Key Points
Question
Does combined sorafenib and hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin improve survival of patients with hepatocellular carcinoma and portal vein invasion compared with sorafenib, the current standard systemic treatment?
Findings
In this randomized clinical trial of 247 patients, those receiving sorafenib plus hepatic arterial infusion chemotherapy had a significantly longer median overall survival time (13.37 vs 7.13 months) and acceptable safety profiles compared with patients receiving sorafenib.
Meaning
First-line sorafenib plus hepatic arterial infusion chemotherapy for patients with hepatocellular carcinoma and portal vein invasion improved survival compared with sorafenib alone.
This randomized clinical trial examines whether combined sorafenib and hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin is associated with improved survival of patients with hepatocellular carcinoma and portal vein invasion compared with sorafenib alone, the current standard systemic treatment.
Abstract
Importance
Sorafenib is the first-line treatment for hepatocellular carcinoma with portal vein invasion; however, it has shown unsatisfactory survival benefit. Sorafenib plus hepatic arterial infusion chemotherapy (HAIC) of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) has shown promising results for these patients in a previous phase 2 study.
Objective
To investigate the efficacy and safety of sorafenib plus HAIC compared with sorafenib for hepatocellular carcinoma with portal vein invasion.
Design, Setting, and Participants
This randomized, open-label clinical trial enrolled 818 screened patients. Of the 818 participants, 247 with hepatocellular carcinoma and portal vein invasion were randomly assigned (1:1) via a computer-generated sequence to receive sorafenib plus HAIC or sorafenib. This trial was conducted at 5 hospitals in China and enrolled patients from April 1, 2016, to October 10, 2017, with a follow-up period of 10 months.
Interventions
Randomization to receive 400 mg sorafenib twice daily (sorafenib group) or 400 mg sorafenib twice daily plus HAIC (SoraHAIC group) (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, fluorouracil bolus 400 mg/m2 on day 1, and fluorouracil infusion 2400 mg/m2 for 46 hours, every 3 weeks).
Main Outcomes and Measures
The primary endpoint was overall survival by intention-to-treat analysis. Safety was assessed in patients who received at least 1 dose of study treatment.
Results
For 247 patients (median age, 49 years; range, 18-75 years; 223 men and 24 women), median overall survival was 13.37 months (95% CI, 10.27-16.46) in the SoraHAIC group vs 7.13 months (95% CI, 6.28-7.98) in the sorafenib group (hazard ratio [HR], 0.35; 95% CI, 0.26-0.48; P < .001). The SoraHAIC group showed a higher response rate than the sorafenib group (51 [40.8%] vs 3 [2.46%]; P < .001), and a longer median progression-free survival (7.03 [95% CI, 6.05-8.02] vs 2.6 [95% CI, 2.15-3.05] months; P < .001). Grade 3/4 adverse events that were more frequent in the SoraHAIC group than in the sorafenib group included neutropenia (12 [9.68%] vs 3 [2.48%]), thrombocytopenia (16 [12.9%] vs 6 [4.96%]), and vomiting (8 [6.45%] vs 1 [0.83%]).
Conclusions and Relevance
Sorafenib plus HAIC of FOLFOX improved overall survival and had acceptable toxic effects compared with sorafenib in patients with hepatocellular carcinoma and portal vein invasion.
Trial Registration
ClinicalTrials.gov identifier: NCT02774187
Introduction
Globally, liver cancer ranked fourth for cancer deaths in 2015, and approximately 90% of those deaths were from hepatocellular carcinoma.1 Transarterial chemoembolization is the first-line treatment for hepatocellular carcinoma without portal vein invasion.2,3,4 Nevertheless, approximately 13% to 32% of patients with hepatocellular carcinoma are diagnosed with portal vein invasion.5,6 These patients have an extremely poor prognosis, with a median survival of 2.7 to 4.0 months with supportive care.7,8 Oral sorafenib is the current standard treatment for these patients.9,10 However, the outcome of these patients treated with sorafenib remains poor, with a median survival time of 5.5 to 7.2 months.11,12
Cisplatin-based hepatic arterial infusion chemotherapy (HAIC) has been widely employed as an alternative therapy to sorafenib for hepatocellular carcinoma patients with portal vein invasion in Japan.13 This procedure provides direct delivery of chemotherapeutic agents into the tumor-feeding arteries and minimizes systemic toxic effects through a first-pass effect in the liver, producing a significantly higher response rate (22%-48%) than systemic chemotherapy (8%-20.9%) or sorafenib (2%-3.3%).9,10,14,15,16,17,18
Recently, it has been proposed that sorafenib combined with HAIC might benefit patients with advanced hepatocellular carcinoma more than sorafenib19,20 because HAIC can effectively reduce the tumor burden, whereas the effectiveness of sorafenib appeared greater in patients with lower tumor burdens.21,22 Furthermore, chemotherapeutic agents have been shown to exert a synergistic anticancer effect with sorafenib in preclinical studies.23,24,25 In 1 randomized phase 2 trial,19 sorafenib plus HAIC of cisplatin extended overall survival by 22% compared with sorafenib. Nevertheless, in another randomized phase 3 trial, the combination of sorafenib and HAIC of cisplatin and fluorouracil failed to demonstrate survival benefit superiority over sorafenib.20 There is insufficient evidence of a survival benefit associated with the addition of cisplatin-based HAIC to sorafenib.
