Abstract
Study Design:
A narrative review of the literature.
Objective:
This article reviews the general principles of treatment and investigation for primary bone tumors of the spine. Furthermore, it explores the emerging alternatives.
Methods:
A review was performed using Medline, Embase, and Cochrane databases.
Results:
Primary bone tumors of the spine are rare entities that general spine surgeons may encounter only a few times in their career. The treatment algorithm of these complex tumors is filled with nuances and is evolving constantly. For these reasons, patients should be referred to experienced tertiary or quaternary centers who can offer a comprehensive multidisciplinary approach. For most malignant spinal bone tumors, surgery remains the cornerstone of treatment. Respecting oncologic principles has been associated with improved survival and decreased local recurrence in multiple settings. However, even in experienced centers, these surgeries carry a significant risk of adverse events and possible long-term neurologic impairment. The associated morbidity of these procedures and the challenges of local recurrence have encouraged professionals caring for these patients to explore alternatives or adjuncts to surgical treatment.
Conclusions:
Over the past few years, several advances have occurred in medical oncology, radiation oncology and interventional radiology, changing the treatment paradigm for some tumors. Other advances still need to be refined before being applied in a clinical setting.
Keywords: en bloc resection, interventional radiology, medical treatment, molecular sequencing, primary spine tumors, radiation oncology, surgery
Introduction
Primary bone tumors of the spine are far less common than metastatic spine disease. According to the Surveillance, Epidemiology and End Results (SEER), the overall incidence of primary bone tumors in the United States is 0.9 per 100 000 men and women per year, including both axial and appendicular lesions.1 An estimated 3450 new cases will be declared this year in the United States. These lesions can harbor different patterns of behavior and represent a heterogeneous group; from benign and latent to malignant and aggressive. Chordoma, chondrosarcoma, osteosarcoma, and Ewing’s sarcomas represent the most frequent malignant bone tumors.1 Some lesions, such as aneurysmal bone cyst (ABC), giant cell tumor (GCT) of the bone, and osteoblastoma are histologically benign but can have aggressive behaviors and recur if not treated properly.
Identifying the rare primary bone tumor of the spine is of paramount importance as their management may differ tremendously from a metastatic spinal lesion. Undergoing inappropriate treatment can negatively affect these patient outcomes and even transform a curable disease to a lethal one. It cannot be overemphasized that vigilant recognition of these lesions is key, and prompt referral to experienced spine tumor centers for investigation and definitive management should be sought. Primary bone tumors of the spine should be treated in dedicated centers with experienced multidisciplinary teams. Although surgery remains a critical part of the treatment for most primary bone tumors, it is only a component of a comprehensive management plan. In this era of rapid medical advances, a multidisciplinary approach is mandatory to optimize these patient outcomes.
This article will review general management principles of primary bone tumors of the spine and will focus on recent advances in the treatment of these unique lesions.
General Principles
Staging
In the presence of a solitary spinal lesion, local and systemic staging should be undertaken. More than 85% of the time, the origin of a lesion can be determined with systematic systemic staging. Investigations include a computed tomography (CT) scan of the chest, abdomen, and pelvis and a bone scan. Positron emission tomography (PET)-CT is also increasingly used for staging.2 After appropriate local and systemic staging a well-planned biopsy to confirm the diagnosis should be done. When a primary bone tumor is suspected, the biopsy should be coordinated by the center where the patient will undergo definitive treatment. Proper biopsy orientation is a key aspect in the management of primary bone tumors. This is highlighted by reports confirming that biopsy performed outside the definitive treating center as well as intralesional resection are associated with greater rates of tumor recurrence.3,4 As a multidisciplinary group of experts caring for patients with spinal tumors, the Spine Oncology Study Group (SOSG) published recommendations that stipulate that the surgeon who will perform the definitive surgery should do or direct the biopsy.5 This ensures suitable orientation of the biopsy allowing resection of its tract within the surgical specimen where feasible.
Classifications
Over the past decades, recognition of the uniqueness of primary bone tumors has led to the acceptance of Enneking’s principles. This classification originated from the appendicular musculoskeletal oncology world.6 A recommended surgical margin is proposed for each stage of this oncological staging system. Tumors are divided into benign and malignant. Benign lesions are classified as S1, latent; S2, active; and S3, aggressive. For benign lesion, observation is suggested for latent lesions and an aggressive curettage or wide/marginal resection is suggested for active or more aggressive lesions. Malignant tumors are classified based on the grade (low vs high), local extension (intra- vs extracompartmental), and the presence of metastasis. For malignant lesions, in the absence of metastases, a wide resection is advocated.
