Key Points
Question
Is the number of anti–vascular endothelial growth factor injections in treatment of neovascular age-related macular degeneration and polypoidal choroidal vasculopathy associated with the patients’ proportions of CD11b+ circulating monocytes?
Findings
In this cohort study of 81 patients with age-related macular degeneration or polypoidal choroidal vasculopathy, anti–vascular endothelial growth factor injections at 12, 24, and 36 months positively estimated and were correlated with the proportions of patients’ CD11b+ circulating monocytes.
Meaning
Although these data suggest that the proportion of CD11b+ circulating monocytes is associated with the number of anti–vascular endothelial growth factor injections, additional longitudinal studies would be needed to determine whether these findings have clinical relevance to influence treatment algorithms or provide novel targets for medical therapy.
This cohort study investigates whether the proportion of CD11b+ circulating monocytes is associated with the number of anti–vascular endothelial growth factor injections given among patients with neovascular age-related macular degeneration or polypoidal choroidal vasculopathy.
Abstract
Importance
CD11b+ immune cells have been implicated in the formation of choroidal neovascularization in experimental studies on animals and disease-association studies on humans. However, the clinical importance of such observations remains unknown.
Objective
To investigate whether the proportion of CD11b+ circulating monocytes is associated with the number of anti–vascular endothelial growth factor (anti-VEGF) injections in neovascular age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV).
Design, Setting, and Participants
These observational cohort studies collected data from January 1, 2010, through December 31, 2013, and from January 1, 2015, through December 31, 2018. Fresh venous blood samples were acquired for flow cytometric immune studies in patients with neovascular AMD or PCV receiving treatment with aflibercept or ranibizumab as needed for 36 months. Patients (n = 81) without immune diseases were consecutively recruited from a single center in Denmark.
Exposures
Proportion of CD11b+ circulating monocytes.
Main Outcomes and Measures
The estimation of the number of intravitreal anti-VEGF injections given at 12, 24, and 36 months by the proportion of CD11b+ circulating monocytes and the correlation between these values. The angiogenic role of CD11b+ circulating monocytes was further evaluated by investigating the expression of the known proangiogenic receptor CCR2.
Results
Eighty-one patients were included in the analysis (54% women; mean [SD] age, 76 [7] years). The proportion of CD11b+ monocytes at baseline positively estimated the future number of anti-VEGF injections at 12 (ρ = 0.77; 95% CI, 0.35-0.93; P = .004), 24 (ρ = 0.82; 95% CI, 0.44-0.95; P = .002), and 36 (ρ = 0.78; 95% CI, 0.34-0.94; P = .005) months. This association was also found retrospectively in a larger sample of patients with neovascular AMD at 12 (ρ = 0.46; 95% CI, 0.16-0.68; P = .004), 24 (ρ = 0.49; 95% CI, 0.20-0.70; P = .002), and 36 (ρ = 0.65; 95% CI, 0.41-0.80; P < .001) months and patients with PCV at 12 (ρ = 0.27; 95% CI, –0.28 to 0.68; P = .30), 24 (ρ = 0.60; 95% CI, 0.12-0.85; P = .02), and 36 (ρ = 0.70; 95% CI, 0.27-0.90; P = .005) months, suggesting that this association is not specific to AMD but rather reflects VEGF activity in neovascularization. CD11b+ monocytes highly coexpressed CCR2, an important monocytic marker of proangiogenic activity.
Conclusions and Relevance
Results of this study demonstrated that the proportion of circulating CD11b+ monocytes estimated and correlated with the number of anti-VEGF injections in patients with neovascular AMD and PCV. Additional longitudinal studies are needed to determine whether these findings have clinical relevance to influence treatment algorithms or provide novel targets for medical therapy.
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible central vision loss in developed countries.1,2,3 A key feature of progression to late AMD is newly formed choroidal neovascularization (CNV) that penetrates through the Bruch membrane into the neuroretina.3 This progression is orchestrated by the neuroretina and the retinal pigment epithelium through secretion of signal molecules, which leads to chorioretinal overexpression of vascular endothelial growth factor (VEGF).4,5 Circulating monocytes play an important role in the neuroretinal injury–facilitated VEGF secretion and VEGF-mediated CNV formation.5,6,7,8,9 Several investigators7,8,9,10,11,12,13,14,15,16,17,18,19,20,21 have characterized circulating monocytes in relation to CNV in animal models of laser-induced CNV and in human patients with neovascular AMD. Important findings include the immunohistochemical documentation of CD11b in areas of experimentally induced CNV and expression by CD11b+ cells of VEGF and other proangiogenic factors.22 CD11b is an integrin expressed on the surface of myeloid cells and facilitates cell adhesion and migration, especially at sites of inflammation. CD11b is also part of complement receptor 3. Patients with neovascular AMD have an increased proportion of CD11b+ circulating monocytes.21 A similar observation is found in patients with polypoidal choroidal vasculopathy (PCV),21 in which VEGF expression and CNV formation are present without the AMD-associated drusenoid characteristics.23,24 Hence, we suspect CD11b+ monocytes are associated with CNV formation rather than immunologic aging or dysfunction.
