This meta-analysis reviews previously published studies to determine the rate of skip metastasis to neck level IV in patients with clinically node-negative neck oral cavity squamous cell carcinoma.
Key Points
Question
What is the rate of skip metastasis to neck level IV in patients with clinically node-negative neck (cN0) oral cavity squamous cell carcinoma (OCSCC)?
Findings
In this meta-analysis of 13 studies, the rate of skip metastasis to neck level IV in patients with cN0 OCSCC was found to be extremely low. The overall rate of level IV involvement was between 0% and 11.4%.
Meaning
Supraomohyoid neck dissection appears to be an adequate treatment for patients with cN0 OCSCC.
Abstract
Importance
The rate of skip metastasis to neck level IV in patients with clinically node-negative neck (cN0) oral cavity squamous cell carcinoma (OCSCC) remains controversial.
Objective
To provide a high level of evidence using a meta-analysis on the rate of skip metastasis to level IV in this subset of patients.
Data Sources
The Embase, PubMed, and Google Scholar databases were searched for articles published during the period of January 1, 1970, through December 31, 2017, using the following key terms: neck dissection, N0 neck, squamous cell carcinoma, skip metastasis, radical neck dissection, lymph node management, neck metastasis, oral cavity cancer, and tongue cancer. Some terms were also used in combination, and the reference section of each article was searched for additional potentially relevant publications. Data were analyzed from January 8 through 11, 2018.
Study Selection
Inclusion criteria were all cohorts, including from any randomized clinical trial, case-control study, case study, and case report; studies of patients with the histopathologic diagnosis of OCSCC; and studies that differentiated data between skip metastasis and sequential metastasis to neck level IV. Of the 115 articles retrieved from the literature, 11 retrospective studies and 2 prospective randomized clinical trials (n = 1359 patients) were included.
Data Extraction and Synthesis
Meta-analysis of Observational Studies in Epidemiology guidelines were followed. Fixed-effects model and 95% CIs were estimated, and data of included studies were pooled using a fixed-effects model.
Main Outcomes and Measures
Overall proportion of neck involvement and the rate of level IV skip metastasis. Subgroup analysis for primary site and tumor staging.
Results
The rate of level IV involvement in patients with cN0 ranged between 0% and 11.40% with a fixed-effects model of 2.53% (95% CI, 1.64%-3.55%). The rate of skip metastasis ranged from 0% to 5.50% with a fixed-effects model of 0.50% (95% CI, 0.09%-1.11%). The rate of level IV skip metastasis did not increase significantly in cases that involved neck levels I through III. Tumor staging and primary site tumor did not significantly affect the rate of skip metastasis.
Conclusions and Relevance
This meta-analysis showed very low rates of skip metastasis to neck level IV in patients diagnosed with cN0 OCSCC. Encountering an allegedly positive lymph node during neck dissection does not portend high rates of level IV involvement. Supraomohyoid neck dissection is therefore adequate for this subset of patients.
Introduction
The treatment of oral cavity squamous cell carcinoma (OCSCC) has changed considerably over the past few decades.1 The pendulum has swung from extensive and radical neck surgeries to modified and selective types of neck dissections (NDs).2,3,4,5 The term supraomohyoid neck dissection (SOHND) refers to the removal of lymph nodes contained in levels I through III of the neck and is currently referred to as a selective ND in levels I through III.6 This type of ND has been frequently used in the management of clinically node-negative neck (cN0) in OCSCC and provides similar control rates as more extensive forms of NDs.7,8,9 However, several studies have concluded that SOHND is inadequate in patients with OCSCC, owing to occult metastasis to neck level IV, and that this level should be routinely dissected.10,11 The designation of the neck level of skip metastasis, the involvement of neck level IV without the involvement of previous levels, in patients with OCSCC remains a matter of controversy.12 Advocates for including level IV in routine NDs claim that it minimizes neck recurrence and improves prognosis, while opponents remain doubtful of its survival benefit and further argue that it harbors added morbidity and longer surgical time.13
The aim of this study is to conduct a meta-analysis of all relevant published literature to scrutinize the rate of skip metastasis to level IV in patients diagnosed with OCSCC without preoperative evidence of neck involvement.
