Abstract
Direct health care costs of illness reflect the costs of medically necessary services and treatments paid for by public and private payers, including hospital-based care, outpatient physician consultations, prescription medications, diagnostic testing, complex continuing care, and home care. The costs of caring for persons with inflammatory bowel disease (IBD) have been rising well above inflation over the past fifteen years in Canada, largely due to the introduction and penetration of expensive biologic therapies. Changing paradigms of care toward frequent patient monitoring and achievement of stricter endpoints for disease control have also increased health services utilization and costs among IBD patients. While the frequency and costs of surgeries and hospitalizations have declined slightly in parallel with increased biologic use (due to better overall disease control), the direct medical costs of care for IBD patients are largely dominated by prescription drug costs. Introduction and penetration of biosimilar agents (at a markedly lower price point than the originator drugs) and increasing gastroenterologist involvement in the care of IBD patients may help to balance rising health care costs while improving health outcomes and quality of life for IBD patients. Ultimately, however, the predicted rise in the prevalence of IBD over the next decade, combined with increasing use of expensive biologic therapies, will likely dictate a continued rise in the direct costs of IBD patient care in Canada for years to come. In 2018, direct health care costs of IBD are estimated to be at least $1 billion Canadian dollars (CAD) and possibly higher than $2 billion CAD.
Highlights
1. In Canada, the direct cost of caring for people living with IBD is estimated in 2018 to be close to $1.28 billion (roughly $4731 per person with IBD).
2. The costs of caring for people living with IBD are dominated by prescription drugs, followed by hospitalization costs. There has been a shift away from hospitalizations and toward pharmaceuticals as the predominant driver of direct health care costs in IBD patients, due to the introduction and widespread use of expensive biologic therapies.
3. The rates of hospitalizations and major abdominal surgeries have been declining in IBD patients in Canada over the past two decades, possibly due to penetration of biologic therapies and advances in patient management paradigms.
4. Inflammatory bowel disease patients cared for by gastroenterologists have better outcomes, including lower risks of surgery and hospitalization. Canadians who live in rural and underserviced areas are less likely to receive gastroenterologist care, potentially due to care preferences or poorer access, which may result in poorer long-term outcomes.
5. Introduction of biosimilar agents at a lower price point than originator biologic therapies, increased gastroenterologist care of IBD patients, and improvements in IBD care paradigms may balance overall treatment costs while improving health outcomes and quality of life for IBD patients. However, in the long-term, direct costs of care may continue to increase, dictated by a rising IBD prevalence and increasing use of biologic therapies.
Key Summary Points
1. The costs of health care for patients with IBD are more than double those without IBD.
2. Prescription drug use accounts for 42% of total direct costs in IBD patients, and costs to treat IBD continue to rise due to increased use of existing biologic therapies and the introduction of several new biologic therapies in recent years.
3. In Manitoba, the mean health care utilization and medication costs for persons with IBD in the year before beginning anti-TNF therapy was $10,206 and increased to $44,786 in the first year of therapy.
4. Biosimilar agents to anti-TNF drugs are now entering the Canadian marketplace and may result in cost savings in patients using biologic agents to treat their IBD.
5. Timely gastroenterologist care has been associated with reduced risks of requiring surgery and emergency care among ambulatory IBD patients and a reduced risk of death among hospitalized patients with ulcerative colitis.
6. Inflammatory bowel disease care provided by gastroenterologists has increased over the past two decades. Even then, the average time from symptom onset to IBD diagnosis exceeds six months, and only one-third of IBD patients receive continuing care with a gastroenterologist during the first five years following diagnosis.
7. Senior (age ≥65), rural-dwelling, and non-immigrant IBD patients have less frequent gastroenterologist care than other groups.
8. About one in five adults with Crohn’s disease and one in eight adults with ulcerative colitis are hospitalized in Ontario every year. Hospitalizations are most common during the first year following IBD diagnosis. Children with IBD (age <18) have the highest rates of hospitalizations and hospital re-admissions.
9. In Canada, 16% of patients hospitalized for Crohn’s disease undergo an intestinal resection, and 11% of patients hospitalized for ulcerative colitis undergo a colectomy during their initial hospitalization. Rates of intestinal resection and colectomy are declining in Canada in persons with Crohn’s disease and ulcerative colitis, respectively.
10. In Ontario, one-third of adult-onset Crohn’s disease patients undergo intestinal resection within ten years of diagnosis. Among Canadian children with Crohn’s disease, approximately one in fifteen children will require intestinal surgery within the first year of diagnosis, and up to one-third will require surgery within ten years of diagnosis.
11. In Ontario, the ten-year colectomy risk following ulcerative colitis diagnosis is 13.3% among young persons and adults and 18.5% among individuals with senior-onset ulcerative colitis. In children with ulcerative colitis, the risk of colectomy is 4.8% to 6% in the first year following diagnosis and increases to 15% to 17% by ten years.
Gaps in Knowledge and Future Directions
1. Forecasting models are necessary to predict the rising costs attributable to biologics associated with increasing prevalence of IBD, more frequent use of these medications, and the introduction of newer agents.
2. Research into ways to minimize the escalating costs associated with increasing use of biologic therapies to treat IBD (and other chronic diseases) is necessary to ensure sustainability of our publicly funded health care system. Biosimilars offer an opportunity to drive down the cost of biologic therapies, and future research should assess the uptake of biosimilars as new biosimilars are introduced into the marketplace.
3. Cost-utility models and budget impact analyses that integrate changes in direct costs (i.e., reduced hospitalizations and increased pharmaceutical costs) with indirect cost savings from improved quality of life are necessary to inform policy decisions.
4. Research into ways to reduce IBD hospitalizations further through targeted outpatient interventions is equally important for health system sustainability and to improve patient quality of life.
5. Research into reasons for reduced gastroenterologist care among rural and underserviced IBD residents would allow targeted interventions to improve specialist care and thereby improve patient health outcomes and quality of life.
Keywords: Costs, Crohn’s disease, Inflammatory bowel disease, Prevalence, Quality of life, Ulcerative colitis
Direct health care costs are the costs associated with medically necessary products and services, including hospital-based care, outpatient physician consultations, prescription medications, diagnostic testing (i.e., laboratory tests and diagnostic imaging), complex continuing care (i.e., rehabilitation, long-term care, etc.), and home care. In Canada, these costs are borne by each province’s public health care system. Because there is no cure for IBD, patients require long-term medical care, including frequent physician visits, multiple medical tests and treatments, hospitalizations, and surgeries. There has also been a push toward more aggressive monitoring and treatment of individuals with IBD over the past decade, with a revised goal of establishing and maintaining complete healing of the bowel (1). This new focus promotes increased use of endoscopy, diagnostic imaging, and laboratory services; increased frequency of specialist visits to monitor disease activity; and more aggressive (and expensive) targeted therapies to achieve strict endpoints. Additionally, “top-down” and “accelerated step-up” approaches to treatment, reflecting earlier introduction of biologic therapies in persons with aggressive disease phenotypes or who are failing early conventional treatments, are being increasingly adopted based on evolving literature and expert opinion (2, 3). Introduction of newer biologic therapies and evolving paradigms of care have increased the complexity of caring for IBD, underscoring the importance of early and continued specialist involvement. Moreover, these factors, which are intended to improve patient outcomes and quality of life, have driven up the costs of IBD care in recent years. This section will focus on the direct cost of care for IBD and health care utilization by patients with IBD. A complete overview of the objectives, working committees, and methodology of creating the report can be found in supplemental file, Technical Document.
