Abstract
Background
Targeted temperature management (TTM) is a recommended treatment modality to improve neurological outcomes in patients with out‐of‐hospital cardiac arrest. The impact of the duration from hospital admission to TTM initiation (door‐to‐TTM; DTT) on clinical outcomes has not been well elucidated. We hypothesized that shorter DTT initiation intervals would be associated with improved survival with favorable neurological outcome.
Methods and Results
We performed a post hoc analysis of nontraumatic paramedic‐treated out‐of‐hospital cardiac arrests. The primary outcome was favorable neurological status at hospital discharge, with a secondary outcome of survival to discharge. We fit a logistic regression analysis to determine the association of early compared with delayed DTT, dichotomized by the median DTT duration, and outcomes. Of 3805 patients enrolled in the CCC (Continuous Chest Compressions) Trial in British Columbia, 570 were included in this analysis. There was substantial variation in DTT among patients receiving TTM. The median DTT duration was 122 minutes (interquartile range 35‐218). Favorable neurological outcomes in the early and delayed DTT groups were 48% and 38%, respectively. Compared with delayed DTT (interquartile range 167‐319 minutes), early DTT (interquartile range 20‐81 minutes) was associated with survival (adjusted odds ratio 1.56, 95% CI 1.02‐2.38) but not with favorable neurological outcomes (adjusted odds ratio 1.45, 95% CI, 0.94‐2.22) at hospital discharge.
Conclusions
There was wide variability in the initiation of TTM among comatose out‐of‐hospital cardiac arrest survivors. Initiation of TTM within 122 minutes of hospital admission was associated with improved survival. These results support in‐hospital efforts to achieve early DTT among out‐of‐hospital cardiac arrest patients admitted to the hospital.
Keywords: cardiac arrest, cardiac arrhythmia, neurocritical care, neuroprotectant, survival
Subject Categories: Mortality/Survival, Quality and Outcomes, Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Health Services
Short abstract
See Editorial Schenone and Menon
Clinical Perspective
What Is New?
Although targeted temperature management is a key component of postresuscitation care among survivors of out‐of‐hospital cardiac arrest, the impact of duration from hospital admission to targeted temperature management initiation (door‐to‐targeted temperature management) has not been well described.
We found that, compared with out‐of‐hospital cardiac arrest patients with delayed door‐to‐targeted temperature management, those with early door‐to‐targeted temperature management (<122 minutes) had increased survival to hospital discharge, including good neurological outcome among those with shockable rhythms.
What Are the Clinical Implications?
These results support the development of protocoled in‐hospital efforts to achieve early initiation of targeted temperature management in patients with out‐of‐hospital cardiac arrest surviving to hospital discharge.
Introduction
Sudden cardiac death continues to be a major public health issue, with only 16% to 23% of patients with out‐of‐hospital cardiac arrest (OHCA) who are admitted to a hospital being discharged with a favorable neurological outcome.1, 2, 3 Based on randomized clinical trials, guidelines recommend the utilization of targeted temperature management (TTM) to improve survival and neurological outcomes among comatose survivors of patients with cardiac arrest.4, 5, 6, 7 As prehospital systems of care yield increasing numbers of resuscitated patients, more OHCA patients survive to hospital admission, further emphasizing the need to optimize in‐hospital strategies to improve survival in this vulnerable population.6
Despite demonstration of improved outcomes with TTM, the optimal timing of TTM initiation remains to be elucidated. Some studies have shown that early initiation of TTM (either prehospital or in the emergency department) may lead to improved neurological outcome and survival, but others have demonstrated inconsistent results.8, 9, 10, 11, 12 Some variation may be due to the mode of TTM initiation (for example, cooled IV saline infusions resulted in worse outcomes in 1 study).7, 8, 9, 10, 11, 12, 13, 14 Furthermore, these prior studies varied in the metrics measured (DTT initiation, door to target temperature) as well as end points. A study by Lee et al examined the relationship between TTM initiation and time to target temperature (induction). The investigators found that induction time was not independently associated with favorable neurological outcomes at hospital discharge.15 To our knowledge, no studies have evaluated the variability in clinical practice, specifically the association between the interval between admission to a hospital and TTM initiation (door‐to‐TTM; DTT) and outcomes among OHCA patients surviving to hospital arrival. It would be of clinical value to have further guidance on the effect of delay of TTM on neurological and survival outcomes using hard clinical outcomes such as favorable neurological status and survival to discharge. This is potentially an attractive metric, allowing in‐hospital providers to objectively assess the quality and efficiency of delivering TTM, and could support protocols to ensure prompt TTM initiation, which is a key priority in the OHCA patient population.
