Abstract
Objectives
Remote ischemic preconditioning has been proposed as a possible potential treatment for ischemic stroke. However, neuroprotective benefits of the pre-procedural administration of remote ischemic preconditioning have not been investigated in patients undergoing an elective endovascular intracranial aneurysm repair procedure. This study investigated the safety and feasibility of remote ischemic preconditioning in patients with an unruptured intracranial aneurysm who undergo elective endovascular treatment.
Methods
In this single-center prospective study, patients with an unruptured intracranial aneurysm undergoing elective endovascular treatment with flow diverters or coiling were recruited. Patients received three intermittent cycles of 5 minutes arm ischemia followed by reperfusion using manual blood cuff inflation/deflation less than 5 hours prior to endovascular treatment. Patients were monitored and followed up for remote ischemic preconditioning-related adverse events and ischemic brain lesions by diffusion-weighted magnetic resonance imaging within 48 hours following endovascular treatment.
Results
A total of seven patients aged 60 ± 5 years with an unruptured intracranial aneurysm successfully completed a total of 21 sessions of remote ischemic preconditioning and the required procedures. Except for two patients who developed skin petechiae over their arms, no other serious procedure-related adverse events were observed as a result of the remote ischemic preconditioning procedure. On follow-up diffusion-weighted magnetic resonance imaging, a total of 19 ischemic brain lesions with a median (interquartile range) volume of 245 (61–466) mm3 were found in four out of seven patients.
Conclusions
The application of remote ischemic preconditioning prior to endovascular intracranial aneurysm repair was well tolerated, safe and clinically feasible. Larger sham-controlled clinical trials are required to determine the safety and efficacy of this therapeutic strategy in mitigating ischemic damage following endovascular treatment of intracranial aneurysms.
Keywords: Preconditioning, ischemic preconditioning, aneurysm, intracranial aneurysm, magnetic resonance imaging
Introduction
Remote ischemic conditioning represents a potential therapeutic intervention in which temporary ischemia in a remote vascular bed offers protection against ischemic and post-ischemic injury in a target vascular bed.1,2 The vast majority of remote ischemic preconditioning (RIPC), which can be done before (pre-conditioning), during (per-conditioning), and after (post-conditioning), has been applied in the setting of coronary ischemia, and were able to mitigate ischemia-related myocardium, kidney and lower extremity injuries following cardiovascular surgeries in several studies.3–7 The mechanism remains yet to be fully elucidated, but most investigators believe that RIPC may protect against ischemia and reperfusion injury by releasing endogenous protective mediators in response to brief periods of induced ischemia, and exerts its underlying protective mechanisms by decreasing free radicals production, apoptosis, excitotoxicity and modulating the immune system.1,3,8,9
The neuroprotective benefits of RIPC against ischemia and reperfusion injuries have been proposed in previous studies. RIPC has been demonstrated to reduce neural damage in experimental models of ischemic stroke,10–13 and mitigate ischemic brain damage and neurological sequelae in humans after cardiac surgery.14 RIPC has recently been applied in the setting of acute stroke, specifically intracranial atherosclerotic stenosis.15,16 Previous studies showed the safety and feasibility of RIPC with extremely low numbers of side effects in aneurysmal subarachnoid hemorrhage,17,18 acute ischemic stroke,19,20 and in patients who undergo mechanical thrombectomy20 and elective carotid endarterectomy.21 The potential efficacy in preventing stroke has also been reported in aneurysmal subarachnoid hemorrhage,22 intracranial atherosclerotic stenosis,16,23 and in patients with severe carotid stenosis who receive carotid artery stenting.24
Despite the high incidence of thromboembolic ischemic brain injuries following endovascular procedures,25 RIPC has been critically understudied in patients with unruptured intracranial aneurysms (IAs) who undergo endovascular treatment (EVT). Here, we report seven patients with an unruptured IA who were treated with RIPC prior to an elective endovascular aneurysm repair procedure in an ideal setting under controlled conditions and constant monitoring. The purpose of our pilot trial was to evaluate the safety and feasibility of RIPC administration before elective endovascular repair of an unruptured IA. Our secondary purpose was to assess the incidence and volume of ischemic lesions, as detected by diffusion-weighted magnetic resonance imaging (MRI), following an endovascular procedure. If this pilot study shows the safety and feasibility of RIPC in this population, we can use these data to design a large randomized clinical trial to evaluate the potential neuroprotective benefits of RIPC in preventing stroke in patients who undergo EVT for IA repair.
