Abstract
CONTEXT:
India experienced pandemic phase of H1N1 in May 2009 to December 2010. The postpandemic phase went on from January 2011 to December 2014. As per the WHO, all countries should immunize their health-care workers as a first priority to protect the essential health infrastructure.
AIMS:
The aim of the study is to assess the level of awareness and acceptance of influenza vaccine among physicians and also the perception of physicians regarding H1N1 infection. This study also examined time of vaccine administration in relation with efficacy concerns based on literature.
SETTINGS AND DESIGN:
A vaccination campaign was conducted for all health-care workers of Seth GSMC and KEM Hospital, Mumbai, in the month of July 2017 based on which a cross-sectional observational study was conducted among the physicians of the same institute.
METHODS:
After ethical clearance, a prevalidated pretested survey based on a pilot survey of 20 physicians was distributed among physicians, which was based on the awareness and acceptance of H1N1 vaccination among physicians and perception of H1N1 infection. Effective sample size was 272.
STATISTICAL ANALYSIS USED:
Descriptive statistics and Chi-square test were generated for the survey responses. All the continuous variables were reported as mean, median, and range. Categorical variables were reported as tables and pie charts. P < 0.05 was taken as significant. Data analysis was done with SPSS version 21.
RESULTS:
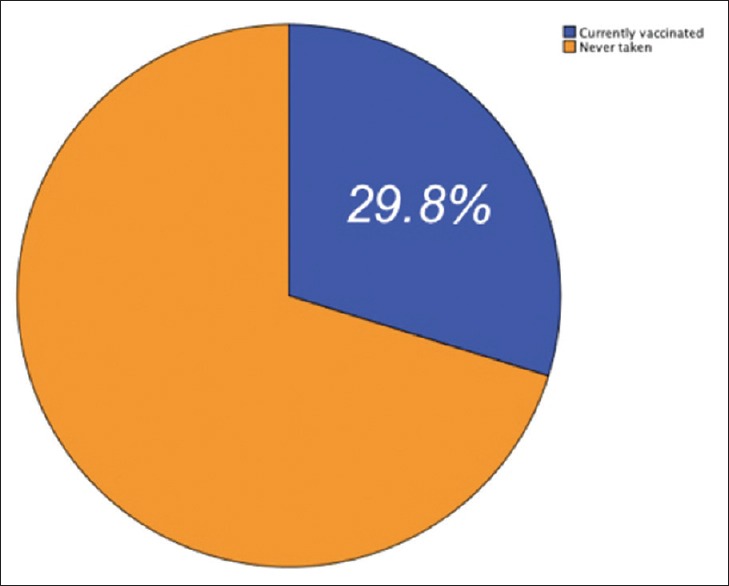
The overall vaccine compliance was 29.8%. This study has found that area of work, deficiency in knowledge about adverse effect of vaccine, misconceptions regarding vaccine, and concerns about efficacy and duration of vaccine are the important factors which lead to decreased vaccine compliance. Furthermore, it is found during the study that timing of vaccination was not given due importance as considering the epidemiological pattern.
CONCLUSIONS:
More emphasis should be given to education sessions and counseling of physicians regarding H1N1 vaccination and oseltamivir therapy. At administrative level, more focus should be given on timing of vaccination and other logistics. Vaccine campaigns should be conducted ideally 1 month before expected rise in cases. Quadrivalent vaccine would be more appropriate over trivalent based on epidemiology of infection in India.