Therefore, with use of data from the patients enrolled in the CCC (Continuous Chest Compressions) trial in British Columbia, the objectives of the current analysis are (1) to determine the association between time of TTM initiation and survival with good neurological outcome and (2) to describe the location and timing (including variability) of TTM initiation from hospital arrival.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Parent Trial
The CCC trial has been detailed previously.16, 17 Briefly, this trial enrolled 26 148 adults with non–trauma‐related OHCA who received chest compressions performed in the field (outside of hospital) between June 2011 and May 2015 at 8 sites.12 Patients in British Columbia comprised 15% (N=3805) of the total subjects enrolled in the trial. Patients were excluded if they had an emergency medical services (EMS)‐witnessed arrest, a written advance directive to not resuscitate, a traumatic injury, an asphyxia cause of arrest, uncontrolled bleeding or exsanguination, were pregnant or incarcerated, had a preexisting tracheostomy, or had initial cardiopulmonary resuscitation (CPR) performed by a nonparticipating EMS provider.
Study Participants
In this study we included patients from British Columbia in the CCC trial who were over the age of 18 years, were treated with hospital TTM, and who survived to admission to a hospital ward. Due to the design of the study, the requirement for subjects to give informed consent was waived.
Outcomes and Definitions
The primary outcome was the proportion of patients with a favorable neurological outcome, defined as those who have 0 to 3 on the modified Rankin scale at hospital discharge; the secondary outcome was overall survival to hospital discharge. DTT was defined as the interval from emergency department admission to initiation of TTM.
Data Collected
CCC Trial research staff prospectively and systematically examined the clinical records of enrolled patients, including patient characteristics, time‐stamped events, and treatments from the prehospital setting, as well as hospital‐based data, as previously described.16, 17
Statistical Analyses
Participants were dichotomized by the median DTT duration (into early and delayed DTT groups). We calculated patient characteristics using means (with standard deviation) if normally distributed, or otherwise using medians (with interquartile range). Continuous variables were compared by use of a 2‐tailed Student t test for continuous variables and a chi‐squared or Fisher exact test for categorical variables. We fit a logistic regression model to evaluate the association between early DTT, compared with delayed DTT, and each outcome while adjusting for patient characteristics and variables known to be associated with OHCA outcomes: age, sex, initial EMS‐recorded rhythm (shockable [ventricular fibrillation, pulseless ventricular tachycardia, or emergency department–advised shock] or nonshockable [asystole, pulseless electrical activity, emergency department–advised no shock or no classified rhythm]), witness status (bystander or not), episode location (public or nonpublic), time from 911 call to EMS arrival on the scene, and duration of EMS CPR to return of spontaneous circulation. We repeated the same logistic regressions in the subgroup of patients with shockable rhythm.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the article as written. This project was conducted in compliance with the protocol and principles laid down in the Declaration of Helsinki, Good Clinical Practice, as defined by the International Conference on Harmonisation where applicable, along with other local regulatory requirements. Before the study initiation, written approval from the Institutional Review Board/Independent Ethics Committee was obtained (H05‐50241).
Results
The study population consisted of 570 of the 3805 British Columbia patients who were enrolled in the CCC trial and who survived to hospital admission (see Figure 1). There was substantial variation in DTT among patients receiving TTM (Figure 2). The median DTT was 122 minutes, which was used to dichotomize the 2 comparison groups. The early‐DTT and delayed‐DTT groups had median DTTs of 35 minutes (interquartile range 20‐81 minutes) and 218 minutes (167‐319 minutes), respectively (Table 1).