Intravitreal anti-VEGF therapy is the cornerstone of therapy against CNV diseases. Despite a large quantity of studies on intravitreal anti-VEGF, no strong factor associated with the long-term need for therapy has been identified.25,26 In this study, we investigated whether circulating CD11b+ monocytes estimate and correlate with the number of anti-VEGF injections in patients with neovascular AMD and PCV.
Methods
Study Design and Ethics
This prospective study follows the tenets of the Declaration of Helsinki and was approved by the Regional Committee of Ethics in Research of the Region of Zealand, Denmark. Oral and written informed consent was obtained from all participants after explaining the nature of the study and before participation. This study was designed as a series of observational studies with immunologic data collected during 2 separate doctoral dissertation studies.20,21 For the present report, we investigated CD11b expression in association with the number of anti-VEGF injections in patients with neovascular AMD and PCV by answering a series of research questions (Table).
Table. Research Questions About CD11b+ Monocytes and the Number of Anti-VEGF Injections.
| Research Question | Data |
|---|---|
| 1 | Do CD11b+ monocytes at day of diagnosis estimate the later number of anti-VEGF injections? |
| Population | Patients with recently diagnosed neovascular AMD and commenced in anti-VEGF treatment using ranibizumab in a regimen as needed in a cohort study in 2010-2013 |
| No. of patients | Of 12 patients, 7 completed all follow-ups; 4 fulfilled predefined exit criteria (all dry macula) at a median 22 (range, 12-29) months (allowed for LOCF); and 1 did not wish to continue treatment after 12 mo (not allowed for LOCF) |
| Study | Whether the proportion of CD11b + circulating monocytes possessed any ability to estimate the total number of anti-VEGF treatments (mean [SD] of 5 [1] injections at 12 mo, 8 [4] at 24 mo, and 11 [6] at 36 mo) |
| 2 | Does the CD11b positivity of monocytes reflect a transient or a permanent state of the systemic immunity? In other words, are correlations maintained several months after the diagnosis? |
| Population | Patients with neovascular AMD already diagnosed and enrolled in anti-VEGF treatment using ranibizumab in a regimen as needed, and treated for several months (for practical purposes, we defined eligible patients as those who had completed a loading dose, hence ≥3 mo after diagnosis) in a cohort study in 2010-2013 |
| No. of patients | Of 37 patients, 30 completed all follow-ups and 7 fulfilled predefined exit criteria (4 fibrosis and 3 dry macula) at a median of 32 (range, 26-35) months (allowed for LOCF) |
| Study | Whether the proportion of CD11b+ circulating monocytes in fresh blood reflected a correlation with the total number of anti-VEGF treatments (mean [SD] of 6 [2] injections at 12 mo, 9 [3] at 24 mo, and 13 [5] at 36 mo) |
| 3 | Is the correlation between CD11b+ monocytes and number of anti-VEGF injections an agent-specific (ranibizumab) phenomenon or is it also present with another anti-VEGF agent (aflibercept)? |
| Population | Patients with neovascular AMD already diagnosed and enrolled in anti-VEGF treatment using aflibercept in a regimen as needed and treated for several months (for practical purposes, we defined eligible patients as those who had completed a loading dose, hence ≥3 mo after diagnosis) in a cohort study in 2015-2018 |
| No. of patients | Of 17 patients, 16 completed all follow-ups; 1 did not wish to continue treatment after 12 mo (not allowed for LOCF) |
| Study | Whether the proportion of CD11b+ circulating monocytes in fresh blood reflected a correlation with the total number of anti-VEGF treatments (mean [SD], 5 [1] injections at 12 mo, 9 [3] at 24 mo, and 13 [4] at 36 mo) |
| 4 | Does correlation between the CD11b expression on monocytes and the number of anti-VEGF injections reflect the presence of a drusenoid environment or VEGF activity? |
| Population | Patients with PCV already diagnosed and enrolled in anti-VEGF treatment using aflibercept in a regimen as needed and treated for several months (for practical purposes, we defined eligible patients as those who had completed a loading dose, hence ≥3 mo after diagnosis) in a cohort study in 2015-2018. |
| No. of patients | Of 15 patients, 11 completed all follow-ups; 3 fulfilled predefined exit criteria (2 dry macula and 1 fibrosis) at a median of 19 (range, 12-19) mo (allowed for LOCF); and 1 did not wish to continue treatment after 26 mo (not allowed for LOCF) |
| Study | Whether the proportion of CD11b+ circulating monocytes in fresh blood indicated the presence of a correlation with the total number of anti-VEGF treatments (mean [SD], 5 [1] injections at 12 months, 8 [3] at 24 mo, and 11 [5] at 36 mo) |
| 5 | Does CD11b expression on monocytes correlate with other known proangiogenic features? |
| Population | Patients recruited for research questions 4 and 5 |
| No. of patients | 32 Patients |
| Study | Expression of the highly proangiogenic chemokine receptor CCR2 on circulating monocytes that were CD11b+ or CD11b−, and the presence of CD11b on circulating monocytes that were CCR2+ or CCR2− |
Abbreviations: AMD, age-related macular degeneration; LOCF, last observation carried forward; PCV, polypoidal choroidal vasculopathy; VEGF, vascular endothelial growth factor.