Methods
Information Sources and Search Strategy
We performed a methodical and comprehensive search for all relevant articles in the English literature published between January 1970 and December 2017 by using the electronic databases Embase, PubMed, and Google Scholar to search the key terms neck dissection, N0 neck, squamous cell carcinoma, skip metastasis, radical neck dissection, lymph node management, neck metastasis, oral cavity cancer, and tongue cancer. Some terms were also used in combination. The reference section of each article was searched for additional potentially relevant publications.
Study Eligibility Criteria
All studies that included patients who underwent an ND of at least levels I through IV and were judged clinically to be preoperatively free of lymph node metastasis were eligible for inclusion in this meta-analysis. The inclusion criteria for the study design were (1) any prospective or retrospective cohort, including from any randomized clinical trial, case-control study, case study, and case report; (2) a study population with the histopathologic diagnosis of OCSCC; and (3) studies that differentiated between true skip metastasis (metastasis solely at neck level IV) and sequential metastasis to neck level IV. Studies that involved mixed populations of cN0 and clinically node-positive (cN+) necks were included only if they enabled sequestration of data that pertained solely to the cN0 necks. Exclusion criteria were (1) studies on patients who had undergone preoperative radiotherapy and chemotherapy, (2) studies on recurrent tumors, and (3) studies that did not enable differentiation between data extracted from patients with cN+ and those with cN0 necks.
Data Extraction
Information regarding study design, patient characteristics, primary tumor treatment, sample size, and average follow-up time was retrieved from the selected articles. Data were initially extracted and evaluated by the 2 principal investigators (A.W. and R.R.) and thereafter rechecked and confirmed by 3 other investigators (N.C., D.M.F., and G.H.). The distributions of the T category, extent of ND, subsite of the primary tumor, and nodal metastasis were recorded (Table 1). A skip metastasis was defined as a positive level IV node on final pathology without the involvement of higher levels (ie, levels I-III). A level IV nodal metastasis coexisting with nodes at other neck levels was assessed separately. Because most of the available studies were retrospective and observational, we followed the guidelines for meta-analysis of observational studies.14
Table 1. Study Characteristics.
| Source | Design | Year of Accrual | Sample Size, No. | Age Range, y | Male/Female, No. | ND Level | Subsite(s) | T Stage | Metastasis to Level IV | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Khafif et al,15 2001 | Retrospective | 1983-1998 | 17a | 24-79 | NA | I-IV | Tongue | 1-3 | 1 | Average, 4.1 y |
| Byers et al,10 1997 | Retrospective | 1970-1990 | 163a | NA | NA | I-IV | Tongue | 1-4 | 9 | >1 y |