TOTAL DIRECT COSTS OF CARING FOR PATIENTS WITH IBD IN CANADA
In population-based studies from Quebec, Manitoba, and Alberta, persons with ulcerative colitis cost the health care system on average between $3552 (Manitoba) and $8900 (Quebec) per person annually, while persons with Crohn’s disease costs the health care system $4232 per person annually (Manitoba) (4–6). On average, caring for older IBD patients costs more than caring for younger IBD patients (4, 5). In Manitoba, the costliest IBD patients are those who are within one year of diagnosis (mean $6611), those who are hospitalized (15% of IBD patients, mean $13,495), those who undergo major surgery (2% of IBD patients, mean $18,749), and those who use anti-TNF therapy (0.7% of IBD patients, mean $31,440) (5). Table 1 provides a comparison of the direct health care costs of IBD across Canadian provinces, the United States, Europe, and Australia.
Table 1.
Summary of the literature on direct health care costs of patients with inflammatory bowel disease
| Country/Province | Study | Time period of study | Type of IBD | Overall cost (mean cost per person per year*) | Cost of outpatient physician visits (% of total) | Cost of emergency department visits (% of total) | Cost of diagnostic procedures (% of total) | Cost of hospitalization (% of total) | Cost of surgical care (% of total) | Cost of medications (% of total) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Canada | |||||||||||
| Alberta | Coward 2015 (4) | Cost per hospitalization | UC | Non-surgical hospitalization: $5499† (IQR 5530) |
Elective colectomy: $14,316† (IQR 6399) Emergent colectomy: $23,698† (IQR 14,404) |
||||||
| Alberta | Loomes 2011 (7) | Costs before and after starting infliximab | CD |
Pre-infliximab
Year 2: $3981 Year 1: $3930 Post-infliximab Year 1: $25,346 Year 2: $20,098 |
Pre-infliximab
Year 2: $264 Year 1: $285 Post-infliximab Year 1: $478 Year 2: $497 |
Pre-infliximab
Year 2: $118 Year 1: $191 Post-infliximab Year 1: $107 Year 2: $131 |
Pre-infliximab:
Year 2 • Colonoscopy: $423 • CT: $99 • MRI: $45 • X-ray: $14 Year 1: • Colonoscopy: $426 • CT: $114 • MRI: $38 • X-ray: $10 Post-infliximab Year 1: • Colonoscopy: $242 • CT: $75 • MRI: $53 • X-ray: $8 Year 2: • Colonoscopy: $255 • CT: $72 • MRI: $32 • X-ray: $7 |
Pre-infliximab
Year 2: $2881‡ Year 1: $2715 ‡ Post-infliximab Year 1: $968 ‡ Year 2: $1037 ‡ |
Pre-infliximab
Year 2: $1895 Year 1: $1504 Post-infliximab Year 1: $263 Year 2: $351 |
Infliximab only (post-infliximab): Year 1: $23,328 Year 2: $17,969 |
|
| Manitoba | Bernstein 2012 (5) | Varying disease duration | IBD (all types) | All patients: $3896 (se 90) Newly diagnosed: $6611 (se 593) Long standing disease (4+ years): $3621 (se 94) |
All patients: 13% Newly diagnosed: 11% Long-standing disease (4+ years): 14% |
All patients: 6% Newly diagnosed: 6% Long-standing disease (4+ years): 6% |
All patients: 39% ‡ Newly diagnosed: 63% ‡ Long-standing disease (4+ years): 35% ‡ |
All patients: 42% Newly diagnosed: 20% Patients with long-standing disease (4+ years): 45% |
|||
| CD | All patients: $4232 (se 137) Newly diagnosed: $6570 (se 686) Long-standing disease (4+ years): 3918 (se 145) |
All patients: 12% Newly diagnosed: 11% Long-standing disease: 13% |
All patients: 5% Newly diagnosed: 7% Long-standing disease: 5% |
All patients: 39% ‡ Newly diagnosed: 63% ‡ Long-standing disease (4+ years): 35% ‡ |
All patients: 44% Newly diagnosed: 19% Long-standing disease (4+ years): 47% |
||||||
| UC | All patients: $3552 (se 117) Newly diagnosed: $6650 (se 958) Long-standing disease (4+ years): $3303 (se 119) |
All patients: 14% Newly diagnosed: 10% Long-standing disease (4+ years): 14% |
All patients: 7% Newly diagnosed: 5% Long-standing disease (4+ years): 7% |
All patients: 39% Newly diagnosed: 63% ‡ Long-standing disease (4+ years): 36% ‡ |
All patients: 40% Newly diagnosed: 21% Long-standing disease (4+ years): 43% |
||||||
| Manitoba | Targownik 2018 (8) | Costs in the year before and after starting anti-TNF | IBD | Pre-anti-TNF: $10,206 Post-anti-TNF: $44,786 |
Pre-anti-TNF: $6419 Post-anti-TNF: $5627 |
Pre-anti-TNF: $1861 Post-anti-TNF: $37,448 (95% of total drug costs) |
|||||
| Quebec | Dan 2017 (6) | Incident cases with a 1-year washout period. Costs are reported per day. | UC | $59.34 (sd 159.29) | GI visits to non-gastroenterologists: $0.38 (sd 0.77) Visits to gastroenterologists: $0.52 (sd 0.76) |
GI-related visits: $1.61 (sd 5.28) | $41.27 (sd 112.74) per day | $9.90 (sd) 110.290 per day | 5-ASA: $1.42 (sd 2.00) per day immunomodulators: $0.25 (0.93) per day anti-TNF: $3.23 (sd 15.67) per day corticosteroids: $0.16 (sd 0.35) per day GPAs: $0.61 (sd 0.87) per day Other UC medications $0.02 (0.17) per day |
||
| United States | Gleason 2013 (9) | Prevalent cases | IBD (all types) | US$22,070 |
Biologic only
$2929 (13.3%) |
||||||
| United States | Karve 2012 (10) | Incident cases with a 6-month washout. Costs incurred in the first 12 months. | IBD (all types) | US$ 18,302 (sd 41,955) | Office visits: US$1013 (1573) | US$366 (1224) | Outpatient visits: US$4063 (sd 9027) | US$10,185 (sd 36,306) | US$2677 (sd 5536) • Steroids: US$67 (sd 276) • Anti-TNFs: US$35 (sd 513) • Immunomodulators: US$151 (sd 397) • Salicylates: US$857 (sd 1308) • Other: $1568 (sd 5040) |
||
| United States | Park 2016 (11) | Prevalent cases | CD | US$18,637 (sd 32,023)§ | MD office: 8.2% | 2.6% | Outpatient hospital procedures: 15.7% Outpatient setting other: 8.6% |
23.1% ‡ | 45.5% | ||
| United States | Wan 2014 (12) | Costs before and after starting infliximab | IBD (all types) |
Pre-infliximab:US$22,463 (sd 25,376) Post-infliximab: Adherent: US$41,713 Non-adherent: US$47,411 |
Pre-infliximab: US$10,264 (sd 12,489) Post-infliximab: Adherent: US$7357 Non-adherent: US$10,909 |
Pre-infliximab:US$7814 (sd 20,092) ‡ Post-infliximab: Adherent: US$2458 ‡ Non-adherent: US$17,634 ‡ |
