Key Points
Question
Is calcium intake associated with age-related macular degeneration (AMD)?
Findings
In secondary analyses of randomized clinical trial participants, baseline calcium intake was found to be associated with AMD; participants with the highest intake of dietary calcium had a lower risk of developing late AMD and geographic atrophy than those in the lowest quintile. The participants in the highest intake of supplementary calcium had a lower risk of developing neovascular AMD compared with those who did not take supplements.
Meaning
Despite these protective associations of dietary and supplementary calcium, the results may be considered hypothesis generating, requiring validation with further investigations.
This secondary analysis of data from the Age-Related Eye Disease Study (AREDS) examines the association of dietary and supplementary calcium intake with age-related macular degeneration onset.
Abstract
Importance
Previous studies of the role of dietary and supplementary calcium in age-related macular degeneration (AMD) have produced mixed results, suggesting that supplementation and decreased dietary intake are both harmful.
Objective
To evaluate the association of baseline dietary and supplementary calcium intake with progression of AMD.
Design, Setting, and Participants
This study involved secondary analyses of participants enrolled in the Age-Related Eye Disease Study (AREDS). The AREDS study (1992-2001) enrolled patients from academic and community-based retinal practices in the United States. Men and women with varying severity of AMD were included. Data analysis for this article occurred from September 2015 to December 2018.
Exposures
Baseline self-reported dietary or supplementary calcium intake.
Main Outcomes and Measures
Development of late AMD, geographic atrophy (central or noncentral), or neovascular AMD detected on centrally graded baseline and annual fundus photographs.
Results
A total of 4751 participants were included (mean [SD] age, 69.4 [5.1] years); 4543 (95.6%) were white, and 2655 (55.9%) were female. Compared with those who were in the lowest quintile, the participants in the highest quintile of dietary calcium intake had a lower risk of developing late AMD (hazard ratio [HR], 0.73 [95% CI, 0.59-0.90]), central geographic atrophy (HR, 0.64 [95% CI, 0.48-0.86]), and any geographic atrophy (HR, 0.80 [95% CI, 0.64-1.00]). The participants in the highest tertile of supplementary calcium intake had a lower risk of developing neovascular AMD (HR, 0.70 [95% CI, 0.50-0.97]) compared with those who did not take calcium supplements. When stratified by sex, women in the highest quintile of dietary calcium intake had a lower risk of developing late AMD (HR, 0.73 [95% CI, 0.56-0.97]) compared with those in the lowest quintile. Women in the highest tertile of calcium supplementation had a lower risk of progression to neovascular AMD (HR, 0.67 [95% CI, 0.48-0.94]) compared with those who did not take calcium supplements. Similar findings were found in men for dietary calcium. Too few men took calcium supplements to allow for analyses.
Conclusions and Relevance
In this secondary analysis, higher levels of dietary and supplementary calcium intake were associated with lower incidence of progression to late AMD in AREDS participants. The results may be owing to uncontrolled confounding or chance and should be considered hypothesis development requiring additional study.
Introduction
Age-related macular degeneration (AMD), a leading cause of blindness in developed countries,1,2 is associated with increasing age. With increasing longevity, the number of people with AMD in the United States is estimated to be approximately 20 million by 2050, a doubling of the number from 2010.3 This disease of considerable public health importance markedly affects the patient’s independence and physical, emotional, and social health.4 Currently, neovascular AMD (nAMD) can be treated with intravitreous anti–vascular endothelial growth factor agents, while there is no effective therapy for the atrophic form of AMD. Additional known risk factors associated with AMD include cigarette smoking, numerous genetic variants,2,5,6,7,8,9,10 and nutritional factors such as the B vitamins; vitamins C, D and E; and zinc, copper, ω-3 fatty acids, lutein, and zeaxanthin, all of which were evaluated in randomized clinical trials5,11 or observational studies.12,13,14,15,16,17
Calcium is commonly used to prevent and treat osteoporosis in the United States, with as many as 50% of men and 65% of women regularly taking calcium supplements.18 Few epidemiological analyses have explored the association between calcium intake and AMD. The Blue Mountains Eye Study,9 a longitudinal (15-year) population-based study conducted in Australia, found that decreased dietary calcium intake was associated with a higher risk for developing late AMD. The participants who were in the lowest quintile of dietary intake of dairy products and calcium had higher odds of developing late AMD than participants in the highest quintile (odds ratio, 2.8 [95% CI, 1.21-3.04]).9 However, a cross-sectional evaluation of the 2007-2008 National Health and Nutrition Examination Survey (NHANES) found an association of self-reported supplementary calcium intake and an increased risk of AMD.10 The pathophysiology of AMD is poorly understood, but previous studies have demonstrated that the drusen contains calcium.19 Hydroxyapatite, a highly insoluble form of calcium phosphate found in bones and teeth, has been observed in spherules in sub–retinal pigment epithelium (RPE) deposits within drusen.20
The purpose of the present study was to retrospectively investigate the associations of dietary and supplemental calcium with the risk of AMD development and progression to intermediate AMD or late AMD (nAMD or geographic atrophy [GA], whether any GA or central GA [CGA]). We evaluated this association in the Age-Related Eye Disease Study (AREDS), a multicenter randomized clinical trial (1992-2001) of nutritional supplements for the treatment of AMD and cataract.8,21 Participants were followed for an additional 5 years in an epidemiologic study, until 2005.
