Background
Epistaxis occurs commonly in the general population, with most people experiencing at least 1 episode in a lifetime. The age of patients most affected by epistaxis follows a bimodal distribution, with the highest rates in those less than 10 years of age as well as those 70 years and older.1 Epistaxis is also commonly observed in clinical practice among patients taking antithrombotic therapies. As numerous cases are self-limiting and do not require patients to seek medical attention, the true incidence is likely not known. In an American study over a 10-year period, epistaxis was responsible for 1 in 200 emergency room (ER) visits, 6% of which required hospitalization.1 Hospitalizations for epistaxis may incur significant costs, with a reported average hospital length of stay (LOS) of 3.24 days, resulting in costs totalling from $6,000 to $17,000 depending on the treatment strategy used.2 However, many cases of epistaxis may be safely managed in the community,3 and providing patients with education on general measures for management and product options may mitigate the need for hospital-based care. In this context, community-based health care practitioners are the first points of contact. In order for practitioners to provide appropriate advice to these patients, it is necessary to have an understanding of the etiology, risk factors and appropriate outpatient treatment strategies for managing epistaxis. As such, our purpose is to provide an overview of community-based management of epistaxis, with a practice tool for health care providers outlining a suggested approach to management in this setting, along with an educational infographic for patients. The treatment of epistaxis in children or in patients with hereditary hemorrhagic telangiectasia is beyond the scope of our review and will not be addressed here.
Nasal anatomy
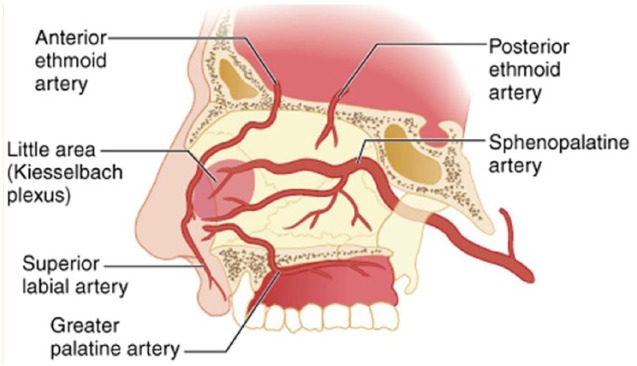
The septum, which laterally divides the nasal cavity, is lined with a mucosal membrane rich in vascular supply.4 Blood supply and vasculature of the nasal cavity is complex. Epistaxis can be classified as posterior or anterior, based on the location of the affected blood vessel (Figure 1).3 Ninety percent of cases of epistaxis are identified as anterior epistaxis.5 The source of anterior epistaxis is most commonly the Kiesselbach plexus (also known as “Little’s area”6), which is located on the anteroinferior region of the nasal septum. Anterior epistaxis is typically self-limiting; however, if medical treatment is required, the source of the bleed is often easily visualized (provided the necessary equipment and knowledge of nasal anatomy are available), which allows for the successful use of localized treatment strategies. In contrast, posterior epistaxis arises from the posterior nasal cavity due to bleeding directly from the sphenopalatine arteries.3 Posterior bleeds may also rarely originate from the internal carotid artery itself, resulting in life-threatening hemorrhage.7 Posterior epistaxis often results in anterior blood flow and may not be as readily identified as in anterior epistaxis. Given this, patients with posterior epistaxis may have symptoms that include nausea, hematemesis, anemia, hemoptysis, melena or hypotension.3 The source of bleeding in posterior epistaxis often cannot be adequately visualized without endoscopy and is therefore more challenging to treat.4
Figure 1.
Nasal blood supply
Source: Tintinalli JE, Stapczynski JS, Ma OJ, et al. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. 2011. New York (NY): McGraw-Hill Education. © McGraw-Hill Education. All rights reserved.
Risk factors
Epistaxis results from damage or rupture of the vasculature in the nasal cavity. While nearly 40% of cases of epistaxis have been reported to be idiopathic,8 there are several potential direct causes, such as trauma (nose picking, facial injury, foreign body, nasogastric tube placement, barotrauma), neoplasms and nasal polyps, nasal dryness, septal perforation, infection and environmental irritants such as cigarette smoke.4,6 Factors that may increase the risk of epistaxis occurrence are listed in Table 1.2,9,10 As our population continues to age, with more people becoming candidates for antithrombotic therapies,11 epistaxis may become more prevalent, given multiple risk factors will be at play.
Table 1.
| Male gender Older age (>65 years) History of nasal surgery History of previous epistaxis Thrombocytopenia (platelets <150 × 109/L) Congestive heart failure Peripheral vascular disease Diabetes Alcohol abuse Coagulopathies Vascular malformations Antiplatelet and anticoagulant medications |
Management
Widely accepted, evidence-based guidelines for the management of epistaxis have not been developed, although various algorithms that reflect local practices have been suggested.12 Overall, literature assessing the pharmacologic management of epistaxis is generally of low quality, consisting largely of prospective cohorts and retrospective chart reviews that lack a comparator or control group. Some have studied agent use in combination, making it problematic to discern the ingredient imparting benefit (e.g., moisturizer base with medicinal ingredient), while others have used agents in combination with mechanical strategies. Differences in the patient populations studied further preclude comparison or combining of data from various studies; for example, some have included only patients with anterior or posterior epistaxis, some have excluded anticoagulated patients whereas others have included only such patients, and some have studied specifically inpatient or outpatient populations. Although most studies assess rebleeding or treatment failure rates, their definitions of these outcomes are variable. Given this, data interpretation and comparison across studies is difficult, only enabling general recommendations to be made.
While most cases of epistaxis are not life threatening and initial self-management strategies are appropriate,5 data indicate that only 40% of patients are able to list a single appropriate general measure to manage a nosebleed.13 Following receipt of verbal and written information specific to the management of epistaxis, this improved to 50% being able to list at least 3 appropriate measures. Patients with multiple risk factors for epistaxis, recurrent epistaxis or those who take antithrombotic agents should be provided with appropriate information for self-management of epistaxis (see “How to manage a nosebleed” infographic). Among all patients amenable to community-based care, a staged approach to epistaxis management should be incorporated that consists of 1) implementing general measures, 2) using products available in the community-based setting that either halt or prevent subsequent episodes of epistaxis and 3) an awareness of measures accessible only within a hospital setting that may be necessary for those with severe epistaxis.