| Balasubramanian et al,16 2012 | Retrospective | 2003-2008 | 52 | 27-80 | 43/9 | I-IV | Tongue | 1-4 | 2 | Average, 2 y |
| Crean et al,13 2003 | Retrospective | 1996-1999 | 49 | Average, 63 | 24/25 | I-IV | Tongue, alveolus, buccal mucosa, FOM, lip, RMT, hard palate | 1-4 | 5 | 1-3 y |
| Feng et al,17 2014 | Retrospective | 1995-2010 | 190a | 19-87 | NA | I-IV | Tongue, alveolus, buccal mucosa, FOM, lip, RMT, hard palate | 1-4 | 6 | 3-18 y |
| Mishra et al,18 2010 | Retrospective | 2006-2008 | 13a | NA | NA | I-IV | Tongue, alveolus, buccal mucosa, FOM, lip, RMT | 1-3 | 0 | Minimum, 1-3 y |
| Vishak and Rohan,19 2014 | Retrospective | 2006-2007 | 57 | 25-65 | 43/14 | I-IV | Tongue | 1 | 2 | NA |
| Cariati et al,20 2018 | Retrospective | 2004-2010 | 88a | 19-81 | NA | I-IV | Tongue | 1-4 | 10 | ≥5 y |
| Dias et al,12 2006 | Retrospective | 1987-1997 | 71a | NA | NA | I-IV | Tongue, FOM | 1-4 | 3 | ≥3 y |
| Shah et al,5 1990 | Retrospective | 1965-1986 | 192a | 17-95 | NA | I-IV | Tongue, alveolus, buccal mucosa, FOM, RMT | 1-4 | 6 | NA |
| Guo et al,21 2014 | Retrospective | 1999-2010 | 160a | NA | 84/76 | I-V | Tongue, alveolus, RMT, buccal mucosa, hard palate | 1-4 | 2 | Median, 76 mo |
| Brazilian Head and Neck Cancer Study Group,9 1998 | Prospective | 1990-1993 | 76a | NA | 58/18 | I-IV | Tongue, alveolus, FOM, RMT | 2-4 | 5 | NA |
| Agarwal et al,22 2018 | Prospective | 2011-2015 | 231 | 22-82 | 190/41 | I-IV | Tongue, alveolus, buccal mucosa, FOM, lip | 1-4 | 0 | Mean, 21 mo |
Abbreviations: FOM, floor of mouth; NA, not available; ND, neck dissection; RMT, retromolar trigone.
Sample size extracted for analysis and is not the entire cohort of the study.
Statistical Analysis
Fixed-effects and random-effects meta-analyses of single proportions were used in conjunction with the inverse variance method to calculate the overall proportion. The Freeman-Tukey double arcsine transformation was implemented to calculate overall proportions. The Clopper-Pearson method (exact binomial) was used to calculate the CI for the individual study results. Result heterogeneity among the studies was quantified using the inconsistency index I2, and a value higher than 75% was considered to be substantial heterogeneity. Statistical analysis was conducted using R statistical software (version 3.3.3, R Foundation).
Results
Study Selection
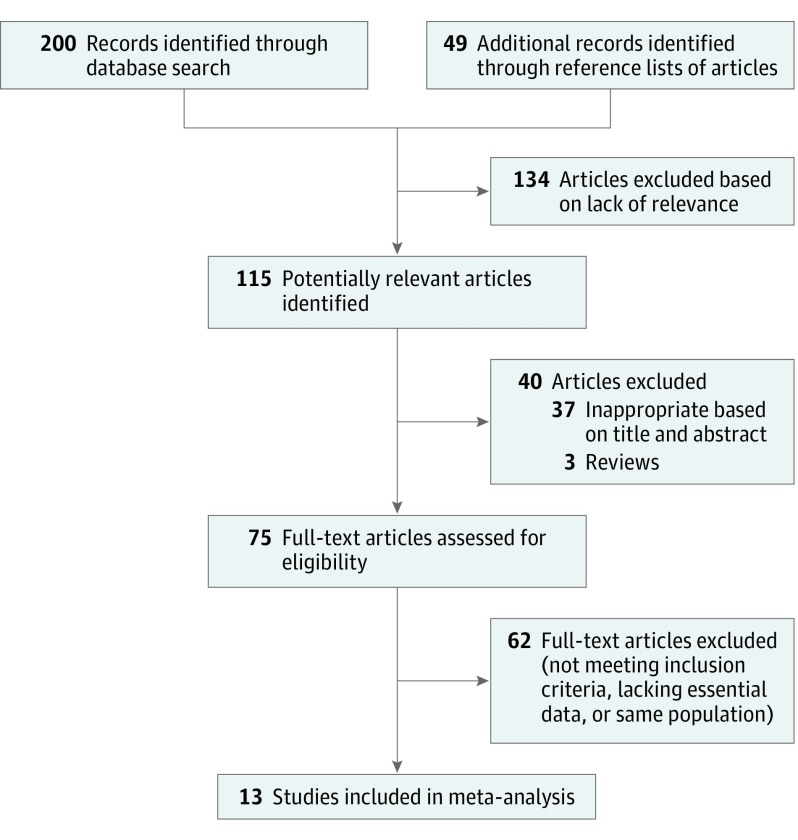
The search strategy identified 115 articles published from January 1, 1970, to December 31, 2017. These articles were selected and transferred into EndNote (Thomson Reuters), and replicates were removed. The various phases of assessing the abstracts and reasons for exclusion from the meta-analysis are depicted in Figure 1. A total of 11 retrospective and 2 prospective randomized clinical studies that met our inclusion criteria, with a total of 1359 patients, were subsequently included in the meta-analysis (Table 1).5,9,10,12,13,15,16,17,18,19,20,21,22
Figure 1. Article Selection.