Keywords: B-strain influenza, H1N1 infection, oseltamivir resistance, quadrivalent vaccine, vaccination timing
Introduction
India experienced pandemic phase of H1N1 in May 2009 to December 2010. The postpandemic phase went on from January 2011 to December 2014. Thereafter, this virus has been circulating as seasonal influenza. As per the Ministry of Health and Family Welfare (MoHFW), India had 8648 cases and 345 swine flu deaths until May 7, 2017. This figure was 1786 cases and 265 deaths in 2016. Forty-one percent of total, i.e., 455 swine flu deaths in India were contributed from Maharashtra. Most of them belonged to 20–50 years’ age group.[1]
As per the WHO, all countries should immunize their health-care workers as a first priority to protect the essential health infrastructure. As vaccine stocks are limited, prioritization of beneficiaries is needed.[2] This strategy proved true in studies conducted in the Western world as it reduces transmission of the virus in health-care settings. This will eventually decrease staff sickness and absenteeism, which will decrease the chance of infection for morbidly ill patients.[3] The Strategic Advisory Group of Experts (SAGE) on immunization meeting held in April 2017 revisited the safety profile of H1N1 vaccine. Cases of Guillain–Barre syndrome and narcolepsy reports following administration of the vaccine for pandemic strain A/California/7/2009 (H1N1) have been rejected by SAGE in the absence of any sufficient evidence.[4] Vaccination is considered a potent tool against antimicrobial resistance. It reduces the use of antibiotics and thereby decreases the chance of developing drug-resistant organisms.[5]
A meta-analysis done over the Western world found that fear of adverse reactions, lack of concern, efficacy concerns, and apprehensions about contraindications are the main causes for noncompliance. Vaccination among young adults has been proved to be effective than for elderly and immune-compromised people.[6] A large retrospective cohort study has found that vaccine acceptance level differs among the different cadre of health care workers. Their concerns and self-interests seem to be significantly associated with vaccine acceptance.[7] Physicians play a crucial role in the control of outbreak of influenza. They educate patients regarding prevention, transmission, and treatment. For understanding the barriers toward vaccination if any, a detailed study on awareness regarding vaccine is essential. Creating awareness and changing attitudes of physicians regarding influenza vaccine is an essential step in providing efficient vaccine delivery. Research regarding their awareness is less studied in this aspect.
A study was undertaken to assess the level of awareness and acceptance of influenza vaccine among physicians and also the perception of physicians regarding H1N1 infection and vaccination. This study also examined time of vaccine administration and also whether the vaccine supplied under the campaign was ideal for the above geographical region based on various strain patterns across the country. These aspects were analyzed and suggestions are put forward for increasing vaccine uptake and ensuring maximum efficacy of vaccine in coming years.
Methods
Ethical clearance was obtained from the Institutional Ethics Committee of Seth GSMC and KEM Hospital, which are guided by the ICH-GCP guidelines, ethical principles set forth in the Declaration of Helsinki, and the ethical guidelines on biomedical research on human participants laid down by the Indian Council of Medical Research. This observational cross-sectional study was conducted over a period of 7 months from October 2017 to April 2018.
A vaccination campaign was conducted for all health-care workers of Seth GSMC and KEM Hospital, Mumbai. The period of the camp was for one month in the month of July 2017 coordinated by the Department of Community Medicine of the same institute. The vaccine which was supplied for immunization was inactivated trivalent vaccine intramuscular dose. As per the government directive, a total of 2000 vials was supplied for Mumbai circle as an initial phase. Out of that, 200 were supplied to the above institute. Circulars were sent to all departments about the details of the camp. The campaign was for all health-care workers.
Most of the studies conducted previously involved health-care workers. However, we selected only physicians as the study participants. This was done intending to get more specific data on mindsets of treating physicians regarding H1N1 vaccination, as they play a vital role in creating awareness about the benefits of vaccination.
The study area is a tertiary care hospital in the city of Mumbai with maximum number of physicians all over the state. Only those physicians who were in direct contact with the patients were selected. The sampling frame consists of 390 faculties and 510 postgraduate residents as a whole. No specific exclusion criteria were applied for selecting participants. A pilot sample study was conducted 2 weeks before the start of the study, among 20 physicians. These physicians were later excluded from the final analysis of the results. The sampling method adopted was systematic random sampling as it reduced selection bias and also ensures that the population is evenly sampled. This also reduced dissimilarity in characteristics of the respondents and nonrespondents. We retrieved the list of faculties and residents from academic section from which every third participant was contacted either by E-mail or phone number. Sampling interval “3” is obtained by dividing total population under study with estimated sample size. Google forms were sent and later a reminder mail was sent to nonrespondents. If no response through mail, a physician was contacted through phone number and asked for the convenient timing for filling physical questionnaire. This was done to improve the response rate of the physicians. Written informed consent was obtained from each of them before survey. Table 1 illustrates the department-wise figure of physicians and their percentage of participation.
