Abstract
IMPORTANCE
Food subsidies are designed to enhance food availability, but whether they promote cardiometabolic health is unclear.
OBJECTIVE
To investigate whether higher consumption of foods derived from subsidized food commodities is associated with adverse cardiometabolic risk among US adults.
DESIGN, SETTING, AND PARTICIPANTS
Cross-sectional analysis of the National Health and Nutrition Examination Survey data from 2001 to 2006. Our final analysis was performed in January 2016. Participants were 10 308 nonpregnant adults 18 to 64 years old in the general community.
EXPOSURE
From a single day of 24-hour dietary recall in the National Health and Nutrition Examination Survey, we calculated an individual-level subsidy score that estimated an individual’s consumption of subsidized food commodities as a percentage of total caloric intake.
MAIN OUTCOMES AND MEASURES
The main outcomes were body mass index (calculated as weight in kilograms divided by height in meters squared), abdominal adiposity, C-reactive protein level, blood pressure, non–high-density lipoprotein cholesterol level, and glycemia.
RESULTS
Among 10 308 participants, the mean (SD) age was 40.2 (0.3) years, and a mean (SD) of 50.5% (0.5%) were male. Overall, 56.2% of calories consumed were from the major subsidized food commodities. United States adults in the highest quartile of the subsidy score (compared with the lowest) had increased probabilities of having a body mass index of at least 30 (prevalence ratio, 1.37; 95% CI, 1.23–1.52), a ratio of waist circumference to height of at least 0.60 (prevalence ratio, 1.41; 95% CI, 1.25–1.59), a C-reactive protein level of at least 0.32 mg/dL (prevalence ratio, 1.34; 95% CI, 1.19–1.51), an elevated non–high-density lipoprotein cholesterol level (prevalence ratio, 1.14; 95% CI, 1.05–1.25), and dysglycemia (prevalence ratio, 1.21; 95% CI, 1.04–1.40). There was no statistically significant association between the subsidy score and blood pressure.
CONCLUSIONS AND RELEVANCE
Among US adults, higher consumption of calories from subsidized food commodities was associated with a greater probability of some cardiometabolic risks. Better alignment of agricultural and nutritional policies may potentially improve population health.
Among the justifications for the 1973 US Farm Bill was to assure consumers a plentiful supply of food at reasonable prices.1 Four decades later, the US population is burdened by substantial obesity and cardiometabolic disease.2,3 Suboptimal diet quality is a leading factor associated with death and disability in the United States.4 Specifically, diets that are high in calories, saturated fats, salt, and sugars but low in fruits and vegetables have been implicated in the development of cardiometabolic risk factors (obesity or adiposity, elevated blood pressure, elevated lipid levels, and diabetes) and diseases.5
The US Department of Agriculture and US Department of Health and Human Services Dietary Guidelines for Americans emphasize consumption of fruits, vegetables, whole grains, protein, and moderate amounts of dairy, while recommending limited consumption of saturated fats, sugars, salt, and refined grains.6 At the same time, current federal agricultural subsidies focus on financing the production of corn, soybeans, wheat, rice, sorghum, dairy, and livestock, the 2 latter of which are in part via subsidies on feed grains.7 From 1995 to 2010, approximately $170 billion was spent on these 7 commodities and programs.7 A large proportion of these subsidized commodities are converted into high-fat meat and dairy products, refined grains, high-calorie juices and soft drinks (sweetened with corn sweeteners), and processed and packaged foods.7 For example, 30% to 40% of the corn, more than half of the soybeans, and almost all of the sorghum grown in the United States are used as feed for US cattle and livestock, while approximately 5% of the corn is converted into high-fructose corn syrup, and the other half of the soybeans are converted into oils.8 Because the US agricultural sector produces approximately 80% of the food that Americans eat (the other 20% comes from imports), the foods that are produced domestically matter for the American diet.4
Commentators have noted that because commodity subsidies are federally funded taxpayers pay for the production of these foods, as well as the potential downstream health expenditures attributable to diet-related cardiometabolic diseases.4,9 However, empirical evidence that the nation’s agricultural policies are misaligned with nutritional recommendations has been limited to ecological assessments.7 To date, no study has examined the associations between consumption of subsidized foods and cardiometabolic health at the individual level. Such evidence may more accurately help characterize the alignment of agricultural policies with nutrition and health. This study aimed to fill that gap using a recently developed scoring system to estimate an individual’s consumption of subsidized foods and their derivatives.10
Methods
Institutional review board approval is not required for secondary analysis using the National Health and Nutrition Examination Survey (NHANES) data. The data collection process of the NHANES has its own institutional review board and written and oral informed consent procedures.