Methods
Study Population, Procedures, and Sample
The AREDS study design has been previously described.21 Briefly, 11 retinal specialty clinics in the United States recruited 4757 participants from November 1992 to April 2001. Adults (aged 55 to 80 years) who had either no AMD, intermediate AMD (bilateral large drusen), and late AMD in 1 eye21,22,23 were enrolled. Each clinical center obtained institutional review board approval for the study, and the study adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
This study, supported by the National Institutes of Health, was required to gather information on race/ethnicity. Using guidelines from the NIH Health Policy on Reporting Race and Ethnicity Data: Subjects in Clinical Research,24 self-reported race and ethnicity were collected with 2 ethnic categories (Hispanic or Latino and not Hispanic or Latino) and 5 racial categories (American Indian or Alaska Native, Asian, black or African American, Native Hawaiian or other Pacific Islander, and white).
Comprehensive eye examinations were conducted at baseline and twice yearly throughout the trial and annual visits through 2005. Stereoscopic fundus photographs were taken at baseline and annually, beginning 2 years after enrollment to assess the progression of AMD.25 These were graded at a centralized University of Wisconsin Fundus Photographic Reading Center by certified personnel who used a standard protocol.
Baseline questionnaires were used to collect demographic, medical, and dietary information.8,25 Nutrient intake, including calcium (dietary), was assessed using a Block food frequency questionnaire,21 which collected data on 80 specific foods and drinks consumed in the participants’ typical dietary intake during the year prior to randomization.26 Participants’ mean daily dietary calcium intake was computed by adding the amounts of calcium present in the foods and beverages reportedly consumed per day. The frequency, duration, and dosage of the calcium supplement were evaluated.
Participants, stratified at baseline by the severity of AMD, were randomly assigned to antioxidant vitamins (vitamins C, E, and beta carotene), zinc plus copper, the combination of both antioxidants and zinc, or placebo.8 At the end of the clinical trial in 2001, 4203 participants were still alive, and 3549 (84.4%) consented to be followed up as part of an epidemiologic study until 2005. Analyses were limited to individuals who had complete data for calcium intake, sex, race, educational attainment, smoking status, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), diabetes mellitus, antacid usage, and nonsteroidal anti-inflammatory drug use.
Measures
Calcium Intake
Baseline dietary calcium intake was determined through food frequency questionnaire, while baseline calcium supplement use was determined through an additional questionnaire. Sex-specific analyses of dietary calcium intake were conducted to account for the differences in daily caloric intake between male and female participants. The amount of dietary calcium intake was energy adjusted. To exclude participants on extreme ends of the calorie-intake spectrum, dietary calcium-intake analyses were calorie restricted from 677 to 1994 kcal in female participants and from 794 to 2771 kcal in male participants.
Measures of Outcomes
The 2 primary outcomes of interest in this study were (1) progression to intermediate AMD (with large drusen) and (2) progression to late AMD consisting of neovascularization, CGA, or any GA. Outcomes were based on standardized grading of annual stereoscopic fundus photographs. In AREDS, large drusen was defined as drusen with diameter of 125 µm or more. An eye was considered to have nAMD if any of the following was present: nondrusenoid RPE detachment, serous hemorrhagic retinal detachment, hemorrhage under the retina or RPE, and/or subretinal fibrosis. An eye was also considered to have nAMD if a clinical center reported treatments for choroidal neovascularization, such as photocoagulation or photodynamic therapy.22,25 Geographical atrophy was defined as an area of partial or complete depigmentation of the RPE in the fundus photographs, measuring at least one-eighth disc diameter, and at least 2 of 3 characteristics: (1) roughly round or oval shape, (2) sharp margins, and (3) visibility of underlying large choroidal vessels. Areas were excluded if there was depigmentation adjacent to disciform scars.8,27,28
Statistical Modeling and Analyses
Cox proportional hazards regression models were used to evaluate progression to intermediate and late AMD among participants consuming different levels of calcium (SAS software version 9.3 [SAS Institute]).29 Analysis was per participant eye. The Wei-Lin-Weissfield method for analyzing repeated measures was used to take into account the correlation between the 2 eyes of each participant. Models were developed separately for different outcomes: late AMD (with nAMD, CGA, or any GA) and large drusen. The risk factors that were considered were age, sex, race, educational attainment, BMI, smoking history, diabetes, antacid usage, anti-inflammatory drug usage, and AREDS treatment assignment. The models for evaluating the quintiles of dietary intake of calcium were also adjusted for calorie intake. Dietary quintiles were obtained separately by sex. To develop the final models, all the variables that were significant in age-adjusted and sex-adjusted models at a P value of .15 were included. In a multistep process, variables were entered or removed from the model by comparing the −2 log of the maximized likelihood between the model with the variable and the model without the variable. A P value of .10 for comparing the models was used, and a final model that best fits the data was obtained. From the final models, hazard ratios (HRs) and 95% CIs were computed to assess the effects of the variables. A P value of .05 was considered statistically significant.