Figure 1.
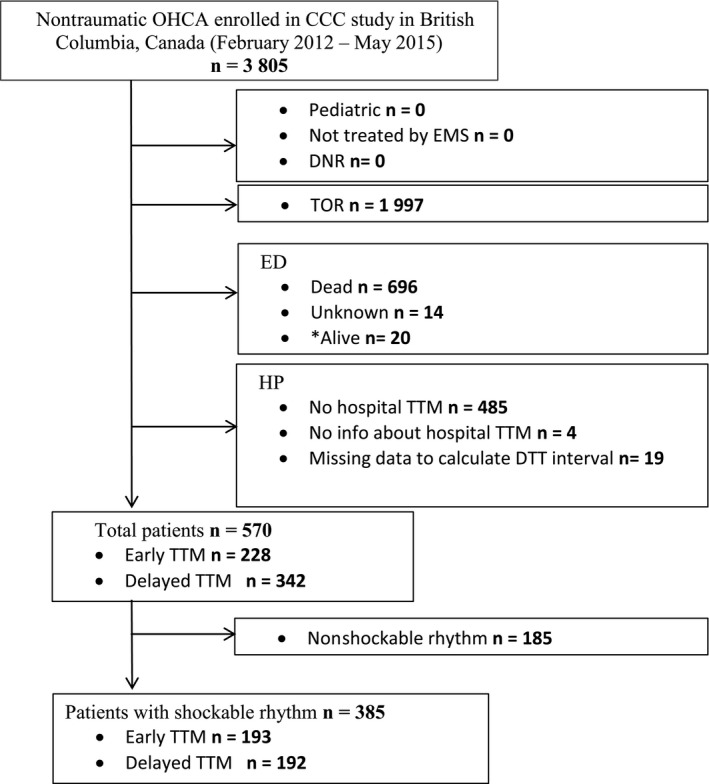
Selection of the study population. *Alive patients—discharged from ED directly, without hospital admission or TTM. CCC indicates continuous chest compression; DNR, Do‐Not‐Resuscitate order; DTT, door‐to‐TTM time; ED, emergency department; EMS, emergency medical services; HP, hospital; OHCA, out‐of‐hospital cardiac arrest; TTM, targeted temperature management; TOR, termination of resuscitation in the field.
Figure 2.
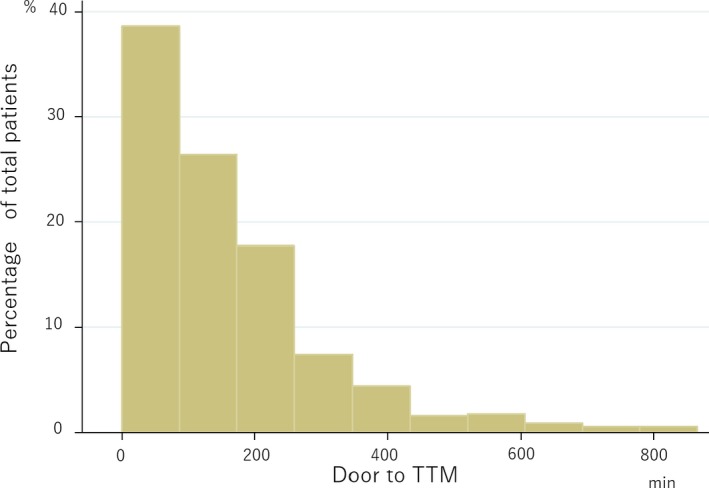
A histogram displaying the variability of DTT in the study population. DTT indicates door‐to‐TTM time; TTM, targeted temperature management.
Table 1.