Post-infliximab:
Adherent: • Infliximab: US$28,289 • Other: US$3373 Non-adherent: • Infliximab: US$14,889 • Other: US$3521 |
||||
| Australia & New Zealand | |||||||||||
| Australia | Niewiadomski 2015 (13) | Incident cases; costs incurred in the first 12 months following diagnosis | CD | A$10,477 (sd 12,737) | A$258 (sd 34) (2%) | A$2196 (sd 956) (21%) | A$6493 (sd 2884) (14%) | A$15283 (sd 18,656) (32%) | A$3366 (sd 5912) (32%) | ||
| UC | A$6292 (sd 6969) | A$242 (sd 37) (4%) | A$1825 (sd 743) (29%) | A$6282 (sd 5276) (18%) | A$35,506 (sd 31,228) (12%) | A$2447 (sd 1898) (39%) | |||||
| Australia | Gibson 2014 (14) | Prevalent cases. Costs are reported per 3-month period | UC | A$2914 (sd 3447, 95% CI 2399 to 3428) | GP: A$371 (4%) Specialist: A$1100 (13%) ED visit: A$46 (<1%) |
A$6643 (82%) ‡ | |||||
| Europe | |||||||||||
| Multi-national | Odes 2010 (15) | Incident cases, followed for up to 10 years. Costs are reported per 3-month period | CD | €569.10 (sd 2188.90) | |||||||
| UC | €324.80 (sd 1659.90) | ||||||||||
| Multi-national | Burisch 2015 (16) | Incident cases. Costs were those incurred in the first 12 months following diagnosis | IBD (all types) | €3956 | €1495 (38%) | €1044 (26%) |
Biologics: €571 (14%) Standard medication: 22% • 5-ASA: €567 • Glucocorticoids: €217 • Immunomodulators: €63 |
||||
| CD | €5942 | €1857 (31%) | €1995 (34%) | Biologics: €1168 (20%) Standard medication: 15% • 5-ASA: €328 • Glucocorticoids: €509 • Immunomodulators: €85 |
|||||||
| UC | €2753 | €1248 (45%) | €476 (17%) | Biologics: 8% Standard medication: 30% • 5-ASA: €734 • Glucocorticoids: €29 • Immunomodulators: €51 |
|||||||
| Netherlands | Severs 2016 (17) | Prevalent cases | IBD | €4866 (95% CI 3290 to 6443) | €58.8 (95% CI 19.5–98.1) | €597.30 (95% CI 188.30–1006.3) | €91.3 (95% CI 4.7–177.8) | Anti-TNF only: €3924.0 (95% CI 2427.0–5420.9) | |||
| Netherlands | van der Valk 2014 (18) | Prevalent cases. Costs are per 3-month interval | CD | €1625.18 (95% CI 1475.87 to 1774.50) | Gastroenterologist: €60.65 (54.70 to 66.59) (3.7%) Specialized nurse: €5.67 (95% CI 4.86 to 6.47) (0.3%) Internist: €5.61 (95% CI 3.66 to 6.66) (0.3%) Dietician: €3.52 (95% CI 2.33 to 4.70) (0.2%) Surgeon: €6.24 (95% CI 3.71 to 8.78) (0.4%) Rheumatologist: €4.06 (95% CI 2.62 to 5.50) (0.2%) Dermatologist: €3.50 (95% CI 1.90 to 5.11) (0.2%) Occupational physician: €1.88 (95% CI 0.87 to 2.88) (0.1%) Psychiatrist: €7.68 (95% CI -5.12 to 20.47) (0.5%) GP visits (evening/weekend): €4.99 (95% CI 3.85 to 6.13) (0.3%) |
€5.83 (95% CI 3.73 to 7.94) (0.4%) | €40.60 (95% CI 33.58–47.56) (2.6%) • Colonoscopy: €24.31 (95% CI 19.71 to 29.46) (1.5%) |
€315.25 (95% CI 231.18 to 399.33) (19.4%) | €9.90 (95% CI 2.71 to 17.10) (0.6%) | €1145.33 (95% CI 1041.80 to 1248.86) (70.5%) • Mesalazine: €54.82 (95% CI 49.27 to 60.38) (3.4%) • Budesonide: €10.83 (8.44 to 13.21) (0.7%) • Prednisone: €0.40 (0.27 to 0.53) (0.0%) • Azathioprine: €23.30 (95% CI 21.15 to 25.44) (1.4%) • 6-mp: €6.13 (4.90 to 7.37) (0.4%) • Methotrexate: €8.12 (95% CI 5.73 to 10.52) (0.5%) • Infliximab: €490.84 (95% CI 411.65–570.03) (30.2%) • Adalimumab: €550.89 (95% CI 427.46–629.33) (33.9%) |
|
| UC | €594.89 (95% CI 504.90 to 684.89) | Gastroenterologist: €41.06 (95% CI 36.22 to 45.90) (6.9%) Specialized nurse: €3.76 (2.97 to 4.56) (1.0%) Internist: €4.26 (95% CI 2.57 to 5.95) (0.7%) Dietician: €2.34 (95% CI 139 to 3.28) (0.4%) Surgeon: €3.06 (95% CI 1.40 to 4.72) (0.5%) Rheumatologist: €1.28 (95% CI 0.30 to 2.27) (0.2%) Dermatologist: €1.71 (95% CI -0.13 to 3.43) (0.3%) Occupational physician: €1.07 (95% CI -0.09 to 2.23) (0.2%) Psychiatrist: €0.36 (95% CI -0.15 to 086) (0.1%) GP (daytime visit): €2.48 (1.71 to 3.25) (0.4%) GP (evening/weekend visit): €4.33 (95% CI 3.11 to 5.55) (0.7%) |
€2.67 (95% CI 1.14 to 4.20) (0.4%) | €29.85 (95% CI 22.97–36.73) (5.1%) • Colonoscopy: €24.31 (18.22 to 30.22) (4.1%) |
€138.64 (83.85 to 193.42) (23.3%) | €8.16 (95% CI 0.78 to 15.54) (1.4%) | €349.86 (95% CI 290.86 to 409.58) (58.8%) • Mesalazine: €136.47 (95% CI 129.9 to 143.01) (22.9%) • Budesonide: €4.86 (95% CI 2.93 to 6.79) (0.8%) • Prednisone: €0.39 (95% CI 0.23 to 0.54) (0.1%) • Azathioprine: €13.83 (95% CI 11.74 to 15.92) (2.3%) • 6-mp: €5.51 (4.12 to 6.90) (0.9%) • Methotrexate: €1.86 (95% CI 0.48 to 3.23) (0.3%) • Infliximab: €145.02 (95% CI 92.2 to 198.02) (24.4%) • Adalimumab: €41.92 (95% CI 14.61 to 69.22) (7.0%) |
||||
| Spain | Aldeguer 2016 (17) | Prevalent cases | UC | €1754.10 (sd 2418.08; 95% CI 1479.37 to 2034.83) | GP visits: €250.52 (sd 203.79; 95% CI 226.86–274.18) GI visits: €54.89 (sd 116.70; 95% CI 41.37–68.44) Mental health visits: €1.19 (sd 11.23, 95% CI -0.11 to 2.49) |
€61.07 (sd 90.77; 95% CI 50.53–71.61) | €50.06 (sd 69.74, 95% CI 41.96 to 58.16) | €853.30 (sd 2157.77; 95% CI 602.79–1103.81) (47.88%) | €596.52 (sd 574.63; 95% CI 429.81 to 563.23) (28.31%) | ||
| United Kingdom | Sprakes 2010 (20) | Costs in the year before and the year after starting infliximab | CD |
Pre-infliximab: £4965.20 §§Post-infliximab: £2214.37§§ |
Pre-infliximab: £479.30 Post-infliximab: £448.45 |
Pre-infliximab: • Radiology: £315.79 • Endoscopy: £397.64 • Lab tests: £48.52 Post-infliximab: • Radiology: £89.46 • Endoscopy: £77.86 • Lab tests: £37.13 |
Pre-infliximab:
£2588.36 Post-infliximab: £670.90 |
Pre-infliximab: £536.88 Post-infliximab: £124.06 |
Pre-infliximab: £598.71§§ Post-infliximab: £448.45§§ Infusion costs: £393.86 |
||
| United Kingdom | Lindsay 2013 (21) | Costs in the year before and the two years after starting infliximab (per year) | CD |
Pre-infliximab: £913.48 Post-infliximab; £823.23 |
Pre-infliximab: £411.00 Post-infliximab: £190.04 |
Pre-infliximab:
£1908.85 Post-infliximab: £1194.01 |
Post-infliximab: £7128.02 |