Table 1.
Department-wise list of physicians and percentage of participation
| Department | Total faculties (percentage responded), n (%) | Total residents (percentage responded), n (%) |
|---|---|---|
| Orthopedics | 18 (12) | 45 (6) |
| Anesthesia | 43 (3) | 121 (6) |
| Dermatology | 14 (1) | 17 (8) |
| Respiratory medicine | 8 (2) | 12 (9) |
| Gynecology | 24 (4) | 28 (9) |
| Psychiatry | 11 (3) | 26 (13) |
| Otorhinolaryngology | 13 (3) | 18 (17) |
| Community medicine | 15 (4) | 57 (19) |
| Ophthalmology | 11 (7) | 18 (17) |
| Surgery | 28 (11) | 36 (24) |
| Pediatrics | 26 (18) | 36 (34) |
| Medicine | 30 (13) | 48 (40) |
Otorhinolaryngology (Ear Nose Throat) Department
Sample size
The sample size was calculated based on the results of the pilot study, where 49% of the physicians were seen to be vaccine compliant. Approximating this to 50%, the sample size was estimated using the formula: Z2 pqN/(e2[N-1] +Z2 pq). The estimated sample size was 269 based on a confidence interval 95% and 5% error. We assumed nonrespondents to be nearly 20% and hence distributed questionnaire to 336 physicians.
The questionnaire was developed after literature search from studies conducted across the globe. Questions were modified; newer questions were added and validated by experts in the field of infection control and public health. It enquired physician's demographic data, perception about H1N1 infection, benefits of vaccination, adverse effects of vaccination, and physician's perspective of barriers to vaccination if any.[3,6,7,8,9] It also enquires physician's knowledge of H1N1 vaccination, motivation if any of those who are vaccine compliant, and source of knowledge of vaccination. A team of specialist from community medicine has tested the questionnaire for accuracy and relevance. One of the panel members was part of the vaccination campaign conducted at the hospital.
Statistical tests
Descriptive statistics and Chi-square test were generated for the survey responses. All the continuous variables were reported as mean, median, and range. Crosstables were created using variables comparing vaccinated and nonvaccinated physicians. Categorical variables were reported as bar diagrams and pie charts. The main outcome variable was vaccine compliance of physicians. Perception regarding H1N1 infection and vaccination were represented as percentages. P < 0.05 was considered as significant. Data entry was done using Excel software. Analysis was performed using SPSS software version 16. Developer: IBM Corporation, Newyork City, Newyork, United States.
Results
Questionnaires were sent to 336 doctors physically and through Google forms. Out of them, 277 responded. Five questionnaires were omitted as they were incomplete. Hence, the effective sample size was 272. The response rate was 78.61%. Table 2 provides an overview of the sociodemographic details of physicians associated with vaccine compliance. Table 1 provides the department-wise list of physicians and percentage of participation. The mean age of vaccinated physicians was 32.05 ± 6.3 years and that of nonvaccinated physicians was 31.96 ± 6.4 years. Figure 1 provides the vaccine complaint percentage among physicians. The mean years of experience of physicians were 7.68 ± 5.79 years. Table 3 compares the department-wise vaccine compliance of physicians within department and within the study group.
Table 2.
