] [Google Scholar]
- 10. Grillo A, Salvi P, Marelli S, Gao L, Salvi L, Faini A, Trifiro G, Carretta R, Pini A, Parati G. Impaired central pulsatile hemodynamics in children and adolescents with Marfan syndrome. J Am Heart Assoc. 2017;6:e006815 DOI: 10.1161/jaha.117.006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst‐Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. [DOI] [PubMed] [Google Scholar]
- 12. Wilkinson IB, McEniery CM, Schillaci G, Boutouyrie P, Segers P, Donald A, Chowienczyk PJ; on behalf of the ARTERY Society . Artery society guidelines for validation of non‐invasive hemodynamic measurement devices: part 1, arterial pulse wave velocity. Artery Res. 2010;4:34–40. [Google Scholar]
- 13. Salvi P, Parati G. Methodological aspects in the measurement of pulse wave velocity by means of applanation tonometry. J Hypertens. 2013;31:35–38. [DOI] [PubMed] [Google Scholar]
- 14. Salvi P, Lio G, Labat C, Ricci E, Pannier B, Benetos A. Validation of a new non‐invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens. 2004;22:2285–2293. [DOI] [PubMed] [Google Scholar]
- 15. Joly L, Perret‐Guillaume C, Kearney‐Schwartz A, Salvi P, Mandry D, Marie PY, Karcher G, Rossignol P, Zannad F, Benetos A. Pulse wave velocity assessment by external noninvasive devices and phase‐contrast magnetic resonance imaging in the obese. Hypertension. 2009;54:421–426. [DOI] [PubMed] [Google Scholar]
- 16. Grillo A, Parati G, Rovina M, Moretti F, Salvi L, Gao L, Baldi C, Sorropago G, Faini A, Millasseau S, Scalise F, Carretta R, Salvi P. Short‐term repeatability of non‐invasive aortic pulse wave velocity assessment: comparison between methods and devices. Am J Hypertens. 2018;31:80–88. [DOI] [PubMed] [Google Scholar]
- 17. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace‐Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445–448. [DOI] [PubMed] [Google Scholar]
- 18. Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens. 2015;33:1023–1031. [DOI] [PubMed] [Google Scholar]
- 19. Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra‐aortic catheter measurements. Blood Press Monit. 2013;18:173–176. [DOI] [PubMed] [Google Scholar]
- 20. Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values.” Eur Heart J. 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens. 2007;25:751–755. [DOI] [PubMed] [Google Scholar]
- 22. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 23. Bland JM. How should I calculate a within‐subject coefficient of variation? https://www-users.york.ac.uk/~mb55/meas/cv.Htm. Accessed October 16, 2006.
- 24. Halme T, Savunen T, Aho H, Vihersaari T, Penttinen R. Elastin and collagen in the aortic wall: changes in the Marfan syndrome and annuloaortic ectasia. Exp Mol Pathol. 1985;43:1–12. [DOI] [PubMed] [Google Scholar]
- 25. Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10:20121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westenberg JJ, Scholte AJ, Vaskova Z, van der Geest RJ, Groenink M, Labadie G, van den Boogaard PJ, Radonic T, Hilhorst‐Hofstee Y, Mulder BJ, Kroft LJ, Reiber JH, de Roos A. Age‐related and regional changes of aortic stiffness in the Marfan syndrome: assessment with velocity‐encoded MRI. J Magn Reson Imaging. 2011;34:526–531. [DOI] [PubMed] [Google Scholar]
- 27. Mariko B, Pezet M, Escoubet B, Bouillot S, Andrieu JP, Starcher B, Quaglino D, Jacob MP, Huber P, Ramirez F, Faury G. Fibrillin‐1 genetic deficiency leads to pathological ageing of arteries in mice. J Pathol. 2011;224:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown‐up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG) . ESC guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 29. Payne RA, Hilling‐Smith RC, Webb DJ, Maxwell SR, Denvir MA. Augmentation index assessed by applanation tonometry is elevated in Marfan syndrome. J Cardiothorac Surg. 2007;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akazawa Y, Motoki N, Tada A, Yamazaki S, Hachiya A, Matsuzaki S, Kamiya M, Nakamura T, Kosho T, Inaba Y. Decreased aortic elasticity in children with Marfan syndrome or Loeys‐Dietz syndrome. Circ J. 2016;80:2369–2375. [DOI] [PubMed] [Google Scholar]
- 31. Kiotsekoglou A, Moggridge JC, Saha SK, Kapetanakis V, Govindan M, Alpendurada F, Mullen MJ, Camm J, Sutherland GR, Bijnens BH, Child AH. Assessment of aortic stiffness in Marfan syndrome using two‐dimensional and Doppler echocardiography. Echocardiography. 2011;28:29–37. [DOI] [PubMed] [Google Scholar]
- 32. Singh P, Almarzooq Z, Codell NCF, Wang Y, Roman MJ, Devereux RB, Weinsaft JW. Cine‐CMR partial voxel segmentation demonstrates increased aortic stiffness among patients with Marfan syndrome. J Thorac Dis. 2017;9:S239–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Canadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome, part 1: pathophysiology and diagnosis. Nat Rev Cardiol. 2010;7:256–265. [DOI] [PubMed] [Google Scholar]
- 34. Forteza A, Evangelista A, Sanchez V, Teixido‐Tura G, Sanz P, Gutierrez L, Gracia T, Centeno J, Rodriguez‐Palomares J, Rufilanchas JJ, Cortina J, Ferreira‐Gonzalez I, Garcia‐Dorado D. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur Heart J. 2016;37:978–985. [DOI] [PubMed] [Google Scholar]
- 35. Milleron O, Arnoult F, Ropers J, Aegerter P, Detaint D, Delorme G, Attias D, Tubach F, Dupuis‐Girod S, Plauchu H, Barthelet M, Sassolas F, Pangaud N, Naudion S, Thomas‐Chabaneix J, Dulac Y, Edouard T, Wolf JE, Faivre L, Odent S, Basquin A, Habib G, Collignon P, Boileau C, Jondeau G. Marfan sartan: a randomized, double‐blind, placebo‐controlled trial. Eur Heart J. 2015;36:2160–2166. [DOI] [PubMed] [Google Scholar]
- 36. Grillo A, Simon G, Salvi P, Rovina M, Baldi C, Prearo I, Bernardi S, Fabris B, Faini A, Parati G, Bardelli M, Carretta R. Influence of carotid atherosclerotic plaques on pulse wave assessment with arterial tonometry. J Hypertens. 2017;35:1609–1617. [DOI] [PubMed] [Google Scholar]
- 37. Alecu C, Gueguen R, Aubry C, Salvi P, Perret‐Guillaume C, Ducrocq X, Vespignani H, Benetos A. Determinants of arterial stiffness in an apparently healthy population over 60 years. J Hum Hypertens. 2006;20:749–756. [DOI] [PubMed] [Google Scholar]
- 38. Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovascular Imaging. 2010;3:1247–1255. [DOI] [PubMed] [Google Scholar]
- 39. McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR; ACCT Investigators . Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 40. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]