Abbreviations: CD: Crohn’s disease; CI: confidence interval; IBD: inflammatory bowel disease; IQR: interquartile range; sd: standard deviation; se: standard error; UC: ulcerative colitis
*Unless otherwise stated; †Median; ‡Includes medical and surgical hospitalizations; §Costs do not include deductibles and other costs to the patient; §§Does not include the cost of infliximab
In Manitoba, the mean and median direct costs of IBD care were more than two times greater in IBD patients than age- and sex-matched controls ($3896 vs $1826 for mean, $1562 vs $448 for median) in 2005 (5). The cost gap between persons with and without IBD was greatest among children. Prescription drug use accounted for 42% and 37% of total direct costs, while hospitalizations were responsible for 39% and 40% of total direct costs among persons with IBD and controls, respectively. In contrast, prescription drugs contributed to just 20% of of total health care costs in Quebec, while hospitalizations (medical and surgical) contributed to 67% of total health care costs (6). In Alberta, the median cost per hospitalization for ulcerative colitis ranged from $5499 (persons with disease flare not requiring colectomy) to $23,698 (persons requiring emergent colectomy) (4). In all three provinces, the costs of biologics were significant drivers of medication costs. For example, while only 6.6% of newly diagnosed patients with ulcerative colitis in Quebec received anti-TNFs, these medications represented 57.4% of the total cost of medications for these patients.
Based on these three studies, there are significant differences in direct costs of IBD care depending on the province and method of ascertaining cost. After the the Manitoba estimates for mean cost per person with IBD have been applied and adjusted for inflation ($4731 CAD per person in 2018), the total direct medical cost of managing the predicted 270,000 patients living with IBD in Canada in 2018 is close to $1.28 billion (range $1.26 to 1.30 billion). However, based on estimates of direct costs in ulcerative colitis patients from Alberta and Quebec, total direct medical costs may be as much as twice this estimate.
Across non-Canadian cohorts, sector-specific expenditures in IBD patients have been highly variable (see Table 1). A cohort study from Australia reported that about 18% and 12% of direct health care costs among persons newly diagnosed with ulcerative colitis were attributable to medical and surgical hospitalization costs, respectively (13). Among those newly diagnosed with Crohn’s disease, 14% and 32% of total direct costs resulted from medical and surgical hospitalizations, respectively (13). This same study reported that 32% and 39% of direct costs of care were related to prescription medications among patients with Crohn’s disease and ulcerative colitis, respectively (13).
In a large European, prospective, population-based, inception cohort of IBD patients, medical therapies and surgeries accounted for 36% and 26% of total health care expenditures, respectively, in the first year following diagnosis (16). Anti-TNF use accounted for just 14% of total health care costs, while standard medical therapy accounted for 22% of total direct costs. Conversely, in a large Dutch cohort, hospitalization costs accounted for less than 20% of health care costs in persons with Crohn’s disease and 23% of health care costs in persons with ulcerative colitis; anti-TNF-α use accounted for 64% and 31% of the total health care cost in Crohn’s disease and ulcerative colitis patients, respectively (18).
SPECIALIST CARE FOR IBD
Early care by gastroenterologists is associated with reduced risks of undergoing surgery (22, 23) and a reduced number of emergency department (ED) visits among IBD patients (24). Admission directly under a gastroenterologist’s supervision has also been associated with a lower risk of dying among hospitalized ulcerative colitis patients (25).
Over the past two decades, there has been a rise in gastroenterologist care for individuals living with IBD in Canada (23, 26). Between 63% and 88% of Crohn’s disease patients see a gastroenterologist at least once in the year following diagnosis (23, 27). However, specialist involvement remains suboptimal because the average time from symptom onset to IBD diagnosis (which is typically made by a specialist) still exceeds six months (22), and only one-third of patients receive continued follow-up care with a gastroenterologist over the first five years following diagnosis (27).
Seniors diagnosed with IBD are less likely to receive ongoing care from a gastroenterologist as compared with persons who are younger at diagnosis. These differences are particularly pronounced for seniors living in rural areas (27). Overall, rural residents with IBD are about one-half as likely to ever see a gastroenterologist in Canada and have 20% fewer annual visits with gastroenterologists compared with their urban counterparts.
In Canada, patients with IBD see a physician for their IBD between two and four times per year (22, 28), with the greatest number of visits occurring in the year following diagnosis. Children with IBD see a physician a median of eleven times per year (interquartile range [IQR] 7), with 90% of these visits being related to their IBD (29). Children with IBD visit a doctor more often than children without IBD for at least five years following diagnosis (29).
HOSPITALIZATIONS
About one in five adults with Crohn’s disease and one in eight adults with ulcerative colitis are hospitalized every year (22, 30). Hospitalizations are most common during the first year after diagnosis. Young adults (18 to 40 years) have higher rates of hospitalization as compared with middle-aged adults (41 to 64 years) and senior (≥65 years) individuals. Roughly one in four Ontario children with IBD are hospitalized in the first year of diagnosis, and almost half are hospitalized within five years of diagnosis (28).