Baseline Patient Characteristics for Early Versus Delayed DTT Initiation
| Variable | Early DTT (n=286) | Delayed DTT (n=284) | P Value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (IQR), y | 63.5 (55‐72) | 63 (52‐73) | 0.37 |
| Male sex (%) | 225 (78.7) | 210 (73.9) | 0.18 |
| Cardiac arrest characteristics | |||
| Shockable rhythm (%) | 206 (72.8) | 179 (63.3) | 0.02 |
| Bystander witness (%) | 209 (73.1) | 200 (70.4) | 0.48 |
| EMS witness (%) | 0 | 0 | ··· |
| Bystander CPR (%) | 196 (68.5) | 184 (64.8) | 0.34 |
| Public location (%) | 97 (34.0) | 94 (33.1) | 0.81 |
| Time from 911 call to EMS arrival (IQR), min | 6.3 (5.2‐7.8) | 6.4 (5.3‐7.7) | 0.23 |
| Time from EMS CPR to ROSC (IQR), min | 13.0 (8.5‐18.0) | 15.0 (10.0‐18.7) | 0.05 |
| Emergency department arrival status | |||
| ROSC present (%) | 270 (94.4) | 264 (93.0) | 0.55 |
| First ED temperature (IQR), °C | 35.8 (34.9‐36.4) | 35.7 (34.8‐36.4) | 0.33 |
| Initial pH within 24 hours | 7.22 (7.11‐7.29) | 7.20 (7.08‐7.29) | 0.34 |
| Methods of TTM | |||
| External TTM (%)a | 285 (99.7) | 283 (99.7) | 1.0 |
| Cooling blankets (%) | 225 (78.7) | 207 (72.9) | ··· |
| Ice packs (%) | 157 (54.9) | 98 (34.5) | ··· |
| Adhesive pads (%) | 1 (0.35) | 0 | ··· |
| Cooling pads (%) | 39 (13.6) | 40 (14.1) | ··· |
| Other (%) | 9 (3.2) | 17 (6.0) | ··· |
| Internal TTM (%) | 21 (7.3) | 22 (7.8) | 0.86 |
| Endovascular methods (%) | 0 | 0 | ··· |
| Intranasal methods (%) | 0 | 0 | ··· |
| Cold IV fluids (%) | 20 (7.0) | 20 (7.0) | ··· |
| Other | 1 (0.3) | 2 (0.7) | ··· |
| DTT (IQR), min | 35.0 (20.0‐81.0) | 218.0 (167.0‐318.5) | <0.01 |
CPR indicates cardiopulmonary resuscitation; DTT, door‐to‐TTM time; ED, emergency department; EMS, emergency medical services; IQR, interquartile range; IV, intravenous; ROSC, return of spontaneous circulation; TTM, targeted temperature management.
Multiple modalities of TTM were used for the majority of patients.
Table 1 lists the baseline demographic information of subjects in both groups. Both groups demonstrated similar baseline characteristics. The patient population in both groups was predominantly composed of middle‐aged men who had a shockable rhythm at time of arrest. The majority of patients had bystander CPR performed. There were no significant differences between the groups in established prognostic factors such as age, prevalence of shockable rhythm at time of arrest, witnessed arrest, or bystander CPR (Table 1). Table S1 demonstrates differences among early versus delayed DTTs among those with shockable rhythms only.
There was no significant difference between groups in incidence of return of spontaneous circulation, initial temperature, and pH at first presentation to the emergency department. TTM in subjects was almost exclusively achieved using external cooling methods (99.7%). This was predominantly achieved with the use of cooling blankets (78.7%) and ice packs (54.9%) (Table 1). Internal cooling methods were used in 7.3% and 7.8% of patients in the early‐DTT and delayed‐DTT groups, respectively. Information about use of feedback control systems was not available.
Favorable neurological outcomes at hospital discharge in the early and delayed DTT groups occurred in 48% and 38%, respectively (P=0.01). Among patients in the early‐DTT group, 147 (52%) survived to discharge, compared with 113 (40%) in the delayed‐DTT group. Compared with delayed DTT, early DTT was associated with increased survival (adjusted odds ratio 1.56, 95% CI 1.02‐2.38). However, there were no significant differences in favorable neurologic outcomes between early‐ and delayed‐DTT groups at hospital discharge (adjusted odds ratio 1.45, 95% CI, 0.94‐2.22) (Table 2).