Meta-analysis Results
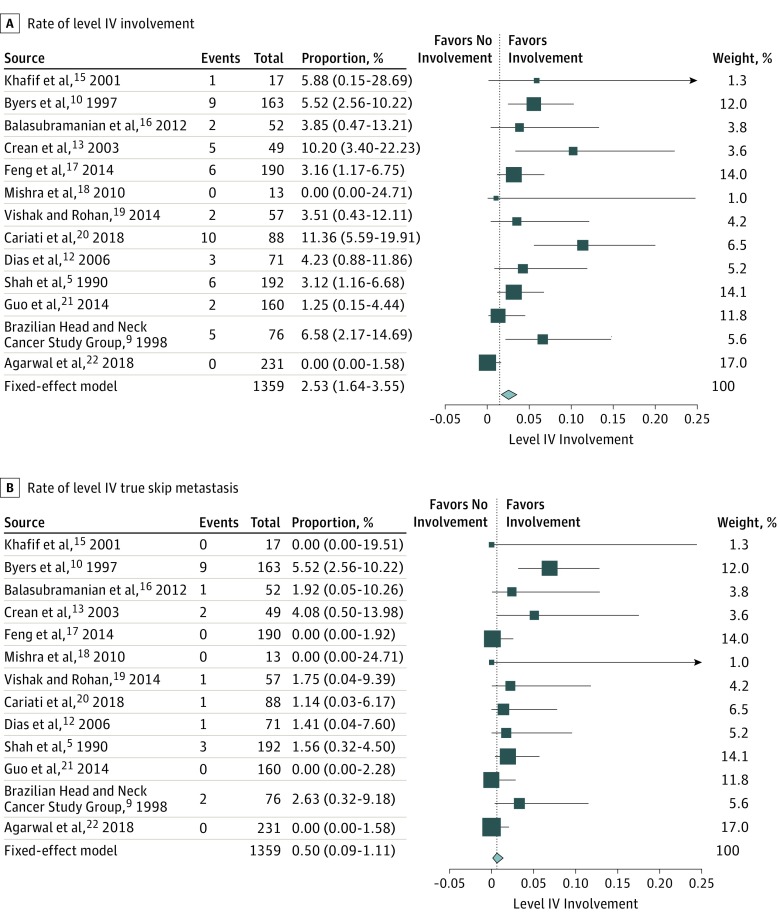
The rate of involvement of level IV among the patients with cN0 was 0% to 11.40% with a fixed-effects model of 2.53% (95% CI, 1.64%-3.55%) (Figure 2A).5,9,10,12,13,15,16,17,18,19,20,21,22 According to results of the meta-analysis, the rate of skip metastasis was extremely low, ranging from 0% to 5.50% with a fixed-effects model of 0.50% (95% CI, 0.09%-1.11%) (Figure 2B).5,9,10,12,13,15,16,17,18,19,20,21,22
Figure 2. Rate of Level IV Involvement and True Skip Metastasis in Preoperative Patients With Clinically Node-Negative Necks.