Surgical Management
An en bloc resection is a surgical resection where the tumor is excised as a single piece as opposed to piecemeal resection. However, in terms of local control and survival, an en bloc resection is meaningless if not accompanied by the pathological description of the margins. Depending on the histological appearance of the margins, they can be classified as intralesional, marginal, or wide. Intralesional means that the tumor capsule has been violated. Wide margins refer to the removal of the tumor along with a shell of healthy tissue contiguous to it. Marginal margins signify that the plane of dissection is in the reactive layer surrounding the tumor or its pseudocapsule. As dura is not excised with the specimen for most cases, margins at the dura are often marginal if the tumor is extending to the epidural space. In 2009, the SOSG issued a strong recommendation based on moderate quality evidence and consensus expert opinion that en bloc resection with wide or marginal margins should be undertaken for surgical treatment of primary malignant bone tumors5 (Table 1). Because of the morbidity especially regarding neural sacrifice controversy remains within some areas of the spinal community. Modern evidence supports en bloc resection with wide/marginal margins as the cornerstone of management for most primary malignant bone tumors and confirms that adhering to evidenced-based oncologic principle results in lower recurrence and mortality rates.7-10 Furthermore, en bloc resection after an Enneking inappropriate surgery (ie, an intralesional procedure for a malignant bone tumor) has been demonstrated to yield inferior outcomes in terms of local recurrence and survival, reinforcing that the first surgery is the best attempt for cure and that these patients should be promptly referred to experienced centres even in a setting of neurological deficit3,4 (Figure 1).
Table 1.
Summary Recommendations From Focus Issues in Spine Oncology for Primary Bone Tumors.
| Focus Issue in Spine Oncology | Question | Recommendations | Strength of Recommendationa (Strong/Weak) | Quality of the Evidence (High/Moderate/Low/Very Low) |
|---|---|---|---|---|
| Benign primary bone tumor | ||||
| Aggressive “benign” primary spine neoplasms: osteoblastoma, aneurysmal bone cyst, and giant cell tumor11 (2009) | What is the optimal treatment for osteoblastomas, ABC, and GCT? | (1) For aggressive osteoblastoma, we recommend en bloc resection when
anatomically feasible. (2) For ABC, we recommend intralesional gross total resection because local recurrence is influenced by the completeness of resection. (3) For GCT, when feasible and based on predicted surgical morbidity (not sacrificing sacral neural function), en bloc resection is recommended. |
Strong Strong Strong |
Very low Very low Very low |
| Benign tumors of the spine: has new chemotherapy and interventional radiology changed the treatment paradigm18 (2016) | (1) What is the role of denosumab in the treatment of GCT? (2) What is the role of selective arterial embolization (SAE) in the treatment of ABC? (3) What is the role of thermal ablation in the treatment of spinal OO? |
Denosumab is indicated for the treatment of inoperable GCT and as neoadjuvant
therapy. SAE might be considered in the treatment of ABC. Percutaneous thermal ablation is indication for selected OO. Absence of intact cortex and close vicinity < 5 mm to neural element warrant precautions. |
Strong Weak Strong |
Very low Very low Very low |
| Malignant primary bone tumor | ||||
| Feasibility and Safety of en bloc resection for primary spine tumors: a systematic review by the Spine Oncology Study Group5 (2009) | (1) What is the effect of incisional biopsy performed before definitive en
bloc resection? 2) Should Enneking principles of en bloc resection of primary tumors be applied to the spine? |
When there is a suspicion of primary spine tumor, the surgeon who performs the
definitive surgery should ideally perform or direct the biopsy
procedure. En bloc resection of primary spine tumors with disease-free margins is achievable if proper oncologic and surgical staging determines that it is feasible. These surgeries should be performed by experienced, multidisciplinary teams. |
Strong Strong |
Low Low |
| Challenges of local recurrence and cure in low grade malignant tumors of the spine37 (2009) | (1) What is the optimal surgical management for chordoma and
chondrosarcoma? (2) What is the role of radiation as an adjuvant treatment, for chordoma and chondrosarcomas? |
En bloc resection with wide or marginal margins (en bloc) is the optimal
surgical treatment. Radiation therapy of at least 60 to 65 Gy equivalents is indicated as an adjuvant treatment when there has been incomplete resection or an intralesional margin. |
Strong Weak |
Moderate Low |
| Ewing and osteogenic sarcoma: evidence for multidisciplinary management60 (2009) | (1) What is the role of chemotherapy in the management for Ewing and
osteogenic sarcoma of the spine? (2) Does the extent of surgical resection affect local control and long-term survival for Ewing and osteogenic sarcoma of the spine? |
Neoadjuvant chemotherapy is recommended for management of both Ewing and
osteogenic sarcoma. (A) En bloc surgical resection for Ewing sarcoma of the spine is recommended it provides improved local control, but not improved overall survival. (B) En bloc surgical resection for osteogenic sarcoma of the spine is recommended as it provides improved local control and potentially improved overall survival. |
Strong Weak Strong |
Moderate Very low Very low |
| Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review61 (2016) | What are the toxicity and local control rates for adjuvant postoperative radiotherapy for spinal and sacral chordoma? | The use of adjuvant high-dose conformal radiotherapy should be used for patients undergoing surgery for the treatment of de novo chordoma when surgical margins are concerning and all recurrent chordoma in the mobile spine and sacrum. | Strong | Low |
| HRQOL | ||||
| Optimizing the adverse event and HRQOL profiles in the management of primary spine tumors53 (2016) | Considering the significant morbidity and potential loss of function primary spinal tumor surgery may ensue, does it result in acceptable quality of life for patients? | We recommend primary spinal tumor surgery be performed with a curative intent whenever possible, even at the expense of greater initial morbidity to optimize long-term HRQOL. | Strong | Very low |
Abbreviations: ABC, aneurysmal bone cyst; GCT, giant cell tumor; OO, osteoid osteoma.