Compared with cisplatin, oxaliplatin has distinct pharmacokinetic, biochemical, cytotoxic, and immunologic properties.26,27,28 Therefore, oxaliplatin might be a better option than cisplatin for HAIC combined with sorafenib. Our previous phase 2 trial of combined treatment with sorafenib and HAIC of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) demonstrated a safe toxic effects profile and a 12-month survival rate of 52.7% in patients with hepatocellular carcinoma and major portal vein invasion.29
Herein, we aimed to compare sorafenib plus HAIC of FOLFOX with sorafenib monotherapy in patients with hepatocellular carcinoma and portal vein invasion.
Methods
Study Design and Participants
This was a randomized, open-label, parallel-group phase 3 trial performed at 5 hospitals in China (eTable 1 in Supplement 1). The trial protocol is available in Supplement 2. Patients were randomly assigned (1:1) to receive either sorafenib (sorafenib group) or sorafenib plus HAIC of FOLFOX (SoraHAIC group). Randomization was performed centrally via a computer-generated randomization sequence and was stratified according to hospital and portal vein invasion grade (Vp1-2, Vp3, and Vp4). The study was approved by each site’s institutional review board, and all patients provided written informed consent.
Eligible patients were 18 years or older with biopsy-confirmed hepatocellular carcinoma (not suitable for curative surgery, or local ablation). Other eligibility criteria were as follows: patients had portal vein invasion confirmed by 2 imaging techniques, Child-Pugh A class liver function, an Eastern Cooperative Oncology Group performance status of 0 to 2, no previous treatment for hepatocellular carcinoma, at least 1 measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,30 and adequate organ function (white blood cell count ≥3.0 × 109/L, absolute neutrophil count ≥1.5 × 109/L, platelet count ≥75 × 109/L, aspartate transaminase and alanine transaminase ≤5 × upper limit of the normal, creatinine clearance rate of ≤1.5 × upper limit of the normal, and left ventricular ejection ≥45%).
The exclusion criteria included hepatic decompensation, including esophageal or gastric variceal bleeding or hepatic encephalopathy; central nervous system metastases; a known medical history of HIV infection; pregnancy or breastfeeding; and other invasive malignant diseases. All recruited patients with hepatitis B virus–related hepatocellular carcinoma received preemptive antiviral therapy. Details of pretreatment evaluation are shown in the eMethods in Supplement 1.
Procedures
Treatment was divided into 3-week cycles. Patients in both groups were treated with 400 mg sorafenib orally twice daily on days 1 through 21, and HAIC regimen was performed every 3 weeks.
In the SoraHAIC group, femoral artery puncture and catheterization were performed on day 1 in every cycle of treatment (eMethods in Supplement 1). The following regimen was administered via hepatic artery: oxaliplatin, 85 mg/m2, from hour 0 to 2 on day 1; leucovorin, 400 mg/ m2, from hour 2 to 3 on day 1; fluorouracil, 400 mg/ m2, bolus at hour 3; and 2400 mg/m2 over 46 hours on days 1 and 2. After HAIC was completed, the catheter and sheath were removed. Repetitive catheterization was performed in the next HAIC cycle.