In a Canadian population-based study, 2.3% of hospitalized IBD patients were re-hospitalized within one month of discharge, while 5.6% and 7.7% were readmitted to hospital within six and twelve months respectively (31). Additionally, 11% of children with IBD were readmitted within one year of hospitalization, compared with 5.3% of senior patients. The average length of Crohn’s disease–related and ulcerative colitis–related hospitalizations were 8.8 (SD 12.3) and 10.2 (SD 11.7) days, respectively. Senior patients stayed in hospital longer than younger patients (thirteen and eight days, respectively) (31).
In a population-based study from Manitoba, 0.74% of patients with IBD were found to be admitted to an intensive care unit every year, which was higher than admission rates for matched controls (hazard ratio [HR], 1.79; 95% confidence interval [CI], 1.58–2.02) (32). The risk of ICU admission was greater for Crohn’s disease patients (HR, 2.31; 95% CI, 1.95–2.75) than ulcerative colitis patients (HR, 1.37; 95% CI, 1.13–1.65) as compared with matched controls.
SURGERIES
Ulcerative Colitis
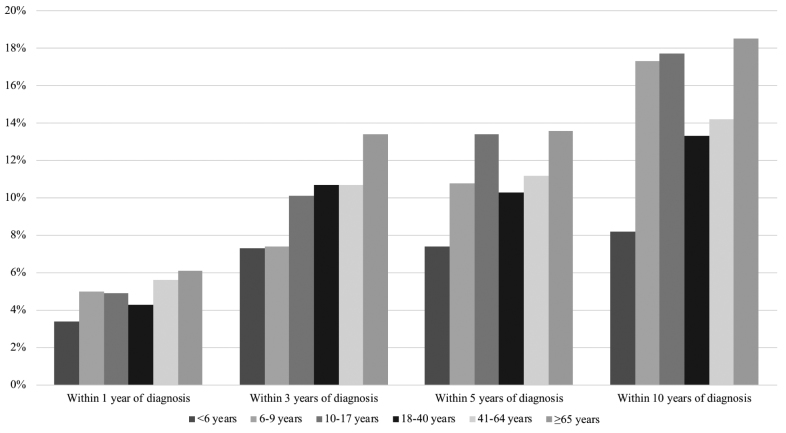
In a systematic review and meta-analysis of population-based studies, the risk of colectomy following ulcerative colitis diagnosis was 4.9% at one year, 11.6% at five years, and 15.6% at ten years (33). Canadian ulcerative colitis patients may have lower colectomy rates based on a recent Manitoba study that reported five-, ten-, and twenty-year risks of colectomy to be 7.5%, 10.4%, and 14.8%, respectively (34). In Ontario, the ten-year risk of colectomy is 13.3% among young adults and 18.5% among individuals with senior-onset ulcerative colitis (see Figure 1) (35). Several studies have shown that colectomy rates have declined in ulcerative colitis over the last two decades, which is possibly related to the introduction of better therapies to treat IBD (29, 36–40).
Figure 1.
Risk of colectomy in patients with ulcerative colitis in Ontario. Data derived from Benchimol et al. and Nguyen et al. (28, 35).
In children, the risk of colectomy is slightly higher than in adults and comparable to senior-onset ulcerative colitis patients (see Figure 1). Colectomy rates within the first year of ulcerative colitis diagnosis range from 4.8% to 6% (28, 29). This number increases to 15% to 17% by ten years following diagnosis. The risk of early surgery is similar across pediatric age groups. However, males diagnosed with ulcerative colitis before six years of age were less likely to undergo colectomy within ten years of diagnosis (28) than males diagnosed at older ages.
Patients hospitalized for acute severe ulcerative colitis are at particular high risk of requiring colectomy. A Canadian population-based study reported that 11% of patients with ulcerative colitis admitted to hospital underwent colectomy during their first hospitalization (31). Another Canadian multicentre study reported that 18% of hospitalizations among patients with ulcerative colitis resulted in colectomy, although colectomy rates varied widely across centres (range 6% to 40%) (41). A separate study conducted in Calgary found that almost 60% of patients with ulcerative colitis admitted to hospital within three years of their diagnosis required a colectomy during their hospitalization (40). It is noteworthy that patients who undergo emergency colectomy have much higher mortality rates than patients who have elective colectomy (5.3% versus 0.7%) (42). Therefore, avoidance of emergency surgery with high-quality and prompt medical and surgical physician support is paramount in the management of refractory colitis.
Crohn’s Disease
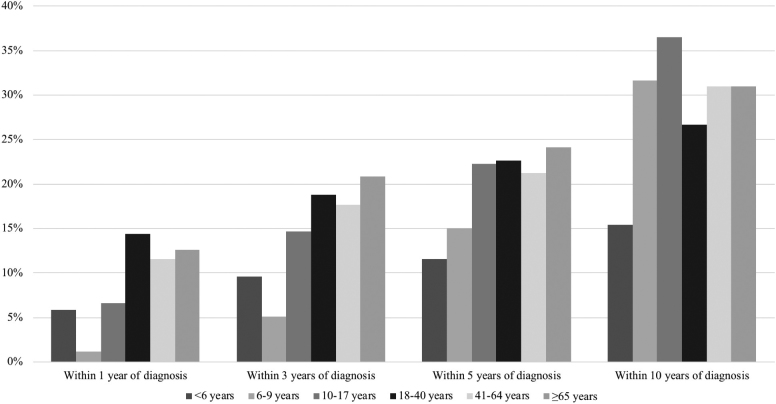
In a systematic review and meta-analysis of population-based studies, 16.3%, 33.3%, and 46.6% of persons with Crohn’s disease required surgery within one, five, and ten years of diagnosis (33), respectively. The most common operation was a limited intestinal resection. Of persons who undergo surgery, 24.2% and 35.0% undergo repeat surgery after five and ten years, respectively (43). This risk is higher among rural Canadians, possibly related to lower use of outpatient specialist care for their IBD (44).
Canadian Crohn’s disease patients may have lower rates of surgery as compared with patients from other jurisdictions. Between 21% and 24% of adult-onset Crohn’s disease patients undergo intestinal resection within five years of diagnosis, and 27% to 31% undergo intestinal resection within ten years (see Figure 2) (35). As with ulcerative colitis, the rates of intestinal resection in patients with Crohn’s disease have decreased over time (29, 45). A study from the Calgary Health Zone reported a decrease of 3.5% per year in the need for intestinal resection between 2002 and 2011 (45). This decrease was driven by a decrease in emergency surgeries (which decreased by 10.1% per year), while the rate of elective surgeries remained constant (45).
Figure 2.
Risk of intestinal resection for Crohn’s disease in Ontario. Data derived from Benchimol et al. and Nguyen et al. (28, 35).
Studies evaluating the risk of surgery in Canadian children with Crohn’s disease estimate that surgical rates are lower in pediatric-onset disease. The one-, five-, and ten-year risks of requiring an intestinal resection are between 6% to 9%, 12% to 23%, and 15% to 36%, respectively (28, 29). The lower risk of surgery in childhood-onset disease is likely due a much lower rate of early fibrostenotic or penetrating disease among children as compared with adults (46). As with adults, the rates of surgery for children with Crohn’s have decreased significantly over the past twenty years (26, 29).
