This cohort study compares cancer detection rate by patient age and breast density as well as cancer detection and prognosis outcomes when using digital breast tomosynthesis vs digital mammography alone for breast cancer screening.
Key Points
Question
Is breast cancer screening with digital breast tomosynthesis (DBT) associated with improved cancer detection rates across all age and breast density groups compared with digital mammography, and are cancer types detected with DBT different from those detected by digital mammography alone?
Findings
In this cohort study of 50 971 breast cancer screening examinations using DBT and 129 369 screening examinations using digital mammography, DBT was associated with increased specificity and increased cancer detection across all age and breast density groups. Invasive cancers detected by DBT were more likely to be smaller and node negative compared with cancers detected by digital mammography, particularly in women aged 40 to 49 years.
Meaning
The findings suggest that screening examinations using DBT detect smaller, node-negative invasive cancers compared with digital mammography, especially among women aged 40 to 49 years; routine mammographic screening may have a favorable risk-benefit ratio for this age group.
Abstract
Importance
Breast cancer screening examinations using digital breast tomosynthesis (DBT) has been shown to be associated with decreased false-positive test results and increased breast cancer detection compared with digital mammography (DM). Little is known regarding the size and stage of breast cancer types detected and their association with age and breast density.
Objective
To determine whether screening examinations using DBT detect breast cancers that are associated with an improved prognosis and to compare the detection rates by patient age and breast density.
Design, Setting, and Participants
This retrospective analysis of prospective cohort data from 3 research centers in the Population-based Research Optimizing Screening Through Personalized Regimens (PROSPR) consortium included data of women aged 40 to 74 years who underwent screening examinations using DM and DBT from January 1, 2011, through September 30, 2014. Statistical analysis was performed from November 8, 2017, to August 14, 2018.
Exposures
Use of DBT as a supplement to DM at breast cancer screening examination.
Main Outcomes and Measures
Recall rate, cancer detection rate, positive predictive value, biopsy rate, and distribution of invasive cancer subtypes.
Results
Among 96 269 women (mean [SD] patient age for all examinations, 55.9 [9.0] years), patient age was 56.4 (9.0) years for DM and 54.6 (8.9) years for DBT. Of 180 340 breast cancer screening examinations, 129 369 examinations (71.7%) used DM and 50 971 examinations (28.3%) used DBT. Screening examination with DBT (73 of 99 women [73.7%]) was associated with the detection of smaller, more often node-negative, HER2–negative, invasive cancers compared with DM (276 of 422 women [65.4%]). Screening examination with DBT was also associated with lower recall (odds ratio, 0.64; 95% CI, 0.57-0.72; P < .001) and higher cancer detection (odds ratio, 1.41; 95% CI, 1.05-1.89; P = .02) compared with DM for all age groups even when stratified by breast density. The largest increase in cancer detection rate and the greatest shift toward smaller, node-negative invasive cancers detected with DBT was for women aged 40 to 49 years. For women aged 40 to 49 years with nondense breasts, the cancer detection rate for examinations using DBT was 1.70 per 1000 women higher compared with the rate using DM; for women with dense breasts, the cancer detection rate was 2.27 per 1000 women higher for DBT. For these younger women, screening with DBT was associated with only 7 of 28 breast cancers (25.0%) categorized as poor prognosis compared with 19 of 47 breast cancers (40.4%) when screening with DM.
Conclusions and Relevance
The findings suggest that screening with DBT is associated with increased specificity and an increased proportion of breast cancers detected with better prognosis compared with DM. In the subgroup of women aged 40 to 49 years, routine DBT screening may have a favorable risk-benefit ratio.
Introduction
Digital breast tomosynthesis (DBT) is often considered the new, better mammogram based on observed increases in specificity and breast cancer detection compared with digital mammography (DM) alone.1,2 However, most of the published studies about DBT, whether from prospective trials or observational studies,3,4,5 use data from first- or prevalent-round screening rather than incident-round screening in which breast cancer detection and recall rates are expected to be lower. In addition, only some of the studies contain patient-level data such as age and breast density,6,7,8,9,10,11 and even fewer contain information about molecular subtypes for both screen-detected and interval cancers.11,12,13,14,15,16 Studies including molecular subtypes are limited but suggest that breast cancers detected by DBT are smaller, less aggressive estrogen receptor–positive cancers compared with cancers detected by DM.15,16 Patient-level factors and tumor subtype data coupled with screening performance and outcome metrics are necessary to estimate the long-term outcomes of DBT, especially for women aged 40 to 49 years, for whom routine screening remains controversial. These data incorporated into simulation modeling approaches, such as those used by the Cancer Intervention and Surveillance Modeling Network consortium,17 will allow population-level estimates of both long-term outcomes and cost-effectiveness of DBT screening.