Sociodemographic details of physicians associated with vaccine compliance (values depicted are percentages of responders)
| Characteristics | Total population (n=272) | Vaccinated (n=81) | Nonvaccinated (n=191) | P |
|---|---|---|---|---|
| Age (years) | ||||
| <30 | 48.1 | 60.49 | 42.93 | 0.05 |
| 30-39 | 37.9 | 33.33 | 39.79 | |
| >40 | 14 | 6.17 | 17.27 | |
| Gender | ||||
| Male | 65.4 | 64.39 | 67.9 | 0.07 |
| Female | 34.6 | 35.60 | 32.09 | |
| Years of experience | ||||
| <3 | 12.5 | 10.19 | 16.04 | 0.06 |
| 3-10 | 66.9 | 68.58 | 62.96 | |
| >10 | 20.6 | 20.41 | 20.98 | |
| Place of residence | ||||
| Mumbai | 94.1 | 91.62 | 100 | 0.07 |
| Outside Mumbai | 5.9 | 8.37 | 0 | |
| Academic position | ||||
| Faculties | 31.25 | 22.35 | 77.64 | 0.07 |
| Residents | 68.75 | 33.15 | 66.84 | |
| Place of posting | ||||
| Only OPD | 34.55 | 37.23 | 62.76 | 0.02 |
| Only ICU | 3.67 | 0 | 100 | |
| All of these areas | 61.76 | 27.38 | 72.61 | |
| Presence of any chronic illness | ||||
| Diabetes | 2.2 | 0 | 100 | 0.05 |
| Hypertension | 2.6 | 0 | 100 | |
| Other chronic illness | 0 | 0 | 0 | |
| No chronic illness | 95.22 | 31.27 | 68.72 | |
| Source of knowledge about vaccination | ||||
| WHO/CDC website | 80.5 | 31.5 | 68.49 | 0.66 |
| Ministry of Health and Family Welfare | 18.8 | 23.52 | 76.47 | |
| Textbooks | 0.35 | 0 | 100 | |
| Friends | 0.35 | 0 | 100 |
OPD=Outpatient department, ICU=Intensive care unit, CDC=Center for Disease Control
Figure 1.
Vaccine compliance of physicians in percent
Table 3.
Department-wise vaccine compliance of physicians
| Department | Total number participated (n=272), n (%) | Vaccinated | |
|---|---|---|---|
| Percentage within department | Percentage of total (n=81) | ||
| Orthopedics | 8 (2.9) | 0 | 0 |
| Anesthesia | 9 (3.3) | 0 | 0 |
| Dermatology | 9 (3.3) | 44.5 | 1.5 |
| Respiratory medicine | 11 (4.0) | 36.4 | 1.5 |
| Gynecology | 13 (4.8) | 15.4 | 0.7 |
| Psychiatry | 15 (5.5) | 60.6 | 3.3 |
| Otorhinolaryngology | 20 (7.4) | 40 | 2.9 |
| Community medicine | 23 (8.5) | 52.2 | 4.4 |
| Ophthalmology | 24 (8.8) | 16.7 | 1.5 |
| Surgery | 35 (12.9) | 11.4 | 1.5 |
| Pediatrics | 52 (19.1) | 36.5 | 7 |
| Medicine | 53 (19.5) | 28.3 | 5.5 |
| Total | 272 (100) | 29.8 | |
Otorhinolaryngology (Ear Nose Throat) Department
Table 4 summarizes physicians’ response regarding the perception of risk of H1N1 and knowledge about vaccination. Regarding infection control of H1N1, 68% adopted regular handwashing and 43.4% wore surgical masks regularly. About 20.2% minimized traveling during peak season, while 7.7% avoided crowded areas. Only 0.7% preferred prophylaxis with oseltamivir as a measure of infection control and 1.1% did not bother to take any specific infection control measure.
Table 4.