In the SoraHAIC group, patients were allowed to have sorafenib as a single agent and were not considered to have discontinued the study treatment when the HAIC was delayed or discontinued in the absence of disease progression. Treatment crossover from sorafenib to sorafenib plus HAIC was allowed after disease progression was confirmed and the investigator judged that a patient in the sorafenib group would not benefit from sorafenib monotherapy. The criteria for terminating study treatment and assessment of tumor response and toxic effects are shown in the eMethods in Supplement 1.
Efficacy and Safety Analyses
Before the study treatment was discontinued, patient visits were scheduled every 3 weeks to monitor safety and clinical and biological parameters. In addition, computed tomographic scans were performed every 6 weeks. An end-of-treatment visit was made within 30 days after the study treatment was stopped.
The primary endpoint was overall survival (defined as the date of randomization to death from any cause). The secondary endpoints were progression-free survival (defined as the date from randomization until progression or death from any cause), overall response (the proportion of patients with complete response or partial response according to RECIST, version 1.1), and adverse events by use of the National Cancer Institute Common Terminology Criteria for adverse events version 4.03. The detailed description of endpoint definition is shown in the eMethods in Supplement 1. In a post hoc analysis, treatment response was assessed according to the modified RECIST (mRECIST) guidelines,31 overall survival was compared in patients with varying portal vein invasion grade (Vp1-2, Vp3, or Vp4), and intrahepatic progression-free survival was compared.
Statistical Analyses
The sample size was based on the assumptions that median overall survival in patients receiving sorafenib would be 6.5 months and that adding HAIC would improve median overall survival to 10 months. To detect this difference with 80% power and a 1-sided α of .05, we calculated that the required number of events would be observed if 218 patients were enrolled, with an enrollment period of 18 months and a follow-up period of 10 months. Based on an estimated dropout rate of 10%, target enrollment was set at 244 patients (122 per group).
The primary efficacy analysis was performed in the intention-to-treat population. The safety analysis comprised all randomized patients who received at least 1 dose of study treatment. The results were compared by student’s t tests or χ2 tests. Survival outcomes were calculated with the Kaplan-Meier method and compared by log-rank tests. Any factors that were statistically significant at P less than .10 in the univariate analysis were candidates for entry into a multivariable Cox proportional hazards model. All P values were 2-sided, with P values less than .05 considered significant. The statistical package used to perform analyses was SAS statistical software (version 9.0; SAS Institute, Inc).
Results
Patient Characteristics and Treatment
Between May 1, 2016, and October 10, 2017, 247 patients (223 men and 24 women; median age, 49 years; range, 18-75 years) were randomly assigned to receive either sorafenib (n = 122) or sorafenib plus HAIC (n = 125). The follow-up ended on August 6, 2018. Two patients (1 per group) were not treated, leaving 245 patients in the safety population (Figure 1). The baseline characteristics were well balanced between the 2 groups (eTable 2 and eTable 3 in Supplement 1). Both hepatitis B virus-related hepatocellular carcinoma and advanced portal vein invasion (Vp3 and Vp4) occurred in most patients (80.6%).
Figure 1. CONSORT Flow Diagram.
HAIC Indicates hepatic arterial infusion chemotherapy; AE, adverse event.
Patients in the SoraHAIC group were treated with a total of 485 cycles of HAIC, and the median HAIC cycle per patient was 4 (eTable 4 in Supplement 1). The median number of positional changes of the microcatheter in subsequent HAIC sessions was 2, and the hepatic-gastroduodenal collateral artery of 67 (54.0%) patients developed collateral branches and were reembolized. The mean and median duration of sorafenib treatment were longer in the SoraHAIC group than in the sorafenib group, but there was no difference in the mean sorafenib dose between the 2 groups (eTable 5 in Supplement 1). Subsequent treatment after discontinuation of study treatment is shown in eTable 6 in Supplement 1. Curative surgical resection was conducted for 16 patients (12.8%) in the SoraHAIC group owing to downstaging, and 3 patients experienced a pathologic complete response. However, 1 patient (0.8%) in the sorafenib group who received treatment crossover underwent resection (1 [0.8%] vs 16 [12.8%], P < .001).