As a complement to observational studies that may be influenced by different underlying risk profiles for women receiving DBT rather than DM, the National Cancer Institute–funded Tomosynthesis Mammographic Imaging Screening Trial (TMIST) began accruing patients in July 2017. This trial will compare screening examination outcomes during a 4.5-year period from approximately 165 000 women aged 45 to 74 years who are randomly assigned to either DM or DBT.18 Rather than comparing only the traditional outcome measures of breast cancer detection, the primary aim of TMIST is a comparison of the number of advanced cancers in each arm of the screening trial. The hypothesis is that screening with DBT will eventually decrease the number of advanced cancers compared with DM. Data on patient age, cancer risk, and breast density, as well as genomic analyses of screen-detected and interval cancers in the TMIST, will hopefully aid in the development of new, more personalized algorithms for breast cancer screening.19
The objectives of our study were to compare outcomes of breast cancer screening examinations with DM vs DBT and to assess whether these outcomes vary by age and breast density. For breast cancers detected from screening, we also evaluated cancer characteristics according to TMIST definitions. These data are also being used by the Cancer Intervention and Surveillance Modeling Network to model and project population-level DBT screening examination outcomes and cost-effectiveness.
Methods
Study Setting
This study was conducted as part of the National Cancer Institute–funded Population-based Research Optimizing Screening Through Personalized Regimens (PROSPR) consortium. The overall aim of PROSPR is to conduct multisite, transdisciplinary research to evaluate and improve cancer screening processes. This study included 3 PROSPR research centers evaluating breast cancer screening examinations—University of Pennsylvania, Philadelphia, an integrated health care delivery system; University of Vermont, Burlington, a statewide breast cancer surveillance system; and Geisel School of Medicine at Dartmouth and the Dartmouth-Hitchcock Health System, Lebanon, New Hampshire, in conjunction with Brigham and Women’s Hospital, Boston, Massachusetts. The institutional review boards at each research center and the PROSPR Statistical Coordinating Center waived individual consent for deidentified data and approved all activities.
Data Collection
Data from the 3 centers were pooled in the PROSPR central repository; a full description of the data collection processes has been previously reported.9 Screening examination data from women aged 40 to 74 years with no known history of breast cancer were included from January 1, 2011, through September 30, 2014. The precise period varied by research center because this was a period of transition from routine DM to DBT use for some of the facilities. All breast examinations were bilateral with an indication of screening, and the women had no other breast imaging within 3 months before the initial (index) screening. All DBT examinations were performed with the same type of equipment (Selenia Dimensions, Hologic), but DM equipment varied by site and was not recorded. Any subsequent diagnostic imaging or short-term follow-up assessments were included as part of the screening episode starting from index screening. To ensure that radiologists (E.F.C. and S.D.H.) had experience with both modalities, we included only examinations interpreted by radiologists who had interpreted at least 50 screening examinations of each type (DM and DBT). We excluded examinations that did not record breast density. From 96 269 women, a total of 50 971 DBT and 129 369 DM screening examinations met these criteria (43 845 women contributed 1 examination, 27 156 women contributed 2 examinations, and 25 268 women contributed 3 or more examinations). In all, 47 radiologists interpreted the examinations with a mean of 3837 screenings per reader (range, 211-16 386 screenings per reader). Breast density was reported using the fourth edition Breast Imaging Reporting and Data System (BI-RADS)20 density categories (1, almost entirely fat; 2, scattered fibroglandular densities; 3, heterogeneously dense; and 4, extremely dense) and were collapsed into nondense (1 or 2) and dense (3 or 4). A first examination was defined as the first known screening examination for a woman with no previous breast imaging records available in PROSPR data and no self-report of previous breast imaging. All other examinations were considered to be subsequent examinations. Information about benign biopsy results was obtained from pathology reports and electronic medical records. Breast cancer clinical or pathologic characteristics were ascertained from pathology reports or from cancer registries covering the geographic location of the facilities and the patients.