Summary of responses of physicians regarding perception of risk of H1N1 and knowledge about vaccination
| Agree (n=272) | Vaccinated (n=81) | Nonvaccinated (n=191) | P | |
|---|---|---|---|---|
| Perceived risk of H1N1 infection | ||||
| Health professionals have high risk for influenza from patients | 98.5 | 95.06 | 100 | 0.02 |
| I may spread infection to my patients even if I am asymptomatic | 92.2 | 97.53 | 90.05 | 0.03 |
| Health professionals are under the highest risk in case of an epidemic | 93.3 | 95.06 | 92.6 | 0.46 |
| I can spread infection to my family even if I am asymptomatic | 93.3 | 93.82 | 93.19 | 0.5 |
| Perception about benefits of vaccination | ||||
| Reduces personal risk | 80.51 | 87.65 | 77.48 | 0.13 |
| Reduces risk of spreading diseases to my family | 93.01 | 96.3 | 91.6 | 0.3 |
| Reduces risk of spreading diseases to patients | 90.8 | 98.7 | 87.4 | 0.01 |
| Reduced workload during an epidemic | 83.8 | 75.3 | 87.4 | 0.01 |
| Perception regarding adverse effect of H1N1 vaccination | ||||
| Only mild local reaction at the injection site | 72.8 | 69.13 | 21.9 | 0.02 |
| There may be an increased risk of neurological complications | 34.92 | 49.3 | 28.7 | 0.005 |
| It may rarely cause seizures if given along with DPT vaccine | 61.4 | 70.3 | 57.6 | 0.08 |
| It may cause vasovagal attack | 96.3 | 95.06 | 96.8 | 0.62 |
| Allergic reaction may occur | 98.52 | 100 | 97.9 | 0.18 |
| Concern regarding efficacy of H1N1 vaccination | ||||
| It may cause flu-like illness in some people | 53.67 | 47 | 99 | 0.3 |
| Vaccine has been tested adequately | 100 | 100 | 100 | 0.6 |
| Vaccine reduce overall infection rate | 98.16 | 97.53 | 98.42 | 0.6 |
| I have no concerns regarding efficacy of vaccine | 70.95 | 80.24 | 67.01 | 0.02 |
| Duration of protection offered by the vaccine | 96.69 | 91.35 | 93.71 | 0.4 |
DPT=Diphtheria pertussis tetanus, H1N1=Swine flu
Only 21% of physicians mentioned that they would start oseltamivir within 48 h of the onset of symptoms. About 52.9% thought that they would start oseltamivir therapy after 2 days of the onset of symptoms, 25% preferred not to take any medicines, and 1.1% preferred to start immediate treatment with oseltamivir if they developed symptoms. Table 5 shows the response of physicians regarding vaccine used in campaign.
Table 5.
Response of physicians regarding vaccine used in campaign
| Type of vaccine | Percentage |
|---|---|
| Inactivated quadrivalent (%) | 86.4 |
| Inactivated trivalent (%) | 4.4 |
| Live attenuated quadrivalent (%) | 9.2 |
| Recombinant trivalent (%) | 0 |
Only 54.6% of physicians disagreed with the common belief that getting flu will be better than taking vaccine, 4.4% agreed to it, and 41% were not sure about it. About 52.6% of physicians knew that H1N1 vaccine is pregnancy safe, while 45.6% were not sure and 1.8% disagreed with it.
The next section of the survey was concerned about perception of adverse effects of influenza vaccination. About 70.2% of physicians correctly mentioned pain at the injection site as the most common adverse effect of influenza vaccination, while 29.8% believed it as allergic reaction.
In response to felt the need of influenza vaccination, 8.1% were of the opinion that they prefer vaccination, as it was supplied free of cost by the hospital administration, while another 8.1% were ready to take vaccination if sufficient knowledge is provided. About 1.1% also preferred a reminder for annual vaccination. About 76.96% of nonvaccinated physicians were disinterested about taking yearly vaccination.
On asking about vaccinating physicians for influenza, 73.5% mentioned right to choice should be given to them. About 23.2% considered physician to patient transfer as not that important mode of transmission. Only 3.3% of physicians believed that the physicians should be vaccinated for continuity of health services.
As an expert opinion for nonuptake of vaccines by health-care workers, 83.19% believed efficacy as the main concern, while 15.8% considered cost as a barrier. About 1.01% believed need for yearly injection as the main reason. Among the nonvaccinated physicians, 77.48% cite efficacy concern as the reason for nonuptake.
Limitations
The study is limited by the lack of information from different tertiary care centers; hence, the findings of the study cannot be generalizable. In spite of this limitation, the study certainly adds to the understanding of decreased acceptance of H1N1 vaccine among physicians.
Discussion
A cross-sectional study in Turkey found that the proportion of vaccine compliant physicians was 34.9% in a tertiary care center. Another study by Seale et al. in China also reported one-fourth percentage of coverage. Vaccine Coverage percentage was 16.5% for Spain; 21.3% and 21.5% for Greece in two separate studies[8] The overall vaccine compliance in this study was 29.8%. The maximum participation was from pediatrics and medicine department, of which compliance was highest among pediatric department. Nature of posting in hospital has found to be significantly associated with the vaccine compliance in our study. Anesthesia and orthopedics who do not have out patient services with respiratory ailments, have poor compliance.