Efficacy
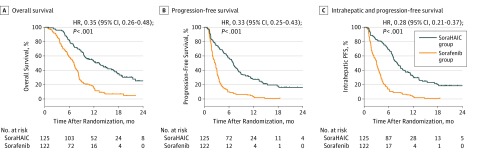
Patients in the SoraHAIC group had a median overall survival of 13.37 months (95% CI, 10.27-16.46) compared with 7.13 months (95% CI, 6.28-7.98) in the sorafenib group (hazard ratio [HR], 0.35; 95% CI, 0.26-0.48; P < .001) (Figure 2A). The overall survival rates at 3, 6, and 9 months were 96%, 82.4%, and 65.6% in the SoraHAIC group and 87.7%, 59.0%, and 24.6% in the sorafenib group, respectively. The median overall survival stratified by portal vein invasion grade in the SoraHAIC group was also significantly longer than that in the sorafenib group (Vp1-2: 18.17 [95% CI, 11.19-25.15] vs 10.87 [95% CI, 6.79-14.95] months, P = .002; Vp3: 13.47 [95% CI, 9.83-17.1] vs 6.27 [95% CI, 4.81-7.73] months, P < .001; Vp4: 9.47 [95% CI, 5.97-12.96] vs 5.5 [95% CI, 3.62-7.38] months, P < .001; eFigures 1-3 in Supplement 1).
Figure 2. Kaplan-Meier Curves.
SoraHAIC group indicates sorafenib plus hepatic arterial infusion chemotherapy group; Sorafenib group, sorafenib monotherapy group; HR, hazard ratio; PFS, progression-free survival.
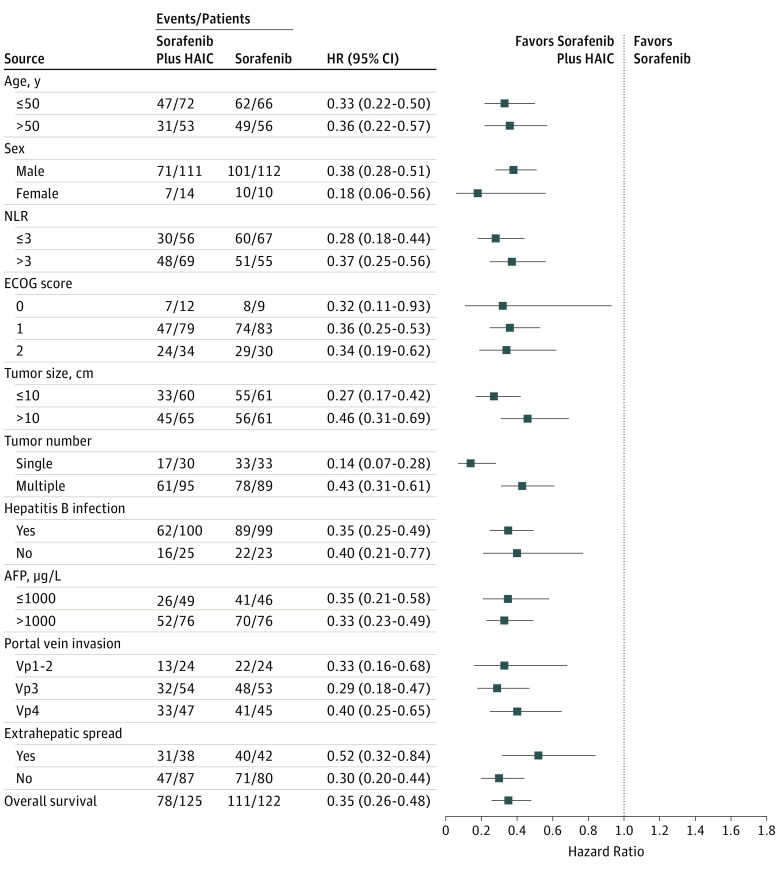
The results of univariable survival analysis are listed in eTable 3 in Supplement 1. In multivariable analysis, independent risk factors of survival were treatment allocation (HR, 3.32; 95% CI, 2.43-4.52; P < .001), neutrophil: lymphocyte ratio (HR, 1.56; 95% CI, 1.15-2.10; P = .004), α-fetoprotein level (HR, 1.52; 95% CI, 1.12-2.07; P = .008), presence or absence of extrahepatic sites (HR, 1.66; 95% CI, 1.23-2.34; P = .001), portal vein invasion grade Vp4 vs Vp1-2 (HR, 2.27; 95% CI, 1.51-3.44; P < .001) and Vp3 vs Vp1-2 (HR, 1.63; 95% CI, 1.08-2.46; P = .02).