Ultimately, all analysis models were adjusted for age, sex, smoking, and AREDS treatment group. The models for large drusen, CGA, nAMD, and late AMD were additionally adjusted for race. The models for late AMD, nAMD, CGA, and any GA were adjusted for BMI. The large drusen model was adjusted for nonsteroidal anti-inflammatory drug usage.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the 4751 AREDS participants. The numbers of participants with complete data included in the analyses of calcium supplement were 4751 for late AMD, CGA, and nAMD; 4680 for any GA; and 3312 for large drusen. A total of 4543 participants (95.6%) were white and 2655 (55.9%) were female. The mean age (SD) was 69.4 (5.1) years, 398 (8.4%) had diabetes, 1540 (32.4%) used nonsteroidal anti-inflammatory drugs, 789 (16.6%) used antacids, and 687 (14.5%) used calcium supplements. Table 2 describes baseline characteristics of individuals who took calcium supplements compared with those who did not. Compared with their peers who did not use supplements, participants who used calcium supplements were more likely to be female (2049 [50.4%] vs 606 [88.2%]) and white (3875 [95.4%] vs 668 [97.2%]), have a lower BMI (≤25.0: 1224 [30.1%] vs 326 [47.5%]), not have diabetes (participants with diabetes: 363 [8.9%] vs 35 [5.1%]), and not have ever smoked (1740 [42.8%] vs 365 [53.1%]). eTable 1 and eTable 2 in the Supplement demonstrate the various levels of dietary calcium intake in quintiles stratified by men and women.
Table 1. Baseline Characteristics of Participants.
| Characteristics | Participants, No. (%) |
|---|---|
| Total | 4751 |
| Age, mean (SD), y | 69.4 (5.1) |
| Sex | |
| Male | 2096 (44.1) |
| Female | 2655 (55.9) |
| Race/ethnicity | |
| White, non-Hispanic | 4543 (95.6) |
| Black, non-Hispanic | 177 (3.7) |
| Hispanic | 13 (0.3) |
| Asian or Pacific Islander | 8 (0.2) |
| Other | 10 (0.2) |
| Educational attainment | |
| ≤High school | 1708 (36.0) |
| Some college | 1409 (29.7) |
| College graduate | 1634 (34.4) |
| Smoking status | |
| Never | 2105 (44.3) |
| Former | 2271 (47.8) |
| Current | 375 (7.9) |
| BMI | |
| ≤25.0 | 1550 (32.6) |
| >25.0 and ≤30 | 1984 (41.8) |
| >30.0 | 1217 (25.6) |
| Diabetes mellitus | |
| Yes | 398 (8.4) |
| No | 4353 (91.6) |
| Age-Related Eye Disease Study treatment assignment | |
| Placebo | 1480 (31.2) |
| Antioxidants | 1481 (31.2) |
| Zinc | 902 (19.0) |
| Antioxidants plus zinc | 888 (18.7) |
| Antacid use | |
| Absent | 3962 (83.4) |
| Present | 789 (16.6) |
| Nonsteroidal anti-inflammatory drug use | |
| Absent | 3211 (67.6) |
| Present | 1540 (32.4) |
| Calcium use | |
| No | 4064 (85.5) |
| Yes | 687 (14.5) |
| Supplementary calcium-intake tertilesa | |
| No calcium use | 4064 (85.5) |
| 1 (Lowest) | 277 (5.8) |
| 2 | 133 (2.8) |
| 3 (Highest) | 277 (5.8) |
| Dietary calcium-intake quintilesa | |
| Missing | 6 (0.1) |
| 1 (Lowest) | 949 (20.0) |
| 2 | 950 (20.0) |
| 3 | 949 (20.0) |
| 4 | 949 (20.0) |
| 5 (Highest) | 948 (20.0) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Numbers in each quintile and tertile include all Age-Related Eye Disease Study participants (N = 4751), and they differ in size from the calorie restriction–defined groups included in disease outcome statistics.