Data Sources and Participant Selection
We used data from 2001 to 2006 from the NHANES, a continuous, cross-sectional study of the noninstitutionalized, civilian US population, with data released in 2-year cycles. Our final analysis was performed in January 2016. Detailed descriptions of the NHANES sampling methods are provided elsewhere.11 We restricted our sample to 10 308 nonpregnant adults 18 to 64 years old in the general community who provided complete dietary dataas determined by the NHANES and had daily caloric intake between 800 and 5000 kcal.12,13
Consumption of Subsidized Foods
Our main exposure of interest was a subsidy score, the proportion of individual-level dietary intake (in calories) derived from the 7 major subsidized food commodities (corn, soybeans, wheat, rice, sorghum, dairy, and livestock). The subsidy score ranges from 0.0 to 1.0, where 0.0 indicates 0% of total energy from subsidized commodities, and 1.0 indicates 100% of total energy from subsidized commodities. This subsidy score variable was estimated using the NHANES dietary recall (first day) data and the following federally sponsored linked databases: MyPyramid Equivalents Database (http://www.ars.usda.gov/SP2UserFiles/Place/12355000/foodlink/mped2/MPED_2.exe), Food Intakes Converted to Retail Commodities (http://www.ars.usda.gov/Services/docs.htm?docid=21993), What We Eat in America (http://www.ars.usda.gov/Services/docs.htm?docid=13793), and National Nutrient Database for Standard Reference (http://www.ars.usda.gov/main/site_main.htm?modecode=80–40-05–25). Detailed methods of the subsidy score calculation are described elsewhere.10 We categorized the subsidy score into quartiles, identified empirically within the sample (Q1 is 0.00–0.47, Q2 is 0.48–0.57, Q3 is 0.58–0.65, and Q4 is 0.66–1.00).
Cardiometabolic Risk Measures
We used the following 6 variables to characterize cardiometabolic risk status: body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), ratio of waist circumference to height, circulating high-sensitivity Creactive protein (CRP) level (a marker of inflammation), blood pressure, non–high-density lipoprotein (HDL) cholesterol level, and glycated hemoglobin level. We categorized each variable into 3 categories using cut points that were defined clinically (BMI, blood pressure, non-HDL cholesterol level, and glycated hemoglobin level) or empirically (ratio of waist circumference to height and CRP level). Table 1 lists these domains and categories.
Table 1.
Categorization of Cardiometabolic Risk Domains
| Risk Domain | Measurement, Units | Categorical Definitions |
|---|---|---|
| Obesity | Body mass index, calculated as weight in kilograms divided by height in meters squared | Normal is <25, overweight is ≥25 to <30, obese is ≥30a |
| Abdominal adiposity | Ratio of waist circumference to height, cm/cm | Tertile 1 is <0.52, tertile 2 is 0.52 to <0.60, tertile 3 is ≥0.60 |
| Elevated C-reactive protein level | High-sensitivity C-reactive protein level, mg/dL | Tertile 1 is 0.01 to 0.09 mg/dL, tertile 2 is >0.09 to <0.32 mg/dL, tertile 3 is ≥0.32 mg/dL |
| Hypertension | SBP and DBP, mm Hg; self-reported diagnosis or antihypertensive medication use | Normal is no self-reported diagnosis and SBP <120 mm Hg and DBP <80 mm Hg, prehypertension is no self-reported diagnosis and SBP 120 to <140 mm Hg or DBP 80 to <90 mm Hg, hypertension is self-reported diagnosis or SBP ≥140 mm Hg or DBP ≥90 mm Hg or antihypertensive medication use |
| Dyslipidemia | Non-HDL cholesterol level, mg/dL; self-reported diagnosis or antihyperlipidemia medication use | Normal is no self-reported diagnosis and non-HDL cholesterol level <130 mg/dL, intermediate dyslipidemia is no self-reported diagnosis and non-HDL cholesterol level 130 to <160 mg/dL, dyslipidemia is self-reported diagnosis or non-HDL cholesterol level ≥160 mg/dL or antihyperlipidemia medication use |
| Dysglycemia | Glycated hemoglobin level, %; self-reported diagnosis of diabetes | Normal is no self-reported diagnosis and glycated hemoglobin level <5.7%, intermediate dysglycemia is no self-reported diagnosis and glycated hemoglobin level ≥5.7%, diagnosed diabetes is self-reported diagnosis |
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol level to millimoles per liter, multiply by 0.0259; C-reactive protein level to nanomoles per liter, multiply by 9.524; and glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Normal includes underweight.
We also created dichotomized categories of each cardiometabolic risk factor. These included obesity (BMI ≥30) vs no obesity, abdominal adiposity (ratio of waist circumference to height ≥0.60) vs no abdominal adiposity, elevated CRP level (≥0.32 mg/dL) vs normal CRP level, hypertension (diagnosed [self-reported] or undiagnosed [no self-reported diagnosis and systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg] hypertension or currently taking antihypertensive medication) vs normotensive, dyslipidemia (diagnosed [self-reported] or undiagnosed [no self-reported diagnosis and non-HDL cholesterol level ≥160 mg/dL] dyslipidemia or currently taking anticholesterolemia medication) vs normal lipid levels, and dysglycemia (self-reported diabetes diagnosis or glycated hemoglobin level ≥5.7%) vs no dysglycemia. To convert CRP level to nanomoles per liter, multiply by 9.524; cholesterol level to millimoles per liter, multiply by 0.0259; and glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Covariates
We categorized age into the following 5 intervals: 18 to 24 years, 25 to 34 years, 35 to 44 years, 45 to 54 years, and 55 to 64 years. Race/ethnicity was categorized as non-Hispanic white, non Hispanic black, Mexican American, and other. Educational attainment was categorized as less than high school graduate, high school graduate, some college, and college graduate or more. We categorized the poverty income ratio according to eligibility for food assistance programs as follows: less than 130% of the poverty level (eligible for the Supplemental Nutrition Assistance Program and free school meals), at least 130% but less than 185% of the poverty level (eligible for the Special Supplemental Nutrition Program for Women, Infants, and Children), and at least 185% of the poverty level. We categorized smoking status (current, past, or never) and at least 10 minutes of leisure time moderate or vigorous physical activity over the past 30 days (yes or no).