Table 2.
Survival and Neurological Outcomes in Early Versus Delayed DTT in All Patients as Well as Those With Only Shockable Rhythms
| All Patients | Patients With Shockable Rhythm | |
|---|---|---|
| Adjusted OR With Early DTT (95% CI) | Adjusted OR With Early DTT (95% CI) | |
| Survival | 1.56 (1.02‐2.38) | 1.82 (1.10‐3.00) |
| Favorable neurological outcome | 1.45 (0.94‐2.22) | 1.79 (1.09‐2.95) |
DTT indicates door‐to‐TTM time; OR, odds ratio.
An a priori analysis among those with initial shockable rhythms found that 63% (113/193) of subjects in the early‐DTT group survived to hospital discharge, and 60% (119/193) survived with a favorable neurologic outcome. In the delayed‐DTT group, 101 subjects (53%) and 95 subjects (50%) had survival to hospital discharge and had favorable neurologic outcome, respectively. Compared with delayed DTT, early DTT was associated with significantly increased survival (adjusted odds ratio 1.82, 95% CI 1.10‐3.00) as well as favorable neurological outcomes (adjusted odds ratio 1.79, 95% CI, 1.09‐2.95) in this subpopulation (Table 2).
Finally, after stratifying patients into quartiles based on DTT, we found that patients with the shortest DTT (quartile 1; median DTT 36 minutes) were most likely to have the greatest adjusted survival compared with those in quartiles 2 to 4 (Figures S1, S2 and Table S2).
Discussion
We aimed to describe the location and timing (including variability) of TTM initiation from hospital arrival and then to determine the association between time of TTM initiation and survival with good neurological outcome. We found there was substantial variation in DTT among patients receiving TTM and that the early initiation of TTM (ie, within 122 minutes of hospital admission) was associated with improved survival. Among those with initial shockable rhythms, early TTM initiation was associated with statistically significant improvement in survival and neurological outcome. These data may help clinicians optimize the outcomes of those resuscitated from OHCA in prioritizing early initiation of TTM.
Guidelines endorse the use of TTM with hopes of optimizing survival with good neurological outcome in comatose survivors of cardiac arrest.6, 7 Although guidelines have continued to recommend use of TTM among survivors of OHCA, less is known about the optimal timing of its implementation. A recent nationwide analysis from the United States found wide hospital‐level variability in TTM use among myocardial infarction patients with OHCA despite few differences in the patient case mix. The body of evidence evaluating the association between hospital arrival and TTM initiation and outcomes has been of poor quality in that the data were observational, and trials were underpowered to detect a clinically meaningful relationship between these variables.15 Multiple factors may have contributed to the high degree of variability observed with DTT, which suggests in‐hospital postarrest system delays, including the lack of adequate protocols to streamline timely initiation of TTM. Possible sources of variability may include delays in TTM application in the context of concomitant invasive coronary angiography, transport of postarrest patients to centers unfamiliar with postarrest care, unclear details around the etiology of arrest and potential contraindications to TTM, or the general lack of high‐quality evidence to justify the dissemination and protocolization of time‐sensitive application of TTM systems in postarrest patients.4, 5, 16, 17, 18 A recent study demonstrated that direct transport to a percutaneous coronary intervention center is associated with better outcomes in OHCA patients, even when bypassing the nearest hospital and regardless of transport time, suggesting that a percutaneous coronary intervention center is a surrogate for the bundle of care that may occur in that center. For example, alongside percutaneous coronary intervention comes neurological expertise, surgical services, and more specialized critical care units.18 Because our study looked at time from emergency department admission to TTM application, it is not directly applicable but allows us to understand 1 of the possible mechanisms in which TTM initiation may be delayed—receiving care in a center lacking expertise in the management of OHCA patients. In any case, optimization of in‐hospital efforts to initiate application of TTM may help to reduce this observed variability.