Age is seen to have significant association with vaccine compliance. Nearly two-third are from <30 age group. A possible explanation for this can be most of them are residents and they are in continuous patient care. Being fresh graduates, better knowledge about vaccine can also be a cause. Gender, years of experience, academic position, place of residence, and source of knowledge about vaccination does not found to have significant association with vaccine compliance. Prior studies have shown the importance of H1N1 vaccination, especially for the extremes of age group. Physicians are supposed to recommend vaccination to common people. As such vaccination percentage of general public is poor. A large community study in Pune shows that vaccine compliance of general population is 8.3%. This study has also found that only 15.8% received advice from doctors regarding influenza vaccination.[10] For this, appropriate knowledge regarding H1N1 vaccination and infection among physicians is important.
Most of the studies found that physicians are considering vaccination as personal protection. However, sense of personal protection and vaccine compliance does not seem to be significantly associated with the study finding. This can be due to uncertainty about efficacy of vaccine as a personal protection. Sickness absenteeism among physician is an important factor affecting continued provision of services during the outbreak periods. Unlike previous studies, only 10% of physicians were unaware that vaccination is also meant to prevent nosocomial infection of vulnerable group.[8]
No single method is found to be fully effective for preventing infection.[11] There is evidence that medical personnel prefer using mask rather than taking vaccination.[8] Our study has found frequent handwashing or wearing surgical mask as the most commonly used protective measure, but nearly half of the participants are not following it.
More than half of the participants believed that flu vaccine causes flu-like syndrome. Injectable flu vaccine contains either inactivated virus or virus-free recombinant vaccine both of which cannot cause infection.[12] This finding reaffirms the widely publicized misconception about vaccination which has been found in previous similar studies in different settings.[8] The study conducted in China found that nearly 60% were concerned about the side effects of the vaccine and around 40% believed that it is not tested adequately.[8] In contrast with these previous studies, few participants reported that vaccine is not tested properly. A possible explanation for this can be previous studies were done during immediate postpandemic phase. Due to the familiarization, knowledge regarding flu vaccination might have improved over the time. Another interesting finding was that 98.2% were apprehensive about the duration of protection offered by the vaccine. This point outs the knowledge
Majority of nonvaccinated physicians expressed their opinion that they are uncomfortable taking yearly vaccination which might have also affected the vaccine compliance. This can be primarily due to inconvenience, but it must be noted that circulating strain of virus may differ every year and possible antigenic drift or shift mechanism is in constant play. This study has also found that nearly one-third of participants believed allergic reaction is the most common adverse reaction; however, pain at injection site and mild local reaction is the most common symptom associated with influenza vaccination. The Centers for Disease Control and Prevention (CDC) clearly mentioned that even people with egg allergy should receive this vaccination under better facility center.[12] These findings emphasize the importance of creating more awareness about influenza vaccination among physicians.
Antiviral medication prophylaxis using oseltamivir may help in prevention, albeit for short term. Currently, oseltamivir and zanamivir are used for treating cases. Considering budgetary benefits, vaccination which provides long-term immunity can be a better alternative.[13] Regarding empirical therapy, CDC recommends starting oseltamivir within 48 h of the onset of symptoms. One of the interesting findings in our study was more than half of the participants prefer to start oseltamivir after failed conservative management. This finding is similar to the reports of district health services that many of the physicians wait for the swab report to start treatment, which will hold the antiviral treatment for 5–6 days.[11,14] This will contribute to resistance to the drug and less efficacy for treating disease.
Regarding timing of vaccination, CDC categorizes countries into two main categories: first, where outbreak starts after the month of October, and second, where outbreaks after April. In countries where multiple episodes of increased reporting of H1N1 infections, vaccination campaign to be conducted before the first episode of such increased activity.[15] This vaccination campaign was conducted at the time when there is a seasonal increased activity of H1N1 at the month of July. Ideally, it will take 1–2 weeks for developing immunity postvaccination. It is a common tendency to see patients and health-related workers taking influenza vaccine during an outbreak. Studies have shown that time of introducing vaccination is one of the most important factors determining the efficacy of vaccine.[3] However, there are reasons to believe that this factor was not considered as that critical. There was a major outbreak of seasonal influenza (H1N1) in Mumbai in 2015 during January–March. The second peak was observed in July and August 2015 resulting in large number of cases and death. In April 2017, the MoHFW notified increased incidence of H1N1 cases in Maharashtra (Circular FTS no. 519324-2017/EMR dated April 20, 2017). Hence, considering the epidemiological pattern, vaccination should have been started 2 months before the proposed campaign. Figure 2 illustrates graphical showing trends in incidence of H1N1 cases in Mumbai.