Of patients hospitalized for Crohn’s disease in Canada, 16% underwent an intestinal resection during their first hospitalization (31). Another study found that 20% of patients admitted to Canadian academic centres between 2011 and 2013 underwent an intestinal resection during hospitalization, although there were significant differences in surgery rates across centres (range 9% to 36%) (41). As with ulcerative colitis, patients who underwent emergency surgery had higher mortality rates than those who underwent elective surgery (3.6% versus 0.6%) (42).
COST OF PRESCRIPTION DRUGS
In the post-biologic era, prescription drugs account for between 30% and 70% of IBD-related health care costs internationally (see Table 1) (11, 13, 16, 18, 19). Before the introduction of biologic therapies to treat IBD, prescription drugs accounted for less than 25% of costs while hospitalizations accounted for more than 50% of direct costs of IBD care (47, 48). Data from Manitoba indicate that prescription drug costs now account for 20% of direct health care costs in newly diagnosed IBD patients and 45% of costs in patients with long-standing disease (5). Therefore, studies evaluating the direct costs of IBD care in the pre-biologic era have little relevance to the present-day circumstance.
Prescription drug costs have continued to rise, relating to the increased use of anti-TNF biologic agents (infliximab, adalimumab, golimumab) as well as the introduction of additional biologic therapies into the marketplace (ustekinumab, vedolizumab). In 2016, seven out of the top ten selling drugs in Canada were biologic drugs (49). Across all approved indications, Canadian sales of immunobiologic drugs has nearly doubled since 2010, reaching $2.2 billion in 2015 (10.3% of the Canadian pharmaceutical market share). This represents a considerably higher share of the pharmaceutical market than what is observed in France, Germany, Italy, Switzerland, the United Kingdom, and the United States (which range from 4.1% to 7.7%) (49). While rising drug costs have been partly offset by fewer IBD-related surgeries (33, 45), likely due to improved disease control, the introduction of biologics has led to a substantial net increase in overall costs of IBD care (20, 50).
An audit of a Canadian national drug claims database (51) in 2012 estimated that public and private drug claims for the year 2011, specific to IBD indications, amounted to $460 million, roughly 84% of which was for the biologic drugs infliximab and adalimumab. There were important differences observed in costs across the country, with per-capita drug costs twice as high in some provinces compared with others. There was also a threefold difference among provinces in the percentage of drug costs that were covered by public plans versus private plans (52).
Based on population-level health administrative data from Manitoba, the mean and median direct costs of IBD care, including prescription pharmaceuticals (imputing drug costs covered by private insurers), hospitalizations (day surgery and inpatient), and physician office visits, were more than two times greater among IBD patients than age- and sex-matched controls ($3896 vs $1826 for mean, $1562 vs $448 for median) for the 2005/2006 fiscal year (5). Prescription drug use accounted for 42% and 37% of total direct costs in IBD patients and controls respectively (see Table 1). In an updated study from this group using population-level data spanning 2004 to 2016, the mean total drug costs and health care utilization costs (including drug costs) for persons with IBD were $1861 CAD and $10,206, respectively, in the year before initiation of anti-TNF therapy and $37,448 and $44,786, respectively, in the year following initiation of anti-TNF therapy (see Table 1) (8). Mean annual hospitalization costs decreased by 12% in the year following anti-TNF initiation, from $6419 to $5627 per person. Similar decreases in inpatient care costs have been observed in Alberta (decreasing from $2715 to $968 in the year before and after infliximab initiation) (7), the United States (12, 53), and the United Kingdom (see Table 1) (20, 21). Outpatient costs appear to be similar before and after treatment with infliximab (12, 20, 21, 53). Overall, 14.2% of adults with Crohn’s disease, 4.1% of adults with ulcerative colitis, and 50% of children are using the anti-TNF biologic agents infliximab and adalimumab (54, 55).
The introduction of biosimilar anti-TNF agents to the marketplace may reduce the cost of treating patients with IBD. Inflectra, the first biosimilar infliximab, was approved for use in Crohn’s disease and ulcerative colitis in Canada on June 14, 2016, and is roughly one-half the cost of the originator infliximab (Remicade) (49, 56). Additional biosimilar infliximab agents and biosimilar adalimumab agents are being developed, which may bend the cost curve even further (57). In 2016, Canadian sales of infliximab (Remicade) and adalimumab (Humira) were roughly $1 billion and $650 million, respectively (56). It is estimated that if even 10% of infliximab usage in Canada was biosimilar infliximab (across all indications), it would translate to more than a $41 million reduction in drug expenditures (49).
Market penetration of biosimilar infliximab has been slow in Canada, reaching just 1% of the total infliximab market share by the end of 2016 (56). However, as a cost savings measure, some provinces now mandate use of biosimilar infliximab for new infliximab starts among persons with IBD requiring provincial drug coverage, which could improve market penetration. Moreover, many private drug insurance companies are starting to follow this example. Unlike in some European nations, forced switching from originator to biosimilar infliximab has not been mandated in Canada, which has also impacted market penetration of biosimilar infliximab.
END-OF-LIFE COSTS
The end-of-life period represents a time of rapidly rising health care demands (58). Health care services provided in the last year of life account for close to 10% ($4.7 billion) of the annual Ontario health care budget, even though decedents constitute only 0.67% of the Canadian population (58, 59). Therefore, the end-of-life period is an important target for the development of cost-effective strategies for health care delivery.
In a population-based cohort study of Ontario decedents between 2010 and 2013, IBD decedents (N = 2214) had $7210 CAD (95% CI, $5005 to $9464) higher adjusted per-patient cost in the last year of life as compared with non-IBD decedents (N = 262,540), including most other decedents with chronic disease (fifteen of the sixteen chronic diseases studied; only renal disease was associated with greater costs).The cost differential was largely related to hospitalizations cost (76% of differential), particularly in the last ninety days of life (60). Improving end-of-life care for IBD patients outside of the hospital setting could substantially reduce health care system costs while improving quality of life.
Conclusions
Inflammatory bowel disease is a costly disease. In Canada, the direct cost of caring for people living with IBD is estimated to be at least $1.28 billion CAD in 2018 and possibly higher than $2 billion CAD based on some estimates. The primary drivers of direct health care costs are hospitalizations and pharmaceuticals. Over time, the source of direct cost of care is shifting from hospitalizations and surgeries to pharmaceutical treatments. The introduction of biologics has improved the care of IBD but at a significant expense to the health care system. Biosimilars will have an impact on reducing the cost of biologics. However, the absolute cost of pharmaceuticals will rise due to an increasing prevalence of IBD and greater penetration of biologics in the treatment of patients with IBD. Several factors may influence health care utilization and the direct costs of caring for IBD. Patients cared for by gastroenterologists have better outcomes, including lower risk of surgery and hospitalization. Improving timely access to gastroenterologist care for Canadians living in rural and underserviced areas who currently are less likely to see gastroenterologists may reduce risks of requiring surgery and emergency care among ambulatory IBD patients.