Patient Educational Handout for Self-Management of Epistaxis
General measures
In the event of epistaxis, patients should be encouraged to take a seated position and remain calm. The anterior aspect of the nose (below the bony bridge) should be pinched continuously for 15 to 20 minutes, resulting in compression of the anterior nasal blood vessels.5,14 This is contrary to the misconception that one should apply pressure on the bony bridge of the nose, wherein compression of blood vessels will not occur. The head should not be tilted backwards and instead should be positioned slightly forward to avoid pooling of blood in the pharynx, which could result in nausea, aspiration, swallowing of blood or airway obstruction.5,15 If bleeding is successfully stopped using the above measures, irritation of the nasal mucosa (e.g., nose picking and blowing) should be avoided for a few hours while the mucosa heals14,15; otherwise, a clot may be dislodged, resulting in recurrent bleeding. For this same reason, patients should not try to pack their nostrils with tissues or dry cotton balls, as eventual removal is likely to disturb any clots that have formed. If bleeding is noticeably reduced following the above steps, it is reasonable to repeat another 15 to 20 minutes of continuous compression. Bleeding refractory to manual compression may be amenable to pharmacologic treatments (see below) or may require emergency care centre assessment for possible invasive treatment measures, especially if bleeding is profuse.3,5,14 Epistaxis associated with head injury should be immediately referred to the ER.
Products for use in the community setting
Numerous products have been used to manage epistaxis (Table 2).16-25 Their role in management is dependent upon the mechanism of action in relation to the underlying cause of epistaxis. Products containing moisturizing agents may serve to mitigate further epistaxis providing dryness is problematic, whereas products that induce a local vasoconstrictor effect or assist in clot stabilization (e.g., antifibrinolytics) offer temporary approaches to halt bleeding yet may not prevent the recurrence of epistaxis, depending on the underlying etiology and subsequent treatment of epistaxis. A practice tool outlining a suggested treatment approach for community-based management of patients with epistaxis is provided in Figure 2. Below is a summary of available data assessing the use of these products. Data are limited and there is currently no evidence to suggest superiority of one agent over another.
Table 2.
Products for the prevention and treatment of epistaxis for use in community settings
| Agent/cost | Prevention or treatment | Mechanism of action | Technique for use | Comments |
|---|---|---|---|---|
| Saline gel16 (e.g., Secaris™) $ |
Prevention | Hydration of nasal mucosa prevents excessive dryness and subsequent irritation. | Using a cotton bud, apply gel into both nostrils daily or as needed for dryness. | • Nonprescription • May be used liberally • No adverse effects |
| Petroleum jelly17 (e.g., Vaseline™) $ |
Prevention | Hydration of nasal mucosa prevents excessive dryness and subsequent irritation. | Using a cotton bud, apply ointment into both nostrils daily or as needed for dryness. Apply at bedtime if presence in nares is bothersome. | • Nonprescription May be used liberally Greasy texture may be unappealing to some patients |
| Triamcinolone 0.025% cream17
$$ |
Prevention | Hydration of nasal mucosa prevents irritation and excessive dryness; triamcinolone may reduce mucosal inflammation. | Using a cotton bud, apply cream into both nostrils once weekly (use with or without daily use of saline gel or petroleum jelly). | • Requires a prescription • Frequent or prolonged use may be associated with adverse effects (e.g., mucosal thinning, septal perforation, epistaxis) Unclear if corticosteroid confers any benefit or if moisturizing effect of cream/vehicle alone is sufficient to prevent epistaxis |
| Oxymetazoline, xylometazoline nasal spray18-21
$ |
Treatment | Alpha-adrenergic agonists stimulate vasoconstriction of the blood vessels in the nasal mucosa to reduce rate and extent of bleeding. | Instil 2-6 sprays into affected nostril(s) during active
bleed, then use 2 sprays 3-4 times daily for 3 days
thereafter. Alternatively, soak gauze or a cotton pledget in the decongestant solution and place in affected nostril(s) for 30 minutes. |
• Nonprescription Use for >3-5 days may lead to rebound congestion May have an unpleasant taste • If the site of bleeding can be visualized, more localized application of the solution may be facilitated through the use of a cotton bud as opposed to placement of a pledget affecting the entire nostril. |
| Topical tranexamic acid (TXA)22-24
$$$ |
Prevention or treatment | Competitively inhibits conversion of plasminogen to plasmin, producing an antifibrinolytic effect. | Using a cotton bud, apply gel into the affected nostril(s)
2-4 times daily. Alternatively, soak gauze or a cotton pledget with TXA 100 mg/mL injectable solution and place into the affected nostril(s) and remove once bleeding is arrested. |
• Requires a prescription No commercial formulation for gel, therefore must be compounded • May have unpleasant taste |
| Glycine and calcium gel25 (e.g., Nozohaem™) $$$ |
Treatment | Exogenous glycine and calcium are thought to facilitate endogenous hemostatic processes at the site of bleeding | Single-use tubes. Insert the tip of the tube into the affected nostril(s) and squeeze the gel into the nostril(s). Leave in place for 30 minutes. | • Commercially
available • Nonprescription • Limited data available |
$, $1 to $10 per 30-day supply; $$, $11 to $20 per 30-day supply; $$$, >$20 per 30-day supply.
Figure 2.