We also examined the distribution of the subsidy score across categories of self-reported household food security status (fully food secure, marginally food secure, food insecure without hunger, or food insecure with hunger). Last, we investigated whether the associations between high consumption of subsidized commodity foods and cardiometabolic risk remained after controlling for overall diet quality using the 2010 Healthy Eating Index (HEI-2010),14 a measure representing adherence to federal dietary guidance (2010 Dietary Guidelines for Americans).
Statistical Analysis
We used statistical analysis software (SAS, version 9.4; SAS Institute and SAS-callable SUDAAN, version 10.0; RTI International). These programs accounted for the NHANES complex design and dietary sampling weights so that characteristics of the represented population could be correctly described.
Using weighted proportions and means with standard errors, weexamined population characteristics overall and across subsidy score quartiles. We used linear regression to estimate the mean (95% CI) cardiometabolic risk factor levels for US adults across subsidy score quartiles, adjusted for age, sex, and race/ ethnicity. We used multivariate logistic regression to estimate the probability of each cardiometabolic risk factor at each quartile of the subsidy score, adjusting for demographic and behavioral covariates (eg, sex, age, race/ethnicity, educational attainment, poverty income ratio, smoking status, moderate or vigorous leisure time physical activity, and total daily caloric intake) and then further adjusting for the HEI-2010. We also used logistic regression to examine the associations between the continuous subsidy score and each dichotomized cardiometabolic risk factor. Individuals with missing data (ranging from <5% to 10% of the total sample) were excluded from the models. P < .05 was considered statistically significant.
Results
Table 2 lists estimated characteristics of the study sample overall and by subsidy score quartiles. Overall, 50.5% of our study sample were male, and this percentage did not vary significantly across quartiles of the subsidy score (P = .77). Overall, 56.2% of calories consumed were from the major subsidized food commodities. On average, individuals eating the highest proportion of subsidized foods (Q4) were younger than individuals in Q1. Quartile 4 also contained a higher proportion of Mexican Americans and a lower proportion of non-Hispanic whites and non-Hispanic blackscomparedwithQ1(P < .001).Comparedwith Q1,individuals in Q4 tended to be significantly less educated, poorer, and less food secure (P < .001 for all). From subsidy score Q1 to Q4, current smoking status increased (P < .001), and leisure time physical activity decreased (P = .006).
Table 2.
Characteristics of 10 308 Adults 18 to 64 Years Old in the National Health and Nutrition Examination Survey From 2001 to 2006 Overall and by Subsidy Score Quartilesa
| Variable | No. | Weighted Distribution | Q1 | Q2 | Q3 | Q4 | P Value |
|---|---|---|---|---|---|---|---|
| Male sex | 5254 | 50.5 (0.5) | 50.8 (1.2) | 49.3 (1.2) | 51.6 (1.4) | 50.2 (1.1) | .77 |
| Age group, y | |||||||
| 18–24 | 2484 | 15.6 (0.7) | 11.0 (0.8) | 12.4 (1.0) | 17.5 (1.1) | 21.4 (1.4) | <.001 |
| 25–34 | 1931 | 20.2 (0.8) | 16.9 (1.3) | 18.7 (1.4) | 20.7 (1.2) | 24.5 (1.3) | |
| 35–44 | 2127 | 24.1 (0.9) | 24.1 (1.2) | 23.5 (1.2) | 25.6 (1.6) | 22.9 (1.5) | |
| 45–54 | 2056 | 24.5 (0.7) | 29.0 (1.4) | 27.5 (1.5) | 22.9 (1.0) | 18.4 (1.2) | |
| 55–64 | 1710 | 15.7 (0.7) | 18.9 (1.2) | 17.9 (1.0) | 13.3 (1.1) | 12.8 (0.9) | |
| Age, mean (SE), y | NA | 40.2 (0.3) | 42.5 (0.4) | 41.7 (0.4) | 39.2 (0.4) | 37.4 (0.4) | <.001 |
| Race/ethnicity | |||||||
| Non-Hispanic white | 4689 | 71.3 (1.8) | 74.7 (1.5) | 72.5 (2.0) | 69.0 (2.5) | 69.2 (2.2) | <.001 |
| Non-Hispanic black | 2465 | 11.7 (1.1) | 11.7 (1.3) | 12.2 (1.2) | 11.5 (1.5) | 11.2 (1.3) | |
| Mexican American | 2385 | 8.3 (0.8) | 5.5 (0.6) | 7.1 (1.0) | 9.9 (1.2) | 10.7 (1.2) | |
| Other | 769 | 8.7 (0.8) | 8.2 (1.1) | 8.2 (1.1) | 9.6 (1.2) | 8.8 (1.1) | |
| Educational attainment | |||||||
| <High school graduate | 2819 | 16.2 (0.7) | 13.2 (1.1) | 13.9 (1.0) | 18.4 (0.9) | 19.3 (1.1) | <.001 |
| High school graduate | 2589 | 25.0 (0.7) | 23.1 (1.0) | 24.4 (1.3) | 25.1 (1.3) | 27.6 (1.1) | |