Our study has several important clinical implications. First, we found that delays in DTT were associated with decreased survival and with worse neurological outcomes, particularly in shockable rhythms. Randomized controlled data have consistently demonstrated that shockable rhythms derive benefit from TTM. This information has long since been disseminated, and societal guidelines provide recommendations to temperature control these patients. Thus, it is not unreasonable to hypothesize that more prompt in‐hospital application of TTM to OHCA patients would result in improved outcomes, specifically in shockable rhythms, although this has not previously been evaluated systematically. Our study lends support to this hypothesis. Second, because increased DTT was demonstrative of a positive clinical outcome, these data should serve as a catalyst to enact more rigid and comprehensive protocols to ensure that prompt TTM initiation is of key priority in the OHCA patient population. In particular, prompt response to OHCA with shockable rhythms is paramount. DTT can now be considered an attractive actionable metric, allowing in‐hospital providers to assess the quality and efficiency of delivering TTM.
There are numerous reasons apart from neuroprotection why implementing TTM early may improve prognosis in patients resuscitated from OHCA. The early use of TTM is often associated with early revascularization in patients with shockable rhythms.4, 5, 18, 19 Numerous therapies are often given in parallel with TTM, including appropriate oxygenation and ventilation strategies, sedation, optimization of glycemic control and hemodynamics, as well as establishment of extracorporeal circulation if required. Access to expert neurological prognostication for withdrawal of care lends benefit to resuscitated patients as well.4, 5, 18, 19 Although it is beyond the scope of this article to delineate which of these guideline‐recommended in‐hospital therapies lend the most benefit to resuscitated patients, it is likely a combination of all of these therapies, including early TTM initiation for neuroprotection, that benefits OHCA patients.
As prehospital systems of care continue to improve, more OHCA patients, independent of rhythm, survive to hospital admission.1, 2, 3 In light of this trend the implementation and optimization of in‐hospital strategies to improve survival in this vulnerable population will continue to grow in importance, and the current study's data support optimization of TTM delivery, including prompt TTM initiation efforts. Future research efforts should aim to evaluate the long‐term effect of early versus delayed DTT on neurological outcome and survival, as this would provide meaningful prognostic information for hospital administrators and critical care providers. Future studies also focusing on the interaction with initial temperature may be important, as it is conceivable that a more severely deranged pre‐TTM temperature may be associated with poorer outcomes, specifically in the setting of increased DTT.
Study Limitations
Our analysis has some limitations. First, due to the methodological design, detailed baseline characteristic data, including history of coronary artery disease, cardiac risk factors, left ventricular ejection fraction, and chronic kidney disease, were not available for collection. However, we were able to extract extensive baseline information with respect to our core variables of interest including cardiac arrest characteristics, emergency department arrival status, and methods of cooling and DTT. This allowed for detailed analysis of DTT. Second, it would have been informative to have stratified outcomes based on baseline temperature of OHCA patients. Many theorize that the true benefit of hypothermia is the avoidance of “hyperthermia.” However, the data were derived from a large clinical trial with detailed and high‐quality data capture about time to TTM initiation, and this also allowed for detailed analysis of DTT. Third, long‐term survival and neurological outcome data are unavailable for the current study; therefore, we cannot comment on the long‐term prognosis of patients who receive early versus delayed TTM. However, recent studies have found that outcomes, including survival, among those patients surviving to hospital discharge following OHCA, are favorable.20, 21, 22, 23, 24
Conclusions
We found wide variability in the initiation of TTM among comatose survivors of OHCA. Initiation of TTM within 122 minutes of hospital admission was associated with improved survival. Among those with initial shockable rhythms, early TTM initiation was associated with improved survival and improved neurological outcomes. These results support in‐hospital efforts to achieve early DTT among OHCA patients admitted to the hospital.
Sources of Funding
This analysis received no funding. The BC Canadian Resuscitation Outcomes Consortium research office is supported by the Heart and Stroke Foundation, Provincial Health Services Authority, and Providence Health Care.
Disclosures
None.
Supporting information
Table S1. Characteristics of Early Versus Delayed DTT Initiation and Methods of Achieving DTT in Patients With Only Shockable Rhythms
Table S2. Survival and Neurological Outcomes in All Rhythms With Door‐to‐TTM in Quartiles
Figure S1. A histogram displaying favorable neurological outcome based on door‐to‐TTM quartile. TTM indicates targeted temperature management.