Figure 2.
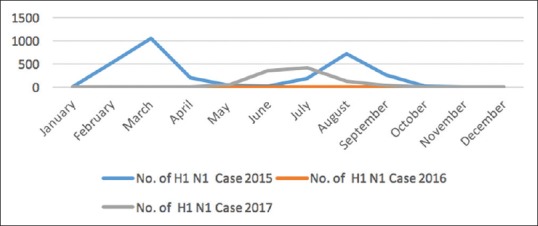
Trends in incidence of H1N1 cases in Mumbai (data obtained from MCGM website); X-axis denotes frequency distribution, Y-axis denotes time
There are mainly three types of influenza vaccines in the market: live-attenuated virus vaccine, trivalent inactivated vaccine, and quadrivalent inactivated vaccine. The trivalent inactivated influenza vaccine which was used for campaign contains the 2009 pandemic strain in addition to H3N2 and one strain of influenza B.[13] Inactivated quadrivalent vaccine contains one additional strain of influenza B. Influenza B causes nearly 25% of flu infections worldwide. The strain of influenza B present in trivalent vaccine may not be matching with the strain circulating during outbreaks. This leads to inadequate protection and leads to reports of vaccine failures.[16] Multiple lineages of B virus have been found in India. In 2012 and 2013, some undetermined B-strain was circulating. Evidence shows that if inactivated quadrivalent vaccine were used for half of the previous seasonal outbreaks, there would have been fewer cases of mismatching. Knowledge about circulating strain and type of vaccine is also important for prescribing. Physicians were not sure about the type of vaccine used for the campaign. Very few opted live attenuated which has its own contraindications. It was unexpected to see that majority opted for inactivated quadrivalent which was not even available in the market at that time. Even though the cost of quadrivalent vaccine is 15% more than trivalent vaccine, cost-effective analysis studies from various parts of the world showed that vaccination with quadrivalent vaccine is more cost-effective than trivalent vaccine. In order to prescribe vaccination, physician's knowledge should be regularly updated.[17,18,19,20,21]
The vaccination campaign was preceded by administrative notice to all departments to take vaccination. All those 81 participants who got vaccinated have mentioned hospital order as the motivation to get vaccinated. This result has significance that informative and stronger educational initiative may lead to better vaccine compliance.
Conclusions
This study has found that area of work, deficiency in knowledge about adverse effect of vaccine, misconceptions regarding vaccine, and concerns about efficacy and duration of vaccine are the important factors which lead to decreased vaccine compliance. Hence, more emphasis should be given to education sessions and efficient logistics support. At administrative level, more focus should be given on timing of vaccination and other logistics. Inactivated quadrivalent vaccine which has been recently launched in India can be provided instead of trivalent as it gives an additional protection against one more strain of influenza B. T his reduces the chances of mismatch that may occur between antigen present in current trivalent vaccine and the antigen circulating during outbreaks. Such mismatches will lead to suboptimal protection.[16]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank the Department of Community Medicine, Seth GSMC and KEMH.
References
- 1.The Hindu. (2018). In the grip of a fever. [online] [Accessed 28 Sep. 2018]. Available at: https://www.thehindu.com/sci-tech/health/in-the-grip-of-a-fever/article25017325.ece .