| Some college | 3000 | 32.6 (0.8) | 33.7 (1.20) | 32.8 (1.4) | 32.2 (1.2) | 31.6 (1.2) | |
| ≥College graduate | 1896 | 26.3 (1.2) | 30.0 (1.5) | 29.0 (2.1) | 24.4 (1.7) | 21.6 (1.4) | |
| Poverty income ratio, % | |||||||
| <130 | 2810 | 19.8 (0.9) | 15.5 (1.1) | 18.2 (1.0) | 23.0 (1.4) | 22.6 (1.4) | <.001 |
| ≥130 to <185 | 1139 | 8.7 (0.4) | 8.1 (0.8) | 8.0 (0.7) | 8.8 (0.8) | 9.9 (0.7) | |
| ≥185 | 5805 | 71.5 (1.1) | 76.4 (1.4) | 73.8 (1.4) | 68.2 (1.4) | 67.5 (1.7) | |
| Food security status | |||||||
| Fully food secure | 7450 | 82.8 (0.7) | 85.6 (1.1) | 85.4 (0.7) | 81.4 (1.0) | 78.6 (1.4) | <.001 |
| Marginally food secure | 945 | 6.6 (0.4) | 4.7 (0.6) | 5.6 (0.5) | 7.5 (0.7) | 8.7 (0.9) | |
| Food insecure without hunger | 962 | 6.5 (0.4) | 5.0 (0.6) | 5.7 (0.5) | 7.2 (0.7) | 8.1 (0.6) | |
| Food insecure with hunger | 542 | 4.1 (0.4) | 4.7 (0.8) | 3.4 (0.5) | 4.0 (0.6) | 4.6 (0.5) | |
| Smoking status | |||||||
| Current | 2949 | 28.5 (0.8) | 28.9 (1.4) | 26.5 (1.3) | 27.6 (1.3) | 31.0 (1.4) | <.001 |
| Past | 1998 | 21.4 (0.7) | 24.9 (1.4) | 22.0 (1.2) | 21.0 (1.3) | 17.8 (1.2) | |
| Never | 5275 | 50.1 (0.9) | 46.2 (1.4) | 51.5 (1.5) | 51.4 (1.6) | 51.2 (1.5) | |
| Leisure time physical activity | |||||||
| Yes | 6760 | 70.3 (0.9) | 73.2 (1.1) | 72.0 (1.5) | 68.4 (1.6) | 67.5 (1.5) | .006 |
| No | 3547 | 29.7 (0.9) | 26.8 (1.1) | 28.0 (1.5) | 31.6 (1.6) | 32.6 (1.5) | |
Abbreviations: NA, not applicable; Q, quartile.
Numbers are proportions (SEs) unless otherwise stated. Because of missing values, the categories do not sum to the total 10 308 for some demographic characteristics. Subsidy score quartiles were defined as follows: Q1 is 0.00 to 0.47, Q2 is 0.48 to 0.57, Q3 is 0.58 to 0.65, and Q4 is 0.66 to 1.00. Poverty income ratio was defined as at least 185% of the poverty level (higher income), at least 130% to less than 185% (eligible for the Special Supplemental Program for Women, Infants, and Children but not the Supplemental Nutrition Assistance Program), and less than 130% of the poverty level (eligible for the Supplemental Nutrition Assistance Program and free school meals). Leisure time physical activity includes moderate and vigorous activity. χ2 Tests were used to determine P values.
In Table 3, the mean (95% CI) cardiometabolic risk factors, adjusted forsex, age, and race/ethnicity, are listed across subsidy score quartiles. Higher mean BMI, ratio of waist circumference to height, CRP level, non-HDL cholesterol level, and glycated hemoglobin level were seen across higher quartiles of subsidy score. Individuals in higher quartiles did not have statistically significantly higher systolic or diastolic blood pressure.
Table 3.
Cardiometabolic Risk Factors by Subsidy Score Quartiles, National Health and Nutrition Examination Survey From 2001 to 2006a
| Variable | Mean (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|
| Overall (N = 10 308) | Q1 (n = 2459) | Q2 (n = 2530) | Q3 (n = 2650) | Q4 (n = 2669) | ||
| Body mass indexb | 28.2 (27.9–28.5) | 27.5 (27.0–27.9) | 27.8 (27.5–28.2) | 28.4 (28.0–28.8) | 29.0 (28.5–29.4) | <.001 |
| Ratio of waist circumference to height | 0.56 (0.56–0.57) | 0.55 (0.55–0.56) | 0.56 (0.56–0.57) | 0.57 (0.56–0.57) | 0.58 (0.57–0.58) | <.001 |
| C-reactive protein level, mg/dL | 2.0 (2.0–2.0) | 1.9 (1.8–1.9) | 2.0 (1.9–2.0) | 2.0 (2.0–2.1) | 2.1 (2.0–2.1) | <.001 |
| SBP, mm Hg | 120.3 (119.6–120.9) | 119.9 (118.9–120.9) | 120.8 (119.8–121.8) | 119. (118.9–120.7) | 120.5 (119.5–121.5) | .13 |
| DBP, mm Hg | 72.3 (71.8–72.8) | 72.3 (71.7–72.9) | 72.8 (72.0–73.6) | 72.4 (71.5–73.2) | 71.8 (71.0–72.6) | .27 |
| Non-HDL cholesterol level, mg/dL | 147.3 (145.9–148.7) | 144.1 (141.3–146.9) | 148.0 (144.8–151.2) | 147.4 (145.2–149.7) | 149.6 (147.1–152.1) | .05 |
| Glycated hemoglobin level, % | 5.41 (5.38–5.44) | 5.36 (5.32–5.41) | 5.37 (5.34–5.41) | 5.45 (5.40–5.50) | 5.47 (5.42–5.53) | <.001 |
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; Q, quintile; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol level to millimoles per liter, multiply by 0.0259; C-reactive protein level to nanomoles per liter, multiply by 9.524; and glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Adjusted for sex, age, and race/ethnicity. Individuals with missing data were excluded from the models.