Various combinations of metastatic patterns with involvement of level IV were analyzed separately in subgroup analyses (Table 2).9,12,13,15,16,17,18,19,21,22 The rate of level IV metastasis did not increase significantly in cases that involved higher levels (ie, levels I-III) of the neck. In fact, the highest fixed-effect model was 0.04% (95% CI, 0%-0.63%) for involvement of levels I, II, and IV. All other fixed-effects models equaled 0% (95% CI, 0%-0.75%). A subgroup analysis according to T stage showed that level IV involvement was 0% (n = 401; 95% CI, 0%-0.63%) for stages I and II and 0% (n = 129; 95% CI, 0%-1.16%) for stages III and IV.
Table 2. Subgroup Analysis Showing Metastatic Patterns With Involvement of Level IV in Preoperative Patients With Clinically Node-Negative Necks.
| Combination of Involved Neck Levels | Source | No. of Patients | Fixed-Effect Model (95% CI), % |
|---|---|---|---|
| I, II, III, IV | Khafif et al,15 2001 | 384 | 0 (0-0.75) |
| Balasubramanian et al,16 2012 | |||
| Mishra et al,18 2010 | |||
| Dias et al,12 2006 | |||
| Agarwal et al,22 2018 | |||
| I, II, IV | Crean et al,13 2003 | 643 | 0.04 (0-0.63) |
| Feng et al,17 2014 | |||
| Mishra et al,18 2010 | |||
| Guo et al,21 2014 | |||
| Agarwal et al,22 2018 | |||
| I, III, IV | Crean et al,13 2003 | 293 | 0 (0-0.23) |
| Mishra et al,18 2010 | |||
| Agarwal et al,22 2018 | |||
| I, IV | Mishra et al,18 2010 | 320 | 0 (0-0.27) |
| Brazilian Head and Neck Cancer Study Group,9 1998 | |||
| Agarwal et al,22 2018 | |||
| II, III, IV | Feng et al,17 2014 | 491 | 0 (0-0.37) |
| Mishra,18 et al 2010 | |||
| Vishak and Rohan,19 2014 | |||
| Agarwal et al,22 2018 | |||
| II, IV | Feng et al,17 2014 | 434 | 0 (0-0.35) |
| Mishra et al,18 2010 | |||
| Agarwal et al,22 2018 | |||
| III, IV | Crean et al,13 2003 | 643 | 0 (0-0.23) |
| Feng et al,17 2014 | |||
| Mishra et al,18 2010 | |||
| Guo et al,21 2014 | |||
| Agarwal et al,22 2018 |
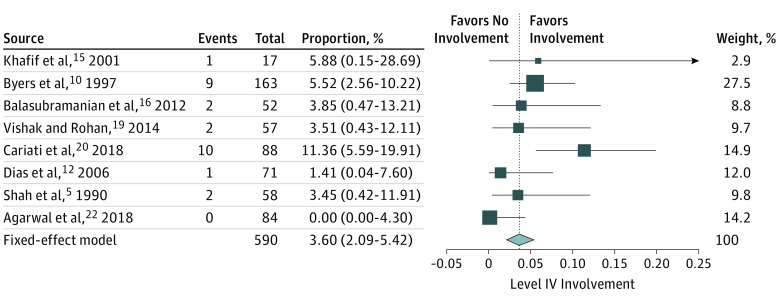
Categorization by oral cavity subsites revealed significant findings only on oral tongue primary lesions. The analysis included 8 studies with 590 patients. The rate of involvement of neck level IV was 0% to 11.40% with a fixed-effects model of 3.60% (95% CI, 2.09%-5.42%) (Figure 3).5,10,12,15,16,19,20,22
Figure 3. Oral Tongue Primary: Rate of Level IV Involvement in Preoperative Patients With Clinically Node-Negative Necks.