Acknowledgements
The authors would like to thank Shabnaz Siddiq who acted as a coordinator for this work, and Joseph Windsor and Fox Underwood who edited the articles. This work was funded by Crohn’s and Colitis Canada. EB and GN were supported by New Investigator Awards from CIHR, Crohn’s and Colitis Canada, and the Canadian Association of Gastroenterology. EB was also supported by the Career Enhancement Program from the Canadian Child Health Clinician Scientist Program. MEK was supported by a post-doctoral fellowship award from CIHR, Crohn’s and Colitis Canada, and the Canadian Association of Gastroenterology. CB was supported in part by the Bingham Chair in Gastroenterology. GN and GK were CIHR Embedded Clinician Research Chairs.
Supplement sponsorship. This article appears as part of the supplement “The Impact of Inflammatory Bowel Disease in Canada 2018,” sponsored by AbbVie Corporation and co-sponsored by Crohn’s and Colitis Canada.
References
- 1. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110(9):1324–38. [DOI] [PubMed] [Google Scholar]
- 2. Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): A cluster randomised controlled trial. Lancet (London, England) 2015;386(10006):1825–34. [DOI] [PubMed] [Google Scholar]
- 3. Hirschmann S, Neurath MF. Top-down approach to biological therapy of Crohn’s disease. Expert Opin Biol Ther 2017;17(3):285–93. [DOI] [PubMed] [Google Scholar]
- 4. Coward S, Heitman SJ, Clement F, et al. Ulcerative colitis-associated hospitalization costs: A population-based study. Can J Gastroenterol Hepatol 2015;29(7):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein CN, Longobardi T, Finlayson G, et al. Direct medical cost of managing IBD patients: A Canadian population-based study. Inflamm Bowel Dis 2012;18(8):1498–508. [DOI] [PubMed] [Google Scholar]
- 6. Dan A, Boutros M, Nedjar H, et al. Cost of ulcerative colitis in Quebec, Canada: A retrospective cohort study. Inflamm Bowel Dis 2017;23(8):1262–71. [DOI] [PubMed] [Google Scholar]
- 7. Loomes DE, Teshima C, Jacobs P, et al. Health care resource use and costs for Crohn’s disease before and after infliximab therapy. Can J Gastroenterol 2011;25(9):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Targownik LE, Witt JC, Singh H, et al. Direct costs of care among patients with inflammatory bowel disease before and after initiation of anti-TNF therapy. Gastroenterology 2018;154(6):S-833. [Google Scholar]
- 9. Gleason PP, Alexander GC, Starner CI, et al. Health plan utilization and costs of specialty drugs with 4 chronic conditions. J Manag Care Pharm 2013;19(7):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karve S, Candrilli S, Kappelman MD, et al. Healthcare utilization and comorbidity burden among children and young adults in the United States with systemic lupus erythematosus or inflammatory bowel disease. J Pediatr 2012;161(4):662–70.e662. [DOI] [PubMed] [Google Scholar]
- 11. Park KT, Colletti RB, Rubin DT, et al. Health insurance paid costs and drivers of costs for patients with Crohn’s disease in the United States. Am J Gastroenterol 2016;111(1):15–23. [DOI] [PubMed] [Google Scholar]
- 12. Wan GJ, Kozma CM, Slaton TL, et al. Inflammatory bowel disease: Healthcare costs for patients who are adherent or non-adherent with infliximab therapy. J Med Econ 2014;17(6):384–93. [DOI] [PubMed] [Google Scholar]
- 13. Niewiadomski O, Studd C, Hair C, et al. Health care cost analysis in a population-based inception cohort of inflammatory bowel disease patients in the first year of diagnosis. J Crohns Colitis 2015;9(11):988–96. [DOI] [PubMed] [Google Scholar]
- 14. Gibson PR, Vaizey C, Black CM, et al. Relationship between disease severity and quality of life and assessment of health care utilization and cost for ulcerative colitis in Australia: A cross-sectional, observational study. J Crohns Colitis 2014;8(7):598–606. [DOI] [PubMed] [Google Scholar]
- 15. Odes S, Vardi H, Friger M, et al. Clinical and economic outcomes in a population-based European cohort of 948 ulcerative colitis and Crohn’s disease patients by Markov analysis. Aliment Pharmacol Ther 2010;31(7):735–44. [DOI] [PubMed] [Google Scholar]
- 16. Burisch J, Vardi H, Pedersen N, et al. Costs and resource utilization for diagnosis and treatment during the initial year in a European inflammatory bowel disease inception cohort: An ECCO-EpiCom Study. Inflamm Bowel Dis 2015;21(1):121–31. [DOI] [PubMed] [Google Scholar]
- 17. Severs M, Petersen RE, Siersema PD, et al. Self-reported health care utilization of patients with inflammatory bowel disease correlates perfectly with medical records. Inflamm Bowel Dis 2016;22(3):688–93. [DOI] [PubMed] [Google Scholar]
- 18. van der Valk ME, Mangen MJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: Results from the COIN study. Gut 2014;63(1):72–9. [DOI] [PubMed] [Google Scholar]
- 19. Aldeguer X, Sicras-Mainar A. Costs of ulcerative colitis from a societal perspective in a regional health care area in Spain: A database study. Gastroenterol Hepatol 2016;39(1):9–19. [DOI] [PubMed] [Google Scholar]
- 20. Sprakes MB, Ford AC, Suares NC, et al. Costs of care for Crohn’s disease following the introduction of infliximab: A single-centre UK experience. Aliment Pharmacol Ther 2010;32(11–12):1357–63. [DOI] [PubMed] [Google Scholar]
- 21. Lindsay JO, Chipperfield R, Giles A, et al. A UK retrospective observational study of clinical outcomes and healthcare resource utilisation of infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther 2013;38(1):52–61. [DOI] [PubMed] [Google Scholar]
- 22. Benchimol EI, Manuel DG, Mojaverian N, et al. Health services utilization, specialist care, and time to diagnosis with inflammatory bowel disease in immigrants to Ontario, Canada: A population-based cohort study. Inflamm Bowel Dis 2016;22(10):2482–90. [DOI] [PubMed] [Google Scholar]
- 23. Nguyen GC, Nugent Z, Shaw S, et al. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011;141(1):90–7. [DOI] [PubMed] [Google Scholar]
- 24. Nugent Z, Singh H, Targownik LE, et al. Predictors of emergency department use by persons with inflammatory bowel diseases: A population-based study. Inflamm Bowel Dis 2016;22(12):2907–16. [DOI] [PubMed] [Google Scholar]
- 25. Murthy SK, Steinhart AH, Tinmouth J, et al. Impact of gastroenterologist care on health outcomes of hospitalised ulcerative colitis patients. Gut 2012;61(10):1410–6. [DOI] [PubMed] [Google Scholar]
- 26. Benchimol EI, Guttmann A, To T, et al. Changes to surgical and hospitalization rates of pediatric inflammatory bowel disease in Ontario, Canada (1994–2007). Inflamm Bowel Dis 2011;17(10):2153–61. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen GC, Sheng L, Benchimol EI. Health care utilization in elderly onset inflammatory bowel disease: A population-based study. Inflamm Bowel Dis 2015;21(4):777–82. [DOI] [PubMed] [Google Scholar]
- 28. Benchimol EI, Mack DR, Nguyen GC, et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 2014;147(4):803–13 e807; quiz e814-805. [DOI] [PubMed] [Google Scholar]