Outcome Measures
We evaluated recall rate from all screening mammograms using a definition of a positive examination as BI-RADS initial assessment category 0, 3, 4, or 5. All other outcome measures were restricted to 126 906 screening mammograms with 1 year of follow-up (101 973 DM and 24 933 DBT) to assess all cancer outcomes. The following outcomes used all screenings with 1-year follow-up as the denominator: (1) biopsy rate per 1000 examinations (number of examinations with biopsies within 1 year); (2) cancer rate per 1000 examinations (number of examinations with cancer diagnosed within 1 year of index screening); and (3) cancer detection rate per 1000 examinations (number of positive screening results with a diagnosis of cancer within 1 year). False-negative rate was not calculated because of limited sample size. We evaluated sensitivity and specificity using standard definitions.20 Positive predictive value–1 was defined as the number of examinations with cancer detected among those with positive screenings, and positive predictive value–3 was defined as the number of examinations with cancer detected among those with biopsies performed. Cancers were classified as invasive or ductal carcinoma in situ based on cancer registry data. Invasive disease was further classified by hormone receptor status (positive or negative), HER2 status (positive or negative), nodal status (positive or negative), tumor size (<1 cm, 1-2 cm, or >2 cm), and tumor grade (1-3). Poor prognosis cancers (called advanced in TMIST) were defined as those with any of the following: metastases, positive nodes and/or invasive tumor size greater than or equal to 2 cm and/or estrogen receptor–negative and progesterone receptor–negative and/or HER2-positive tissue equal to 1 cm or larger.
Statistical Analysis
Statistical analysis was performed from November 8, 2017, to August 14, 2018. We calculated estimates of outcomes by age, breast density, and first vs subsequent examination by mammogram screening type (DM vs DBT). We also considered the statistical significance of mammogram screening type in a model of each outcome adjusting for the 3 research centers, breast density, and age group. Thus, known sources of variation were controlled for before assessing the association of screening modality. For recall, we adjusted for first vs subsequent mammogram examination, but data were too sparse to include this term in models of true-positive and false-negative outcomes. Testing was done by logistic regression using a generalized estimating equation approach adjusting for clustering by radiologists within each research center. We used standard logistic regression to estimate odds ratios (ORs) and 95% CIs for comparing breast cancer features because clustering by the radiologist was unlikely. For all analyses, SAS, version 9.4 (SAS Institute Inc) was used, and 2-sided α = .05 was used for statistical significance.
Results
In a previous report,9 demographic characteristics of the population screened were shown. A description of important characteristics of women being screened by each modality are described in eTable 1 in the Supplement. Among 96 269 women, the mean (SD) patient age was 55.9 (9.0) years for all examinations; patient age was 56.4 (9.0) years for DM and was 54.6 (8.9) years for DBT. Of 180 340 examinations, 129 369 examinations used DM (71.7%) and 50 971 examinations used DBT (28.3%). In this population, use of DBT was slightly more common among younger women, women with dense breasts, and those undergoing their first screening. These factors were adjusted when comparing the 2 modalities.
Consistently, younger women, women with dense breasts, and those at first screening had higher recall, but recall was lower for DBT compared with DM (Table 1). The original unadjusted rates are shown in Table 1 and Table 2. The generalized estimating equation analysis confirmed the lower recall rate for DBT (OR, 0.64; 95% CI, 0.57-0.72; P < .001) after adjustment for age group, breast density, first or subsequent screening, and research center. This pattern remained within every age group with similar ORs across age groups. Screening examinations among women with nondense breasts had lower recall for DBT vs DM (OR, 0.62; 95% CI, 0.54-0.72; P < .001), as did those among women with dense breasts (OR, 0.65; 95% CI, 0.58-0.73; P < .001).