a A strong recommendation allows clinicians to confidently apply an intervention “to all or almost all the patients in all or almost all the circumstances without thorough review of the underlying evidence and without a detailed discussion with the patient.”62 A consensus weak recommendation is an endorsement of the intervention, but the magnitude is less and circumstances altered compared with a strong recommendation.
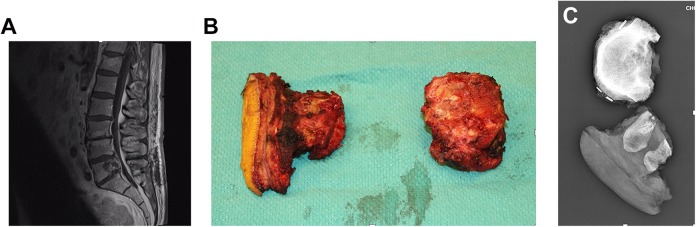
Figure 1.
En bloc resection of a L5 chordoma after inappropriate intralesional resection. (A) L5 chordoma at initial presentation. This patient underwent a decompressive laminectomy and intralesional resection. (B) Pathological specimen. Because of inappropriate intralesional resection, a skin ellipse was removed to excised tumor contaminated tissue when the Enneking appropriate en bloc resection was performed. This required a complex plastic closure. (C) Specimen x-ray.
In the appendicular skeleton, benign aggressive lesions such as ABC, GCT, and osteoblastoma are usually treated with intralesional curettage with or without local adjuvant such as phenol and liquid nitrogen. However, intralesional resection carries a significant risk of local recurrence, which can be challenging in the spine. Furthermore, local adjuvants usually cannot be used around the spine due to the risk of thermal injury. The SOSG issued strong recommendation with a very-low-quality evidence to perform en bloc resection for stage 3 osteoblastoma and GCT when feasible based on the staging and predicted surgical morbidity. For ABC, a gross total intralesional resection (very-low-quality evidence) is recommended.11 More recently, data favoring en bloc resection over intralesional resection for GCT of the spine with regard to local recurrence have been published.12
As surgical treatment of primary bone tumors is associated with significant morbidity and mortality, alternative therapies and adjuvant treatments have emerged with the goal of facilitating the surgery or, in some cases, replacing surgery altogether while achieving similar outcomes. This evolving field involves medical treatment, percutaneous techniques, radiation therapy, and the emergence of precision medicine with molecular sequencing. Furthermore, interest in patient reported outcomes has come to the forefront, acknowledging that local recurrence and survival are not the only outcomes that need consideration.
Medical Oncology and Interventional Radiology
Giant cell tumors harbor 3 types of cells: the multinucleated giant cell, the stromal cell and the mononuclear monocyte. The hallmark of GCT is the multinucleated giant cells, which express high levels of the receptor of activator nuclear factor κ-B ligand (RANKL). Activation of the RANKL leads to bony resorption. Denosumab is a monoclonal antibody that inhibits RANKL and it was postulated that this medication could halt progression in inoperable GCT. The first clinical trial funded by industry yield promising results with clinical response in more than 85% of the patients at 6 months (37 patients).13 On histopathological analysis of that same cohort, a marked reduction of the multinucleated giant cells was observed (>90%).14 The second clinical trial (282 patients) results were in line with the first one: an overall 75% objective tumor response, however, mostly partial responses.15 Of note, these clinical trials included axial and appendicular GCT. This led to the Food and Drug Administration approval of denosumab for the treatment of inoperable GCT in 2013. Specific to the spine, in 2015, Goldschlager et al16 published the first case-series of neoadjuvant denosumab with all patients presenting a favorable clinical and radiological response to denosumab. One patient out of 5 presented with histopathological failure to treatment.16 Decreased epidural disease as well as tumor calcification was reported with this treatment (Figure 2). Tumor angiogenesis has been shown to be reduced with denosumab.17 In 2016, the AOSpine Knowledge Forum Tumor (AOSKFT) recommended denosumab either as a stand-alone for treatment of inoperable GCT or as an adjuvant prior to surgical resection.18 Preoperative treatment duration recommendation was either 6 months or to maximal calcification/tumor reduction. However, uncertainty regarding long-term response and side effects remains. Concerns regarding rate of recurrence after discontinuation have been reported, especially after intralesional procedure.19,20 This can be explained by the findings of Mak et al21 that although the multinucleated giant cells are effectively eliminated with denosumab, the true neoplastic cells (the stromal cells) persist. Short-term treatment is associated with mild toxicities, but prolonged treatment is associated with a 9% risk of osteonecrosis of the jaw as well as a 4% risk of atypical femoral fracture in the most recent series.19 Furthermore, case reports of malignant transformation following this treatment have been published although causal relationship cannot be made.22-24 Denosumab is definitely a valuable treatment, but as with any newer treatment, vigilance is mandatory.