Discussion
Although it is well established that patients with OCSCC are at high risk for lymph node metastasis, the extent of nodal involvement for each neck level remains controversial.12 One of the main confounders is the heterogeneity of the study groups, which results in the lack of data stratification by T stages, subsites, and involvement of other neck levels. Another bias in many publications stems from combining the results of the primary neck surgery with those of revision surgeries for neck recurrences. These drawbacks became apparent during the process of data extraction, and they were addressed by excluding all patients with revision NDs and by omitting all groups lacking this information.
After performing a meta-analysis of all extracted data, we found that the actual rate of skip metastasis to level IV was only 0.5%. We also performed several subgroup analyses, the first of which was designed to account for the various primary tumor sites. Unfortunately, data in almost all of the analyzed articles failed to report the relations between the primary tumor site and the neck levels involved by metastatic tumor. Only primary lesions of the tongue could be accurately assessed. That analysis again confirmed a relatively low rate of skip metastasis, with a fixed-effects model of 3.60% (range, 0%-11.40%; 95% CI, 2.09%-5.42%). Another subgroup analysis was made on the various T stages. Once again, the majority of articles reviewed did not provide the rates of lymph node involvement according to the pathological T staging. Many articles did not differentiate between the various T stages or combined them into low (I-II) and high (III-IV) stages. Even so, the rate of the various T stages had relatively low influence on the rate of skip metastasis. Specifically, the rate of skip metastasis was 0% for advanced stage disease as well as for early stage disease, which was surprising considering that higher T stages are associated with infamously high rates of lymph node involvement. However, this figure probably represents a selection bias. Because only a small number of articles provided the correlation between T stages and skip metastasis, these articles shift the statistical analysis to manifest negligible level IV metastasis. On the same note, a recently published study on the Surveillance, Epidemiology, and End Results Program database23 did find a linear correlation between the initial T stage and neck involvement, including level IV, but did not differentiate patients deemed preoperative as clinically cN0. This article also provided data on the relation between the primary site tumor and neck metastasis. As for both parameters (T stage and primary site), neck level IV involvement did not exceed 11.2%, even for patients with a cN+ neck on presentation.
The idea of skip metastasis was initially described by Byers et al10 and refers to the condition in which OCSCC bypasses levels I, II, or both and goes directly to levels III or IV. Those authors reported a 15.8% rate of skip metastasis and therefore recommended routine dissection at neck level IV. Careful analysis of their data, however, revealed that only 5.5% of patients with clinical cN0 disease had skip metastasis to level IV in the initial ND specimen. Moreover, they described another 9 patients (9.9%) with recurrences at neck level IV, which had not been included in an earlier ND. Accounting for neck recurrence as a missed pathological lymph node in the primary surgery is problematic. The neck has now lost its anatomical lymphatic drainage and, in many cases, radiated. Because no data on recurrence in other dissected levels were provided, it is impossible to accurately calculate the rate of regional recurrence. Notwithstanding, even when accounting for all of the cases mentioned in this study, the incidence of skip metastasis or subsequent recurrence in level IV was only 4.8% (13 of 270). As such, concluding that neck level IV should be routinely dissected is open to question, even with a low tolerance threshold for adding neck levels to diminish recurrences.
Crean et al13 demonstrated that 5 of 49 (10%) patients had involvement of neck level IV despite having been preoperatively diagnosed with a cN0 neck. Only one of those patients (2%) had a true skip metastasis to level IV. After revisiting the database, our main criticism is the obvious inclination of the study population toward advanced disease, with 39% of the patients at stage T4 and only 6% at stage T1. However, the results of the study raise the question of the necessity of performing a level IV ND in advanced stage disease. The results of the present study failed to find advanced stage disease as an indication for extending the ND.
Another article, by Cariati et al,20 was recently published and demonstrated double-figure recurrences in the neck. Notably, the true rate of level IV involvement, as clearly stated by the authors, was 7.4%. There was only 1 case (1.2%) of skip metastasis to level IV. Although they did report the relationship between various risk factors and neck involvement, they did not provide a subgroup analysis of skip metastasis with regard to the initial T stage. We therefore disagree with those authors’ opinion that all NDs must include level IV. Our rebuttal is based on the results of the present meta-analysis, which is almost 20-fold larger and reveals an overall involvement rate of 2.53% and skip metastasis rate of 0.50%, which does not justify routinely dissecting neck level IV. Additionally, patients diagnosed with a cN0 neck prior to surgery still had relatively low rates of skip metastasis, including patients with advanced stage tumors.