Table 2. Baseline Characteristics of Participants Who Took Calcium Supplements vs Those Who Did Not.
| Baseline Characteristic | Patients, No. (%) (N = 4751) | |
|---|---|---|
| Without Supplements (n = 4064) | With Supplements (n = 687) | |
| Age, mean (SD), y | 69.4 (5.1) | 69.4 (5.1) |
| Sex | ||
| Male | 2015 (49.6) | 81 (11.8) |
| Female | 2049 (50.4) | 606 (88.2) |
| Race | ||
| White, non-Hispanic origin | 3875 (95.3) | 668 (97.2) |
| Black, non-Hispanic origin | 161 (4.0) | 16 (2.3) |
| Hispanic | 11 (0.3) | 2 (0.3) |
| Asian or Pacific Islander | 8 (0.2) | 0 |
| Other | 9 (0.2) | 1 (0.1) |
| Educational attainment | ||
| ≤High school | 1494 (36.8) | 214 (31.1) |
| Some college | 1172 (28.8) | 237 (34.5) |
| College graduate | 1398 (34.4) | 236 (34.4) |
| Smoking status | ||
| Never | 1740 (42.8) | 365 (53.1) |
| Former | 1985 (48.8) | 286 (41.6) |
| Current | 339 (8.3) | 36 (5.2) |
| BMI | ||
| ≤25.0 | 1224 (30.1) | 326 (47.5) |
| >25.0 and ≤30 | 1751 (43.1) | 233 (33.9) |
| >30.0 | 1089 (26.8) | 128 (18.6) |
| Diabetes mellitus | ||
| Yes | 363 (8.9) | 35 (5.1) |
| No | 3701 (91.1) | 652 (94.9) |
| Age-Related Eye Disease Study treatment assignment | ||
| Placebo | 1262 (31.1) | 218 (31.7) |
| Antioxidants | 1271 (31.3) | 210 (30.6) |
| Zinc | 764 (18.8) | 138 (20.1) |
| Antioxidants plus zinc | 767 (18.9) | 121 (17.6) |
| Antacid use | ||
| Absent | 3406 (83.8) | 556 (80.9) |
| Present | 658 (16.2) | 131 (19.1) |
| Nonsteroidal anti-inflammatory drug use | ||
| Absent | 2766 (68.1) | 445 (64.8) |
| Present | 1298 | 242 (35.2) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Significant Associations With Incident AMD