Patients in the SoraHAIC group had a significantly longer median progression-free survival (7.03 months; 95% CI, 6.05-8.02) than those in the sorafenib group (2.6 months; 95% CI, 2.15-3.05; HR, 0.33; 95% CI, 0.25-0.43; P < .001) (Figure 2B). The median intrahepatic progression-free survival was 8.07 months (95% CI, 6.69-9.44) in the SoraHAIC group and 3.1 months (95% CI, 2.66-3.54) in the sorafenib group (HR, 0.28; 95% CI, 0.21-0.37; P < .001) (Figure 2C). Based on RECIST, the overall response was significantly higher in the SoraHAIC group (40.8%) than in the sorafenib group (2.46%, P < .001) (Table). In the SoraHAIC group, 14 patients achieved complete response of intrahepatic lesions based on mRECIST. Forest plot analysis of factors associated with overall survival showed that sorafenib plus HAIC provided a clinical benefit in all preplanned subgroup analyses (Figure 3).
Table. Summary of Best Responsea.
| Variable | RECIST | mRECIST | ||||
|---|---|---|---|---|---|---|
| Group, No. (%) | P Value | Group, No. (%) | P Value | |||
| SoraHAIC | Sorafenib | SoraHAIC | Sorafenib | |||
| Overall Responseb | ||||||
| Complete response | 0 | 0 | NA | 10 (8) | 0 | .002 |
| Partial response | 51 (40.8) | 3 (2.5) | <.001 | 58 (46.4) | 7 (5.7) | <.001 |
| Stable disease | 43 (34.4) | 57 (46.7) | .05 | 27 (21.6) | 55 (45.1) | <.001 |
| Progressive disease | 25 (20.0) | 52 (42.6) | <.001 | 24 (19.2) | 50 (41.0) | <.001 |
| Not assessablec | 6 (4.8) | 10 (8.2) | .31 | 6 (4.8) | 10 (8.2) | .31 |
| Disease control rate | 94 (75.2) | 60 (49.2) | <.001 | 95 (76.0) | 62 (50.8) | <.001 |
| Overall response rate | 51 (40.8) | 3 (2.5) | <.001 | 68 (54.4) | 7 (5.7) | <.001 |
| Intrahepatic Responsed | ||||||
| Complete response | 0 | 0 | NA | 14 (11.2) | 0 | <.001 |
| Partial response | 60 (48.0) | 4 (3.3) | <.001 | 60 (48.0) | 8 (6.6) | <.001 |
| Stable disease | 42 (33.6) | 64 (52.5) | .003 | 29 (23.2) | 62 (50.8) | <.001 |
| Progressive disease | 17 (13.6) | 44 (36.1) | <.001 | 16 (12.8) | 42 (34.4) | <.001 |
| Not assessablec | 6 (4.8) | 10 (8.2) | .31 | 6 (4.8) | 10 (8.2) | .31 |
| Disease control rate | 102 (81.6) | 68 (55.7) | <.001 | 103 (82.4) | 70 (57.4) | <.001 |
| Overall response rate | 60 (48.0) | 4 (3.3) | <.001 | 74 (59.2) | 8 (6.6) | <.001 |
Abbreviations: mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECIST, Response Evaluation Criteria in Solid Tumors; NA, not applicable; Sorafenib group, sorafenib monotherapy group; SoraHAIC group, sorafenib plus hepatic arterial infusion chemotherapy group.
Statistical significance was assessed with the χ2 test.
Overall response included assessment of the change in tumor burden inside and outside the liver.
There were 16 patients who could not be evaluated for treatment response because of death, poor performance status, or patients’ refusal of computed tomography scan.
Intrahepatic response only included assessment of the change in tumor burden inside the liver.
Figure 3. Forest Plot for Overall Survival.
HAIC Indicates hepatic arterial infusion chemotherapy; HR, hazard ratio; NLR, neutrophil:lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; AFP, α-fetoprotein; Vp4, main portal vein invasion; Vp3, first branch portal vein invasion; Vp2, second branch portal vein invasion; Vp1, third branch portal vein invasion.
Furthermore, 35 patients in the sorafenib group crossed over to the SoraHAIC group. The median overall survival of these 35 patients was 9.47 months (95% CI, 8.19-10.74), whereas the median overall survival of patients who did not undergo treatment crossover in the sorafenib group was 5.53 months (95% CI, 4.69-6.38).