Calculated as weight in kilograms divided by height in meters squared.
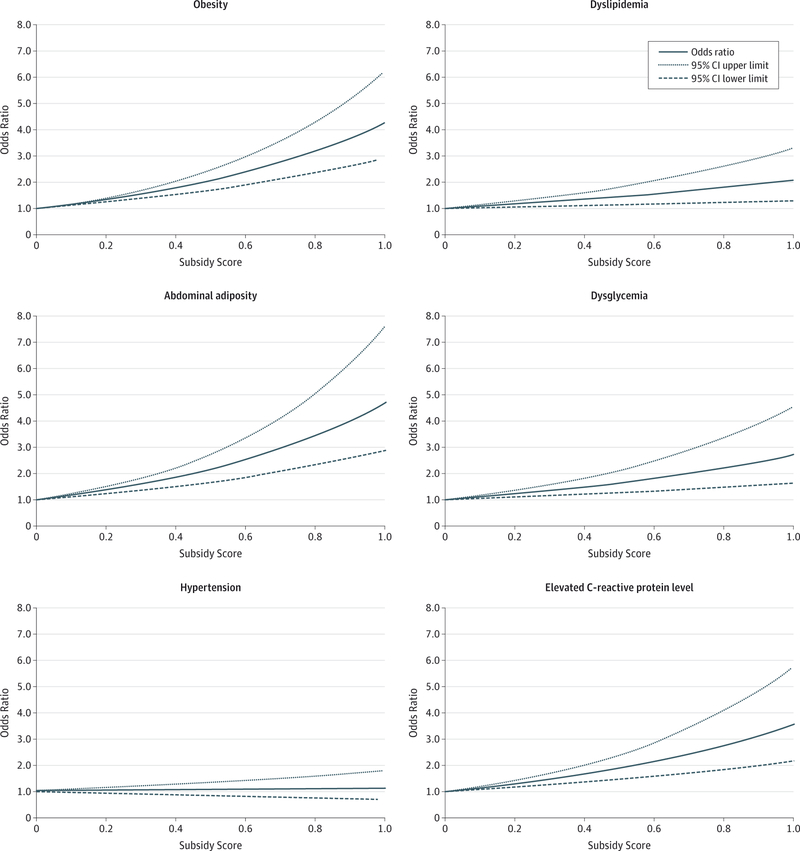
Table 4 lists the predicted marginal probability of each dichotomizedcardiometabolic risk factoracross subsidy scorequartiles and the corresponding prevalence ratios after adjusting for sociodemographic factors. The eTable in the Supplement lists the predicted marginal probability and prevalence ratios of the 3-level cardiometabolic risk factors across subsidy score quartiles. In the fully adjusted model in Table 4, compared with Q1, those in Q4 had a 37% higher probability of being obese (prevalence ratio, 1.37; 95% CI, 1.23–1.52), a 41% higher probability of having abdominal adiposity (prevalence ratio, 1.41; 95% CI, 1.25–1.59), a 34% higher probability of having an elevated CRP level (prevalence ratio, 1.34; 95% CI, 1.19–1.51), a 14% higher probability of having dyslipidemia (prevalence ratio, 1.14; 95% CI, 1.05–1.25), and a 21% higher probability of having dysglycemia (prevalence ratio, 1.21; 95% CI, 1.04 1.40). Wefound no statistically significant associations between blood pressure and the subsidy score. Moreover, further adjusting for overall diet quality using the HEI-2010 did not significantly change the results. The Figure shows the associations between the continuous subsidy score and the dichotomized cardiometabolic risk factors.
Table 4.