Table 1. Recall Rates by Age, Breast Density, First and Subsequent Mammography Screening, and Mammography Typea.
| Mammography Type, Age | Overall | Recall Rate, % (No. of Mammography Screening Examinations) | |||||
|---|---|---|---|---|---|---|---|
| First Screening Examination | Subsequent Screening Examination | ||||||
| Overall | Nondense Breasts | Dense Breasts | Overall | Nondense Breasts | Dense Breasts | ||
| Digital mammographyb | |||||||
| 40-49 y | 16.3 | 28.5 | 28.5 (2228) | 28.6 (2396) | 14.4 | 12.8 (14 417) | 15.9 (14 043) |
| 50-64 y | 9.8 | 20.1 | 17.8 (2049) | 24.7 (1069) | 9.3 | 8.8 (44 696) | 10.6 (20 417) |
| 65-74 y | 8.4 | 16.3 | 16.5 (735) | 16.0 (257) | 8.1 | 7.8 (21 150) | 9.2 (5912) |
| Total breast tomosynthesisb | |||||||
| 40-49 y | 11.7 | 17.1 | 15.9 (1617) | 18.5 (1604) | 14.4 | 9.0 (6410) | 11.7 (7133) |
| 50-64 y | 7.6 | 14.8 | 15.8 (967) | 13.1 (578) | 7.1 | 6.3 (15 534) | 8.6 (8718) |
| 65-74 y | 6.2 | 13.9 | 12.4 (298) | 17.5 (120) | 5.8 | 5.5 (6013) | 6.8 (1917) |
Breast density was based on fourth edition Breast Imaging Reporting and Data System (BI-RADS)20 categories (1, almost entirely fat; 2, scattered fibroglandular densities; 3, heterogeneously dense; and 4, extremely dense) collapsed into nondense (BI-RADS 1 or 2) and dense (BI-RADS 3 or 4).
The overall recall rate for digital mammography was 11.2% and for digital breast tomosynthesis was 8.7%.
Table 2. Outcomes by Age, Breast Density, and Mammography Typea.
| Mammography Type, Age, Breast Density | Patients With 1 y of Follow-up, No. | Biopsy Rate, Per 1000 Examinations | Cancers, No. | Cancer Rate, per 1000 Examinations | Cancer Detection Rate, per 1000 Examinations | Sensitivity, % | Specificity, % | Positive Predictive Value–1, %b | Positive Predictive Value–3, %b |
|---|---|---|---|---|---|---|---|---|---|
| Digital Mammography | |||||||||
| 40-49 y | |||||||||
| Nondense | 13 634 | 17.68 | 39 | 2.86 | 2.71 | 94.9 | 84.9 | 1.78 | 15.35 |
| Dense | 13 655 | 29.00 | 51 | 3.73 | 2.93 | 78.4 | 82.3 | 1.64 | 11.62 |
| 50-64 y | |||||||||
| Nondense | 36 729 | 14.40 | 146 | 3.98 | 3.68 | 92.5 | 91.1 | 3.97 | 26.47 |
| Dense | 17 065 | 21.68 | 100 | 5.86 | 5.51 | 94.0 | 89.1 | 4.82 | 26.49 |
| 65-74 y | |||||||||
| Nondense | 16 301 | 16.99 | 116 | 7.12 | 6.75 | 94.8 | 92.2 | 8.03 | 41.16 |
| Dense | 4589 | 26.15 | 41 | 8.93 | 7.63 | 85.4 | 90.6 | 7.53 | 33.33 |
| All | 101 973 | 18.96 | 493 | 4.83 | 4.42 | 91.5 | 88.9 | 3.85 | 24.57 |
| Digital Breast Tomosynthesis | |||||||||
| 40-49 y | |||||||||
| Nondense | 4305 | 28.34 | 19 | 4.41 | 4.41 | 100 | 89.6 | 4.09 | 15.57 |
| Dense | 4037 | 35.42 | 28 | 6.94 | 5.20 | 75.0 | 86.0 | 3.60 | 19.58 |
| 50-64 y | |||||||||
| Nondense | 8194 | 23.92 | 35 | 4.27 | 4.03 | 94.3 | 93.2 | 5.61 | 17.35 |
| Dense | 4345 | 28.31 | 36 | 8.29 | 7.59 | 91.7 | 94.4 | 8.25 | 27.64 |
| 65-74 y | |||||||||
| Nondense | 3113 | 27.95 | 33 | 10.60 | 9.64 | 90.9 | 94.4 | 14.78 | 36.78 |
| Dense | 939 | 30.88 | 9 | 9.58 | 9.58 | 100 | 93.9 | 13.64 | 31.03 |
| All | 24 933 | 28.08 | 160 | 6.42 | 5.82 | 90.6 | 91.3 | 6.29 | 22.29 |
Breast density was based on fourth edition Breast Imaging Reporting and Data System (BI-RADS)20 categories (1, almost entirely fat; 2, scattered fibroglandular densities; 3, heterogeneously dense; and 4, extremely dense) collapsed into nondense (BI-RADS 1 or 2) and dense (BI-RADS 3 or 4).