Retinal Diagnosis and Patient Eligibility
All participants were examined using slitlamp biomicroscopy, digital fundus photography, and spectral-domain optical coherence tomography. Retinal angiography using fluorescein and indocyanine green was performed for documenting and subtyping CNV. Neovascular AMD was diagnosed in the presence of fibrovascular pigment epithelium detachments and evidence of active CNV membrane on retinal angiography.27 Polypoidal choroidal vasculopathy was diagnosed in the presence of at least 1 polyp in the early phase of indocyanine green angiography.24 Supportive evidence of PCV included the presence of a branching vascular network, polyp pulsation, orange-red focal polyplike structures, and a dome-shaped choroid protrusion elevating the retinal pigment epithelium.24
All participants completed a semistructured interview to obtain a complete list of medical conditions, medications, and any ongoing treatments. These data were cross-checked with each patient’s electronic medical record for accuracy. Eligible participants included patients with neovascular AMD or PCV that was deemed treatable with intravitreal anti-VEGF (eg, no fibrotic scar), who agreed to commence treatment at the time of diagnosis, and who adhered to recommended treatment. Because of the immunologic nature of the study, we restricted our study population to those without any immune disease (no participants had autoimmune diseases, infectious diseases, any cancer, immune dysfunction, or history of chemotherapy or immune therapy) to avoid blurring our results. We measured plasma C-reactive protein levels to ensure that no participants were recruited with an ongoing acute-phase response (plasma C-reactive protein level, >15 mg/L [to convert to nanomoles per liter, multiply by 9.524]),28 which is known to alter the systemic immune system. To avoid any potential interaction of systemic antibodies with antibodies in flow cytometry, we did not include participants who had received anti-VEGF therapy recently (<4 weeks for ranibizumab and <8 weeks for aflibercept). Each of the 5 research questions had additional criteria for inclusion (Table). Because of potential interaction between dyes used in retinal angiography and antibodies in flow cytometry, blood was sampled before retinal angiography. For research question 1, we sampled blood from participants with suspected neovascular AMD based on results of the fundus examination and retinal optical coherence tomography scan and awaited confirmation of diagnosis using retinal angiography immediately after blood sampling.
All participants were recruited from a single center (Zealand University Hospital) in Denmark, where access to retinal care is free of charge, including transportation to the hospital and all costs related to anti-VEGF therapy. The recruitment in all substudies was consecutive to avoid any selection bias. Patients initially recruited but who—against our best advice—refused treatment at any point, who diverged from recommended treatment with the first 12 months, or for whom we did not have sufficient data on follow-up were not considered eligible for inclusion in this study. Patients who diverged from recommended treatment after the first 12 months were included but only while the patient followed the recommended treatment regimen.
Clinical Measurements and Anti-VEGF Treatment
Best-corrected visual acuity (BCVA) and mean central retinal thickness (CRT) were measured at time of diagnosis and at all follow-up visits. Best-corrected visual acuity was tested using an LED monitor system (CC-100 charts; Topcon) by personnel specifically trained in Early Treatment of Diabetic Retinopathy Study (ETDRS) charts. Retinal optical coherence tomography was used to measure CRT (Heidelberg Eye Explorer; Heidelberg Engineering).
Eyes received intravitreal injections with 0.05 mL of ranibizumab (Lucentis) or 0.05 mL of aflibercept (Eylea). All injections were given by physicians or specially trained nurses.29 We did not allocate patients to ranibizumab or aflibercept, but were able to study them separately by performing the 2 doctoral dissertation studies consecutively (from January 1, 2010, through December 31, 2013, and from January 1, 2015, through December 31, 2018). Ranibizumab was the only option available at our clinic before introduction of aflibercept in 2013; after introduction of aflibercept, the Danish national guidelines recommended aflibercept as the first choice of treatment. Treatment was administered as needed, with retreatment criteria based on the Danish Ophthalmological Society guideline and recommendations (eTable 1 in the Supplement).30 In patients with PCV, supplementary photodynamic therapy was provided for polyp closure.
Blood Sampling and Flow Cytometry
Venous blood was sampled from antecubital veins in tubes coated with lithium heparin for routine measurement of plasma C-reactive protein levels and EDTA for the flow cytometry protocol within 6 hours of sampling (eTable 2 in the Supplement) using target-specific monoclonal fluorescent antibodies and fluorochrome-matched negative isotypes (eTable 3 in the Supplement). Flow cytometric data were exported for analysis with Kaluza Analysis software (version 1.5.20365.16139; Beckman Coulter). We identified monocytes using forward vs side scatter and then determined the proportion of identified monocytes that were CD11b+ (eFigure 1 in the Supplement). Using CD14 and CD16 labeling, studies by Subhi et al19,21 have previously validated that our protocol using forward vs side scatter indeed identifies monocytes. In research question 5, we also studied the expression of the chemokine receptor CCR2.