Suggested treatment approach for patients in the community setting with epistaxis
Unmedicated and medicated local moisturizers
Regardless of a medicinal ingredient, these products have vehicles that are moisturizing in nature, affording benefit to those with dry nares. Saline nasal gel for the prevention of recurrent epistaxis has only been studied in a cohort of 74 anticoagulated patients (mean international normalized ratio [INR] 2.4) for the prevention of recurrent anterior epistaxis.16 Ninety-three percent of patients had resolution of their chronic epistaxis after 3 months of saline gel use. However, it is unclear how patients used the gel (including frequency and technique) or if any adjunct treatment or preventative strategies were employed. Given the benign risk profile, it is reasonable to recommend liberal use of saline gel for any patient at risk for or experiencing recurrent epistaxis, particularly if nasal dryness is an underlying risk factor. Of note, saline nasal sprays or irrigations have not been studied in patients with epistaxis and are not expected to confer the same moisturizing effects that a gel provides; in fact, saline solution may irritate the nasal mucosa, thereby exacerbating the risk for epistaxis.26 Thought to work by a similar mechanism of hydrating the nasal mucosa, the use of triamcinolone 0.025% cream applied once weekly to the nares along with daily topical petroleum jelly has been studied in patients with recurrent mild anterior epistaxis (criteria for “mild” epistaxis not provided).17 Although there was no control group for comparison, 89% of these patients had resolution of their epistaxis at 6 weeks’ follow-up. It is not possible to differentiate whether this benefit was derived from either agent alone or if it was a combined effect of both products. Also, given that epistaxis is generally not thought to be an inflammatory condition, the benefits of a topical corticosteroid beyond the moisturizing effects of its vehicle cream alone are uncertain. If a steroid-based product is used, application should be limited (perhaps once weekly as studied) due to the ability of topical steroids to thin the mucosa with prolonged use as well as the concern regarding epistaxis being a common adverse effect of intranasal corticosteroids.17
Topical decongestants
Topical nasal decongestants are thought to be valuable agents in the management of acute epistaxis because of their direct and localized vasoconstriction of the blood vessels in the nasal cavity. Their place in therapy is largely limited to the acute phase of management, including prevention of acute recurrence in the days following the bleed. Both oxymetazoline and xylometazoline have been studied in this setting.18 One study found a 75% success rate with the use of oxymetazoline (strength not specified) 4 to 6 sprays per nostril given once followed by 2 sprays every 6 hours for 1 to 3 days in patients with posterior epistaxis,18 and another reported a 65% success rate with the use of 2 sprays per nostril 3 times daily for 3 to 5 days.19 Mattoo et al.20 saw even higher rates of epistaxis resolution: 86% and 90% with topical xylometazoline 0.5% and epinephrine 1:10,000, respectively. These products were applied by packing the affected nostril(s) for 30 minutes with cotton packs soaked in the decongestant solution and are used in combination with other strategies. In 1 study,20 all cases received silver nitrate cautery following the decongestant, regardless of bleeding status. In 2 of the 3 above studies,19,20 treatment was followed by nasal packing (without any embedded active ingredients) in cases where bleeding persisted despite the use of decongestants. Topical decongestants are a safe and accessible alternative for outpatients; however, continuous use should not persist beyond 3 to 5 days to reduce the risk of developing rebound nasal congestion.21 If heavy bleeding persists despite the initial dose (2-6 sprays or 30 minutes of soaked packs), referral for assessment and further intervention would be prudent.
Antifibrinolytics
Tranexamic acid (TXA), an antifibrinolytic, has been the most extensively studied of all of the products available for patient self-treatment. However, high-quality, robust data to support its routine use in patients with epistaxis are still lacking.27 A randomized, double-blind, parallel group trial of 68 patients has found no difference in bleeding arrest at 30 minutes between TXA 10% gel and placebo (60% TXA vs 76% placebo, p = 0.16).22 The high rate of bleeding arrest observed with the placebo gel may be partially explained in that fewer patients randomized to placebo had severe epistaxis relative to those receiving TXA. In contrast, an unblinded randomized trial in patients with anterior epistaxis (n = 216) observed a significantly higher rate of bleeding cessation with placement of TXA 100 mg/mL-soaked gauze compared to those treated with epinephrine-soaked gauze followed by anterior nasal packing (71% vs 31%, p < 0.001).23 This also led to significantly more TXA patients being discharged from the ER within 2 hours compared to control (95% vs 6.4%, p < 0.001). A recent open-label randomized trial among 124 patients with anterior epistaxis taking acetyl salicylic acid (ASA), clopidogrel, or both, compared TXA-soaked gauze to anterior nasal packing and demonstrated superiority of topical TXA over nasal packing.24 Lastly, a randomized placebo controlled trial (n = 89) used oral TXA 1 g 3 times daily as an adjunct to nasal packing and/or cautery in patients with epistaxis.28 Those with thromboembolic disease or who were taking antithrombotic medications were excluded. Oral TXA did not produce any benefit with respect to rebleeding rates (47% TXA vs 57% placebo, p > 0.50) or hospital LOS (mean LOS: 5.42 days TXA vs 5.02 days placebo, modal LOS: 3 days TXA vs 4 days placebo). The rate of adverse effects was also not different between oral TXA and placebo. Despite the inconclusive evidence for the efficacy of TXA in epistaxis, topical TXA appears to pose few to no risks to patients. A Cochrane review of 29 randomized-controlled trials involving 2612 participants comparing topical TXA used intra- or postoperatively for surgical indications (and including only 1 trial of patients with epistaxis) to control groups found no increase in thromboembolic events, including myocardial infarction, stroke, deep vein thrombosis and pulmonary embolism.29 However, most of the studies were underpowered to detect a meaningful difference for this outcome. The same inferences regarding benefit/risk profile cannot be made for oral TXA, lending support to offer only the topical TXA as a reasonable therapeutic alternative. Topical TXA has also been used as a preventative agent in patients prone to recurrent epistaxis, particularly those taking anticoagulants.