Adjusted Prevalence and Prevalence Ratio of Cardiometabolic Risk Factors by Subsidy Score Quartiles, National Health and Nutrition Examination Survey From 2001 to 2006a
| Subsidy Score | Adjusted Prevalence and Prevalence Ratio (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity | Adiposity Adiposity | Protein Level Protein Level | Hypertension | Dyslipidemia | Dysglycemia | |||||||
| Model 1b | ||||||||||||
| Q1 | 26.3 | 1 [Reference] | 25.3 | 1 [Reference] | 27.1 | 1 [Reference] | 27.3 | 1 [Reference] | 40.0 | 1 [Reference] | 14.6 | 1 [Reference] |
| Q2 | 29.7 | 1.13 (1.02–1.24) | 29.3 | 1.16 (1.04–1.30) | 31.3 | 1.15 (1.01–1.32) | 30.1 | 1.10 (0.98–1.24) | 43.2 | 1.08 (0.99–1.18) | 14.7 | 1.01 (0.85–1.19) |
| Q3 | 34.9 | 1.33 (1.18–1.49) | 32.7 | 1.29 (1.17–1.43) | 35.7 | 1.32 (1.19–1.46) | 28.5 | 1.04 (0.93–1.18) | 46.0 | 1.15 (1.06–1.25) | 18.7 | 1.29 (1.13–1.46) |
| Q4 | 37.4 | 1.42 (1.27–1.59) | 37.3 | 1.48 (1.30–1.68) | 37.8 | 1.39 (1.01–1.32) | 30.2 | 1.11 (0.99–1.23) | 46.0 | 1.15 (1.06–1.25) | 19.0 | 1.30 (1.13–1.51) |
| Model 2c | ||||||||||||
| Q1 | 27.0 | 1 [Reference] | 25.9 | 1 [Reference] | 27.4 | 1 [Reference] | 28.1 | 1 [Reference] | 40.4 | 1 [Reference] | 14.9 | 1 [Reference] |
| Q2 | 29.5 | 1.10 (0.99–1.21) | 29.1 | 1.12 (1.01–1.25) | 31.9 | 1.16 (1.02–1.32) | 30.3 | 1.08 (0.95–1.22) | 43.4 | 1.07 (0.97–1.19) | 14.3 | 0.96 (0.81–1.15) |
| Q3 | 34.8 | 1.29 (1.14–1.46) | 32.4 | 1.25 (1.14–1.38) | 35.3 | 1.29 (1.17–1.42) | 28.3 | 1.01 (0.89–1.14) | 46.2 | 1.14 (1.04–1.25) | 18.0 | 1.21 (1.06–1.38) |
| Q4 | 36.9 | 1.37 (1.23–1.52) | 36.4 | 1.41 (1.25–1.59) | 36.9 | 1.34 (1.19–1.51) | 30.2 | 1.07 (0.96–1.21) | 46.3 | 1.14 (1.05–1.25) | 17.9 | 1.21 (1.04–1.40) |
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; Q, quartile; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol level to millimoles per liter, multiply by 0.0259; C-reactive protein level to nanomoles per liter, multiply by 9.524; and glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Subsidy score quartiles were defined as follows: Q1 is 0.00 to 0.47, Q2 is 0.48 to 0.57, Q3 is 0.58 to 0.65, and Q4 is 0.66 to 1.00. Obesity was defined as body mass index (calculated as weight in kilograms divided by height in meters squared) of at least 30. Abdominal adiposity was defined as a ratio of waist circumference to height of at least 0.60. Elevated C-reactive protein level was defined as at least 0.32 mg/dL. Hypertension was defined as diagnosed (self-reported) or undiagnosed (no self-reported diagnosis and SBP ≥140 mm Hg or DBP ≥90 mm Hg) hypertension or currently taking antihypertensive medication. Dyslipidemia was defined as diagnosed (self-reported) or undiagnosed (no self-reported diagnosis and non-HDL cholesterol level ≥160 mg/dL) dyslipidemia or currently taking anticholesterolemia medication. Dysglycemia was defined as self-reported diabetes diagnosis or glycated hemoglobin level of at least 5.7%. Individuals with missing data were excluded from the models.
Model adjusted for sex, age, and race/ethnicity.
Model adjusted for sex, age, race/ethnicity, educational attainment, poverty income ratio, smoking status, moderate and vigorous leisure time physical activity, and total daily caloric intake.
Discussion
More than half of all calories consumed by nonelderly adults in the United States during the 6-year period from 2001 to 2006 originated from subsidized food commodities. Adjusted forso ciodemographic and lifestyle factors, being among the highest quartile of subsidized food consumers was associated with having a 14% to 41% higher probability of cardiometabolic risk as measured by BMI, abdominal adiposity, CRP level, and lipid levels. These associations remained robust to adjustment for overall diet quality.
Our findings suggest that better alignment of agricultural and nutritional policies may have the potential to improve the distribution of risk factors forcardiometabolic disease and may help clarify the ongoing debate about the role of agricultural subsidies on health. Public health and nutrition professionals have noted a link between agricultural policy and obesity and cardiometabolic risk and have called for elimination of agricultural subsidies or at least a shift to include healthier crops.15–18 However, it has also been argued that farm policies do not contribute to obesity and that their elimination would actually increase caloric intake inthe United States,19–21 but 1 noteworthy limitation of that work is that it considers total calories (and obesity) rather than quality of calories (and cardiometabolic risk). Therefore, a key strength and contribution of our analysis is the consideration of diet quality (composition) rather than just quantity of calories. In a previous publication, our group showed that diets of individuals with higher subsidy scores tended to be rich in high-fat dairy, grains, and meat products and poor in fruits and vegetables and overall diet quality (as measured by the HEI-2010).10
Moreover, previous research has described the effect of socioeconomic status on cardiometabolic health in the United States, with poorer and less educated individuals more likely to have poor cardiometabolic health.22,23 Our group has previously shown that younger, poorer, less educated, and less food-secure individuals consumed diets withdisproportionately higher proportions of subsidized food commodities.10 The present finding that higher subsidy scores are associated with adverse cardiometabolic risk highlights the effect that agricultural subsidies may be having on health disparities in the United States, in part due to the lower cost per calorie of unhealthier food and the higher cost per calorie of healthier food.24 This observation has implications forfood security because these same population groups may also be restricted by the amount of money they have available to meet their nutritional requirements. For example, higher prices for healthy foods have been found to be associated with increased blood glucose level among people with type 2 diabetes, and this association is especially pronounced among low-income individuals with diabetes.25
Our findings, taken together with our group’s previous publication showing that diets of individuals with a higher subsidy score tend to be of lower nutritional quality,10 support previous calls torealign agricultural policies with nutritional needs in the modern era of increasing cardiometabolic diseases.7 But what canbe done? One potential policy lever foraddressing this need may be to shift agricultural subsidies toward the production of healthier crops, such as fruits and vegetables. A successful example comes from Finland’s berry project in the latter 20th century.26,27 By molding a collaboration among berry farmers, industry, commercial sectors, and health authorities with financing from the Ministry of Agriculture and the Ministry of Commerce, many farmers switched from dairy to berry production, dairy consumption declined, and local berry consumption gradually rose in Finland.27–29 This berry project was part of the larger North Karelia Project,29 which by the year 2000 helped reduce countrywide cardiovascular disease mortality by 80% (attributed to dietary changes and dramatic reductions in cardiometabolic risk factors like hypertension, hypercholesterolemia, and smoking) and all-cause mortality by 45%, aswell as increase male life expectancy by 7 years.30 Although since our period of study (2001–2006) food subsidies in the United States have changed in scope and there are now several initiatives to increase fruit and vegetable production,31 there is still much more that can be done.