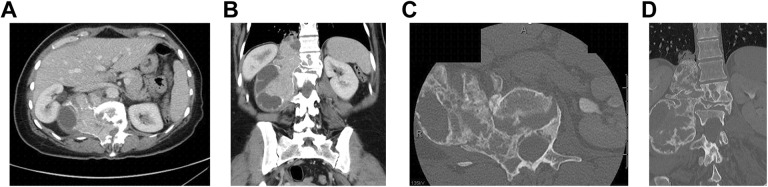
Figure 2.
Denosumab response in a L1-L2 giant cell tumor after 6 months of treatment. (A) Axial computed tomography (CT) pretreatment. (B) Coronal CT pretreatment. (C) Axial CT after 6 months of denosumab. (D) Coronal CT after 6 months of denosumab.
Selective arterial embolization as a stand-alone treatment has been reported with ABC. Initially, promising results were observed with tumor calcification and regression.25,26 The main problematic issue with this technique was that up to 35% of the patients in these series required more than 6 treatments, which is a concern from a radiation exposure standpoint.26 The most recent series published by the same group tempered the initial enthusiasm with a rate of failure approaching 30%.27 Nonetheless, their results showed a reasonable safety profile and this technique can be considered as an option when the morbidity of other procedures appears to be too high. As an alternative, exploratory research on denosumab use for ABC has been published with a handful of case reports.28-31 As of now, evidences to support its use in ABC are lacking.
Thermal ablation for osteoid osteoma (OO) is currently the gold standard treatment in the appendicular skeleton with a failure rate of 5%.32 The potential proximity of the OO to the spinal cord has naturally raised concerns about the safety of thermal ablation in the spine. With radiofrequency ablation, a temperature of 90°C is usually applied for 6 minutes to achieve a satisfactory ablation of the nidus. Generally, lesions with an absent cortex or located within 5 mm of a neural structure are considered at risk of thermal injury.18 Nonetheless, Yu et al33 have performed radiofrequency ablation successfully with only 1 mm of cerebrospinal fluid space between the lesion and the neural element. Techniques with either air or saline insufflation have been proposed to reduce the risk of injury.34,35
Radiation Therapy
Even in experienced hands, achieving en bloc resection with marginal or wide margins is challenging with a failure rate of 21% to be Enneking appropriate.36 Consequently, radiation therapy has been recommended to increase local control. Chordoma and chondrosarcoma are known to be radioresistant, necessitating doses of approximately 70 Gy.37 A recent survey by the AOKFT revealed large variations across some of the world’s most experienced cancer centers in the use of radiation modalities in the setting of newly diagnosed spinal chordomas.38 For tumors with which en bloc resection is feasible with acceptable morbidity, some centers are giving neoadjuvant radiation as a standard of care, other centers are administering routine postoperative radiation regardless of the surgical margins, and some centers are not pursuing any radiation treatment when wide/marginal margins are obtained. On the other hand, when en bloc resection would result in significant morbidity, some centers rely more heavily on neoadjuvant and adjuvant therapies with variation in the surgical procedure. These findings highlight that the optimal radiation therapy regimen remains unsolved.
Radiation oncology has undergone a major transformation over the past decade as a consequence of on-board image-guidance systems, incorporation of multimodal imaging, sophisticated treatment planning software, and delivery hardware that permits millimetric precision. These technical developments have also resulted in a new technique known as spine stereotactic body radiotherapy (SBRT), which refers to the delivery of tumor ablative doses within the diseased vertebral segment, in a single or few high dose fractions, while sparing the surrounding organs-at-risk (primarily the spinal cord). The intent is to maximize local control.39 Although the main application has been in the treatment of metastatic spine disease, spine SBRT is evolving into the management of primary spinal tumors. For spinal chordoma, treatment with high-dose SBRT has been reported with encouraging local tumor control results40; however, it represents a major departure from established practice of protracted radiation delivery with intensity modulated photon radiotherapy or proton particle–based radiation (eg, 78 Gy in 39 fractions).41 At present the use of spine SBRT remains investigational for chordoma and should be performed on clinical trial.