A commercially available gel (Nozohaem™) containing glycine and calcium—thought to facilitate clot formation through activation of endogenous coagulation processes—has been marketed for the acute management of epistaxis. A single-centre randomized trial of 40 patients receiving this gel versus 60 patients getting anterior nasal packaging found the gel group superior to the packing group in terms of bleeding cessation time, discharge time, pain score and side effect profile.25
ER/hospital-based management strategies
Severe and life-threatening blood loss can occur in select cases of epistaxis and should be managed in a hospital-based setting where rapid assessment and monitoring of blood loss can occur through serial measurements of complete blood cell counts and hemoglobin levels. The stepwise treatment approach for severe epistaxis may vary by institution but generally includes the use of mechanical strategies (Table 3)3-5,7,10,30-37 and/or pharmacologic agents (Table 4).18-20,22-24,28,38-50 Topical pharmacologic products, including hemostatic agents such as gelatin-thrombin matrix, fibrin glue or vasoconstrictors, may be used alone or in conjunction with mechanical strategies where temporary cessation or reduction of bleeding is necessary to facilitate the intervention, such as with cautery and anterior or posterior nasal packing. Nasal packing, particularly posterior packing, can be extremely uncomfortable for patients and requires patients to be hospitalized due to the risk of airway compromise. Systemic antibiotics or gauze impregnated with antibiotics may be used in cases of prolonged packing for prevention of Staphylococcal infection; however, the benefits of this practice are uncertain.46,50 More invasive surgical procedures, such as arterial ligation or embolization, may be employed when all other interventions have failed and where the appropriate medical specialists (such as ear/nose/throat [ENT] physicians) are available.
Table 3.
Mechanical strategies for management of epistaxis in the acute care setting
| Method* | Description | Place in therapy |
|---|---|---|
| Cautery5 | Application of a chemical (e.g., silver nitrate) or electrical device directly to site of bleeding on the nasal mucosa to halt bleeding. Following cauterization, it generally takes 4-6 weeks for the nasal mucosa to heal. | Indicated for those with continued epistaxis despite general measures or use of topical products. |
| Anterior nasal packing3-5,7 (e.g., Merocel™, Rapid Rhino™, nasal tampon, inflatable tampon, ribbon gauze) | The placement of an intranasal device within the affected
nare(s) that is designed to apply constant pressure and
enable clot formation at the affected site. Usually removed
within 48 hours of placement. If bleeding site was not visualized, ongoing epistaxis following removal of packing may indicate a posterior source of bleeding. |
Indicated for those with continued epistaxis despite general measures, use of topical products and/or cautery. |
| Posterior nasal packing3-5,7 (e.g., Double-Balloon, Foley catheter) | Procedure involves placement of packing in the posterior nasal cavity followed by placement in the anterior cavity. Very uncomfortable for the patient and ideally performed by a specialist. Patients with posterior packing are admitted to hospital for observation due to the risk of airway compromise or bradydysrythmias. Packing is removed in 72-96 hours. | May be inserted upon failure of anterior nasal packing. May be a temporizing measure until more definitive therapy may be implemented (e.g., ligation). |
| Nasal arterial ligation30-33,36,37 | Best performed by a specialist, endoscopically targeting ligation close to the bleeding vessel. This operative procedure is performed under local or general anesthesia wherein 1 or more of the 4 vascular systems supplying the nose is ligated with a metal clip or by electrocautery. | Reserved for complicated epistaxis that has not responded to less invasive strategies. Serious complications, including stroke, blindness and facial numbness, may occur. |
| Nasal arterial embolization10,34-37 | Procedure performed by a specialist, usually under local anesthesia. Angiography of nasal vasculature is performed with microcatheters inserted into affected vasculature via femoral artery access to release microparticles (e.g., polyvinyl alcohol particles, platinum coils, or gelfoam material) targeted to decrease arterial blood flow within the affected vessel(s) and facilitate endothelial repair. Does not aim to devascularize the nose. | Invasive procedure reserved for intractable epistaxis that has not responded to less invasive measures. Serious complications, including stroke, tissue necrosis, facial paralysis, temporofacial pain and headache, may occur. |
Some of these methods are performed in combination or conjunction with local decongestants or antifibrinolytics.
Table 4.
Pharmacologic agents for treatment of epistaxis in the acute care setting
| Agent | Mechanism of action | Technique for use |
|---|---|---|
| Gelatin-thrombin matrix38-43,47 (e.g., FloSeal™) | Supplied as 2 separate components: human thrombin and gelatin matrix. When mixed and applied to active bleed, thrombin in the gel converts fibrinogen in blood into insoluble fibrin. This initiates the development of a hemostatic plug, forming a clot at the site of bleeding. | For topical use only. May be applied following application
of topical vasoconstrictors. Mix thrombin solution and gelatin matrix as per manufacturer instructions. With or without endoscopic guidance, using the supplied syringe and applicator, the matrix is applied directly to the source of bleeding. Reapplication may be necessary if bleeding persists. |
| Fibrin glue44,48,49 | Supplied as separate components of sealer protein component (containing fibrinogen) and thrombin-calcium component. When mixed, thrombin activates the fibrinogen into fibrin. Direct application of fibrin allows for placement of an exogenous clot to the site of bleeding. | May be applied following application of topical
vasoconstrictors. Separate components are loaded into 2 syringes and placed into a 2-channel syringe device, as per manufacturer instructions. As the syringes are depressed, the fibrin precipitates in the mixing chamber and is then deposited directly onto the site of bleeding. |
| Topical vasoconstrictors (e.g., oxymetazoline, xylometazoline18-20) | Alpha-adrenergic agonists stimulate vasoconstriction of the blood vessels in the nasal mucosa to reduce rate and extent of bleeding. | Various doses and techniques have been studied. Some administer in a nasal spray (dose range 2-6 sprays per nostril), whereas others place a cotton pledget soaked with a solution of decongestant drug in the nasal cavity for 30 minutes. |
| Desmopressin45 | A synthetic analogue of antidiuretic hormone. Increases plasma factor VIII, thereby increasing overall coagulation activity. Thought to have a direct and local effect on blood vessel walls that enhances platelet adhesion. | In a cohort of patients with postoperative epistaxis, a single dose of desmopressin 0.3 μg/kg intravenously (IV) was used. |
| Systemic antibiotics46,50 | Purported use is adjunct in nature. May be used in prolonged retention of nasal packing for prevention of toxic shock syndrome secondary to Staphylococcus aureus infection and/or sinusitis or bacteremia secondary to nasal mucosa manipulation. | Agents used: - Amoxicillin/clavulanate (dose not specified) - Cefazolin 1 g IV q8h May be used with or without concurrent use of antibiotic-impregnated nasal packing. |
| Tranexamic acid22-24,28 | Competitively inhibits the conversion of plasminogen to plasmin, thereby producing an antifibrinolytic effect. | Various routes of administration have been
studied: - Cotton pledget soaked in 100 mg/mL tranexamic acid injectable solution placed in nasal cavity - 10% tranexamic acid gel, filling the entire affected nostril - Tranexamic acid 1 g PO TID × 10 days |
Concomitant antithrombotic therapy
Patients with epistaxis on anticoagulant or antiplatelet therapies are generally treated no differently from those not taking such agents, in that self-management strategies should be tried initially and if bleeding persists, then patients should be referred for further assessment. One may have a lower threshold for referral to acute care in patients taking antithrombotics due to the increased likelihood that they may require invasive measures to manage bleeding.11,51-55 The major difference in managing these patients lies in the consideration of modifying the antithrombotic regimen when the antithrombotic drug is thought to be a major contributor to the nosebleed. Of note, consultation with or referral to the clinician most responsible for managing a patient’s antithrombotic therapy should be sought prior to recommending a modification in therapy to the patient.