Materials and methods
Patient selection
This single-center prospective study was conducted from May 2017 to September 2017 at the Saint Mary’s Hospital, Rochester, Minnesota. The protocol of this trial was approved by the Mayo Clinic institutional review board. Fifteen consecutive patients with an angiography confirmed diagnosis of an unruptured IA scheduled to undergo an elective neuroendovascular repair procedure using endosaccular coiling or flow diversion by pipeline embolization device (PED) were assessed for eligibility. Patients who consented to participate in this study and who conformed to the protocol requirements were screened and included. Exclusion criteria were: age less than 18 years, pregnancy, abnormal ECG with clinically significant findings, pre-existing ischemic stroke, history of myocardial infarction within one month of the enrollment, peripheral vascular disease affecting the upper limbs, any orthopedic, surgical, vascular, or soft tissue injury of upper extremity, systolic blood pressure above 200 mmHg, chronic kidney disease, and current use of sulfonamide or nicorandil drugs. The study was performed in accordance with Mayo Clinic policies and the ethical standards laid down in the Declaration of Helsinki. After a full explanation of the study, signed written informed consent was obtained from all patients. Among 15 patients, seven patients completed the study and follow-up.
Procedures
Subjects were hospitalized the day of endovascular therapy and remained in the hospital for at least 12 hours after the procedure. Demographic and clinical data including age, sex, dual antiplatelet therapy (DAPT) with aspirin and clopidogrel, and cardiovascular risk factors such as history of hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, atrial fibrillation, congestive heart disease, and smoking were assessed during enrollment procedures (Table 1). The first RIPC cycle was administered by a nurse using a medium or large manual blood pressure cuff in the outpatient area before transferring patients to the preoperative area in the angiography suite for the endovascular procedure. The cuff was placed around the patient’s arm, and three cycles of 5-minute inflation of a blood pressure cuff to 200 mmHg or at least to a pressure 50 mmHg higher than the patient’s systolic blood pressure were applied to one upper arm followed by 5-minute cuff deflation. After the completion of the RIPC procedure and removing the cuff, the arm was examined for any evidence of bruise or damage to the limb. The perioperative care and endovascular procedure were conducted according to the standard of care.
Table 1.
Patient baseline demographic and clinical characteristics.
| Variables | RIPC (n = 7) |
|---|---|
| Age, median (IQR), years | 59 (56–64) |
| Race, white (%) | 6 (86%) |
| Intracranial aneurysm treatment | |
| PED (flow diversion) | 2 (29%) |
| Coil (endosaccular coiling) | 4 (57%) |
| Combined coiling and PED | 1 (14%) |
| Cuff-related complications | 2 (29%) |
| AF, N (%) | 0 (0) |
| DM, N (%) | 0 (0) |
| Hypertension, N (%) | 2 (29%) |
| Current smoking, N (%) | 5 (71%) |
| CHF, N (%) | 0 (0) |
| CAD, N (%) | 0 (0) |
| Hyperlipidemia, N (%) | 1 (14%) |
| Median (IQR) time from RIPC administration to endovascular procedure (minutes) | 180 (76–265) |
| Interval between the endovascular procedure and post-endovascular imaging (hours) | 28 (24.26–31.25) |
| Pre-EVT DAPT | 7 (100%) |
| Post-EVT DAPT | 7 (100%) |
AF: atrial fibrillation; CAD: coronary artery disease; CHF: congestive heart failure; DAPT: dual antiplatelet therapy (aspirin (325 mg) plus clopidogrel); DM: diabetes mellitus; EVT: endovascular treatment; IQR: interquartile range; PED: pipeline embolization device; RIPC: remote ischemic preconditioning.