Positive predictive value–1 was defined as the number of examinations with cancer detected among those with positive screenings, and positive predictive value–3 was defined as the number of examinations with cancer detected among those with biopsies performed.
The breast cancer detection rates were higher with DBT compared with DM (OR, 1.41; 95% CI, 1.05-1.89; P = .02) for all age groups. For women aged 40 to 49 years with nondense breasts, the cancer detection rate for examinations using DBT was 1.70 per 1000 women higher compared with the rate using DM; for women with dense breasts, the cancer detection rate was 2.27 per 1000 women higher for DBT. Use of DBT was associated with lower recall but also with higher cancer detection across all age groups. When stratified by women with nondense and dense breasts, the same pattern was observed (Table 2). For example, for subsequent examinations of nondense breasts, recall was consistently lower for DBT (ages 40-49 years [9.0%], 50-64 years [6.3%], and 65-74 years [5.5%]) than DM (40-49 y [12.8%], 50-64 y [8.8%], and 65-74 y [7.8%]). A similar pattern of consistently lower recall was observed for subsequent examinations of dense breasts (DBT: 40-49 y [11.7%], 50-64 y [8.6%], and 65-74 y [6.8%]; DM: 40-49 y [15.9%], 50-64 y [10.6%], and 65-74 y [9.2%]). Breast cancer detection rates (per 1000 examinations) were higher for nondense breasts screened with DBT (40-49 y [4.41], 50-64 y [4.03], and 65-74 y [9.64]) than DM (40-49 y [2.71], 50-64 y [3.68], and 65-74 y [6.75]). For dense breasts, the rates were similarly higher for DBT (40-49 y [5.20], 50-64 y [7.59], and 65-74 y [9.58]) than DM (40-49 y [2.93], 50-64 y [5.51], and 65-74 y [7.63]).
Overall breast cancer rates were higher in the population screened with DBT (OR, 1.47; 95% CI, 1.12-1.94; P = .01) compared with those screened with DM. This association was greatest among women aged 40 to 49 years, with higher cancer rates among women screened with DBT vs DM (OR, 1.70; 95% CI, 1.04-2.77; P = .03). In the 2 older age groups, overall cancer rates were more modestly increased for patients screened with DBT vs DM, but the differences were not statistically significant (50-64 years: OR, 1.33 [95% CI, 0.83-2.12], P = .23; 65-74 years: OR, 1.55 [95% CI, 0.87-2.74], P = .14). Among women with nondense breasts, those screened with DBT had higher cancer rates vs those screened with DM (OR, 1.55; 95% CI, 1.05-2.27; P = .03); a smaller difference was observed among women with dense breasts and was not statistically significant (OR, 1.39; 95% CI, 0.98-1.97; P = .06).
Because sensitivity was computed for cancer cases and specificity was computed for noncancer cases and although breast cancer detection rates for DBT were higher, sensitivity overall was slightly higher for DM (91.5% vs 90.6%); however, after adjustment for age and breast density, there was no significant difference (OR, 0.69; 95% CI, 0.38-1.24; P = .21). Screening examinations with DBT were associated with higher specificity (OR, 1.46; 95% CI, 1.30-165; P < .001) after adjustment for research center, age group, and breast density; DBT was similarly associated with higher specificity in every age group and at each level of breast density (all P < .001).
Positive predictive value–1 was greater for DBT than for DM (OR, 2.00; 95% CI, 1.47-2.72; P < .001), and positive predictive value–3 was nonsignificantly elevated favoring DBT (OR, 1.33; 95% CI, 0.99-1.78; P = .06) after adjustment for age, breast density, and research center (Table 2). In the entire population, the likelihood of women requiring a biopsy was increased for DBT (OR, 1.23; 95% CI, 1.08-1.40; P = .002) after adjustment for age group, breast density, and health system (Table 2). This finding differed little within each age group, although the difference was no longer significant because of smaller sample sizes. The increased biopsy rate with DBT was comparable among women with nondense breasts (OR, 1.29; 95% CI, 1.05-1.58; P = .01) and women with dense breasts (OR, 1.17; 95% CI, 1.00-1.37; P = .06).