Three key factors must be evaluated when determining whether or not an antithrombotic agent is a major contributor to the epistaxis and subsequently if modification in therapy should be considered. They are the severity of bleeding, the degree of anticoagulation/platelet inhibition at the time of bleeding and the patient’s thrombotic risk. First, the severity of bleeding must be assessed. Though there is no validated tool to stratify epistaxis severity, community-based clinicians may use objective parameters to estimate severity such as duration and history of epistaxis, volume of blood loss, whether a patient is symptomatic for blood loss (e.g., dizziness, hypotension, tachycardia, lightheadedness or syncope) and, for patients with recurrent epistaxis, assessment of hemoglobin for a downward trend over time may be helpful.
Second, anticoagulation status or degree of platelet inhibition at the time of bleeding should be estimated. It should be noted that therapeutic anticoagulation (e.g., patients on warfarin with an INR within target range or appropriately dosed direct oral anticoagulants [DOACs] for renal function) should not cause overt bleeding but rather might uncover preexisting tendencies or anomalies for bleeding. For platelet inhibition, one would infer a greater degree of inhibition with larger doses of antiplatelets or dual antiplatelet therapy (DAPT). Therefore, excessive anticoagulation or platelet inhibition should be ruled out as a contributor for epistaxis, including an assessment of factors that may contribute to an enhanced inability to clot.
For patients taking warfarin: INR should be assessed, including an assessment of factors known to increase INR, such as the presence of a drug interaction, deterioration in health status or changes in lifestyle (e.g., reduction in vitamin K intake or excessive alcohol consumption).56 In patients with a therapeutic INR and epistaxis suitable for self-management, continuation of warfarin therapy is recommended,11,57-59 as data have indicated that there is no increase in bleeding complications when warfarin therapy is uninterrupted in such patients.11,59,60
For patients taking DOACs: The patient’s current renal function, age and weight should be assessed to ensure appropriateness of the agent and dose. Patients should also be assessed for any new interacting medications that may increase DOAC levels. Several case reports of epistaxis in patients taking dabigatran or rivaroxaban have been published,61-66 but the management strategies employed were variable (including temporarily or permanently stopping the DOAC, changing the DOAC to a vitamin K antagonist or using topical tranexamic acid), offering little insight into general recommendations.
For patients taking antiplatelets: Again, patients should be assessed for drug interactions that could increase antiplatelet levels. Patients taking DAPT are indeed at a higher risk of bleeding due to the additive platelet inhibition; however, this risk is generally acceptable in light of the significant thrombotic risk for which it is indicated (e.g., recent placement of coronary artery stents). There are no routinely available laboratory parameters that quantify platelet inhibition.
For patients taking combination anticoagulant/antiplatelet therapy, the above factors should be assessed, as applicable. While this combination is appropriate for specific indications, one should be vigilant in estimating the overall degree of thrombotic inhibition in such patients given the cumulative effect of anticoagulants and antiplatelets on hemostatic processes.
Third, the patient’s indication for antithrombotic therapy must be evaluated, given that this defines the specific thrombotic event the drug is intended to prevent. An understanding of thrombotic risk will affect the threshold at which therapy modification is considered. Certain disease states have more refined risk stratification schemes to estimate risk, such as with atrial fibrillation, the CHADS2 score (1 point for each of Congestive heart failure, Hypertension, Age 75 years or older, Diabetes mellitus and 2 points for history of Stroke or transient ischemic attack) offers a validated approach to estimating risk of stroke.67 The greater the CHADS2 score, the higher the thrombotic risk and hence the more reluctant one is to withhold antithrombotic therapy. For example, in a patient with epistaxis anticoagulated for atrial fibrillation, a clinician may be more willing to withhold therapy in a patient with a CHADS2 score of 1 (annual stroke risk ~2.8%) than in a patient with a CHADS2 score of 6 (annual stroke risk ~18.2%).67 In such circumstances, consultation with the clinician managing the antithrombotic therapy is advised because inappropriate dose reduction or cessation of therapy may result in thrombotic events with potentially devastating consequences.
Prevention
Preventative measures include avoiding inciting factors such as trauma to the nasal mucosa (e.g., nose picking). Keeping the nasal mucosa hydrated, especially during winter months, by the use of humidifiers and/or saline nasal gels may mitigate the occurrence or severity of epistaxis.14,15 Provision of verbal education for patient self-management of epistaxis supplemented by written information has been shown to significantly improve patients’ recall and understanding of appropriate self-management and first aid techniques.13,68 A good understanding of the potential etiology, risk factors and available outpatient products used to prevent and treat epistaxis will better prepare the clinician to counsel appropriate patients for strategies to manage epistaxis and to recognize when patients with recurrent epistaxis may benefit from referral to ENT specialists for more definitive preventive treatment strategies such as cautery or septoplasty.