Safety assessment
Patients were examined and monitored for any adverse events (AEs) associated with RIPC administration by study staff during the entire study period. AEs, abnormal shifts from baseline, and collected data were recorded and reported to the principal investigator. Safety was assessed based on the frequency of AEs. AEs were defined as: (a) discontinuation of study due to inability to tolerate the RIPC procedure; (b) signs and symptoms of neurovascular and tissue or skin injuries such as any skin lesions, local edema, erythema, and tenderness as a result of RIPC procedure; (c) complications that are not routinely observed during standard EVT of IAs. Major AEs were defined as complications resulting in death, life-threatening sequel, increased length of hospital stay, or substantial disability.
Imaging
Patients underwent angiography during the procedure and follow-up brain MRI including axial T1/T2, susceptibility weighted imaging, diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery sequences within 48 hours after completion of the interventional procedure. As research MRI could not be performed in the inpatient setting, all follow-up MRIs were performed in an outpatient setting located at the Mayo Clinic’s downtown campus. All MRIs were obtained using a 3 Tesla scanner (4 mm slice thickness, with no gap between the slices), except for one patient in whom 1.5 Tesla MRI was used. Lesion volumes were calculated manually by multiplication of the area and slice thickness (4 mm in all cases). Brain images were reviewed for the presence of restricted diffusion lesions. A 25-year experienced subspecialty trained neuroradiologist (DFK) blinded to the patient information and treatment independently reviewed the MRI result.
Statistical analysis
Statistical analyses were performed using JMP 13.0 statistical software (SAS Institute, Inc.) and descriptive values were reported as the number (percentage) and median (interquartile range; IQR).
Results
Demographic
Among the 15 patients who were eligible for inclusion and received the RIPC procedure, four patients did not undergo EVT, and four patients refused to undergo the follow-up MRI. The main reason given for refusal to undergo a follow-up MRI was related to the inconvenience of having the MRI performed in the outpatient setting. The research MRI could not be performed in the inpatient setting. Finally, a total of seven patients accomplished all required procedures and were included in our final analysis. The median (IQR) age was 59 (56–64) years. The baseline demographic and clinical characteristics of patients are summarized in Table 1. Four patients were treated with endosaccular coiling alone, two with flow diverter, and one with both coiling and flow diverter. All patients received DAPT including aspirin (325 mg) and clopidogrel within the 7 days before and after EVT.
Safety and tolerability
Out of the eight patients who did not complete all requirements of the study, the RIPC-related data of two patients were not recorded. The remaining six patients completed all inflation and deflation cycles of RIPC and tolerated the procedure well. Only one patient experienced cyanosis in her lower arm with cuff inflation, as well as petechiae on her hand and forearm and three small bruises in her brachial fold.
All seven patients who completed the study tolerated the RIPC procedure well including all of the cycles of inflation and deflation. In total, very few AEs were observed (Table 2), and no patients developed life-threatening serious or severe AEs. Two patients developed small petechiae that were limited to the upper right arm and anterior cubital fossa in one patient, and to the upper arm skin in the other patient. No other cuff-related severe symptomatic systemic or local AEs such as bruising, edema, tenderness, ecchymosis or other skin lesions were detected. During RIPC administration, no patients experienced cardiovascular events, infections or stroke prior to the endovascular procedure.
Table 2.