Data Collection
Patient-specific data on treatment outcomes (change in BCVA and CRT from baseline to follow-up) were extracted in January 2018 for all patients (baseline, first follow-up [3-4 months after treatment start], and 12-, 24-, and 36-month follow-up). For each patient, we extracted the number of anti-VEGF treatments at 12, 24, and 36 months from electronic medical records. We used the last observation carried forward to account for missing data on all patients who followed our treatment regimen and discontinued owing to predefined exit criteria (untreatable lesion or dry macula). We did not use the last observation carried forward in cases where the patient opted out of treatment owing to high self-perceived burden of monthly or bimonthly injections or if the patient died before the follow-up, because these cases would have required additional injections if the patient had followed the recommended treatment or been alive. If the patient had 2 eyes eligible for the study, we included the first eye enrolled in treatment.31 If both eyes were enrolled at the same time, then the right eye was included.31
Statistical Analysis
Statistical analyses were performed using SPSS software (version 23; IBM Corporation). The presence of normal distribution was evaluated visually on histograms and statistically using the Kolmogorov-Smirnov test. Because normal distribution was present, data were presented as mean (SD) values, unless otherwise indicated, and tested using parametric tests. Changes in BCVA and CRT were tested statistically using the paired-sample t test. The association between the proportion of CD11b+ monocytes and the number of treatments was investigated using linear regression analysis to estimate (research question 1) (including a multiple regression model with forward-selection method to explore and adjust for the variables age, sex, and baseline BCVA and CRT) and Pearson correlation analysis to correlate (research questions 2, 3, and 4). Sensitivity analyses were restricted to participants with 36 months of follow-up and investigated the association with injections per year until patient dropout. Diagnostic ability in estimating at least 12 injections at 36 months used the area under the receiver operating characteristics curve (AUC). Statistical tests were 2-sided and P < .05 was interpreted as statistically significant.
Results
Study Participants
We included a total of 81 participants (44 women [54%] and 37 men [46%]; mean [SD] age, 76 [7] years) (eTable 4 in the Supplement). Mean BCVA on treatment-naive eyes was 64 (14) ETDRS letters; mean CRT, 385 (107) μm. Overall, anti-VEGF treatment improved BCVA at 3 months (mean difference [Δ], 2.8 [95% CI, 0.5-5.0] ETDRS letters; P = .02) and stabilized BCVA at 12 (mean Δ, 2.3 [95% CI, –0.8 to 5.3] ETDRS letters; P = .10), 24 (mean Δ, –0.4 [95% CI, −3.9 to 3.1] ETDRS letters; P = .80), and 36 (mean Δ, –1.7 [95% CI, –5.7 to 2.4] ETDRS letters; P = .40) months (eFigure 2 in the Supplement). Clinically significant improvement (≥15 ETDRS letters) was observed in 12 participants (15%) at 3 months, 14 (17%) at 12 months, 15 (19%) at 24 months, and 12 (15%) at 36 months; clinically significant worsening (≤15 ETDRS letters) was observed in 4 participants (5%) at 3 months, 8 (10%) at 12 months, 11 (14%) at 24 months, and 16 (20%) at 36 months. The CRT significantly decreased at 3 months (mean Δ, –87 [95% CI, –109 to –64] μm; P < .001) and remained decreased at 12 (mean Δ, –77 [95% CI, –104 to –50] μm; P < .001), 24 (mean Δ, –81 [95% CI, –107 to –54] μm; P < .001), and 36 (mean Δ, –74 [95% CI, –101 to –46] μm; P < .001) months (eFigure 2 in the Supplement). Participant characteristics and clinical results are also available per study sample (eTable 4 and eFigure 3 in the Supplement).
Proportion of CD11b+ Monocytes at Day of Diagnosis and Estimation of Later Number of Intravitreal Ranibizumab Injections
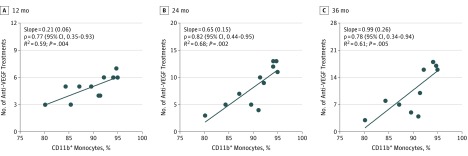
The mean proportion of CD11b+ monocytes at the day of diagnosis was 89.9% (4.7%). The proportion of CD11b+ monocytes estimated the future number of anti-VEGF injections at 12 (ρ = 0.77; 95% CI, 0.35-0.93; P = .004), 24 (ρ = 0.82; 95% CI, 0.44-0.95; P = .002), and 36 (ρ = 0.78; 95% CI, 0.34-0.94; P = .005) months in a univariate model (Figure 1). In a multiple regression model, CD11b+ monocytes continued to estimate the number of anti-VEGF treatments at 12 (β = .77; P = .004), 24 (β = .94; P < .001), and 36 (β = .78; P = .005) months. The only other factor included in the multiple regression model was baseline BCVA, which coestimated the number of anti-VEGF treatments (a higher baseline BCVA lightly estimated more anti-VEGF treatments) at 24 months (β = .38; P = .05). Sensitivity analyses suggested similar associations apart from no statistically significant estimation at 12 months (eTable 5 in the Supplement). The AUC was 1.00 (95% CI, 1.00 to 1.00) (eFigure 4 in the Supplement).
Figure 1. Estimation of the Number of Anti–Vascular Endothelial Growth Factor (Anti-VEGF) Injections Using the Proportion of CD11b+ Monocytes at Time of Diagnosis of Neovascular Age-Related Macular Degeneration.