- 29. Singh H, Nugent Z, Targownik LE, et al. Health care use by a population-based cohort of children with inflammatory bowel disease. Clin Gastroenterol Hepatol 2015;13(7):1302–9.e1303. [DOI] [PubMed] [Google Scholar]
- 30. Longobardi T, Walker JR, Graff LA, et al. Health service utilization in IBD: Comparison of self-report and administrative data. BMC Health Serv Res 2011;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen GC, Bollegala N, Chong CA. Factors associated with readmissions and outcomes of patients hospitalized for inflammatory bowel disease. Clin Gastroenterol Hepatol 2014;12(11):1897–904.e1891. [DOI] [PubMed] [Google Scholar]
- 32. Marrie RA, Garland A, Peschken CA, et al. Increased incidence of critical illness among patients with inflammatory bowel disease: A population-based study. Clin Gastroenterol Hepatol 2014;12(12):2063–70.e2064. [DOI] [PubMed] [Google Scholar]
- 33. Frolkis AD, Dykeman J, Negron ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145(5):996–1006. [DOI] [PubMed] [Google Scholar]
- 34. Targownik LE, Singh H, Nugent Z, et al. The epidemiology of colectomy in ulcerative colitis: Results from a population-based cohort. Am J Gastroenterol 2012;107(8):1228–35. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen GC, Bernstein CN, Benchimol EI. Risk of surgery and mortality in elderly-onset inflammatory bowel disease: A population-based cohort study. Inflamm Bowel Dis 2017;23(2):218–23. [DOI] [PubMed] [Google Scholar]
- 36. Abou Khalil M, Boutros M, Nedjar H, et al. Incidence rates and predictors of colectomy for ulcerative colitis in the era of biologics: Results from a provincial database. J Gastrointest Surg 2018;22(1):124–32. [DOI] [PubMed] [Google Scholar]
- 37. Moore SE, McGrail KM, Peterson S, et al. Infliximab in ulcerative colitis: The impact of preoperative treatment on rates of colectomy and prescribing practices in the province of British Columbia, Canada. Dis Colon Rectum 2014;57:83–90. [DOI] [PubMed] [Google Scholar]
- 38. Reich KM, Chang HJ, Rezaie A, et al. The incidence rate of colectomy for medically refractory ulcerative colitis has declined in parallel with increasing anti-TNF use: A time-trend study. Aliment Pharmacol Ther 2014;40(6):629–38. [DOI] [PubMed] [Google Scholar]
- 39. Kaplan GG, Seow CH, Ghosh S, et al. Decreasing colectomy rates for ulcerative colitis: A population-based time trend study. Am J Gastroenterol 2012;107(12):1879–87. [DOI] [PubMed] [Google Scholar]
- 40. Al-Darmaki A, Hubbard J, Seow CH, et al. Clinical predictors of the risk of early colectomy in ulcerative colitis: A population-based study. Inflamm Bowel Dis 2017;23(8):1272–7. [DOI] [PubMed] [Google Scholar]
- 41. Nguyen GC, Murthy SK, Bressler B, et al. Quality of care and outcomes among hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis 2017;23(5):695–701. [DOI] [PubMed] [Google Scholar]
- 42. Singh S, Al-Darmaki A, Frolkis AD, et al. Postoperative mortality among patients with inflammatory bowel diseases: A systematic review and meta-analysis of population-based studies. Gastroenterology 2015;149(4):928–37. [DOI] [PubMed] [Google Scholar]
- 43. Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: A systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109(11):1739–48. [DOI] [PubMed] [Google Scholar]
- 44. Benchimol EI, Bernstein CN, Nguyen GC, et al. Disparities in the care of rural and urban Canadians with inflammatory bowel disease: A population-based study (abstract). J Can Assoc Gastroenterol 2018;1(Suppl 2):51–2. [Google Scholar]
- 45. Ma C, Moran GW, Benchimol EI, et al. Surgical rates for Crohn’s disease are decreasing: A population-based time trend analysis and validation study. Am J Gastroenterol 2017;112(12):1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135(4):1114–22. [DOI] [PubMed] [Google Scholar]
- 47. Bassi A, Dodd S, Williamson P, et al. Cost of illness of inflammatory bowel disease in the UK: A single centre retrospective study. Gut 2004;53(10):1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blomqvist P, Ekbom A. Inflammatory bowel diseases: Health care and costs in Sweden in 1994. Scand J Gastroenterol 1997;32(11):1134–9. [DOI] [PubMed] [Google Scholar]
- 49. Patented Medicine Prices Review Board. News: The most expensive biologic treatments for chronic inflammatory disease dominate the Canadian market. 2016; <http://www.pmprb-cepmb.gc.ca/news.asp?a=view&id=188> (Accessed March 16, 2018).
- 50. Pillai N, Dusheiko M, Burnand B, et al. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS One 2017;12(10):e0185500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. IMS Brogan Inc. Pharmastat Prescription Database. Ottawa: IMG Brogan Solutions, 2012. [Google Scholar]
- 52. Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: A Canadian burden of illness review. Can J Gastroenterol 2012;26(11):811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Waters HC, Vanderpoel JE, Nejadnik B, et al. Resource utilization before and during infliximab therapy in patients with inflammatory bowel disease. J Med Econ 2012;15(1):45–52. [DOI] [PubMed] [Google Scholar]
- 54. Church P, Walters T, Benchimol E, et al. Steroid-free remission among canadian pediatric inflammatory bowel disease patients. Can J Gastroenterol Hepatol 2016;2016(4792898):7–8. [Google Scholar]
- 55. Targownik LE, Tennakoon A, Leung S, et al. Temporal trends in initiation of therapy with tumor necrosis factor antagonists for patients with inflammatory bowel disease: A population-based analysis. Clin Gastroenterol Hepatol 2017;15(7):1061–70 e1061. [DOI] [PubMed] [Google Scholar]
- 56. Lungu E, Warwick G. Potential Savings from Biosimilars in Canada Patented Medicine Prices Review Board presentation in CADTH Symposium 2017. [Presentation] 2017. <https://www.cadth.ca/sites/default/files/symp-2017/presentations/april24-2017/Concurrent-Session-B4-Gary-Warwick.pdf> (Accessed March 16, 2018). [Google Scholar]
- 57. Murray S. New data on biosimilars of the big three anti-TNF drugs presented at EULAR 2016. Pharmafile. 2016. <http://www.pharmafile.com/news/505016/new-data-biosimilars-big-three-anti-tnf-drugs-presented-eular-2016> (Accessed June 8, 2016). [Google Scholar]
- 58. Tanuseputro P, Wodchis WP, Fowler R, et al. The health care cost of dying: A population-based retrospective cohort study of the last year of life in Ontario, Canada. PLoS One 2015;10(3):e0121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drummond D; Commission on the Reform of Ontario’s Public Services Public Services for Ontarians: A Path to Sustainability and Excellence. Toronto: Queen’s Printer for Ontario, 2012. [Google Scholar]
- 60. Murthy SK, James PD, Antonova L, et al. High end of life health care costs and hospitalization burden in inflammatory bowel disease patients: A population-based study. PLoS One 2017;12(5):e0177211. [DOI] [PMC free article] [PubMed] [Google Scholar]