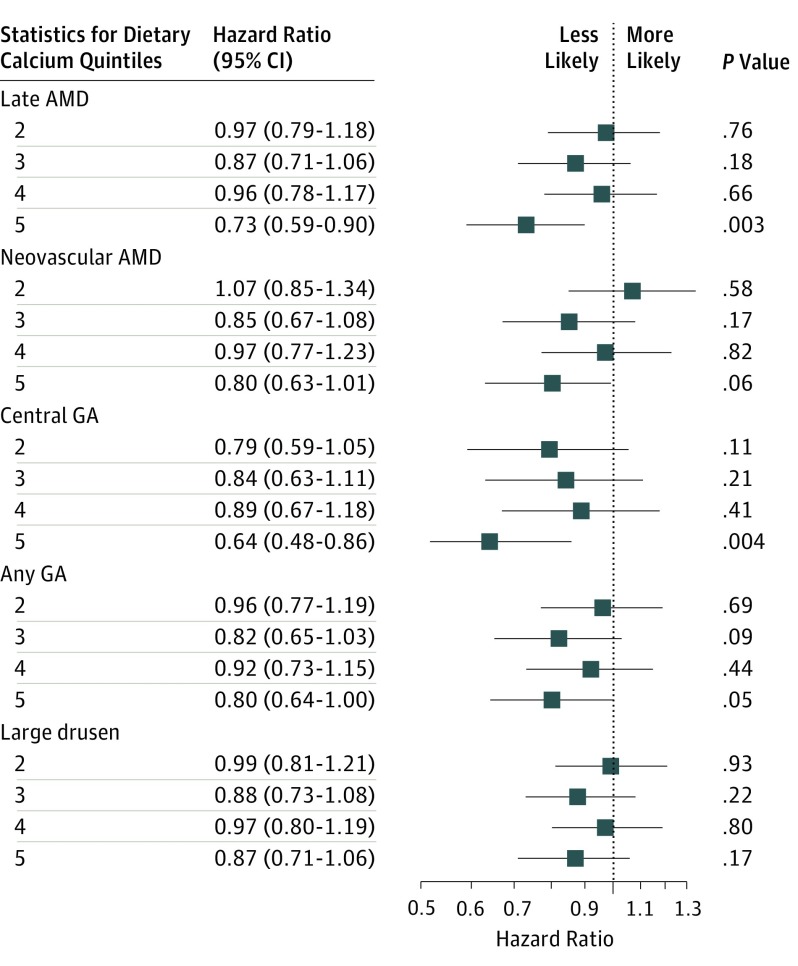
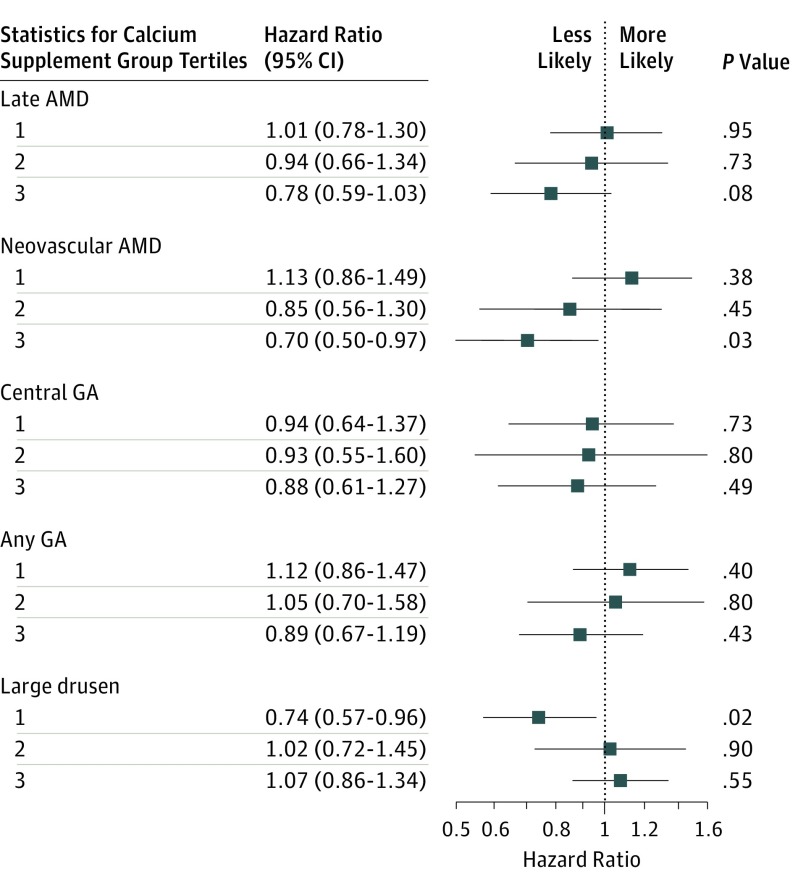
Hazard ratios (HRs) and 95% CIs are shown from the final adjusted models for dietary calcium intake (Figure 1). The same values are shown for supplementary calcium intake (Figure 2).
Figure 1. Comparison of Self-reported Dietary Calcium Intake (Highest vs Lowest Quintiles) for Incidence of Age-Related Macular Degeneration (AMD).
The reference group is composed of participants in quintile 1 of self-reported calcium intake levels; quintile 1 represents the lowest calcium intake, and quintile 5, the highest. GA indicates geographic atrophy.
Figure 2. Comparison of Self-reported Supplementary Calcium (Highest Tertile vs No Supplementation) for Incidence of Age-Related Macular Degeneration (AMD).
The reference group includes participants who do not take calcium supplementation; tertile 1 represents the lowest calcium intake, and tertile 3, the highest. GA indicates geographic atrophy.
Dietary Calcium
Comparing participants (n = 778) in the highest quintile of dietary calcium intake with participants in the lowest quintile of calcium intake (n = 839) resulted in findings of a lower risk of developing late AMD (HR, 0.73 [95% CI, 0.59-0.90]), CGA (HR, 0.64 [95% CI, 0.48-0.86]), and any GA (HR, 0.80 [95% CI, 0.64-1.00]). Participants in the highest quintile of dietary calcium intake did not have a statistically significant lower risk than participants in the lowest quintile of calcium intake for developing nAMD (HR, 0.80 [95% CI, 0.63-1.01]) or large drusen (HR, 0.87 [95% CI, 0.71-1.06]; Figure 1).