Figure S2. A histogram displaying survival based on door‐to‐TTM quartile. TTM indicates targeted temperature management.
Acknowledgments
We are indebted to the tireless work of the study coordinators who tracked down each case and entered data, the paramedics who managed each OHCA, the health care workers who provided postarrest care, and to our patients who participated to advance our understanding of cardiac arrest and clinical outcomes. The authors had full access to the data and take full responsibility for its integrity. All authors have read and agreed to the article as written.
(J Am Heart Assoc. 2019;8:e012001 DOI: 10.1161/JAHA.119.012001.)
References
- 1. Uribarri A, Bueno H, Pérez‐Castellanos A, Loughlin G, Sousa I, Viana‐Tejedor A, Fernandez‐Aviles F. Impact of time to cooling initiation and time to target temperature in patients treated with hypothermia after cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2015;4:365–372. [DOI] [PubMed] [Google Scholar]
- 2. Grunau B, Kawano T, Dick W, Straight R, Connolly H, Schlamp R, Scheuermeyer F, Fordyce CF, Barbic D, Tallon J, Christenson J. Trends in care processes and survival following prehospital resuscitation improvement initiatives for out‐of‐hospital cardiac arrest in British Columbia, 2006–2012. Resuscitation. 2018;125:118–125. [DOI] [PubMed] [Google Scholar]
- 3. Lai CY, Lin FH, Chu H, Ku CH. Survival factors of hospitalized out‐of‐hospital cardiac arrest patients in Taiwan: a retrospective study. PLoS One. 2018;13:e0191954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hypothermia After Cardiac Arrest Study Group . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- 5. Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutterridge G, Smith K. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- 6. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VRM, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post‐resuscitation care 2015. Resuscitation. 2015;95:202–222. [DOI] [PubMed] [Google Scholar]
- 7. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez‐Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post–cardiac arrest syndrome epidemiology, pathophysiology, treatment, and prognostication: a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:24522483. [DOI] [PubMed] [Google Scholar]
- 8. Fordyce CB. Reduced critical care utilization: another victory for effective bystander interventions in cardiac arrest. Resuscitation. 2017;119:A4–A5. [DOI] [PubMed] [Google Scholar]
- 9. Haugk M, Testori C, Sterz F, Uranitsch M, Holzer M, Behringer W, Herkner H. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Crit Care. 2011;15:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sendelbach S, Hearst MO, Johnson PJ, Unger BT, Mooney MR. Effects of variation in temperature management on cerebral performance category scores in patients who received therapeutic hypothermia post cardiac arrest. Resuscitation. 2012;83:829–834. [DOI] [PubMed] [Google Scholar]
- 11. Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly AM, Silvester W; Rapid Infusion of Cold Hartmanns Investigators . Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest. Crit Care Med. 2012;40:747–753. [DOI] [PubMed] [Google Scholar]
- 12. Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, Eichwede F, Mols P, Schwab T, Vergnion M, Storm C, Pesenti A, Pachl J, Guérisse F, Elste T, Roessler M, Fritz H, Durnez P, Busch HJ, Inderbitzen B, Barbut D. Intra‐arrest transnasal evaporative cooling a randomized, prehospital, multicenter study (PRINCE: Pre‐ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122:729–736. [DOI] [PubMed] [Google Scholar]
- 13. Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, Copass MK, Carlbom D, Deem S, Longstreth WT Jr, Olsufka M, Cobb L. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311:45–52. [DOI] [PubMed] [Google Scholar]