Because the present study is cross-sectional, future research is still needed to investigate whether there isatemporal relationship between consumption of subsidized foods and cardiometabolic risks and diseases. In addition, we need robust modeling of how changes tocurrent subsidy structures would alter the production and consumption of various foods and resulting health outcomes. Although other related diet quality indexes (eg, the HEI-2010) exist, the subsidy score provides additional benefit for better understanding the role of subsidized foods on health independent of overall diet quality.
There are some limitations to our analysis. First, a single day of 24-hour dietary recall in the NHANES was used to assess diet and create the subsidy score, and the residual intrapersonal variability may decrease differences between demographic subgroups. However, a single 24-hour recall provides greater detail on the specific types and amounts of food eaten than afood-frequency questionnaire. Second, the subsidy score has its limitations. For example, it was not possible to directly calculate the amount of high-fructose corn syrup in foods or the exact proportion of subsidized meat that is consumed as processed vs unprocessed due to incomplete nutritional and ingredient information for foods reported in the NHANES. In addition, some by-products of subsidized commodities (eg, soy lecithin) are not captured by our analysis because these byproducts are not traced through the food system. However, the amount of these by-products in foods is negligible, and their exclusion is unlikely to affect the results significantly. Third, the cross-sectional nature of this study does not allow inference of causality. We have not demonstrated that the agricultural subsidies themselves are responsible for the current cardiometabolic risk burden in the United States, but rather that agricultural subsidies are one part of the entire panoply of cardiometabolic risk factors, some of which include poverty, cheap food, poor dietary choices, and fewer options. Fourth, a limitation of the study is the potential for unmeasured confounding. Although we controlled for known demographic and lifestyle risk factors, many important risk factors, such as smoking, physical activity, poverty, and food insecurity, increased across subsidy score quartiles, suggesting that there may be other relevant risk factors for which we were unable to control.
Conclusions
The cost of treating obesity-related cardiometabolic diseases in the United States is estimated to range from $150 billion per year to as much as $300 billion per year if indirect costs are included, an amount that exceeds government spending on either farm support or nutrition assistance programs.4 During the period of our data collection, estimated Medicare spending would have been approximately 8% lower and Medicaid spending approximately 12% lower in the absence of obesity.32 Takentogether with data in the present article, the government from 1995 to 2010 spent $170 billion on subsidizing the production of foods that were associated with obesity,7 a poor health outcome that in turn was associated withexpanded expenditures for health services covered by Medicare and Medicaid. Although eating fewer subsidized foods will not eradicate obesity, our results suggest that individuals whose diets consist of a lower proportion of subsidized foods have alower probability of being obese. Nutritional guidelines are focused on the population’s needs for healthier foods, but to date food and agricultural policies that influence food production and availability have not yet done the same.
Supplementary Material
Figure.
Association Between the Continuous Subsidy Score and Cardiometabolic Risk Factors
Obesity was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of at least 30. Abdominal adiposity was defined as a ratio of waist circumference to height of at least 0.60. Hypertension was defined as diagnosed (self-reported) or undiagnosed (no self-reported diagnosis and systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg) hypertension or currently taking antihypertensive medication. Dyslipidemia was defined as diagnosed (self-reported) or undiagnosed (no self-reported diagnosis and non–high-density lipoprotein cholesterol level ≥160 mg/dL) dyslipidemia or currently taking anticholesterolemia medication. Dysglycemia was defined as self-reported diabetes diagnosis or glycated hemoglobin level of at least 5.7%. Elevated C-reactive protein level was defined as at least 0.32 mg/dL. To convert cholesterol level to millimoles per liter, multiply by 0.0259; C-reactive protein level to nanomoles per liter, multiply by 9.524; and glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Key Points.
Question
Is an individual’s consumption of foods derived from subsidized food commodities associated with adverse cardiometabolic risk?
Findings
In this cross-sectional analysis of adults in the National Health and Nutrition Examination Survey, being among the highest quartile of subsidized food consumers was associated with having a higher probability of cardiometabolic risk.
Meaning
Food commodity subsidies support the production of foods associated with adverse cardiometabolic risk, and supporting the production of foods associated with cardiometabolic health may help improve population health.
Acknowledgments
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest Disclosures: None reported.
Contributor Information
Karen R. Siegel, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Kai McKeever Bullard, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Giuseppina Imperatore, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Henry S. Kahn, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Aryeh D. Stein, Hubert Department of Global Health, Rollins School of Public Health, Emory University; Nutrition and Health Sciences Program, Laney Graduate School, Emory University, Atlanta, Georgia.
Mohammed K. Ali, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; Hubert Department of Global Health, Rollins School of Public Health, Emory University; Nutrition and Health Sciences Program, Laney Graduate School, Emory University Atlanta, Georgia.