Summary
Epistaxis will occur in the majority of people at least once in their lifetime; however, those with risk factors are more predisposed. The mode of treatment depends on the location and severity of the bleed, although most cases can be appropriately self-managed by patients. Overall, the literature to support different treatment modalities is derived from heterogeneous patient populations and is generally of low quality. Community-based treatment approaches should always begin with general measures, including manual compression of the nares, with the potential for application of topical vasoconstrictors and/or tranexamic acid. If medical attention is sought, more invasive measures such as nasal packing, cautery or surgical approaches may be considered. In patients on antithrombotic medications with active epistaxis, an evaluation of bleeding severity, anticoagulation or platelet inhibition status and overall thrombotic risk should be done to determine whether therapy modification and consultation with the clinician managing the therapy is warranted. Lastly, in patients with recurrent epistaxis, preventive measures ensuring adequate hydration and avoidance of trauma to the nares should be implemented. Front-line community-based pharmacists are well positioned to identify patients suitable for self-management of epistaxis and provide them with the appropriate education and tools to treat and prevent future episodes.
Footnotes
Author Contributions:J. Smith participated in the design of the work, drafted the work, critically reviewed the work and approved the final version of the work to be published. J. Hanson critically reviewed the work for important intellectual content and approved the final version of the work to be published. R. Chowdhury critically reviewed the work for important intellectual content and approved the final version of the work to be published. T. J. Bungard designed the work, critically reviewed the work for important intellectual content and approved the final version of the work to be published.
Financial Acknowledgment:No funding was received for this manuscript.
Statement of Conflicting Interest:JS, JH and RC have nothing to disclose. TJB reports no conflicts related to this manuscript; she has received honoraria for an advisory board from Boehringer-Ingelheim and speaker honoraria from Bayer. TJB has unrestricted research grants from Leo Pharma and Pfizer Canada.
References
- 1. Pallin DJ, Chng YM, McKay MP, Emond JA, Pelletier AJ, Camargo CA., Jr. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med 2005;46(1):77-81. [DOI] [PubMed] [Google Scholar]
- 2. Goljo E, Dang R, Iloreta AM, Govindaraj S. Cost of management in epistaxis admission: impact of patient and hospital characteristics. Laryngoscope 2015;125(12):2642-7. [DOI] [PubMed] [Google Scholar]
- 3. Kucik CJ, Clenney T. Management of epistaxis. Am Fam Physician 2005;71(2):305-11. [PubMed] [Google Scholar]
- 4. Diamond L. Managing epistaxis. JAAPA 2014;27(11):35-9. [DOI] [PubMed] [Google Scholar]
- 5. Schlosser RJ. Clinical practice: epistaxis. N Engl J Med 2009;360(8):784-9. [DOI] [PubMed] [Google Scholar]
- 6. Koh E, Frazzini VI, Kagetsu NJ. Epistaxis: vascular anatomy, origins and endovascular treatment. AJR Am J Roentgenol 2000;174(3):845-51. [DOI] [PubMed] [Google Scholar]
- 7. Kasperek ZA, Pollock GF. Epistaxis: an overview. Emerg Med Clin North Am 2013;31(2):443-54. [DOI] [PubMed] [Google Scholar]
- 8. Kotecha B, Fowler S, Harkness P, Walmsley J, Brown P, Topham J. Management of epistaxis: a national survey. Ann R Coll Surg Engl 1996;78(5):444-6. [PMC free article] [PubMed] [Google Scholar]
- 9. Corte FC, Orfao T, Dias CC, Moura CP, Santos M. Risk factors for the occurrence of epistaxis: prospective study. Auris Nasus Larynx 2018;45(3):471-5. [DOI] [PubMed] [Google Scholar]
- 10. Cohen JE, Moscovici S, Gomori JM, Eliashar R, Weinberger J, Itshayek E. Selective endovascular embolization for refractory idiopathic epistaxis is a safe and effective therapeutic option: technique, complications and outcomes. J Clin Neurosci 2012;19(5):687-90. [DOI] [PubMed] [Google Scholar]
- 11. Bola S, Marsh R, Braggins S, Potter C, Hickey S. Does the continuation of warfarin change management outcomes in epistaxis patients? J Laryngol Otol 2016;130(3):256-60. [DOI] [PubMed] [Google Scholar]
- 12. Escabasse V, Malard O, Crampette L, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL). Managing epistaxis under coagulation disorder due to antithrombotic therapy. Eur Ann Otorhinolaryngol Head Neck Dis 2017;134(3):195-9. [DOI] [PubMed] [Google Scholar]
- 13. Lavy J. Epistaxis in anticoagulated patients: educating an at-risk population. Br J Haematol 1996;95(1):195-7. [DOI] [PubMed] [Google Scholar]
- 14. American Academy of Family Physicians. Information from your family doctor. Nosebleeds. Am Fam Physician 2005;71(2):312. [PubMed] [Google Scholar]
- 15. Mayo Clinic. Nosebleeds: when to see your doctor. 2018. Available: https://www.mayoclinic.org/symptoms/nosebleeds/basics/when-to-see-doctor/sym-20050914 (accessed Oct. 3, 2017).
- 16. Massick D, Hurtuk A. Effectiveness of a nasal saline gel in the treatment of recurrent anterior epistaxis in anticoagulated patients. Ear Nose Throat J 2011;90(9):E4. [DOI] [PubMed] [Google Scholar]
- 17. London SD, Lindsey WH. A reliable medical treatment for recurrent mild anterior epistaxis. Laryngoscope 1999;109(9):1535-7. [DOI] [PubMed] [Google Scholar]
- 18. Doo G, Johnson DS. Oxymetazoline in the treatment of posterior epistaxis. Hawaii Med J 1999;58(8):210-2. [PubMed] [Google Scholar]
- 19. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol 1995;104(9 Pt 1):704-6. [DOI] [PubMed] [Google Scholar]
- 20. Mattoo O, Yousuf A, Mir A, Muzaffar R, Pampori R. Control of anterior epistaxis: a comparative analysis of the decongestive effect of xylometazoline and adrenaline in idiopathic epistaxis in emergency settings. Clin Rhinol 2011;4(3):130-5. [Google Scholar]
- 21. Canadian Pharmacists Association. Xylometazoline, CPhA Monograph. 2015. Available: www.e-therapeutics.ca (accessed October 3, 2017).