Univariate linear regression analysis between the proportion of CD11b+ monocytes at time of diagnosis shows a positive and statistically significant ability to estimate the total number of anti-VEGF injections at 12, 24, and 36 months in a treatment regimen consisting of ranibizumab as needed. Data points indicate the association between the proportion of CD11b+ monocytes and the number of anti-VEGF injections at different points for each patient.
Correlation Between CD11b+ Monocytes and Number of Intravitreal Ranibizumab Injections
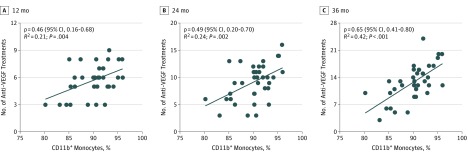
Time of blood sampling ranged from 3 to 35 months after time of diagnosis. Mean proportion of CD11b+ monocytes for ranibizumab treatment was 90.1% (3.7%) and correlated moderately to strongly with the number of anti-VEGF injections at 12 (ρ = 0.46; 95% CI, 0.16-0.68; P = .004), 24 (ρ = 0.49; 95% CI, 0.20-0.70; P = .002), and 36 (ρ = 0.65; 95% CI, 0.41-0.80; P < .001) months (Figure 2). Sensitivity analyses suggested similar associations (eTable 6 in the Supplement). The AUC was 0.84 (95% CI, 0.70-0.98) (eFigure 4 in the Supplement).
Figure 2. Correlation Between the Number of Anti–Vascular Endothelial Growth Factor (Anti-VEGF) Injections and the Proportion of CD11b+ Monocytes.
Monocyte proportions were measured several months after time of diagnosis in patients with neovascular age-related macular degeneration. Pearson correlation analysis shows a positive and statistically significant correlation between the proportion of CD11b+ monocytes and the total number of anti-VEGF injections at 12, 24, and 36 months in a treatment regimen consisting of ranibizumab as needed. Data points indicate the association between the proportion of CD11b+ monocytes and the number of anti-VEGF injections at different points for each patient.
Correlation Between CD11b+ Monocytes and Number of Intravitreal Aflibercept Injections
Mean proportion of CD11b+ monocytes for aflibercept treatment was 95.2% (2.7%). Although the proportion of CD11b+ monocytes did not correlate significantly with the number of anti-VEGF injections at 12 months (ρ = 0.24; 95% CI, –0.27 to 0.65; P = .35), significant correlations emerged at later follow-ups. The proportion of CD11b+ monocytes correlated moderately with the number of anti-VEGF injections at 24 months (ρ = 0.52; 95% CI, 0.03-0.81; P = .04) and strongly correlated with the number of anti-VEGF injections at 36 months (ρ = 0.62; 95% CI, 0.17-0.85; P = .01). Sensitivity analyses suggested similar associations (eTable 7 in the Supplement). The AUC was 0.92 (95% CI, 0.76-1.00) (eFigure 4 in the Supplement). This agent-independent correlation suggests that CD11b+ may correlate more fundamentally to the VEGF activity at sites of injury.
Correlations in PCV
In patients with PCV, mean proportion of CD11b+ monocytes was 92.2% (3.0%). We saw a similar pattern of a nonsignificant weak correlation between proportion of CD11b+ monocytes and number of anti-VEGF injections at 12 months (ρ = 0.27; 95% CI, –0.28 to 0.68; P = .30) and significant, strong correlations at 24 (ρ = 0.60; 95% CI, 0.12-0.85; P = .02) and 36 (ρ = 0.70; 95% CI, 0.27-0.90; P = .005) months. Sensitivity analyses suggested similar associations (eTable 8 in the Supplement). The AUC was 0.86 (95% CI, 0.66-1.00) (eFigure 4 in the Supplement). These correlations in PCV—a nondrusenoid VEGF-mediated disease—suggest that the association may be more fundamentally related to the systemic contribution to CNV.
A Profile of Chemotaxis and Proangiogenesis
We found that most CD11b+ monocytes expressed CCR2, whereas the proportion of CCR2-expressing monocytes were much smaller in CD11b− monocytes (P < .001) (Figure 3). Almost all CCR2+ monocytes expressed CD11b, whereas only approximately half of CCR2− monocytes expressed CD11b (P < .001) (Figure 3).
Figure 3. Association Between CD11b and Proangiogenic Receptor CCR2.
The strong association between CD11b and CCR2 provides further evidence of a chemotaxic and proangiogenic profile for CD11b+ monocytes. CD11b+ monocytes are highly positive for CCR2, whereas only a small proportion of CD11b− monocytes are positive for CCR2. Almost all CCR2+ monocytes are CD11b+, whereas only half of CCR2− monocytes are CD11b+. Bars indicate mean; whiskers, 95% CI.