Safety
Treatment-related adverse events, which occurred in 10% or more of patients, are shown in eTable 7 in Supplement 1. The overall incidence of treatment-related adverse events was similar between the SoraHAIC group and the sorafenib group (any grade: 118 [95.16%] vs 109 [90.08%], P = .15; grade 3/4: 66 [53.23%] vs 51 [42.15%], P = .10; serious adverse events: 40 [32.26%] vs 42 [34.71%], P = .69; eTable 8 in Supplement 1). The following grade 3 to 4 adverse events were more frequent in the SoraHAIC group than in the sorafenib group: neutropenia (12 [9.68%] vs 3 [2.48%]), thrombocytopenia (16 [12.9%] vs 6 [4.96%]), and vomiting (8 [6.45%] vs 1 [0.83%]). Furthermore, a particular abdominal pain associated with the HAIC of oxaliplatin was observed in 34 patients in the SoraHAIC group. This pain could be acute and severe but was quickly relieved by slowing or stopping the infusion of oxaliplatin, after which the infusion would continue. Ten patients with thrombosis or dislocation of the catheter tip in the SoraHAIC group were transferred to the digital subtraction angiography room to undergo repositioning of the catheter, and no severe vascular complications were observed. Within 30 days after study treatment was stopped, 3 treatment-related deaths that were not attributed to disease progression were observed: 2 in the SoraHAIC group and 1 in the sorafenib group.
The frequencies of dose reductions, dose interruptions, and discontinuations of HAIC owing to adverse events are shown in eTable 4 in Supplement 1. In addition, there were no differences between groups in the frequency of dose reductions, dose interruptions, or discontinuations of sorafenib owing to adverse events (eTable 5 in Supplement 1).
Discussion
This randomized phase 3 trial demonstrated an association of sorafenib plus HAIC with extended overall survival by 87.5% or 6.24 months compared with sorafenib among patients with hepatocellular carcinoma and portal vein invasion, with consistent results among all subgroup analyses. Sorafenib plus HAIC was also associated with a significant improvement in the overall response and progression-free survival compared with sorafenib. Both sorafenib plus HAIC and sorafenib had acceptable safety profiles. Notably, in 1 trial (REFLECT),32 lenvatinib produced comparable tumor response and overall survival to HAIC plus sorafenib as demonstrated in this trial. But the current study population may be considered to have a worse prognosis than those of the REFLECT trial because all patients included in this study had portal vein invasion. Further studies are needed to compare lenvatinib plus HAIC with sorafenib plus HAIC as the first-line treatment for advanced hepatocellular carcinoma.
Patients with hepatocellular carcinoma and portal vein invasion were selected because most studies of HAIC focused on these patients and HAIC was recommended as first-line treatment for hepatocellular carcinoma with portal vein invasion in Japan.13 These patients were more likely to benefit from additional HAIC.33 In addition, to avoid the confounding effect of deaths unrelated to tumor progression, we selected patients with Child-Pugh class A.
In this trial, overall survival in the SoraHAIC group was consistent with that observed in our phase 2 trial.29 The overall survival in the sorafenib group was consistent with that observed in the Asia-Pacific trial.10 The survival benefit demonstrated in this study may be partly because oxaliplatin-based HAIC is more effective than cisplatin-based HAIC against hepatocellular carcinoma. First, oxaliplatin kills cancer cells by inducing ribosome biogenesis stress rather than by engaging a DNA damage response.26 Second, immunogenic tumor cell death induced by oxaliplatin (but not cisplatin) can promote a permanent antitumor immune response.28 Furthermore, compared with cisplatin, hepatic arterial infusion of oxaliplatin have higher concentration ratios between tumor and peripheral tissue.27
On the other hand, the survival benefit observed in this study may also be partly owing to the synergistic antitumor effect of sorafenib and FOLFOX agents. Sorafenib has multiple targets, including Raf-1, Braf, VEGFR-1-3, and PDGFR-β, and the inhibition of Raf-1 by sorafenib can induce apoptosis and help to overcome resistance to FOLFOX agents.23 In particular, the synergism between oxaliplatin and fluorouracil is, in part, owing to the fluorouracil-mediated suppression of drug transporters.34 Sorafenib can further inhibit the activity of multidrug resistance–associated transporters.24
Finally, the arterial infusion technique used in this study may also help to capture the survival benefits of additional HAIC. These results showed that the change of the microcatheter tip position (median 2) in the subsequent cycle of HAIC and reembolization of the newly developed hepatic-gastroduodenal collateral artery (54.03% patients) was frequently needed. Therefore, compared with an implanted port catheter system, repetitive catheterization and digital subtraction angiography before starting each session of HAIC is more reliable for concentrating the chemotherapy dose in the targeted area and avoiding anticancer drug exposure in other organs. Notably, 1 randomized trial19 comparing sorafenib plus cisplatin-based HAIC to sorafenib using a repetitive catheterization procedure yielded positive results, whereas another20 using an implanted port catheter system reported negative results.