Demographic, clinical, imaging and procedural characteristics of patients.
| Patient no. | Decade of life | Site of aneurysm | Aneurysm largest diameter size (mm) | Treatment | Time from RIPC to EVT (minutes) | Time from EVT to postoperative MRI (hours) | Complication | No. of DWI positive lesions | Volume of DWI lesions (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6th | Vertebrobasilar junction | 14.7 | Flow diversion | 61 | 31.25 | Abdominal pain due to retroperitoneal hematomaa | 6 | 302.34 |
| 2 | 6th | Left paraclinoid ICA | 16 | Flow diversion and coiling | 279 | 22.35 | – | 3 | 18.29 |
| 3 | 7th | Vertebrobasilar junction | 6.5 | Coiling | 180 | 28 | Petechiae on upper right arm and at anterior cubital | – | – |
| 4 | 7th | Right MCA aneurysm | 5.3 | Coiling | 265 | 24.26 | Coil migration without neurological sequelae | – | – |
| 5 | 6th | Right paraclinoid ICA | 13 | Coiling | 156 | 43.65 | Small petechiae on right upper arm | – | – |
| 6 | 6th | Left ICA | 5 | Flow diversion | 76 | 24.86 | Thrombosis of PED device, recanalized with Reopro infusion | 8 | 188.59 |
| 7 | 7th | Left MCA | 7.2 | Coiling | 185 | 30.37 | – | 2 | 520.18 |
EVT: endovascular treatment; ICA: internal carotid artery; MCA: middle cerebral artery; PED: pipeline embolization device.
The patient developed abdominal pain and was revealed to have a retroperitoneal hematoma on computed tomography imaging. Serial hemoglobin levels were followed and remained stable. The patient’s abdominal pain improved, and she was discharged the following day.
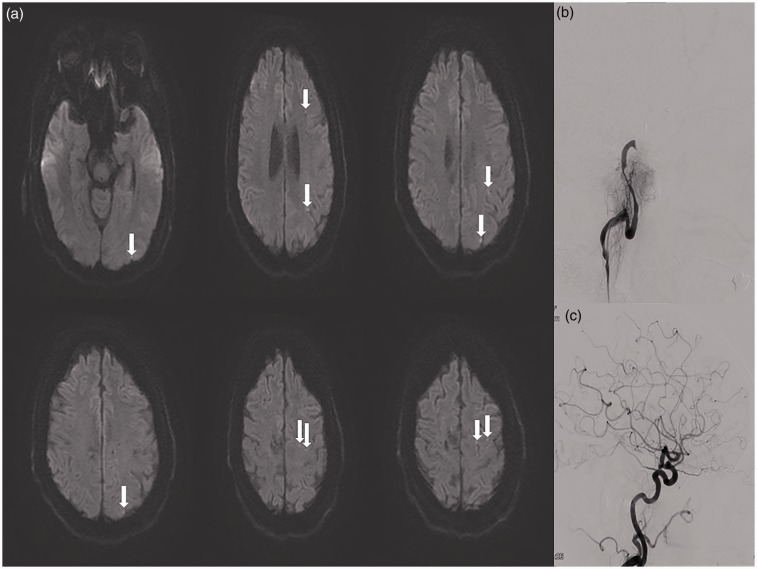
At the termination of the endovascular procedure and after transferring to the postoperative area, one patient was not able to move her right upper extremity and was noted to have minimal right lower extremity movement. Angiography revealed thrombosis of the PED. The occlusion of the PED was recanalized by immediate infusion of Reopro and the patient’s neurological symptoms improved. No acute infarction or hemorrhage was seen in the post-procedure head computed tomography, and the patient was admitted to the neurological intensive care unit for monitoring. The postoperative magnetic resonance angiography and MRI demonstrated stent patency and the punctate areas of acute infarction, respectively (Figure 1); however, no large ischemic lesion was observed. The patient was discharged and had an uncomplicated recovery. In another patient, the coil was dislodged into a posterior branch of the right middle cerebral artery (MCA) following placement. The patient was given a bolus of intravenous Reopro immediately and did not develop any resultant neurological deficits. The patient’s follow-up MRI was negative for ischemic lesions.
Figure 1.
(a) Arrows show multiple tiny positive diffusion-weighted imaging ischemic brain lesions detected on the magnetic resonance imaging of a patient after endovascular treatment. (b) The patient experienced acute focal neurological deficit after completion of endovascular treatment. The left internal carotid artery (ICA) angiography shows complete ICA occlusion after pipeline embolization device stent placement. (c) The ICA was re-canalized after intra-arterial administration of Reopro. The patient did not experience any neurological deficits.