Examination with DBT was associated with a higher proportion of screening-detected invasive breast cancers that were 1 cm or smaller and node negative (73 of 99 [73.7%]) compared with DM (276 of 422 [65.4%]) (eTable 2 in the Supplement). In addition, screening examinations using DM were associated with a higher frequency of cancers considered to have a poorer prognosis than those using DBT based on TMIST criteria (OR, 2.28; 95% CI, 1.15-4.52; P = .02). Among women aged 40 to 49 years, breast cancers detected by DBT were less often classified as advanced cancers by TMIST criteria compared with cancers detected by DM (7 of 28 [25.0%] vs 19 of 47 [40.4%]), although the difference was not statistically significant (P = .17).
Discussion
The controversy about routine mammographic screening examinations centers around the risk-benefit balance of the procedure. A screening examination that minimizes false-positive results while maintaining or even increasing the detection of clinically significant breast cancers compared with conventional 2-dimensional mammography may have a favorable risk-benefit ratio. Our results supported the evolving literature showing that screening with DBT simultaneously improves breast cancer detection while reducing false-positive recalls.1,2,3,4,5,6,7,8 Of importance, reductions in recalls for DBT were significant across all age groups and breast density categories in our study.
The ongoing controversy surrounding the age at which to begin routine mammographic screening is driven in part by the lower incidence of breast cancer and the lower detection rate (often because of increased breast density) in younger women. McCarthy et al,6 in a subgroup analysis of data presented here, showed that mammographic screening using DBT resulted in significant reductions in recalls across all age groups; however, the largest reduction for women aged 40 to 49 years was coupled with a statistically significant increase in cancer detection. This combination of recall reduction and increased cancer detection has been shown previously, even when correcting for the association of breast density with age.21
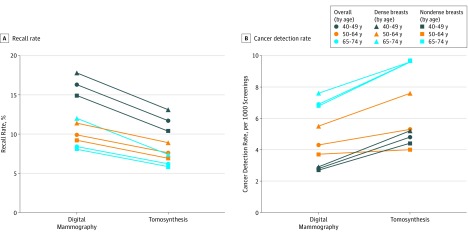
When recall and cancer detection rates were plotted by age and breast density, the outcomes for women aged 40 to 49 years who were screened with DBT shifted toward the outcomes for women aged 50 to 59 years who were screened with DM (Figure 1A and B). Although the cancer detection rate was higher among women aged 40 to 49 years who were screened with DBT (4.8 per 1000 screenings) compared with women aged 50 to 59 years who were screened with DM (4.3 per 1000 screenings), the recall rate in this younger DBT cohort was higher than the recall rate in the older DM cohort. The higher recall and cancer detection rate in the younger women were probably attributable to more younger women undergoing baseline screening examinations, in which both recall and cancer detection is expected to be higher.22 This shift in the risk-benefit balance of outcomes with DBT for women aged 40 to 49 years with outcomes considered to be acceptable for DM for women aged 50 to 59 years by systematic reviews (eg, US Preventive Services Task Force) is compelling evidence to support reconsideration of routine mammographic screening with DBT for these younger women. A similar shift in the combined gains of outcomes was seen when results were plotted by dense and nondense breasts (Figure). Further research is needed to confirm these results in other study populations, particularly to assess whether the increased cancer detection rate of DBT persists during multiple rounds of screening.
Figure. Comparison of Recalls and Cancer Detection Rates Between Digital Mammography and Digital Breast Tomosynthesis Overall and for Nondense Breasts and Dense Breasts by Age Group and Modality.
The higher sensitivity of screening examinations using DM compared with using DBT in our data was counterintuitive because the cancer detection rate for DBT was higher in all age groups. This finding may have been attributable to the increasing use of supplemental screening examinations with ultrasonography or magnetic resonance imaging during the study period and more supplemental screening occurring during the later period of DBT. It is also possible that women undergoing screening examination with DBT were more likely to have elevated cancer risk compared with women undergoing screening examination with DM. Unfortunately, detailed information about family history of breast cancer, BRCA mutation status, and other risk factors were not available for the full cohort.