Moreover, the adverse events in the sorafenib group were consistent with those observed in our phase 2 study,29 and all sorafenib-related adverse events were consistent with those in previous trials of sorafenib.9,10 Although patients treated with sorafenib plus HAIC had significantly elevated frequencies of grade 3 to 4 neutropenia, thrombocytopenia, and vomiting, probably owing to the cytotoxic agents oxaliplatin and fluorouracil, these adverse events were not unexpected and were manageable by treatment interruption or dose modification. In addition, there was no significant difference in the overall incidence of any grade or grade 3/4 adverse events between groups. In both this trial and our previous phase 2 study, no severe vascular complications were observed despite repeated puncture and catheterization.29 According to previous reports, the incidence of implanted port catheter system-related grade 3 to 4 adverse events (such as dislocation of catheter tip, thrombosis, and port-related infection) ranged from 7% to 12%.20,35
Limitations
The major limitation of this study was its open-label nature. The subsequent treatments for patients who had stopped study treatment might be influenced by investigator and patient decisions. In addition, the number of patients in the SoraHAIC group who underwent subsequent hepatic resection was greater than that in the sorafenib group. However, this can be explained by the better treatment response in the SoraHAIC group resulting in more translation to resectable hepatocellular carcinoma. Second, this study was performed in an endemic area. The predominant etiology of hepatocellular carcinoma in China was hepatitis B virus. Therefore, whether the results could be applied to Western countries, where the etiology of hepatocellular carcinoma is mainly hepatitis C virus, remains to be elucidated.
Conclusions
Compared with sorafenib alone, the addition of HAIC of FOLFOX improved overall survival among patients with hepatocellular carcinoma and portal vein invasion. The safety results suggest that sorafenib plus HAIC has acceptable treatment-related toxic effects. Future studies are warranted to evaluate molecular targeted agent plus HAIC for hepatocellular carcinoma without vascular invasion, especially for tumors beyond up-to-7 criteria36 because hepatocellular carcinomas of this kind commonly showed resistance to transarterial chemoembolization (the current standard of care) and poor outcome.37
eTable 1. Study institutes
eTable 2. Baseline characteristics of the intention-to-treat population
eTable 3. Univariable analysis for all recruited patients
eTable 4. HAIC administration
eTable 5. Sorafenib administration
eTable 6. Number of patients who received subsequent treatments after discontinuation of study treatment.
eTable 7. Treatment Related Adverse Events
eTable 8. Incidence of serious treatment-emergent adverse events
eFigure 1. Kaplan-Meier analysis of overall survival in patients with Vp 1-2
eFigure 2. Kaplan-Meier analysis of overall survival in patients with Vp 3
eFigure 3. Kaplan-Meier analysis of overall survival in patients with Vp 4
eMethods. Additional Methods
Trial Protocol.
Data sharing statement.