Clinical and imaging outcome
Four patients showed abnormalities on their diffusion-weighted MRI. In total, 19 ischemic lesions were found on post-treatment DWI scans of four out of the seven patients; of those, all had more than one high signal intensity tiny DWI lesion. However, none of the patients experienced post-procedural clinically evident ischemic cerebrovascular events, transient ischemic attacks, or hemorrhagic strokes in the follow-up visit. The total and median (IQR) volumes of all DWI lesions in the four patients following endovascular intervention were 1029.4 and 245 (61–466) mm3, respectively. Moreover, the volume of the smallest and biggest single lesions were 1.43 and 400.44 mm3, respectively.
Discussion
In this study, in the setting of a small, prospective pilot study, we demonstrated the safety and feasibility of RIPC in patients undergoing elective aneurysm repair. RIPC was readily applied and quite well tolerated by the patients. Disruption in workflow was modest to start, but then minimal in later patients. Three treated patients demonstrated no DWI evidence of ischemia, including one patient who had a permanent MCA branch occlusion from a coil embolus. One patient had an acute internal carotid artery (ICA) occlusion that responded angiographically to Reopro, and was left with tiny, distal DWI abnormalities but remained neurologically normal. Several other patients had tiny DWI lesions without neurological deficit. This study is important as it provides evidence that RIPC may have utility in the setting of elective aneurysm repair.
Previous studies have shown that up to 70% of the normal population develops new DWI brain lesions following endovascular aneurysm treatment. Although the effects of these tiny and mainly asymptomatic ischemic brain lesions are not fully clear, these so-called ‘clinically silent infarcts’ have been associated with further cognitive impairment and strokes.26,27 In our study, four patients had DWI brain lesions which were all tiny and clinically silent; however, none of them experienced neurological deficit. Interestingly, all three patients who were treated with flow diverters in our study had at least one post-procedure DWI lesion while only one patient out of four who was treated with coiling alone had two DWI lesions. These findings are: (a) consistent with recent meta-analysis findings25 that demonstrated a significantly higher rate of DWI-positive lesions following endovascular flow diversion compared with coiling alone, possibly due to the high ratio of metal coverage in flow diverters that might increase the risk of intra-luminal thrombosis; (b) in contrary to most previous studies25,28,29 that reported a higher occurrence rate of DWI lesions after endovascular coiling. The low rate of DWI lesions on post-procedure MRI in our patients who were treated with coiling is interesting because it might shed new light on the possible potential neuroprotective benefits of RIPC for the prevention and treatment of stroke in patients who are at high risk of ischemic events. Large controlled trials are needed to validate this interesting finding.
Similar to the findings of most previous clinical trials,15,17–19,24 the administration of RIPC was safe and feasible in our study. Previous authors, using a prospective randomized controlled clinical trial in 58 patients with symptomatic intracranial arterial stenosis, have found no adverse effects of the twice daily administration of 5 × 5 minutes bilateral arm RIPC for 180 consecutive days.23 Our case series supports the current literature by providing information regarding the safety of RIPC in a different critical setting. Only two individuals experienced local mild petechiae on their arms, and no other RIPC procedure-induced local or systemic AEs, including bruising, symptomatic DVT, were observed. No serious RIPC procedure-induced AEs have been detected in our study, and none of the patients withdrew from the RIPC procedure because of inconvenience, intolerance or AEs. Only one patient developed neurological deficit due to the occlusion of PED following an endovascular procedure which was recovered by the immediate infusion of Reopro. As acute thrombosis formation commonly happens intra or post-procedurally following coiling or PED30,31 it is difficult to attribute this sequel to the RIPC procedure. The potential neuroprotective benefits of RIPC in the treatment of stroke have been suggested in previous studies.15,19 In a pilot randomized placebo controlled phase II trial by England et al. (RECAST study),15 which evaluated the safety and efficacy of RIPC, the administration of an intermittent four cycles of 5 minutes limb ischemia in the non-paretic arm of patients with acute ischemic stroke was safe and well tolerated by patients and was associated with improved neurological outcome without any peri or post-procedural vascular complications. However, four patients in the sham group experienced vascular events including two myocardial infarctions and two ischemic strokes. In a recent study, Zhao et al.24 assessed the effects of RIPC in reducing ischemic injury following carotid artery stenting in 189 patients with severe carotid artery stenosis. The administration of five cycles of RIPC consisted of 5 minutes bilateral upper arm cuff inflation to 200 mmHg followed by 5 minutes deflation using an auto control device, twice daily for 2 weeks prior to carotid artery stenting was feasible, safe and well tolerated without any severe AEs. Patients in RIPC showed a significantly smaller volume of lesions and lower incidence of new ischemic DWI lesions (15.87%) than patients in the sham and control groups (36.51% and 41.27%, respectively). Therefore, our study extends the current literature by addressing the clinical applicability of RIPC in preventing ischemic injury and the unmet need for effective prophylactic (neuroprotective) treatment in patients with an unruptured IA who undergo EVT.