In principle, proton therapy offers a substantial clinical advantage over conventional photon therapy with regard to limiting dosage to surrounding tissues. Using a combination of photons and protons, 5-year local control rate as high as 94% has been published for primary spinal sarcomas.42-44 Combination of neoadjuvant radiation therapy, en bloc resection, and a postoperative radiation course has been postulated to result in the highest rate of local tumor control.43 However, Houdek et al45 recently challenged this concept. In their retrospective study of 239 patients, neoadjuvant radiation therapy did not reduce mortality, local recurrence or metastasis.45 Furthermore, it was associated with significant wound complications and sacral fracture.
A disruptive technology that has been reported for resistant tumors like chordoma is carbon ion particle radiation. This particle is biologically distinct from protons, represents a potential for a greater biologic effect, and has inherent unique radiobiological characteristics that may explain the high rates of local tumor control reported for sacral chordoma.46 For example, one of the largest series for sacral chordoma outcomes with carbon ion was reported by Imai et al.46 They reported a 5-year local control rate of 77%, which is impressive as compared with historic data. The technique is still experimental and evolving with only few installations globally.
Mobile spine chordoma is a separate entity and the optimal technology is debatable especially as the precision afforded by modern image-guided photon therapy may allow less uncertainty in margins compared to proton or carbon ion therapy. The high precision allows for tighter and more reproducible dose gradients at the spinal cord–tumor interface, which may provide an advantage in this clinical scenario. Ultimately, we need comparative data for these radiation modalities to understand what is best and cost-effective, given the far greater costs associated with particles as compared with photons and the greater availability for photon therapy globally.
Molecular Sequencing
Precision medicine with molecular sequencing is changing the face of oncology. Knowing the molecular signature of a specific tumor for a specific patient opens the horizon for potential treatment that could never have been envisioned before. This area of translational medicine is still in its infancy but is definitely promising. Given the rarity of primary bone tumors, collaborative approaches have to be advocated. An example of this is that through the AOSKFT, Bettegowda et al47 were able to extract DNA from 109 paraffin-embedded chordoma specimens. They were able to demonstrate association with the single nucleotide polymorphism (SNP) rs2305089 in the T gene and survival.47 Overexpression of the T (brachyury) gene is known to be the hallmark of chordoma. Other collaborative networks are expanding our knowledge on the genetic landscape of chordoma.48
Tumor surveillance would benefit from new methods to allow early detection of tumor recurrence and/or metastasis. Liquid biopsy, taken from a blood sample, are potentially prognostic or predictive marker in multiple cancers.49 Liquid biopsies inform on circulating tumor cells as well as tumor-derived cell-free nucleic acids, exosomes, and platelets. Because of the rarity of primary bone tumor and the absence of specific markers expressed by most primary bone tumors, the characterization of primary bone tumor circulating tumor cells has to date been relatively limited.50 Recently, techniques have been described to monitor Ewing sarcoma through liquid biopsy.51,52
Health-Related Quality of Life
En bloc resection is associated with significant morbidity (13%-73.7%) and mortality (0%-7.7%), even in the most experienced centres. Because of their unique anatomical relation and because of the potential bowel and bladder dysfunction, sacral resection are considered highly morbid procedure with a complication rate approaching 100%.53 The impact of these extensive and potentially impairment-producing procedures on health-related quality of life (HRQOL) is definitely a critically valuable piece of information.
At the present time, there is paucity of published data regarding HRQOL following these surgeries.54-58 Nonetheless, when combining the available literature, HRQOL after surgery for primary bone tumor of the spine is acceptable with HRQOL reaching close to normative values over time.53 Furthermore, tumor recurrence seems to be correlated with worse HRQOL, reinforcing the application of oncologic principles.57
Clinical Resources
Guidelines have been developed in the management of appendicular sarcomas. Although spine has its own specificities, the overall management shares similarities. The American National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines (www.nccn.org) and its European counterpart, the ESMO/European Network Working Group, Clinical Practice Guidelines for Bone Sarcomas59 are available tools that provide guidance for diagnosis, treatment, and surveillance for most common primary bone tumors.
The Chordoma Foundation (www.chordomafoundation.org) is a nonprofit organization that promote research and provide counseling for patients with diagnosed chordoma. Patient educational vignettes and peer support are offered through the chordoma foundation. Furthermore, their website provides comprehensive information regarding diagnosis, management and offers a list of centers in Europe and North America with recognized expertise in primary bone tumors.
Conclusion
Treating primary bone tumors of the spine is challenging and this field has been evolving rapidly over the past few years, especially due to international and local collaborative networks. Respecting oncologic principles is the foundation of the treatment of these tumors. However, new advances in medical oncology, radiation therapy and interventional radiology are now emerging and may be changing the treatment paradigm. Some of these advances are very promising and might one day become standard of care. Until then, these patients should be treated according to the best available evidence. For the primary bone tumor population, this means referral to an experienced center where they will be treated according to the latest standard of care and hopefully, will be able to contribute to scientific advances.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was organized and funded by AOSpine International through the AOSpine Knowledge Forum Tumor, a focused group of international spine oncology experts acting on behalf of AOSpine. Study support was provided directly through the AOSpine Research Department.