Discussion
Circulating monocytes play an important role in CNV formation in experimental models of laser-induced CNV and are an important source of VEGF in CNV.7,8,9 Sakurai et al8 demonstrated that depletion of circulating monocytes leads to a significant reduction (approximately 90%) of CNV size. McLeod et al32 investigated postmortem eyes from patients with neovascular AMD and found numerous macrophages in the choroid near areas of CNV. Histopathologic studies of surgically excised PCV specimens also describe the presence of macrophages and mononuclear cells in the choroid near the PCV lesions.33,34 Kumar et al35 recently demonstrated that PCV may be a disease with 2 stages: an initiation stage in which local choroidal vascular factors lead to polyp formation and a progression stage in which monocytes and macrophages lead to exudative changes through VEGF expression and angiogenesis. Together this evidence supports that dynamics of circulating monocytes contribute to VEGF-mediated CNV formation. Our study supports the evidence behind this mechanism and demonstrates that phenotypical characteristics of these circulating monocytes may have important clinical implications and can estimate the number of anti-VEGF injections. The number of injections varies more with time, which may explain why correlations generally become stronger with time. However, the association may not be related to BCVA or CRT (eTable 9 in the Supplement) because these clinical outcomes were more strongly associated with treatment adherence, retinal injury, and subretinal fibrosis.
Proangiogenic potential and specific characteristics of monocytes and macrophages have been the attention of CNV-related laboratory and clinical studies.10,11,12,13,14,15,16,17,18,19,20,21 Cousins et al36 found that circulating monocytes with a high production of proinflammatory and proangiogenic cytokines are more prevalent in patients with AMD. Chen et al15 found that circulating monocytes from patients with neovascular AMD secrete VEGF at a much higher level when compared with samples from healthy controls. Subhi et al21 previously identified that patients with neovascular AMD and PCV have increased proportions of CD11b+ circulating monocytes. Interestingly, Calippe et al37 found that CD11b+ mononuclear phagocytes, including circulating monocytes, interact directly with complement factor H and play an important role in AMD. Homeostatic elimination of mononuclear phagocytes from the subretinal space is mediated by thrombospondin 1 binding; however, this process is obstructed by complement factor H binding on CD11b, and thrombospondin 1 binding is more potent in the AMD-associated complement factor H (Y402H) variant.3,37 Overall, ample evidence describes how CD11b+ monocytes are proangiogenic in CNV diseases such as neovascular AMD. We wondered whether the proangiogenic activity of CD11b+ immune cells was based on the local resident CD11b+ macrophages and microglia, on the CD11b+ circulating monocytes, or both. This aspect was clarified in a recent study by Brockmann et al,38 who found that inhibition of the local resident CD11b+ macrophages and microglia did not provide any significant reduction of CNV size and concluded that the CNV-related activity of CD11b+ cells may come from the systemic circulation. This concluding hypothesis by Brockmann et al38 aligns with the findings of our study.
Strengths and Limitations
One important strength of this study is that we investigated the association between CD11b+ circulating monocytes and number of anti-VEGF injections from multiple angles by answering different research questions. Consistent findings when investigating a phenomenon from different angles suggest validity evidence. This evidence includes using different fluorochromes (eTable 3 in the Supplement), leading to slight differences in mean CD11b+ in different study samples. Limitations include not considering patients with any ongoing immune disease or therapy as eligible. On one hand, we did not want to mix such immune states with underlying CNV-related immunology; on the other, investigating patients with CNV who have immune diseases or are in ongoing immune therapy may help understanding the relationship between systemic alterations of the immune system and CNV formation.39 Therefore, we can only generalize our results to patients without any immune disease or therapy. Although methods for handling missing data allow analysis, they do not actually provide the missing data, and this factor introduces some uncertainty to the results. Whether characteristics and function of circulating monocytes undergo short- and long-term fluctuation during anti-VEGF treatment is relatively unexplored.18 Finally, we performed a small single-center study, and larger studies with replications of our findings are warranted.
Conclusions
This study found evidence that the proportion of CD11b+ monocytes in a patient with neovascular AMD or PCV can potentially estimate the long-term number of anti-VEGF injections needed. We propose that CD11b+ on circulating monocytes may provide insight into disease prognosis and give patients some insight into what can be expected in terms of long-term need for intravitreal therapy. Although these data suggest measuring the proportion of CD11b+ circulating monocytes to estimate the number of anti-VEGF injections, additional longitudinal studies would be needed to determine whether these findings have clinical relevance to influence treatment algorithms or provide novel targets for medical therapy. The association identified in this investigation between the application of anti-VEGF injections and the proportion of circulating CD11b+ monocytes suggests that targeting these monocytes, directly or indirectly, may help identify novel therapeutics against CNV-dominated diseases.