K. M. Narayan, Hubert Department of Global Health, Rollins School of Public Health, Emory University; Nutrition and Health Sciences Program, Laney Graduate School, Emory University, Atlanta, Georgia.
REFERENCES
- 1. Agriculture and Consumer Protection Act of 1973. Pub L No. 93–86, 87 Stat 221.
- 2.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA 2014;312(2): 189–190. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2015 update: a report from the American Heart Association [published correction appears in Circulation. 2015;131(24):e535.]. Circulation 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Glickman D, Veneman AM. The essential role of food and farm policy in improving health. Health Aff (Millwood) 2013;32(9):1519–1521. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Fahimi S, Singh GM, et al. ; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014;371(7):624–634. [DOI] [PubMed] [Google Scholar]
- 6.McGuire S U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010 7th ed. Washington, DC: US Government Printing Office; January 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franck C, Grandi SM, Eisenberg MJ. Agricultural subsidies and the American obesity epidemic. Am J Prev Med 2013;45(3):327–333. [DOI] [PubMed] [Google Scholar]
- 8.Economic Research Service, US Department of Agriculture. Feed Grains Database http://www.ers.usda.gov/data-products/feed-grains-database.aspx. Accessed August 2012.
- 9.Basu S, Seligman HK, Gardner C, Bhattacharya J. Ending SNAP subsidies for sugar-sweetened beverages could reduce obesity and type 2 diabetes. Health Aff (Millwood) 2014;33(6):1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel KR, McKeever Bullard K, Ali MK, et al. The contribution of subsidized food commodities to total energy intake among US adults. Public Health Nutr 2016;19(8):1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics, Centers for Disease Control and Prevention. Key concepts about NHANES survey design http://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/SampleDesign/Info1.htm. Accessed August 2012.
- 12.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69(2):243–249. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Energy. In: Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: National Academies Press; 2005:chap 5. [Google Scholar]
- 14.Center for Nutrition Policy and Promotion, US Department of Agriculture. Healthy Eating Index http://www.cnpp.usda.gov/healthyeatingindex. Published in 2010. Accessed January 2016.
- 15.Pollan M The (agri)cultural contradictions of obesity. New York Times Magazine October 12, 2003. [Google Scholar]
- 16.Nestle M The ironic politics of obesity. Science 2003;299(5608):781. [DOI] [PubMed] [Google Scholar]
- 17.Russo M Apples to Twinkies: comparing federal subsidies of fresh produce and junk food http://www.uspirg.org/reports/xxp/apples-twinkies. Published 2011. Accessed August 2012.
- 18.Yach D Nutritional change is not a simple answer to non-communicable diseases. BMJ 2011; 343:d5097. [DOI] [PubMed] [Google Scholar]
- 19.Alston JM, Summer DA, Vosti SA. Are agricultural policies making us fat? likely links between agricultural policies and human nutrition and obesity, and their policy implications. Rev Agric Econ 2006;28(3):313–322. [Google Scholar]
- 20.Rickard BJ, Okrent AM, Alston JM. How have agricultural policies influenced caloric consumption in the United States? Health Econ 2013;22(3):316–339. [DOI] [PubMed] [Google Scholar]
- 21.Alston JM, Summer DA, Vosti SA. Farm subsidies and obesity in the United States: national evidence and international comparisons. Food Policy 2008;33(6):470–479. [Google Scholar]
- 22.Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States [published correction appears in PLoS Med. 2006;3(12):e545]. PLoS Med 2006;3(9):e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290(14):1884–1890. [DOI] [PubMed] [Google Scholar]
- 24.Drewnowski A. Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med 2004;27(3)(suppl):154–162. [DOI] [PubMed] [Google Scholar]
- 25.Anekwe TD, Rahkovsky I. The association between food prices and the blood glucose level of US adults with type 2 diabetes. Am J Public Health 2014;104(4):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietinen P, Lahti-Koski M, Vartiainen E, Puska P. Nutrition and cardiovascular disease in Finland since the early 1970s: a success story. J Nutr Health Aging 2001;5(3):150–154. [PubMed] [Google Scholar]
- 27.Pekka P, Pirjo P, Ulla U. Influencing public nutrition for non-communicable disease prevention: from community intervention to national programme: experiences from Finland. Public Health Nutr 2002;5(1A):245–251. [PubMed] [Google Scholar]
- 28.Kuusipalo J, Mikkola M, Moisio S, Puska P. The East Finland Berry and Vegetable Project: a health-related structural intervention programme. Health Promot Int 1986;1(3):385–391. [Google Scholar]
- 29.Puska P The North Karelia Project: nearly 20 years of successful prevention of CVD in Finland. Hygie 1992;11(1):33–35. [PubMed] [Google Scholar]
- 30.Puska P, Vartiainen E, Tuomilehto J, Salomaa V, Nissinen A. Changes in premature deaths in Finland: successful long-term prevention of cardiovascular diseases. Bull World Health Organ 1998;76(4):419–425. [PMC free article] [PubMed] [Google Scholar]
- 31.Steinhauer J Farm Bill reflects shifting American menu and a senator’s persistent tilling. New York Times March 8, 2014. [Google Scholar]
- 32.Trogdon JG, Finkelstein EA, Feagan CW, Cohen JW. State and payer-specific estimates of annual medical expenditures attributable to obesity. Obesity (Silver Spring) 2012;20(1):214–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.