ORCID iD: Raphaële Charest-Morin  https://orcid.org/0000-0002-7689-1087
https://orcid.org/0000-0002-7689-1087
References
- 1. Mukherjee D, Chaichana KL, Gokaslan ZL, Aaronson O, Cheng JS, McGirt MJ. Survival of patients with malignant primary osseous spinal neoplasms: results from the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2003. J Neurosurg Spine. 2011;14:143–150. doi:10.3171/2010.10.SPINE10189 [DOI] [PubMed] [Google Scholar]
- 2. Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med. 2012;53:1244–1252. doi:10.2967/jnumed.112.109306 [DOI] [PubMed] [Google Scholar]
- 3. Fourney DR, Rhines LD, Hentschel SJ, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–122. doi:10.3171/spi.2005.3.2.0111 [DOI] [PubMed] [Google Scholar]
- 4. Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–2134. [DOI] [PubMed] [Google Scholar]
- 5. Yamazaki T, McLoughlin GS, Patel S, Rhines LD, Fourney DR. Feasibility and safety of en bloc resection for primary spine tumors: a systematic review by the Spine Oncology Study Group. Spine (Phila Pa 1976). 2009;34(22 suppl):S31–S38. doi:10.1097/BRS.0b013e3181b8b796 [DOI] [PubMed] [Google Scholar]
- 6. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;(153):106–120. [PubMed] [Google Scholar]
- 7. Fisher CG, Versteeg AL, Dea N, et al. Surgical management of spinal chondrosarcomas. Spine (Phila Pa 1976). 2016;41:678–685. doi:10.1097/BRS.0000000000001485 [DOI] [PubMed] [Google Scholar]
- 8. Charest-Morin R, Dirks MS, Patel S, et al. Ewing sarcoma of the spine: prognostic variables for survival and local control in surgically treated patients. Spine (Phila Pa 1976). 2018;43:622–629. doi:10.1097/BRS.0000000000002386 [DOI] [PubMed] [Google Scholar]
- 9. Varga PP, Szövérfi Z, Fisher CG, et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J. 2015;24:1092–1101. doi:10.1007/s00586-014-3728-6 [DOI] [PubMed] [Google Scholar]
- 10. Gokaslan ZL, Zadnik PL, Sciubba DM, et al. Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine. 2016;24:644–651. doi:10.3171/2015.7.SPINE15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive “benign” primary spine neoplasms: osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine (Phila Pa 1976). 2009;34(22 suppl):S39–S47. doi:10.1097/BRS.0b013e3181ba0024 [DOI] [PubMed] [Google Scholar]
- 12. Charest-Morin R, Fisher CG, Varga PP, et al. ; AOSpine Knowledge Forum Tumor. En bloc resection versus intralesional surgery in the treatment of giant cell tumor of the spine. Spine (Phila Pa 1976). 2017;42:1383–1390. doi:10.1097/BRS.0000000000002094 [DOI] [PubMed] [Google Scholar]
- 13. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi:10.1016/S1470-2045(10)70010-3 [DOI] [PubMed] [Google Scholar]
- 14. Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18:4415–4424. doi:10.1158/1078-0432.CCR-12-0578 [DOI] [PubMed] [Google Scholar]
- 15. Rutkowski P, Ferrari S, Grimer RJ, et al. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22:2860–2868. doi:10.1245/s10434-015-4634-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldschlager T, Dea N, Boyd M, et al. Giant cell tumors of the spine: has denosumab changed the treatment paradigm? J Neurosurg Spine. 2017;91:526–533. doi:10.3171/2014.10.SPINE13937 [DOI] [PubMed] [Google Scholar]
- 17. Girolami I, Mancini I, Simoni A, et al. Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol. 2016;69:240–247. doi:10.1136/jclinpath-2015-203248 [DOI] [PubMed] [Google Scholar]
- 18. Charest-Morin R, Boriani S, Fisher CG, et al. Benign tumors of the spine: has new chemotherapy and interventional radiology changed the treatment paradigm? Spine (Phila Pa 1976). 2016;41(suppl 20):S178–S185. doi:10.1097/BRS.0000000000001818 [DOI] [PubMed] [Google Scholar]
- 19. Palmerini E, Chawla NS, Ferrari S, et al. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer. 2017;76:118–124. doi:10.1016/j.ejca.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 20. Errani C, Tsukamoto S, Leone G, et al. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018;100:496–504. doi:10.2106/JBJS.17.00057 [DOI] [PubMed] [Google Scholar]
- 21. Mak IWY, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am. 2014;96:e127 doi:10.2106/JBJS.M.01332 [DOI] [PubMed] [Google Scholar]
- 22. Tsukamoto S, Righi A, Vanel D, Honoki K, Donati DM, Errani C. Development of high-grade osteosarcoma in a patient with recurrent giant cell tumor of the ischium while receiving treatment with denosumab. Jpn J Clin Oncol. 2017;47:1090–1096. doi:10.1093/jjco/hyx112 [DOI] [PubMed] [Google Scholar]
