Abstract
Importance
Confirmation of long-term comparability between subcutaneous and intravenous trastuzumab is essential.
Objective
To evaluate efficacy and safety of subcutaneous trastuzumab compared with that of intravenous trastuzumab for patients with ERBB2 (HER2)–positive early breast cancer after 6 years’ follow-up in the HannaH (Enhanced Treatment With Neoadjuvant Herceptin) trial.
Design, Setting, and Participants
Open-label, prospective, multicenter, international, neoadjuvant-adjuvant, randomized, phase 3 noninferiority clinical trial (primary end points: pathologic complete response and serum trough concentration predose cycle 8) conducted for 596 patients with ERBB2-positive early breast cancer enrolled from October 19, 2009, to December 1, 2010.
Interventions
Eligible patients received 8 cycles of chemotherapy (4 cycles of docetaxel, 75 mg/m2, followed by 4 cycles of fluorouracil, 500 mg/m2, epirubicin, 75 mg/m2, and cyclophosphamide, 500 mg/m2) with either fixed-dose subcutaneous trastuzumab, 600 mg, or intravenous trastuzumab (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) every 3 weeks in the neoadjuvant setting. Patients received an additional 10 cycles of subcutaneous trastuzumab or intravenous trastuzumab (according to their initial randomization) after surgery in the adjuvant setting to complete 1 year of anti-ERBB2 therapy.
Main Outcomes and Measures
Event-free and overall survival rates were calculated using the Kaplan-Meier method. Hazard ratios were estimated by Cox proportional hazards regression. Adverse events and serious adverse events were graded per standard criteria.
Results
In total, 294 women (mean [SD] age, 50.3 [11.1] years) treated with subcutaneous trastuzumab and 297 women (mean [SD] age, 49.5 [10.8] years) treated with intravenous trastuzumab were included in respective intention-to-treat populations. Six-year event-free survival rates (65% in both study groups; hazard ratio, 0.98; 95% CI, 0.74-1.29) and overall survival rates (84% in both study groups; hazard ratio, 0.94; 95% CI, 0.61-1.45) were similar between the subcutaneous and intravenous trastuzumab groups. Patients achieving a total pathologic complete response had longer event-free survival and higher 6-year overall survival rates than those with residual disease. Incidence of adverse events (290 of 297 [97.6%] vs 282 of 298 [94.6%]), grade 3 or higher adverse events (158 of 297 [53.2%] vs 160 of 298 [53.7%]), cardiac events (44 of 297 [14.8%] vs 42 of 298 [14.1%]), and serious adverse events (65 of 297 [21.9%] vs 45 of 298 [15.1%]) was comparable between the subcutaneous and intravenous trastuzumab treatment groups.
Conclusions and Relevance
This final analysis of the HannaH trial further confirms the comparable efficacy and safety of subcutaneous and intravenous trastuzumab and highlights the suitability of subcutaneous trastuzumab as an alternative route of administration for patients with ERBB2-positive early breast cancer.
Trial Registration
ClinicalTrials.gov identifier: NCT00950300
This final analysis of the phase 3 randomized clinical trial HannaH evaluates the efficacy and safety of subcutaneous trastuzumab compared with that of intravenous trastuzumab for patients with ERBB2-positive early breast cancer after 6 years’ follow-up.
Key Points
Question
Do subcutaneous trastuzumab and intravenous trastuzumab have comparable long-term efficacy and safety for patients with ERBB2 (HER2)–positive early breast cancer?
Findings
In this final analysis of a randomized clinical trial of 596 patients, 6-year event-free survival rates were 65% with subcutaneous and intravenous trastuzumab, and 6-year overall survival rates were 84% in both study groups. Safety was comparable between subcutaneous and intravenous treatment.
Meaning
Subcutaneous trastuzumab has long-term efficacy and safety comparable to that of intravenous trastuzumab, thus confirming the suitability of subcutaneous trastuzumab treatment for patients with ERBB2-positive early breast cancer.