- 2.World Health Organization. WHO Recommendations on Pandemic (H1N1) 2009 Vaccines. World Health Organization; 2015. [Google Scholar]
- 3.Torun SD, Torun F. Vaccination against pandemic influenza A/H1N1 among healthcare workers and reasons for refusing vaccination in Istanbul in last pandemic alert phase. Vaccine. 2010;28:5703–10. doi: 10.1016/j.vaccine.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control. What is Guillain-Barré syndrome (GBS)? What causes GBS? Center for Disease Control. 2017 [Google Scholar]
- 5.Apps.who.int. (2017). Weekly epidemiological record. [online] [Accessed 20 Sep. 2018]. Available at: http://apps.who.int/iris/bitstream/handle/10665/255611/WER9222.pdf; jsessionid=FAB745EC928F5B1B48B07AAA869DF0C2?sequence=1 .
- 6.Hollmeyer HG, Hayden F, Poland G, Buchholz U. Influenza vaccination of health care workers in hospitals – A review of studies on attitudes and predictors. Vaccine. 2009;27:3935–44. doi: 10.1016/j.vaccine.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 7.Hidiroglu S, Ay P, Topuzoglu A, Kalafat C, Karavus M. Resistance to vaccination: The attitudes and practices of primary healthcare workers confronting the H1N1 pandemic. Vaccine. 2010;28:8120–4. doi: 10.1016/j.vaccine.2010.09.104. [DOI] [PubMed] [Google Scholar]
- 8.Seale H, Kaur R, Wang Q, Yang P, Zhang Y, Wang X, et al. Acceptance of a vaccine against pandemic influenza A (H1N1) virus amongst healthcare workers in Beijing, China. Vaccine. 2011;29:1605–10. doi: 10.1016/j.vaccine.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 9.Najimi A, Golshiri P. Knowledge, beliefs and preventive behaviors regarding influenza A in students: A test of the health belief model. J Educ Health Promot. 2013;2:23. doi: 10.4103/2277-9531.112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundaram N, Purohit V, Schaetti C, Kudale A, Joseph S, Weiss MG, et al. Community awareness, use and preference for pandemic influenza vaccines in Pune, India. Hum Vaccin Immunother. 2015;11:2376–88. doi: 10.1080/21645515.2015.1062956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza – Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 12.Center for Disease Control. Misconceptions about Seasonal Flu and Flu Vaccines. Seas Influ. Center for Disease Control. 2013:18–21. [Google Scholar]
- 13.Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: Formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251–63. doi: 10.1016/j.ejpb.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Swine Flu Catching them Young. The Hindu; 2017. [Last accessed on 2017 Aug 28]. Available from: http://www.thehindu.com/news/cities/mumbai/swine-flu-catching-them-young/article19571938.ece . [Google Scholar]
- 15.WHO. Vaccination Timing. 2018. [Last accessed on 2018 Mar 13]. pp. 14–5. Available from: http://www.who.int/influenza/vaccines/tropics/vaccination_timing/en/
- 16.Tisa V, Barberis I, Faccio V, Paganino C, Trucchi C, Martini M, et al. Quadrivalent influenza vaccine: A new opportunity to reduce the influenza burden. J Prev Med Hyg. 2016;57:E28–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Brogan AJ, Talbird SE, Davis AE, Thommes EW, Meier G. Cost-effectiveness of seasonal quadrivalent versus trivalent influenza vaccination in the United States: A dynamic transmission modeling approach. Hum Vaccin Immunother. 2017;13:533–42. doi: 10.1080/21645515.2016.1242541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang MC, Tan EC, Su JJ. Cost-effectiveness analysis of quadrivalent versus trivalent influenza vaccine in Taiwan: A lifetime multi-cohort model. Hum Vaccin Immunother. 2017;13:81–9. doi: 10.1080/21645515.2016.1225636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You JH, Ming WK, Chan PK. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong – A decision analysis. Hum Vaccin Immunother. 2015;11:564–71. doi: 10.1080/21645515.2015.1011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorrington D, van Leeuwen E, Ramsay M, Pebody R, Baguelin M. Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England. BMC Med. 2017;15:166. doi: 10.1186/s12916-017-0932-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García A, Ortiz de Lejarazu R, Reina J, Callejo D, Cuervo J, Morano Larragueta R, et al. Cost-effectiveness analysis of quadrivalent influenza vaccine in Spain. Hum Vaccin Immunother. 2016;12:2269–77. doi: 10.1080/21645515.2016.1182275. [DOI] [PMC free article] [PubMed] [Google Scholar]