- 23. Park A, Cipriano CA, Hill K, Kyriakos M, McDonald DJ. Malignant transformation of a giant cell tumor of bone treated with denosumab. JBJS Case Connect. 2016;6:e78 doi:10.2106/JBJS.CC.16.00024 [DOI] [PubMed] [Google Scholar]
- 24. Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473:3050–3055. doi:10.1007/s11999-015-4249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amendola L, Simonetti L, Simoes CE, Bandiera S, De Iure F, Boriani S. Aneurysmal bone cyst of the mobile spine: the therapeutic role of embolization. Eur Spine J. 2012;22:533–541. doi:10.1007/s00586-012-2566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boriani S, Lo SF, Puvanesarajah V, et al. ; AOSpine Knowledge Forum Tumor. Aneurysmal bone cysts of the spine: treatment options and considerations. J Neurooncol. 2014;120:171–178. doi:10.1007/s11060-014-1540-0 [DOI] [PubMed] [Google Scholar]
- 27. Terzi S, Gasbarrini A, Fuiano M, et al. Efficacy and safety of selective arterial embolization in the treatment of aneurysmal bone cyst of the mobile spine: a retrospective observational study. Spine (Phila pa 1976). 2017;42:1130–1138. doi:10.1097/BRS.0000000000002017 [DOI] [PubMed] [Google Scholar]
- 28. Lange T, Stehling C, Fröhlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22:1417–1422. doi:10.1007/s00586-013-2715-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pelle DW, Ringler JW, Peacock JD, et al. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res. 2014;164:139–148. doi:10.1016/j.trsl.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 30. Dubory A, Missenard G, Domont J, Court C. Interest of denosumab for the treatment of giant-cells tumors and aneurysmal bone cysts of the spine. About nine cases. Spine (Phila Pa 1976). 2016;41:E654–E660. doi:10.1097/BRS.0000000000001350 [DOI] [PubMed] [Google Scholar]
- 31. Skubitz KM, Peltola JC, Santos ER, Cheng EY. Response of aneurysmal bone cyst to denosumab. Spine (Phila Pa 1976). 2015;40:E1201–E1204. doi:10.1097/BRS.0000000000001027 [DOI] [PubMed] [Google Scholar]
- 32. Lanza E, Thouvenin Y, Viala P, et al. Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol. 2013;37:1530–1539. doi:10.1007/s00270-013-0815-8 [DOI] [PubMed] [Google Scholar]
- 33. Yu X, Wang B, Yang S, et al. Percutaneous radiofrequency ablation versus open surgical resection for spinal osteoid osteoma [published online July 25, 2018]. Spine J. doi:10.1016/j.spinee.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 34. Rybak LD, Gangi A, Buy X, La Rocca Vieira R, Wittig J. Thermal ablation of spinal osteoid osteomas close to neural elements: technical considerations. AJR Am J Roentgenol. 2010;195:W293–W298. doi:10.2214/AJR.10.4192 [DOI] [PubMed] [Google Scholar]
- 35. Klass D, Marshall T, Toms A. CT-guided radiofrequency ablation of spinal osteoid osteomas with concomitant perineural and epidural irrigation for neuroprotection. Eur Radiol. 2009;19:2238–2243. doi:10.1007/s00330-009-1404-8 [DOI] [PubMed] [Google Scholar]
- 36. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976). 2006;31:493–503. doi:10.1097/01.brs.0000200038.30869.27 [DOI] [PubMed] [Google Scholar]
- 37. Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG. Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine (Phila Pa 1976). 2009;34(22 suppl):S48–S57. doi:10.1097/BRS.0b013e3181b969ac [DOI] [PubMed] [Google Scholar]
- 38. AOSpine Knowledge Forum Tumor. Current treatment strategy for newly diagnosed chordoma of the mobile spine and sacrum: results of an international survey. J Neurosurg Spine. 2018;1:1–7. doi:10.3171/2018.6.SPINE18362 [DOI] [PubMed] [Google Scholar]
- 39. Tseng CL, Eppinga W, Charest-Morin R, et al. Spine stereotactic body radiotherapy: indications, outcomes, and points of caution. Global Spine J. 2017;7:179–197. doi:10.1177/2192568217694016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lockney DT, Shub T, Hopkins B, et al. Spinal stereotactic body radiotherapy following intralesional curettage with separation surgery for initial or salvage chordoma treatment. Neurosurg Focus. 2017;42:E4 doi:10.3171/2016.9.FOCUS16373 [DOI] [PubMed] [Google Scholar]
- 41. Stacchiotti S, Sommer J; Chordoma Global Consensus Group. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16:e71–e83. doi:10.1016/S1470-2045(14)71190-8 [DOI] [PubMed] [Google Scholar]
- 42. DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115–122. doi:10.1002/jso.23617 [DOI] [PubMed] [Google Scholar]