Another reported surgical option was to extend the neck dissection to level IV nodes whenever suspicious nodes at levels II and III were encountered during SOHND.15,24 The results in the current meta-analysis do not support this protocol. After performing statistical analyses for combinations of various neck level involvement, the rate of level IV involvement remained extremely low (Table 2). However, this data was based on smaller cohorts compared with the entire meta-analysis. This is because many articles did not provide the entire data set on the involvement of the various neck levels. We therefore concur that the subgroup analysis on the metastatic patterns of lymph node involvement in the neck (Table 2) should be addressed with caution.
Limitations
There are obvious limitations to this study. The retrospective nature of most studies included in this meta-analysis is one. The fact that many studies did not match the tumor staging and tumor subsite to the extent of neck disease per patient also hampered statistics and subgroup analysis. However, we found very low rates of skip metastasis.
Conclusions
The results of this meta-analysis demonstrate very low rates of skip metastasis to neck level IV in patients diagnosed with cN0 OCSCC. Various tumor stages and subsites had similarly low rates of skip metastasis. These findings oppose routine dissection of neck level IV in these patients. Encountering a lymph node suspected as being positive during ND does not portend high rates of level IV involvement, and SOHND is adequate for this subset of patients.
References
- 1.Huang SF, Kang CJ, Lin CY, et al. Neck treatment of patients with early stage oral tongue cancer: comparison between observation, supraomohyoid dissection, and extended dissection. Cancer. 2008;112(5):1066-1075. doi: 10.1002/cncr.23278 [DOI] [PubMed] [Google Scholar]
- 2.Lindberg R. Distribution of cervical lymph node metastasis from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446-1449. doi: [DOI] [PubMed] [Google Scholar]
- 3.Byers RM. Modified neck dissection: a study of 967 cases from 1970 to 1980. Am J Surg. 1985;150(4):414-421. doi: 10.1016/0002-9610(85)90146-1 [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne AJ. Neck dissection for cancer. Curr Probl Cancer. 1985;9(8):1-34. doi: 10.1016/S0147-0272(85)80024-6 [DOI] [PubMed] [Google Scholar]
- 5.Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastasis from squamous carcinoma of the oral cavity. Cancer. 1990;66(1):109-113. doi: [DOI] [PubMed] [Google Scholar]
- 6.Ferlito A, Robbins KT, Shah JP, et al. Proposal for a rational classification of neck dissections. Head Neck. 2011;33(3):445-450. doi: 10.1002/hed.21614 [DOI] [PubMed] [Google Scholar]
- 7.Medina JE, Byers RM. Supraomohyoid neck dissection: rationale, indications, and surgical technique. Head Neck. 1989;11(2):111-122. doi: 10.1002/hed.2880110203 [DOI] [PubMed] [Google Scholar]
- 8.Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989;158(4):309-313. doi: 10.1016/0002-9610(89)90122-0 [DOI] [PubMed] [Google Scholar]
- 9.Brazilian Head and Neck Cancer Study Group Results of a prospective trial on elective modified radical classical versus supraomohyoid neck dissection in the management of oral squamous carcinoma. Am J Surg. 1998;176(5):422-427. doi: 10.1016/S0002-9610(98)00230-X [DOI] [PubMed] [Google Scholar]
- 10.Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of “skip metastasis” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19(1):14-19. doi: [DOI] [PubMed] [Google Scholar]