This study has several potential limitations. First, because of small sample size and lack of a control group, we cannot definitely generalize our results to all patients who undergo neuro-endovascular IA repair. Second, there was a relativity long time interval from RIPC administration to embolization, which may prevent the maximum effect of RIPC during its short-lived first protection window. Therefore, the administration of RIPC at a time closer to EVT in the angiography suite (i.e. within less than 1 hour) is recommended for future studies. Third, because we did not obtain pre-procedural MRI, we may not be able to differentiate between old versus new ischemic lesions. Fourth, the postoperative diffusion-weighted MRI scan was performed between 22 and 43 hours after EVT. For more accurate estimation of the effect of RIPC on ischemic brain changes, obtaining brain images within a narrower time window from EVT is therefore suggested for further studies. Fifth, as the adequate induction of ischemia to the limb following blood pressure cuff inflation has not been evaluated in our study, using bedside Doppler sonography is recommended to confirm blood flow cessation to the limb during RIPC administration.
Conclusion
The administration of RIPC in patients who undergo a neuro-interventional procedure for IA repair was easy, inexpensive, feasible, and safe. Large-scale sham-controlled blinded clinical trials are needed to evaluate the efficacy of RIPC in mitigating ischemic injury in patients susceptible to ischemic brain damage and decrease the risk of procedurally induced stroke.
Acknowledgements
The authors are grateful to all the patients for their participation in this study. The authors are also thankful to Ms Roanna Vine for editing the manuscript.
Author contribution
All authors have made substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting of the work or revising it critically for important intellectual content.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was partially supported by the Mayo Clinic Radiology Department internal funding through grant number RDCRKALL.
References
- 1.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008; 79: 377–386. [DOI] [PubMed] [Google Scholar]
- 2.Bell R, Yellon D. Surgery: remote ischaemic conditioning – approaching prime time? Nat Rev Cardiol 2013; 10: 619–621. [DOI] [PubMed] [Google Scholar]
- 3.Heusch G, Botker HE, Przyklenk K, et al. Remote ischemic conditioning. J Am Coll Cardiol 2015; 65: 177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo SJ, Zhou YJ, Shi DM, et al. Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can J Cardiol 2013; 29: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 5.Eitel I, Stiermaier T, Rommel KP, et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 2015; 36: 3049–3057. [DOI] [PubMed] [Google Scholar]
- 6.Sloth AD, Schmidt MR, Munk K, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 2014; 35: 168–175. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Kellum JA, Van Aken H, et al. Long-term effects of remote ischemic preconditioning on kidney function in high-risk cardiac surgery patients: follow-up results from the RenalRIP Trial. Anesthesiology 2017; 126: 787–798. [DOI] [PubMed] [Google Scholar]
- 8.Hess DC, Hoda MN, Bhatia K. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke 2013; 44: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 9.Saxena P, Newman MA, Shehatha JS, et al. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Cardiac Surg 2010; 25: 127–134. [DOI] [PubMed] [Google Scholar]
- 10.Ren C, Gao X, Steinberg GK, et al. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 2008; 151: 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn CD, Manlhiot C, Schmidt MR, et al. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke 2011; 42: 2960–2962. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Kim YJ, Lee KM, et al. Ischemic preconditioning enhances neurogenesis in the subventricular zone. Neuroscience 2007; 146: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Liu X, Yan F, et al. Protective effects of remote ischemic preconditioning in rat hindlimb on ischemia–reperfusion injury. Neural Regen Res 2012; 7: 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparovic H, Kopjar T, Rados M, et al. Remote ischemic preconditioning preceding coronary artery bypass grafting reduces the volume of ischemic brain lesions. Circulation 2017; 136 (suppl_1): A17967–A17967.