- 43. Rotondo RL, Folkert W, Liebsch NJ, et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J Neurosurg Spine. 2015;23:788–797. doi:10.3171/2015.3.SPINE14716 [DOI] [PubMed] [Google Scholar]
- 44. Indelicato DJ, Rotondo RL, Begosh-Mayne D, et al. A prospective outcomes study of proton therapy for chordomas and chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys. 2016;95:297–303. doi:10.1016/j.ijrobp.2016.01.057 [DOI] [PubMed] [Google Scholar]
- 45. Houdek MT, Rose PS, Schwab JH, et al. A comparison of outcomes of treatment paradigms for sacral chordoma: does preoperative radiation improve prognosis? Paper presented at: AAOS 2018 Annual Meeting; March 6-10, 2018; New Orleans, LA: http://aaos2018.conferencespot.org/66451aaos-1.4066572/2-1.4076032/t004-1.4076285/a056-1.4077344/106-1.4077378 . Accessed January 18, 2019. [Google Scholar]
- 46. Imai R, Kamada T, Araki N; Working Group for Bone and Soft Tissue Sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys. 2016;95:322–327. doi:10.1016/j.ijrobp.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 47. Bettegowda C, Yip S, Lo SL, et al. ; AOSpine Knowledge Forum Tumor. Spinal column chordoma: prognostic significance of clinical variables and T (brachyury) gene SNP rs2305089 for local recurrence and overall survival. Neuro Oncol. 2017;19:405–413. doi:10.1093/neuonc/now156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tarpey PS, Behjati S, Young MD, et al. The driver landscape of sporadic chordoma. Nat Commun. 2017;8:890 doi:10.1038/s41467-017-01026-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi:10.1126/science.aar3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tellez-Gabriel M, Brown HK, Young R, Heymann MF, Heymann D. The challenges of detecting circulating tumor cells in sarcoma. Front Oncol. 2016;6:202 doi:10.3389/fonc.2016.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benini S, Gamberi G, Cocchi S, et al. Detection of circulating tumor cells in liquid biopsy from Ewing sarcoma patients. Cancer Manag Res. 2018;10:49–60. doi:10.2147/CMAR.S141623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang P, Samuel G, Crow J, Godwin AK, Zeng Y. Molecular assessment of circulating exosomes toward liquid biopsy diagnosis of Ewing sarcoma family of tumors. Transl Res. 2018;201:136–153. doi:10.1016/j.trsl.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dea N, Charest-Morin R, Sciubba DM, et al. Optimizing the adverse event and HRQOL profiles in the management of primary spine tumors. Spine (Phila Pa 1976). 2016;41(suppl 20):S212–S217. doi:10.1097/BRS.0000000000001821 [DOI] [PubMed] [Google Scholar]
- 54. Colman MW, Karim SM, Lozano-Calderon SA, et al. Quality of life after en bloc resection of tumors in the mobile spine. Spine J. 2015;15:1728–1737. doi:10.1016/j.spinee.2015.03.042 [DOI] [PubMed] [Google Scholar]
- 55. Kato S, Murakami H, Demura S, et al. Patient-reported outcome and quality of life after total en bloc spondylectomy for a primary spinal tumour. Bone Joint J. 2014;96-B:1693–1698. doi:10.1302/0301-620X.96B12.33832 [DOI] [PubMed] [Google Scholar]
- 56. Mazel C, Owona P, Cogan A, Balabaud L, Grunenwald D. Long-term quality of life after en-bloc vertebrectomy: 25 patients followed up for 9 years. Orthop Traumatol Surg Res. 2014;100:119–126. doi:10.1016/j.otsr.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 57. Schwab JH, Janssen SJ, Pereira NRP, et al. Quality of life after resection of a chordoma of the mobile spine. Bone Joint J. 2017;99-B:979–986. doi:10.1302/0301-620X.99B7.BJJ-2016-1126.R1 [DOI] [PubMed] [Google Scholar]
- 58. Liljenqvist U, Lerner T, Halm H, Buerger H, Gosheger G, Winkelmann W. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008;17:600–609. doi:10.1007/s00586-008-0599-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Casali PG, Bielack S, Abecassis N, et al. ; ESMO Guidelines Committee, PaedCan and ERN EURACAN. Bone sarcomas: ESMO-PaedCan-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl_4):iv79–iv95. doi:10.1093/annonc/mdy310 [DOI] [PubMed] [Google Scholar]
- 60. Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine. 2009;34(22 Suppl):S58–S68. doi:10.1097/BRS.0b013e3181ba6436 [DOI] [PubMed] [Google Scholar]
- 61. Pennicooke B, Laufer I, Sahgal A, et al. Safety and Local Control of Radiation Therapy for Chordoma of the Spine and Sacrum: A Systematic Review. Spine. 2016;41(Suppl 20):S186–S192. doi:10.1097/BRS.0000000000001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129(1):174–181. doi:10.1378/chest.129.1.174 [DOI] [PubMed] [Google Scholar]