In our analysis, the incorporation of outcomes was based on cancer prognosis categories as defined by TMIST.18 As breast cancer detection continues to improve with new screening modalities, there is an emphasis on detecting cancers considered to be of poorer prognosis because these cancers may be treated before there is an opportunity to metastasize and potentially become lethal.19 A few studies15,16 have suggested that cancers detected by DBT tend to be smaller, less aggressive cancers with better prognosis than those detected by DM. Our data showed that cancers detected by DBT overall were smaller, less often node positive, and less often HER-2 enriched (all factors associated with poor prognosis) than cancers detected by DM. A study has previously shown23 that among women diagnosed with breast cancer after negative mammogram results, younger age was most strongly associated with cancers of poor prognosis. In our subgroup analysis among women aged 40 to 49 years, screening with DBT was associated with only 25.0% of cancers being categorized as advanced cancers compared with 40.4% of cancers detected by DM. Although the smaller number of cancers with poor prognosis detected with DBT may have been the result of a stage shift attributed to earlier detection, it is unclear whether these lower-grade cancers with better prognosis might have become clinically significant before the next few screenings if screening had been with DM. However, the difference in cancer with poor prognosis detected by DBT compared with DM in women aged 40 to 49 years was larger than in any other age group. When coupled with the reduction in recall and the increased overall detection of cancer, our data further support the consideration of routine screening using DBT in this age group. The randomized design of TMIST will provide further insight into the biologic nature and long-term outcomes for both DM and DBT with fewer concerns regarding differential underlying risk in the women screened by different modalities.
Limitations
Several limitations should be considered when interpreting our findings. All data came from health care systems in the northeastern United States. Minority and Hispanic women were underrepresented, and most women had health insurance. Each PROSPR site adopted and implemented DBT at different times and rates, and consequently, the radiologists may differ in experience with DBT. However, unlike many of the published studies6,7,8,10,11,15,16 about screening examination outcomes of DBT, our study was multi-institutional and included all screening mammograms meeting the requirements. Although our data included the important patient-level characteristics of age, breast density, and first or subsequent screening, we did not have complete breast cancer risk assessment data for analysis. In addition, although we had tumor registry data including size, cancer stage, receptor type, and nodal status, the number of cancers was small; thus, assessment for statistical significance was limited in subgroup analyses, including false-negative results.
Conclusions
In our study, across multiple, diverse research centers in the northeastern United States, DBT was associated with an improvement in specificity across all age and breast density groups and an increase in the proportion of breast cancers found that were smaller and less often node positive. These smaller-sized cancers, which may not have de-differentiated into more aggressive subtypes, are expected to be associated with a better long-term prognosis. These findings suggest that, in the subgroup of women aged 40 to 49 years, routine mammographic screening may be associated with a favorable risk-benefit ratio.
eTable 1. Screening Examination Characteristics
eTable 2. Description of Detected Invasive Cancers
References
- 1.Houssami N, Miglioretti DL. Digital breast tomosynthesis: a brave new world of mammography screening. JAMA Oncol. 2016;2(6):725-727. doi: 10.1001/jamaoncol.2015.5569 [DOI] [PubMed] [Google Scholar]
- 2.Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J Natl Cancer Inst. 2018;110(9):942-949. doi: 10.1093/jnci/djy121 [DOI] [PubMed] [Google Scholar]
- 3.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583-589. doi: 10.1016/S1470-2045(13)70134-7 [DOI] [PubMed] [Google Scholar]
- 4.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47-56. doi: 10.1148/radiol.12121373 [DOI] [PubMed] [Google Scholar]