- 15.England TJ, Hedstrom A, O’Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke 2017; 48: 1412–1415. [DOI] [PubMed] [Google Scholar]
- 16.Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012; 79: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez NR, Connolly M, Dusick JR, et al. Phase I clinical trial for the feasibility and safety of remote ischemic conditioning for aneurysmal subarachnoid hemorrhage. Neurosurgery 2014; 75: 590–598. discussion 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch S, Katsnelson M, Dong C, et al. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke 2011; 42: 1387–13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014; 45: 159–167. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Che R, Li S, et al. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann Clin Transl Neurol 2018; 5: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh SR, Nouraei S, Tang TY, et al. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovasc Surg 2010; 44: 434–439. [DOI] [PubMed] [Google Scholar]
- 22.Laiwalla AN, Ooi YC, Liou R, et al. Matched cohort analysis of the effects of limb remote ischemic conditioning in patients with aneurysmal subarachnoid hemorrhage. Translat Stroke Res 2016; 7: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng R, Ding Y, Asmaro K, et al. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics 2015; 12: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Meng R, Ma C, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation 2017; 135: 1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bond KM, Brinjikji W, Murad MH, et al. Diffusion-weighted imaging-detected ischemic lesions following endovascular treatment of cerebral aneurysms: a systematic review and meta-analysis. Am J Neuroradiol 2017; 38: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasiliense LBC, Stanley MA, Grewal SS, et al. Silent ischemic events after Pipeline embolization device: a prospective evaluation with MR diffusion-weighted imaging. J Neurointerv Surg 2016; 8: 1136–1139. [DOI] [PubMed] [Google Scholar]
- 27.Hahnemann ML, Ringelstein A, Sandalcioglu IE, et al. Silent embolism after stent-assisted coiling of cerebral aneurysms: diffusion-weighted MRI study of 75 cases. J Neurointerv Surg 2014; 6: 461–465. [DOI] [PubMed] [Google Scholar]
- 28.Iosif C, Lecomte J-C, Pedrolo-Silveira E, et al. Evaluation of ischemic lesion prevalence after endovascular treatment of intracranial aneurysms, as documented by 3-T diffusion-weighted imaging: a 2-year, single-center cohort study. J Neurosurg 2017; 128: 982–991. [DOI] [PubMed] [Google Scholar]
- 29.Platz J, Wagner M, Güresir E, et al. Early diffusion-weighted MRI lesions after treatment of unruptured intracranial aneurysms: a prospective study. J Neurosurg 2017; 126: 1070–1078. [DOI] [PubMed] [Google Scholar]
- 30.Patel A, Miller TR, Shivashankar R, et al. Early angiographic signs of acute thrombus formation following cerebral aneurysm treatment with the Pipeline embolization device. J Neurointerv Surg 2017; 9: 1125–1130. [DOI] [PubMed] [Google Scholar]
- 31.Adeeb N, Griessenauer CJ, Moore JM, et al. Ischemic stroke after treatment of intraprocedural thrombosis during stent-assisted coiling and flow diversion. Stroke 2017; 48: 1098–1100. [DOI] [PubMed] [Google Scholar]