- 14. Debaty G, Maignan M, Savary D, Koch FX, Ruckly S, Durand M, Picard J, Escallier C, Chouquer R, Santre C, Minet C, Guergour D, Hammer L, Bouvaist H, Belle L, Adrie C, Payen JF, Carpentier F, Gueugniaud PY, Danel V, Timsit J. Impact of intra‐arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: a randomized controlled trial. Intensive Care Med. 2014;40:1832–1842. [DOI] [PubMed] [Google Scholar]
- 15. Lee BK, Jeung KW, Jung YH, Lee DH, Lee SM, Cho YS, Heo T, Yun JG, Min YI. Relationship between timing of cooling and outcomes in adult comatose cardiac arrest patients treated with targeted temperature management. Resuscitation. 2017;113:135–141. [DOI] [PubMed] [Google Scholar]
- 16. Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, Christenson J, Kudenchuk P, Vaillancourt C, Rea TD, Idris AH, Colella R, Isaacs M, Straight R, Stephens S, Richardson J, Condle J, Schmicker RH, Egan D, May S, Ornato JP; ROC Investigators . Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373:2203–2214. [DOI] [PubMed] [Google Scholar]
- 17. Brown S, Wang H, Aufderheide T. A randomized trial of continuous versus interrupted chest compressions in out‐of‐hospital cardiac arrest: rationale for and design of the Resuscitation Outcomes Consortium CCC Trial. Am Heart J. 2015;169:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fordyce CB, Chen AY, Wang TY, Lucas J, Goyal A, Wong GC, Diepen SV, Kontos MC, Henry TD, Granger CB, Roe MT. Patterns of use of targeted temperature management for acute myocardial infarction patients following out‐of‐hospital cardiac arrest: insights from the National Cardiovascular Data Registry. Am Heart J. 2018;206:131–133. [DOI] [PubMed] [Google Scholar]
- 19. Kragholm K, Malta Hansen C, Dupre ME, Xian Y, Strauss B, Tyson C, Monk L, Corbett C, Fordyce CB, Pearson DA, Fosbøl EL, Jollis JG, Abella BS, McNally B, Granger CB. Direct transport to a percutaneous cardiac intervention center and outcomes in patients with out‐of‐hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2017;10:e003414. [DOI] [PubMed] [Google Scholar]
- 20. Vyas A, Chan PS, Cram P, Nallamothu BK, Mcnally B, Girotra S. Early coronary angiography and survival after out‐of‐hospital cardiac arrest. Circ Cardiovasc Interv. 2015;8:e002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stub D, Smith K, Bray JE, Bernard S, Duffy SJ, Kaye DM. Hospital characteristics are associated with patient outcomes following out‐of‐hospital cardiac arrest. Heart. 2011;97:1489–1494. [DOI] [PubMed] [Google Scholar]
- 22. Mooney MR, Unger BT, Boland LL, Burke MN, Kebed KY, Graham KJ, Henry TD, Katsiyiannis WT, Satterlee PA, Sendelbach S, Hodges JS, Parham WM. Therapeutic hypothermia after out‐of‐hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124:206–214. [DOI] [PubMed] [Google Scholar]
- 23. Kragholm K, Wissenberg M, Mortensen RN, Hansen SM, Malta Hansen C, Thorsteinsson K, Rajan S, Lippert F, Folke F, Gislason G, Køber L, Fonager K, Jensen SE, Gerds TA, Torp‐Pedersen C, Rasmussen BS. Bystander efforts and 1‐year outcomes in out‐of‐hospital cardiac arrest. N Engl J Med. 2017;376:1737–1747. [DOI] [PubMed] [Google Scholar]
- 24. Fordyce CB, Wang TY, Chen AY, Thomas L, Granger CB, Scirica BM, Henry TD, Wong GC, Ramanathan K, Hansen CM, Kragholm K, Peterson ED, Anderson ML. Long‐term post‐discharge risks in older survivors of myocardial infarction with and without out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2016;67:1981–1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Early Versus Delayed DTT Initiation and Methods of Achieving DTT in Patients With Only Shockable Rhythms
Table S2. Survival and Neurological Outcomes in All Rhythms With Door‐to‐TTM in Quartiles
Figure S1. A histogram displaying favorable neurological outcome based on door‐to‐TTM quartile. TTM indicates targeted temperature management.
Figure S2. A histogram displaying survival based on door‐to‐TTM quartile. TTM indicates targeted temperature management.