- 22. Tibbelin A, Aust R, Bende M, et al. Effect of local tranexamic acid gel in the treatment of epistaxis. ORL J Otorhinolaryngol Relat Spec 1995;57(4):207-9. [DOI] [PubMed] [Google Scholar]
- 23. Zahed R, Moharamzadeh P, Alizadeharasi S, Ghasemi A, Saeedi M. A new and rapid method for epistaxis treatment using injectable form of tranexamic acid topically: a randomized controlled trial. Am J Emerg Med 2013;31(9):1389-92. [DOI] [PubMed] [Google Scholar]
- 24. Zahed R, Mousavi Jazayeri MH, Naderi A, Naderpour Z, Saeedi M. Topical tranexamic acid compared with anterior nasal packing for treatment of epistaxis in patients taking antiplatelet drugs: randomized controlled trial. Acad Emerg Med 2018;25(3):261-6. [DOI] [PubMed] [Google Scholar]
- 25. Torabi M, Esfahani A, Baneshi M. A new method of epistaxis management using nasal gel: a single centre, randomized clinical trial. Asian J Pharm Clin Res 2018;11(3):104-8. [Google Scholar]
- 26. Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg 2007;137(5):815-21. [DOI] [PubMed] [Google Scholar]
- 27. Hilton L, Reuben A. Best evidence topic reports. BET 3: Topical intranasal tranexamic acid for spontaneous epistaxis. Emerg Med J 2014;31(5):436-7. [DOI] [PubMed] [Google Scholar]
- 28. White A, O’Reilly BF. Oral tranexamic acid in the management of epistaxis. Clin Otolaryngol Allied Sci 1988;13(1):11-6. [DOI] [PubMed] [Google Scholar]
- 29. Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev 2013;(7):CD010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asanau A, Timoshenko AP, Vercherin P, Martin C, Prades J. Sphenopalatine and anterior ethmoidal artery ligation for severe epistaxis. Ann Otol Rhinol Laryngol 2009;118(9):639-44. [DOI] [PubMed] [Google Scholar]
- 31. Cooke ET. An evaluation and clinical study of severe epistaxis treated by arterial ligation. J Laryngol Otol 1985;99(8):745-9. [DOI] [PubMed] [Google Scholar]
- 32. Sylvester MJ, Chung SY, Guinand LA, Govindan A, Baredes S, Eloy JA. Arterial ligation versus embolization in epistaxis management: counterintuitive national trends. Laryngoscope 2017;127(5):1017-20. [DOI] [PubMed] [Google Scholar]
- 33. McDermott AM, O’Cathain E, Carey BW, O’Sullivan P, Sheahan P. Sphenopalatine artery ligation for epistaxis: factors influencing outcome and impact of timing of surgery. Otolaryngol Head Neck Surg 2016;154(3):547-52. [DOI] [PubMed] [Google Scholar]
- 34. Christensen NP, Smith DS, Barnwell SL, Wax MK. Arterial embolization in the management of posterior epistaxis. Otolaryngol Head Neck Surg 2005;133(5):748-53. [DOI] [PubMed] [Google Scholar]
- 35. Sadri M, Midwinter K, Ahmed A, Parker A. Assessment of safety and efficacy of arterial embolisation in the management of intractable epistaxis. Eur Arch Otorhinolaryngol 2006;263(6):560-6. [DOI] [PubMed] [Google Scholar]
- 36. Strach K, Schrock A, Wilhelm K, et al. Endovascular treatment of epistaxis: indications, management and outcome. Cardiovasc Intervent Radiol 2011;34(6):1190-8. [DOI] [PubMed] [Google Scholar]
- 37. Strong EB, Bell DA, Johnson LP, Jacobs JM. Intractable epistaxis: transantral ligation vs. embolization: efficacy review and cost analysis. Otolaryngol Head Neck Surg 1995;113(6):674-8. [DOI] [PubMed] [Google Scholar]
- 38. Cote D, Barber B, Diamond C, Wright E. FloSeal hemostatic matrix in persistent epistaxis: prospective clinical trial. J Otolaryngol Head Neck Surg 2010;39(3):304-8. [PubMed] [Google Scholar]
- 39. Khan MK, Reda El, Badawey M, Powell J, Idris M. The utility of FloSeal haemostatic agent in the management of epistaxis. J Laryngol Otol 2015;129(4):353-7. [DOI] [PubMed] [Google Scholar]
- 40. Kilty SJ, AlHajry M, AlMutairi D, et al. Prospective clinical trial of gelatin-thrombin matrix as first line treatment of posterior epistaxis. Laryngoscope 2014;124(1):38-42. [DOI] [PubMed] [Google Scholar]
- 41. Lau AS, Upile NS, Lazarova L, Swift AC. Evaluating the use of Floseal haemostatic matrix in the treatment of epistaxis: a prospective, control-matched longitudinal study. Eur Arch Otorhinolaryngol 2016;273(9):2579-84. [DOI] [PubMed] [Google Scholar]
- 42. Mathiasen RA, Cruz RM. Prospective, randomized, controlled clinical trial of a novel matrix hemostatic sealant in patients with acute anterior epistaxis. Laryngoscope 2005;115(5):899-902. [DOI] [PubMed] [Google Scholar]
- 43. Wakelam OC, Dimitriadis PA, Stephens J. The use of FloSeal haemostatic sealant in the management of epistaxis: a prospective clinical study and literature review. Ann R Coll Surg Engl 2017;99(1):28-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walshe P. The use of fibrin glue to arrest epistaxis in the presence of a coagulopathy. Laryngoscope 2002;112(6):1126-8. [DOI] [PubMed] [Google Scholar]
- 45. Faber C, Larson K, Amirlak B, Guyuron B. Use of desmopressin for unremitting epistaxis following septorhinoplasty and turbinectomy. Plast Reconstr Surg 2011;128(6):728e-32e. [DOI] [PubMed] [Google Scholar]
- 46. Derkay CS, Hirsch BE, Johnson JT, Wagner RL. Posterior nasal packing: are intravenous antibiotics really necessary? Arch Otolaryngol Head Neck Surg 1989;115(4):439-41. [DOI] [PubMed] [Google Scholar]
- 47. Baxter II. Floseal Hemostatic matrix: instructions for use. 2014. Available: http://www.baxter.ca/en_CA/assets/downloads/monographs/Floseal%20FinalArtwork_0726933_23FEB2015_footer%20removed.pdf (accessed October 3, 2017).