eTable 1. Summary of the pro re nata Treatment Regimen in this Study
eTable 2. Protocol for Blood Preparation for Flow Cytometry
eTable 3. Antibodies Used for Flow Cytometry for Different Study Parts
eTable 4. Participant Characteristics
eTable 5. Sensitivity Analyses for Linear Regression Statistics Made in Research Question 1
eTable 6. Sensitivity Analyses for Correlation Statistics Made in Research Question 2
eTable 7. Sensitivity Analyses for Correlation Statistics Made in Research Question 3
eTable 8. Sensitivity Analyses for Correlation Statistics Made in Research Question 4
eTable 9. Correlation Between CD11b+ Monocytes and Best-Corrected Visual Acuity (BCVA) in ETDRS Letters and Mean Central Retinal Thickness (CRT)
eFigure 1. Methods for Investigating the Proportion of CD11b+ Monocytes (Research Questions 1-4) and Its Relationship to CCR2 Expression (Research Question 5)
eFigure 2. Eighty-one Eyes of 81 Patients Were Treated in a pro re nata Regimen and Followed for 36 Months
eFigure 3. Anti-VEGF Treatment Results in Terms of Best-Corrected Visual Acuity (BCVA) and Central Retinal Thickness (CRT) Stratified by Study Population
eFigure 4. ROC Curves for CD11b+ Monocytes’ Predictive Ability of Number of Anti-VEGF Injections During a Period of 36 Months (<12 Injections vs ≥12 Injections)
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. ; Vision Loss Expert Group of the Global Burden of Disease Study . Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 2.Sedeh FB, Scott DAR, Subhi Y, Sørensen TL. Prevalence of neovascular age-related macular degeneration and geographic atrophy in Denmark. Dan Med J. 2017;64(11):A5422. [PubMed] [Google Scholar]
- 3.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. doi: 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 4.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001;20(3):385-414. doi: 10.1016/S1350-9462(00)00025-2 [DOI] [PubMed] [Google Scholar]
- 5.Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F. On phagocytes and macular degeneration. Prog Retin Eye Res. 2017;61:98-128. doi: 10.1016/j.preteyeres.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 6.Grunin M, Hagbi-Levi S, Chowers I. The role of monocytes and macrophages in age-related macular degeneration. Adv Exp Med Biol. 2014;801:199-205. doi: 10.1007/978-1-4614-3209-8_26 [DOI] [PubMed] [Google Scholar]
- 7.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3586-3592. doi: 10.1167/iovs.03-0038 [DOI] [PubMed] [Google Scholar]
- 8.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3578-3585. doi: 10.1167/iovs.03-0097 [DOI] [PubMed] [Google Scholar]
- 9.Ma W, Zhang Y, Gao C, Fariss RN, Tam J, Wong WT. Monocyte infiltration and proliferation reestablish myeloid cell homeostasis in the mouse retina following retinal pigment epithelial cell injury. Sci Rep. 2017;7(1):8433. doi: 10.1038/s41598-017-08702-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas P, Aggermann T, Nagl M, Steindl-Kuscher K, Krugluger W, Binder S. Implication of CD21, CD35, and CD55 in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2011;152(3):396-399.e1. doi: 10.1016/j.ajo.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 11.Sennlaub F, Auvynet C, Calippe B, et al. CCR2+ monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013;5(11):1775-1793. doi: 10.1002/emmm.201302692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunin M, Burstyn-Cohen T, Hagbi-Levi S, Peled A, Chowers I. Chemokine receptor expression in peripheral blood monocytes from patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(9):5292-5300. doi: 10.1167/iovs.11-9165 [DOI] [PubMed] [Google Scholar]
- 13.Hagbi-Levi S, Grunin M, Jaouni T, et al. Proangiogenic characteristics of activated macrophages from patients with age-related macular degeneration. Neurobiol Aging. 2017;51:71-82. doi: 10.1016/j.neurobiolaging.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 14.Grunin M, Hagbi-Levi S, Rinsky B, Smith Y, Chowers I. Transcriptome analysis on monocytes from patients with neovascular age-related macular degeneration. Sci Rep. 2016;6:29046. doi: 10.1038/srep29046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Lechner J, Zhao J, et al. STAT3 activation in circulating monocytes contributes to neovascular age-related macular degeneration. Curr Mol Med. 2016;16(4):412-423. doi: 10.2174/1566524016666160324130031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner J, Chen M, Hogg RE, et al. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8). J Neuroinflammation. 2017;14(1):42. doi: 10.1186/s12974-017-0820-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, Faber C, Falk M, Nissen MH, Hviid TV, Sørensen TL. Altered expression of CD46 and CD59 on leukocytes in neovascular age-related macular degeneration. Am J Ophthalmol. 2012;154(1):193-199.e2. doi: 10.1016/j.ajo.2012.01.036 [DOI] [PubMed] [Google Scholar]
- 18.Subhi Y, Lykke Sørensen T. New neovascular age-related macular degeneration is associated with systemic leucocyte activity. Acta Ophthalmol. 2017;95(5):472-480. doi: 10.1111/aos.13330 [DOI] [PubMed] [Google Scholar]
- 19.Subhi Y, Krogh Nielsen M, Molbech CR, Sørensen TL. Altered proportion of CCR2+ and CX3CR1+ circulating monocytes in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Clin Exp Ophthalmol. 2018;46(6):661-669. doi: 10.1111/ceo.13152 [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Falk MK, Hviid TV, Sørensen TL. Increased expression of CD200 on circulating CD11b+ monocytes in patients with neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1029-1037. doi: 10.1016/j.ophtha.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Subhi Y, Krogh Nielsen M, Molbech CR, et al. CD11b and CD200 on circulating monocytes differentiate two angiographic subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2017;58(12):5242-5250. doi: 10.1167/iovs.17-22479 [DOI] [PubMed] [Google Scholar]
- 22.Li L, Heiduschka P, Alex AF, Niekämper D, Eter N. Behaviour of CD11b-positive cells in an animal model of laser-induced choroidal neovascularisation. Ophthalmologica. 2017;237(1):29-41. doi: 10.1159/000453550 [DOI] [PubMed] [Google Scholar]
- 23.Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29(1):19-29. doi: 10.1016/j.preteyeres.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 24.Lorentzen TD, Subhi Y, Sørensen TL. Prevalence of polypoidal choroidal vasculopathy in white patients with exudative age-related macular degeneration: systematic review and meta-analysis. Retina. 2018;38(12):2363-2371. doi: 10.1097/IAE.0000000000001872 [DOI] [PubMed] [Google Scholar]
- 25.The 2017 Jackson Memorial Lecture, Daniel F. Martin [press release]. New Orleans, LA: American Academy of Ophthalmology; November 12, 2017. https://www.aao.org/eyenet/academy-live/detail/reshaping-retina-practice-2017-jackson-memorial-le. Accessed May 22, 2018.