When participants were stratified by sex, women in the highest quintile of self-reported dietary calcium intake (n = 453; 0.71-2.04 mg/kcal/day) had a lower risk of developing late AMD than women in the lowest quintile of dietary calcium intake (n = 410; 0.15-0.36 mg/kcal/day; HR, 0.73 [95% CI, 0.56-0.97]). Differences noted between women in the highest quintile of dietary calcium intake and women in the lowest quintile of dietary calcium intake were not significant in the nAMD, CGA, any GA, and large drusen analyses. Men in the highest quintile of dietary calcium intake (n = 381; 0.60-1.58 mg/kcal/day) had a lower risk of developing late AMD (HR, 0.71 [95% CI, 0.51-0.99]), CGA (HR, 0.56 [95% CI, 0.34-0.90]), and any GA (HR, 0.62 [95% CI, 0.43-0.88]) compared with men in the lowest quintile of dietary calcium intake (n = 368; 0.12-0.34 mg/kcal/day). Differences between men in the highest quintile of dietary calcium intake and men in the lowest quintile of dietary calcium intake were not significant in the neovascularization or large drusen analyses.
Calcium Supplementation
Participants in the highest tertile of self-reported calcium supplementation (n = 277 [5.8%]) had a lower risk of developing neovascularization than participants who did not take calcium supplements (n = 4064 [85.5%]; HR, 0.70 [95% CI, 0.50-0.97]). Participants in the highest tertile of self-reported calcium supplementation were found to have a nonsignificant lower risk of late AMD (HR, 0.78 [95% CI, 0.59-1.03]), CGA (HR, 0.88 [95% CI, 0.61-1.27]), and any GA (HR, 0.89 [95% CI, 0.67-1.19]) than participants that did not take calcium supplements. Participants in the highest tertile of self-reported calcium supplementation compared with those who did not take calcium supplements were not found to have different risks of developing large drusen (HR, 1.07 [95% CI, 0.86-1.34]; Figure 2). Since antacids may contain calcium, additional analyses compared the participants who took antacids (n = 3962 [83.4%]) vs those who did not (n = 789 [16.6%]) were performed, and no differences in calcium intake were not found. Analyses conducted to evaluate the effect of calcium supplementation in those in the lowest quintile of dietary calcium intake showed no significant differences for all the AMD outcomes.
When stratified by sex, women in the highest tertile of self-reported supplementary calcium intake (mean [SD], 0.898 [0.191] mg/kcal/day) had a lower risk of developing nAMD compared with women who did not take calcium supplements (HR, 0.67 [95% CI, 0.48-0.94]). Differences noted between women in the highest tertile of self-reported supplementary calcium intake and women in the lowest quintile of tertile of self-reported supplementary calcium intake were not significant in the late AMD, CGA, any GA, and large drusen analyses. Too few men (n = 81) took calcium supplements to allow for analysis of association between calcium supplementation and risk for AMD.
Other Associated Dietary Risk Factors
Higher dietary intake of lutein and fish were also evaluated in this study. Dietary intake of calcium was found to be highly associated with increased lutein intake (both in quintiles; χ216, 43.2; P < .001) but not with increased fish intake (in quartiles; χ212, 14.81; P = .25).
Discussion
We found a significant association between calcium intake and late AMD development but did not find a significant association between calcium intake and intermediate AMD development. Few epidemiological analyses have explored the association of calcium intake with AMD, and results have been mixed. The Australian-based Blue Mountains Eye Study9 found decreased dietary calcium intake to be associated with a higher risk for developing late AMD over a 15-year follow-up period. Participants in the lowest quintile of dairy product and dietary calcium intake had a higher odds of developing late AMD than participants in the highest quintile (odds ratio, 2.8 [95% CI, 1.21-3.04]).9 The analyses of the 2007-2008 NHANES,10 which was a cross-sectional study, suggested increased calcium intake is associated with a higher odds of having any AMD. Of the 248 participants in the NHANES, only 28 with AMD had late AMD, and the remaining number were considered to have early AMD. The participants in the NHANES who self-reported supplementary calcium consumption of more than 800 mg per day had a higher odds of AMD diagnosis compared with those who did not report calcium supplementation (OR, 1.85 [95% CI, 1.25-2.75]).10 Contrary to the NHANES study and similar to the Blue Mountains Eye Study, this study found that increased calcium intake is associated with a lower risk of AMD progression.