- 48. Omrix Biopharmaceuticals L. Evicel: product monograph. 2017. Available: https://pdf.hres.ca/dpd_pm/00041607.PDF (accessed Oct. 2017).
- 49. Baxter C. Tisseel: product monograph. 2016. Available: https://pdf.hres.ca/dpd_pm/00035795.PDF (accessed Oct. 2017).
- 50. Biswas D, Mal RK. Are systemic prophylactic antibiotics indicated with anterior nasal packing for spontaneous epistaxis? Acta Otolaryngol 2009;129(2):179-81. [DOI] [PubMed] [Google Scholar]
- 51. Soyka MB, Rufibach K, Huber A, Holzmann D. Is severe epistaxis associated with acetylsalicylic acid intake? Laryngoscope 2010;120(1):200-7. [DOI] [PubMed] [Google Scholar]
- 52. Stadler RR, Kindler R, Holzmann D, Soyka MB. The long-term fate of epistaxis patients with exposure to antithrombotic medication. Eur Arch Otorhinolaryngol 2016;273(9):2561-7. [DOI] [PubMed] [Google Scholar]
- 53. Smith J, Siddiq S, Dyer C, Rainsbury J, Kim D. Epistaxis in patients taking oral anticoagulant and antiplatelet medication: prospective cohort study. J Laryngol Otol 2011;125(1):38-42. [DOI] [PubMed] [Google Scholar]
- 54. Tay HL, McMahon AD, Evans JMM, MacDonald TM. Aspirin, nonsteroidal anti-inflammatory drugs and epistaxis: a regional record linkage case control study. Ann Otol Rhinol Laryngol 1998;107(8):671-4. [DOI] [PubMed] [Google Scholar]
- 55. Garcia Callejo FJ, Becares Martinez C, Calvo Gonzalez J, Martinez Beneyto P, Marco Sanz M, Marco Algarra J. Epistaxis and dabigatran, a new non-vitamin K antagonist oral anticoagulant. Acta Otorrinolaringol Esp 2014;65(6):346-54. [DOI] [PubMed] [Google Scholar]
- 56. White PJ. Patient factors that influence warfarin dose response. J Pharm Pract 2010;23(3):194-204. [DOI] [PubMed] [Google Scholar]
- 57. Choudhury N, Sharp HR, Mir N, Salama NY. Epistaxis and oral anticoagulant therapy. Rhinology 2004;42(2):92-7. [PubMed] [Google Scholar]
- 58. Musgrave KM, Powell J. A systematic review of anti-thrombotic therapy in epistaxis. Rhinology 2016;54(4):292-391. [DOI] [PubMed] [Google Scholar]
- 59. Srinivasan V, Patel H, John DG, Worsley A. Warfarin and epistaxis: should warfarin always be discontinued? Clin Otolaryngol Allied Sci 1997;22(6):542-4. [PubMed] [Google Scholar]
- 60. Biggs TC, Baruah P, Mainwaring J, Harries PG, Salib RJ. Treatment algorithm for oral anticoagulant and antiplatelet therapy in epistaxis patients. J Laryngol Otol 2013;127(5):483-8. [DOI] [PubMed] [Google Scholar]
- 61. Utkewicz MD, Brunetti L, Awad NI. Epistaxis complicated by rivaroxaban managed with topical tranexamic acid. Am J Emerg Med 2015;33(9):1329.e5-e7. [DOI] [PubMed] [Google Scholar]
- 62. Chen BC, Viny AD, Garlich FM, et al. Hemorrhagic complications associated with dabigatran use. Clin Toxicol 2012;50(9):854-7. [DOI] [PubMed] [Google Scholar]
- 63. Sennesael AL, Dogne JM, Spinewine A. Optimizing the safe use of direct oral anticoagulants in older patients: a teachable moment. JAMA Intern Med 2015;175(10):1608-9. [DOI] [PubMed] [Google Scholar]
- 64. Stollberger C, Krutisch G, Finsterer J. Life-threatening epistaxis and red blood cell polyagglutination under dabigatran. Blood Coagul Fibrinolysis 2014;25(4):384-6. [DOI] [PubMed] [Google Scholar]
- 65. Freshour JE, Hudson JQ, Stevens AB, Franks AS. Epistaxis associated with dabigatran in an elderly patient with reduced creatinine clearance. Am J Health Syst Pharm 2012;69(14):1184-6. [DOI] [PubMed] [Google Scholar]
- 66. Gruenebaum DD, Alsarah A, Alsara O, Laird-Fick H. Bleeding complication of triple therapy of rivaroxaban, prasugrel and aspirin: a case report and general discussion. Case Rep Cardiol 2014;2014:293476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285(22):2864-70. [DOI] [PubMed] [Google Scholar]
- 68. Hassenplug KL, Burkiewicz JS, Jackson TL, Peppers LR. Educating patients in self-management of epistaxis in an anticoagulation clinic. Am J Health Syst Pharm 2006;63(10):909-11. [DOI] [PubMed] [Google Scholar]