- 5.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499-2507. doi: 10.1001/jama.2014.6095 [DOI] [PubMed] [Google Scholar]
- 6.McCarthy AM, Kontos D, Synnestvedt M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst. 2014;106(11):dju316. doi: 10.1093/jnci/dju316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R Jr. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol. 2013;200(6):1401-1408. doi: 10.2214/AJR.12.9672 [DOI] [PubMed] [Google Scholar]
- 8.Durand MA, Haas BM, Yao X, et al. Early clinical experience with digital breast tomosynthesis for screening mammography. Radiology. 2015;274(1):85-92. doi: 10.1148/radiol.14131319 [DOI] [PubMed] [Google Scholar]
- 9.Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat. 2016;156(1):109-116. doi: 10.1007/s10549-016-3695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald ES, Oustimov A, Weinstein SP, Synnestvedt MB, Schnall M, Conant EF. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol. 2016;2(6):737-743. doi: 10.1001/jamaoncol.2015.5536 [DOI] [PubMed] [Google Scholar]
- 11.Bahl M, Gaffney S, McCarthy AM, Lowry KP, Dang PA, Lehman CD. Breast cancer characteristics associated with 2D digital mammography versus digital breast tomosynthesis for screening-detected and interval cancers. Radiology. 2018;287(1):49-57. doi: 10.1148/radiol.2017171148 [DOI] [PubMed] [Google Scholar]
- 12.Caumo F, Romanucci G, Hunter K, et al. Comparison of breast cancers detected in the Verona screening program following transition to digital breast tomosynthesis screening with cancers detected at digital mammography screening. Breast Cancer Res Treat. 2018;170(2):391-397. doi: 10.1007/s10549-018-4756-4 [DOI] [PubMed] [Google Scholar]
- 13.Houssami N, Bernardi D, Caumo F, et al. Interval breast cancers in the ‘screening with tomosynthesis or standard mammography’ (STORM) population-based trial. Breast. 2018;38:150-153. doi: 10.1016/j.breast.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 14.Yun SJ, Ryu CW, Rhee SJ, Ryu JK, Oh JY. Benefit of adding digital breast tomosynthesis to digital mammography for breast cancer screening focused on cancer characteristics: a meta-analysis. Breast Cancer Res Treat. 2017;164(3):557-569. doi: 10.1007/s10549-017-4298-1 [DOI] [PubMed] [Google Scholar]
- 15.Wang WS, Hardesty L, Borgstede J, Takahashi J, Sams S. Breast cancers found with digital breast tomosynthesis: a comparison of pathology and histologic grade. Breast J. 2016;22(6):651-656. doi: 10.1111/tbj.12649 [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Kang HJ, Shin JK, et al. Biologic profiles of invasive breast cancers detected only with digital breast tomosynthesis. AJR Am J Roentgenol. 2017;209(6):1411-1418. doi: 10.2214/AJR.17.18195 [DOI] [PubMed] [Google Scholar]
- 17.Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA. 2018;319(2):154-164. doi: 10.1001/jama.2017.19130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ECOG-ACRIN Cancer Research Group. TMIST Breast Cancer Screening Trial. https://ecog-acrin.org/tmist. Accessed July 28, 2018.
- 19.Pisano ED. Is tomosynthesis the future of breast cancer screening? Radiology. 2018;287(1):47-48. doi: 10.1148/radiol.2018172953 [DOI] [PubMed] [Google Scholar]
- 20.D’Orsi CJ, Bassett LW, Berg WA, et al. BI-RADS: mammography In: D’Orsi CJ, Mendelson EB, Ikeda DM, et al, eds. Breast Imaging Reporting and Data System: ACR BI-RADS–Breast Imaging Atlas. 4th ed Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 21.Rafferty EA, Rose SL, Miller DP, et al. Effect of age on breast cancer screening using tomosynthesis in combination with digital mammography. Breast Cancer Res Treat. 2017;164(3):659-666. doi: 10.1007/s10549-017-4299-0 [DOI] [PubMed] [Google Scholar]
- 22.McDonald ES, McCarthy AM, Akhtar AL, Synnestvedt MB, Schnall M, Conant EF. Baseline screening mammography: performance of full-field digital mammography versus digital breast tomosynthesis. AJR Am J Roentgenol. 2015;205(5):1143-1148. doi: 10.2214/AJR.15.14406 [DOI] [PubMed] [Google Scholar]
- 23.McCarthy AM, Barlow WE, Conant EF, et al. ; PROSPR Consortium . Breast cancer with a poor prognosis diagnosed after screening mammography with negative results. JAMA Oncol. 2018;4(7):998-1001. doi: 10.1001/jamaoncol.2018.0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Screening Examination Characteristics
eTable 2. Description of Detected Invasive Cancers