- 26.Bogunovic H, Waldstein SM, Schlegl T, et al. Prediction of anti-VEGF treatment requirements in neovascular and using a machine learning approach. Invest Ophthalmol Vis Sci. 2017;58(7):3240-3248. doi: 10.1167/iovs.16-21053 [DOI] [PubMed] [Google Scholar]
- 27.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113(2):260-266. doi: 10.1016/j.ophtha.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 28.Rifai N, Ridker PM. Proposed cardiovascular risk assessment algorithm using high-sensitivity C-reactive protein and lipid screening. Clin Chem. 2001;47(1):28-30. [PubMed] [Google Scholar]
- 29.Chakouri S, Mundt LP, Subhi Y, Sørensen TL. Physician assistants and nurse practitioners in ophthalmology: has the time come? Am J Ophthalmol. 2018;186:174-175. doi: 10.1016/j.ajo.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 30.Danish Ophthalmological Society Treatment recommendation including medicine recommendation for treatment of wet age-related macular degeneration [society guideline]. http://www.dansk-oftalmologisk-selskab.dk/arkiver/502. Published May 2, 2018. Accessed May 22, 2018.
- 31.Bunce C, Patel KV, Xing W, Freemantle N, Doré CJ; Ophthalmic Statistics Group . Ophthalmic statistics note 1: unit of analysis. Br J Ophthalmol. 2014;98(3):408-412. doi: 10.1136/bjophthalmol-2013-304587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLeod DS, Bhutto I, Edwards MM, Silver RE, Seddon JM, Lutty GA. Distribution and quantification of choroidal macrophages in human eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(14):5843-5855. doi: 10.1167/iovs.16-20049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49(11):4729-4737. doi: 10.1167/iovs.08-2134 [DOI] [PubMed] [Google Scholar]
- 34.Tso MOM, Suarez MJ, Eberhart CG. Pathologic study of early manifestations of polypoidal choroidal vasculopathy and pathogenesis of choroidal neo-vascularization. Am J Ophthalmol Case Rep. 2017;11:176-180. doi: 10.1016/j.ajoc.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Nakashizuka H, Jones A, et al. Proteolytic degradation and inflammation play critical roles in polypoidal choroidal vasculopathy. Am J Pathol. 2017;187(12):2841-2857. doi: 10.1016/j.ajpath.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122(7):1013-1018. doi: 10.1001/archopht.122.7.1013 [DOI] [PubMed] [Google Scholar]
- 37.Calippe B, Augustin S, Beguier F, et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity. 2017;46(2):261-272. doi: 10.1016/j.immuni.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 38.Brockmann C, Kociok N, Dege S, et al. Local partial depletion of CD11b+ cells and their influence on choroidal neovascularization using the CD11b-HSVTK mouse model. Acta Ophthalmol. 2018;96(7):e789-e796. doi: 10.1111/aos.13716 [DOI] [PubMed] [Google Scholar]
- 39.Bak M, Sørensen TL, Flachs EM, et al. Age-related macular degeneration in patients with chronic myeloproliferative neoplasms. JAMA Ophthalmol. 2017;135(8):835-843. doi: 10.1001/jamaophthalmol.2017.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of the pro re nata Treatment Regimen in this Study
eTable 2. Protocol for Blood Preparation for Flow Cytometry
eTable 3. Antibodies Used for Flow Cytometry for Different Study Parts
eTable 4. Participant Characteristics
eTable 5. Sensitivity Analyses for Linear Regression Statistics Made in Research Question 1
eTable 6. Sensitivity Analyses for Correlation Statistics Made in Research Question 2
eTable 7. Sensitivity Analyses for Correlation Statistics Made in Research Question 3
eTable 8. Sensitivity Analyses for Correlation Statistics Made in Research Question 4
eTable 9. Correlation Between CD11b+ Monocytes and Best-Corrected Visual Acuity (BCVA) in ETDRS Letters and Mean Central Retinal Thickness (CRT)
eFigure 1. Methods for Investigating the Proportion of CD11b+ Monocytes (Research Questions 1-4) and Its Relationship to CCR2 Expression (Research Question 5)
eFigure 2. Eighty-one Eyes of 81 Patients Were Treated in a pro re nata Regimen and Followed for 36 Months
eFigure 3. Anti-VEGF Treatment Results in Terms of Best-Corrected Visual Acuity (BCVA) and Central Retinal Thickness (CRT) Stratified by Study Population
eFigure 4. ROC Curves for CD11b+ Monocytes’ Predictive Ability of Number of Anti-VEGF Injections During a Period of 36 Months (<12 Injections vs ≥12 Injections)