We found an inconsistent association between calcium consumption and the different stages of AMD. The Blue Mountains Eye Study found a similar inconsistency for increased fish consumption, which reduced the risk for late AMD but was not associated with a reduced risk of developing early AMD.9 A 2011 systematic review and meta-analysis found that lutein and zeaxanthin are protective against late AMD but not early AMD.30 As stated by Blue Mountains Eye Study authors, it is possible that the pathophysiological processes that underlie development of early AMD are different from those involved in the transition from early to late AMD.9 We also believe that the pathways that lead to drusen may be quite different than the development of late AMD, as reflected by genetic associations.31 The role of dietary and supplementary calcium in the development of drusen is unknown. Investigators have found hydroxyapatite, a highly insoluble form of calcium phosphate that is normally found in bones and teeth, in spherules of sub-RPE deposits within drusen. The association of dietary calcium or supplementation with the later stages of AMD development may just reflect other health habits of this population. For example, those who have a higher calcium intake and supplementation may have a healthier diet in general and may be more compliant about exercising and taking prescribed medications. Higher dietary intake of lutein and fish have been found to be associated with decreased risk of AMD. These 2 dietary factors were evaluated in this study, and we found dietary intake of calcium (in quintiles) is highly associated with increased lutein intake (in quintiles; χ216, 43.2; P < .001) but not with increased fish intake (in quartiles; χ212, 14.81; P = .25).
The strengths of our study include the large number of participants. In addition, this study had a long-term follow-up period with adequate power to evaluate the association of calcium with AMD, albeit somewhat limited power for evaluation of the subgroup of men supplementing with calcium.
Limitations
This study is a secondary analysis, and results may arise by chance or owing to uncontrolled confounding, so they should be considered as hypothesis development. In addition, the results of the current study may have limited generalizability because participants of the AREDS were mainly white, with few black, Hispanic, and Asian participants. In addition, AREDS participants were volunteers for the clinical trial, and such volunteers are often healthier and better educated than the general population.32
Recall bias may be another limitation of this study, because we depended on self-reported data for the baseline food frequency questionnaires, which required highly detailed recall of food intake over the past year, including the frequency and serving size of 80 food items. Similarly, the questionnaire evaluated vitamin and mineral supplementation, which included the dosage and frequency over the past year. However, although recall bias may occur, we speculate that there may be less bias in estimating the extreme ends of the scale (ie, the lowest and highest tertiles and quintiles).
Another limitation is the lack of information on the length of dietary intake and supplementation. Data for calcium supplementation were available on follow-up in approximately 25% of the study population. Most of the participants (99.4%) who had reported taking calcium at baseline were continuing to take the supplements at the end of the study.
Conclusions
Age-related macular degeneration is a condition that will continue to affect the visual health of the public for decades to come; therefore, it is important to determine factors that affect the risk for AMD. We found no evidence that calcium intake might be harmful in the development of AMD, although further studies would be necessary to further define this association. A randomized clinical trial of calcium is unlikely to occur, because a large proportion of this elderly population is already taking supplementation for the prevention of osteoporosis. Further support will also be required to establish such an association in other observational data from prospective studies of cohorts with sufficient development of AMD. As noted, this association may simply reflect the potential for increased calcium intake and supplementation to be a surrogate for better health habits. These results do not change current clinical management of the calcium intake of individuals in the general population who have other medical indications for taking calcium.
eTable 1. Calorie-adjusted Dietary Calcium Intake for Female AREDS Participants
eTable 2. Calorie-adjusted Dietary Calcium Intake for Male AREDS Participants
References
- 1.Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 2.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. doi: 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 3.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J; Vision Health Cost-Effectiveness Study Group . Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533-540. doi: 10.1001/archophthalmol.2009.58 [DOI] [PubMed] [Google Scholar]
- 4.Rovner BW, Casten RJ, Hegel MT, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2014;121(11):2204-2211. doi: 10.1016/j.ophtha.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman HR, Chan CC, Ferris FL III, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835-1845. doi: 10.1016/S0140-6736(08)61759-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280-1287. doi: 10.1016/j.ophtha.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 7.Guymer RH, Chong EW. Modifiable risk factors for age-related macular degeneration. Med J Aust. 2006;184(9):455-458. [DOI] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group Risk factors associated with age-related macular degeneration: A case-control study in the age-related eye disease study, Age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107(12):2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinath B, Flood VM, Louie JC, et al. Consumption of dairy products and the 15-year incidence of age-related macular degeneration. Br J Nutr. 2014;111(9):1673-1679. doi: 10.1017/S000711451300408X [DOI] [PubMed] [Google Scholar]