References
- 1.Fitzmaurice C, Allen C, Barber RM, et al. ; Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2017;3(4):524-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 4.Verslype C, Rosmorduc O, Rougier P; ESMO Guidelines Working Group . Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii41-vii48. [DOI] [PubMed] [Google Scholar]
- 5.Chan SL, Johnson PJ, Mo F, et al. International validation of the Chinese university prognostic index for staging of hepatocellular carcinoma: a joint United Kingdom and Hong Kong study. Chin J Cancer. 2014;33(10):481-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Izumi N, Ichida T, et al. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2016;46(5):372-390. doi: 10.1111/hepr.12697 [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62-67. doi: 10.1002/hep.510290145 [DOI] [PubMed] [Google Scholar]
- 8.Villa E, Moles A, Ferretti I, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32(2):233-238. doi: 10.1053/jhep.2000.9603 [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, et al. ; SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 10.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 11.Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50(4):445-454. doi: 10.1007/s00535-014-0978-3 [DOI] [PubMed] [Google Scholar]
- 12.Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469-478. doi: 10.1007/s00280-018-3638-0 [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Matsui O, Izumi N, et al. ; Liver Cancer Study Group of Japan . JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3(3-4):458-468. doi: 10.1159/000343875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501-3508. doi: 10.1200/JCO.2012.44.5643 [DOI] [PubMed] [Google Scholar]
- 15.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532-1538. doi: 10.1093/jnci/dji315 [DOI] [PubMed] [Google Scholar]
- 16.Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95(3):588-595. doi: 10.1002/cncr.10694 [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Ahn SH, Yoon YJ, et al. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110(1):129-137. doi: 10.1002/cncr.22759 [DOI] [PubMed] [Google Scholar]
- 18.Patt YZ, Charnsangavej C, Yoffe B, et al. Hepatic arterial infusion of floxuridine, leucovorin, doxorubicin, and cisplatin for hepatocellular carcinoma: effects of hepatitis B and C viral infection on drug toxicity and patient survival. J Clin Oncol. 1994;12(6):1204-1211. doi: 10.1200/JCO.1994.12.6.1204 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27(11):2090-2096. doi: 10.1093/annonc/mdw323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo M, Ueshima K, Yokosuka O, et al. ; SILIUS study group . Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424-432. doi: 10.1016/S2468-1253(18)30078-5 [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999-1008. doi: 10.1016/j.jhep.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 22.Sanoff HK, Chang Y, Lund JL, O’Neil BH, Dusetzina SB. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21(9):1113-1120. doi: 10.1634/theoncologist.2015-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma SQ, Cao BR, Zhang H, et al. The lack of Raf-1 kinase feedback regulation enhances antiapoptosis in cancer cells. Oncogene. 2017;36(14):2014-2022. [DOI] [PubMed] [Google Scholar]
- 24.Malofeeva EV, Domanitskaya N, Gudima M, Hopper-Borge EA. Modulation of the ATPase and transport activities of broad-acting multidrug resistance factor ABCC10 (MRP7). Cancer Res. 2012;72(24):6457-6467. doi: 10.1158/0008-5472.CAN-12-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin M, Fartoux L, Vignaud A, Arrivé L, Menu Y, Rosmorduc O. The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: a pilot study. Eur Radiol. 2011;21(2):281-290. doi: 10.1007/s00330-010-1914-4 [DOI] [PubMed] [Google Scholar]
- 26.Bruno PM, Liu Y, Park GY, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. 2017;23(4):461-471. doi: 10.1038/nm.4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzodic R, Gomez-Abuin G, Rougier P, et al. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anticancer Drugs. 2004;15(6):647-650. doi: 10.1097/01.cad.0000131684.06390.fe [DOI] [PubMed] [Google Scholar]
- 28.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482-491. doi: 10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 29.He MK, Zou RH, Li QJ, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol. 2018;41(5):734-743. [DOI] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 31.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 32.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 33.Obi S, Sato S, Kawai T. Current status of hepatic arterial infusion chemotherapy. Liver Cancer. 2015;4(3):188-199. doi: 10.1159/000367746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theile D, Grebhardt S, Haefeli WE, Weiss J. Involvement of drug transporters in the synergistic action of FOLFOX combination chemotherapy. Biochem Pharmacol. 2009;78(11):1366-1373. doi: 10.1016/j.bcp.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 35.Sinn M, Nicolaou A, Ricke J, et al. Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with primary liver cancer: a phase II-study (NCT00356161). BMC Gastroenterol. 2013;13:125. doi: 10.1186/1471-230X-13-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018;7(1):1-19. doi: 10.1159/000487148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified bolondi’s subclassification (Kinki Criteria). Dig Dis. 2015;33(6):751-758. doi: 10.1159/000439290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Study institutes
eTable 2. Baseline characteristics of the intention-to-treat population
eTable 3. Univariable analysis for all recruited patients
eTable 4. HAIC administration
eTable 5. Sorafenib administration
eTable 6. Number of patients who received subsequent treatments after discontinuation of study treatment.
eTable 7. Treatment Related Adverse Events
eTable 8. Incidence of serious treatment-emergent adverse events
eFigure 1. Kaplan-Meier analysis of overall survival in patients with Vp 1-2
eFigure 2. Kaplan-Meier analysis of overall survival in patients with Vp 3
eFigure 3. Kaplan-Meier analysis of overall survival in patients with Vp 4
eMethods. Additional Methods
Trial Protocol.
Data sharing statement.