- 11.De Zinis LOR, Bolzoni A, Piazza C, Nicolai P. Prevalence and localization of nodal metastasis in squamous cell carcinoma of the oral cavity: role and extension of neck dissection. Eur Arch Otorhinolaryngol. 2006;263(12):1131-1135. doi: 10.1007/s00405-006-0128-5 [DOI] [PubMed] [Google Scholar]
- 12.Dias FL, Lima RA, Kligerman J, et al. Relevance of skip metastasis for squamous cell carcinoma of the oral tongue and the floor of the mouth. Otolaryngol Head Neck Surg. 2006;134(3):460-465. doi: 10.1016/j.otohns.2005.09.025 [DOI] [PubMed] [Google Scholar]
- 13.Crean SJ, Hoffman A, Potts J, Fardy MJ. Reduction of occult metastatic disease by extension of the supraomohyoid neck dissection to include level IV. Head Neck. 2003;25(9):758-762. doi: 10.1002/hed.10282 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khafif A, Lopez-Garza JR, Medina JE. Is dissection of level IV necessary in patients with T1-T3 N0 tongue cancer? Laryngoscope. 2001;111(6):1088-1090. doi: 10.1097/00005537-200106000-00029 [DOI] [PubMed] [Google Scholar]
- 16.Balasubramanian D, Thankappan K, Battoo AJ, Rajapurkar M, Kuriakose MA, Iyer S. Isolated skip nodal metastasis is rare in T1 and T2 oral tongue squamous cell carcinoma. Otolaryngol Head Neck Surg. 2012;147(2):275-277. doi: 10.1177/0194599812439664 [DOI] [PubMed] [Google Scholar]
- 17.Feng Z, Li JN, Niu LX, Guo CB. Supraomohyoid neck dissection in the management of oral squamous cell carcinoma: special consideration for skip metastasis at level IV or V. J Oral Maxillofac Surg. 2014;72(6):1203-1211. doi: 10.1016/j.joms.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Mishra P, Sharma AK. A 3-year study of supraomohyoid neck dissection and modified radical neck dissection type I in oral cancer: with special reference to involvement of level IV node metastasis. Eur Arch Otorhinolaryngol. 2010;267(6):933-938. doi: 10.1007/s00405-009-1155-9 [DOI] [PubMed] [Google Scholar]
- 19.Vishak S, Rohan V. Cervical node metastasis in T1 squamous cell carcinoma of oral tongue: pattern and the predictive factors. Indian J Surg Oncol. 2014;5(2):104-108. doi: 10.1007/s13193-014-0301-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cariati P, Cabello Serrano A, Fernandez Solis J, Martinez Lara I. Distribution of cervical metastasis in tongue cancer: are occult metastasis predictable? A retrospective study of 117 oral tongue carcinomas. J Craniomaxillofac Surg. 2018;46(1):155-161. doi: 10.1016/j.jcms.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 21.Guo CB, Feng Z, Zhang JG, et al. Supraomohyoid neck dissection and modified radical neck dissection for clinically node-negative oral squamous cell carcinoma: a prospective study of prognosis, complications and quality of life. J Craniomaxillofac Surg. 2014;42(8):1885-1890. doi: 10.1016/j.jcms.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 22.Agarwal SK, Akali NR, Sarin D. Prospective analysis of 231 elective neck dissections in oral squamous cell carcinoma with node negative neck—to decide the extent of neck dissection. Auris Nasus Larynx. 2018;45(1):156-161. doi: 10.1016/j.anl.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 23.Marchiano E, Patel TD, Eloy JA, Baredes S, Park RCW. Impact of nodal level distribution on survival in oral cavity squamous cell carcinoma: a population-based study. Otolaryngol Head Neck Surg. 2016;155(1):99-105. doi: 10.1177/0194599816636356 [DOI] [PubMed] [Google Scholar]
- 24.Ambrosch P, Freudenberg L, Kron M, Steiner W. Selective neck dissection in the management of squamous cell carcinoma of the upper digestive tract. Eur Arch Otorhinolaryngol. 1996;253(6):329-335. doi: 10.1007/BF00178287 [DOI] [PubMed] [Google Scholar]