- 10.Kakigi CL, Singh K, Wang SY, Enanoria WT, Lin SC. Self-reported calcium supplementation and age-related macular degeneration. JAMA Ophthalmol. 2015;133(7):746-754. doi: 10.1001/jamaophthalmol.2015.0514 [DOI] [PubMed] [Google Scholar]
- 11.Chew EY, Clemons TE, Sangiovanni JP, et al. ; Age-Related Eye Disease Study 2 (AREDS2) Research Group . Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142-149. doi: 10.1001/jamaophthalmol.2013.7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh N, Chappell RJ, Millen AE, Albert DM, Mares JA. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch Ophthalmol. 2007;125(5):661-669. doi: 10.1001/archopht.125.5.661 [DOI] [PubMed] [Google Scholar]
- 13.Merle BMJ, Silver RE, Rosner B, Seddon JM. Associations between vitamin D intake and progression to incident advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(11):4569-4578. doi: 10.1167/iovs.17-21673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millen AE, Voland R, Sondel SA, et al. ; CAREDS Study Group . Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch Ophthalmol. 2011;129(4):481-489. doi: 10.1001/archophthalmol.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cougnard-Grégoire A, Merle BM, Korobelnik JF, et al. Vitamin D deficiency in community-dwelling elderly is not associated with age-related macular degeneration. J Nutr. 2015;145(8):1865-1872. doi: 10.3945/jn.115.214387 [DOI] [PubMed] [Google Scholar]
- 16.Seddon JM, Reynolds R, Shah HR, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011;118(7):1386-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki A, Inoue M, Nguyen E, et al. Dietary n-3 fatty acid, α-tocopherol, zinc, vitamin d, vitamin c, and β-carotene are associated with age-related macular degeneration in Japan. Sci Rep. 2016;6:20723. doi: 10.1038/srep20723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817-822. doi: 10.3945/jn.109.118539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulshafer RJ, Allen CB, Nicolaissen B Jr, Rubin ML. Scanning electron microscopy of human drusen. Invest Ophthalmol Vis Sci. 1987;28(4):683-689. [PubMed] [Google Scholar]
- 20.Thompson RB, Reffatto V, Bundy JG, et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc Natl Acad Sci U S A. 2015;112(5):1565-1570. doi: 10.1073/pnas.1413347112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study (AREDS): design implications, AREDS report No. 1. Control Clin Trials. 1999;20(6):573-600. doi: 10.1016/S0197-2456(99)00031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL III; Age-Related Eye Disease Study Research Group . Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112(4):533-539. doi: 10.1016/j.ophtha.2004.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JR, Koo E, Agrón E, et al. ; Age-Related Eye Disease Study Group . Risk factors associated with incident cataracts and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology. 2011;118(11):2113-2119. doi: 10.1016/j.ophtha.2011.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institutes of Health NIH policy on reporting race and ethnicity data: subjects in clinical research. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-01-053.html. Published August 8, 2001. Accessed February 19, 2019.
- 25.Chew EY, Clemons TE, Agrón E, et al. ; Age-Related Eye Disease Study Research Group . Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 2014;132(3):272-277. doi: 10.1001/jamaophthalmol.2013.6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SanGiovanni JP, Chew EY, Agrón E, et al. ; Age-Related Eye Disease Study Research Group . The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126(9):1274-1279. doi: 10.1001/archopht.126.9.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report number 6. Am J Ophthalmol. 2001;132(5):668-681. doi: 10.1016/S0002-9394(01)01218-1 [DOI] [PubMed] [Google Scholar]
- 28.Davis MD, Gangnon RE, Lee LY, et al. ; Age-Related Eye Disease Study Group . The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report No. 17. Arch Ophthalmol. 2005;123(11):1484-1498. doi: 10.1001/archopht.123.11.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox DR, Oakes D. Analysis of Survival Data: Monographs on Statistics and Applied Probability. New York, NY: Chapman and Hall; 1984. [Google Scholar]
- 30.Ma L, Dou HL, Wu YQ, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. 2012;107(3):350-359. doi: 10.1017/S0007114511004260 [DOI] [PubMed] [Google Scholar]
- 31.van Asten F, Simmons M, Singhal A, et al. ; Age-Related Eye Disease Study 2 Research Group . A deep phenotype association study reveals specific phenotype associations with genetic variants in age-related macular degeneration: Age-Related Eye Disease Study 2 (AREDS2) report No. 14. Ophthalmology. 2018;125(4):559-568. doi: 10.1016/j.ophtha.2017.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganguli M, Lytle ME, Reynolds MD, Dodge HH. Random versus volunteer selection for a community-based study. J Gerontol A Biol Sci Med Sci. 1998;53(1):M39-M46. doi: 10.1093/gerona/53A.1.M39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Calorie-adjusted Dietary Calcium Intake for Female AREDS Participants
eTable 2. Calorie-adjusted Dietary Calcium Intake for Male AREDS Participants