Introduction
Subcutaneous trastuzumab may offer several advantages compared with intravenous trastuzumab, including shorter treatment times, a reduction in the use of health care resources, increased convenience for patients, and greater patient preference.1,2,3,4 In the primary analysis of the HannaH (Enhanced Treatment With Neoadjuvant Herceptin) trial,5 subcutaneous trastuzumab was shown to be noninferior to intravenous trastuzumab for patients with ERBB2 (HER2)–positive early breast cancer (EBC), based on the coprimary end points of pathologic complete response (absence of invasive neoplastic cells in the breast; remaining ductal carcinoma in situ was accepted) and serum trough concentration predose dose cycle 8. The 2-year follow-up analyses demonstrated similar 3-year event-free survival (EFS) rates6 and confirmed the comparability of the safety profiles for subcutaneous trastuzumab and intravenous trastuzumab observed in the primary analysis.5,6,7 The overall safety profile for subcutaneous trastuzumab in the HannaH trial was also shown to be consistent with the known safety profile for subcutaneous trastuzumab for patients with ERBB2-positive EBC in both SafeHer, which had a study population of more than 2500 patients,8 and PrefHer.1,4,9
In this final analysis of the HannaH trial, we report the long-term efficacy and safety outcomes at a median follow-up of approximately 6 years. We also examine the effect of hormone receptor status and total pathologic complete response (tpCR; absence of invasive neoplastic cells in the breast and ipsilateral axillary lymph nodes, regardless of ductal carcinoma in situ) status on long-term efficacy.
Methods
Study Design and Patients
The HannaH trial was an open-label, prospective, multicenter, international, neoadjuvant-adjuvant, randomized, phase 3 noninferiority clinical trial. Details of the study design have been published5 and can also be found in Supplement 1. The study was performed in accordance with the Declaration of Helsinki10 and Good Clinical Practice guidelines. Approval for the study protocol and all material provided to the patients was obtained from the independent ethics committees at the participating institutions. All patients provided written informed consent.
Eligible patients (N = 596) were randomized 1:1 to receive fixed-dose 600-mg subcutaneous trastuzumab or intravenous trastuzumab (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg), administered concurrently with 8 cycles of chemotherapy (4 cycles of docetaxel, 75 mg/m2, followed by 4 cycles of fluorouracil, 500 mg/m2, epirubicin, 75 mg/m2, and cyclophosphamide, 500 mg/m2) every 3 weeks in the neoadjuvant setting. After surgery, patients received an additional 10 cycles of subcutaneous trastuzumab or intravenous trastuzumab (according to their initial randomization) in the adjuvant setting to complete 1 year of trastuzumab-based anti-ERBB2 therapy.5 A protocol amendment extended the posttreatment observation period to 5 years, as requested by the authorities.
Outcomes
The coprimary end points were serum trough concentration predose cycle 8 and pathologic complete response of patients receiving subcutaneous trastuzumab vs those receiving intravenous trastuzumab.5 Secondary end points included EFS, overall survival (OS), and safety.5 Exploratory subgroup analyses included EFS by hormone receptor and tpCR status.6
Statistical Analysis
Target sample sizes and power calculations for the primary analysis have been reported.5 Statistical analyses were performed with SAS, version 8.2 (SAS Institute Inc). Efficacy analyses were conducted for all patients in the intention-to-treat population who had at least 1 efficacy assessment after the first administration of the study drug.6 Safety analyses were conducted for all patients who received at least 1 dose of subcutaneous trastuzumab or intravenous trastuzumab, including information from the neoadjuvant and adjuvant settings.5 The Kaplan-Meier method was used to calculate EFS and OS rates. Hazard ratios (HRs) from timewise comparisons of results are unstratified and were estimated by Cox proportional hazards regression. Adverse events (AEs) were graded per standard criteria.
Results
Patients
Of the 596 enrolled patients from 81 centers across 24 countries between October 19, 2009, and December 1, 2010,5 297 were randomized to receive subcutaneous trastuzumab and 299 were randomized to receive intravenous trastuzumab.5 The intention-to-treat population comprised 294 patients treated with subcutaneous trastuzumab and 297 patients treated with intravenous trastuzumab.5 Patient dispositions are shown in the eFigure in Supplement 2. At the clinical cutoff (January 24, 2017), the median duration of follow-up was 5.9 years (range, 0.03-6.3 years) in the subcutaneous trastuzumab group and 6.0 years (range, 0.08-6.8 years) in the intravenous trastuzumab group, including the treatment-free follow-up period.
Efficacy
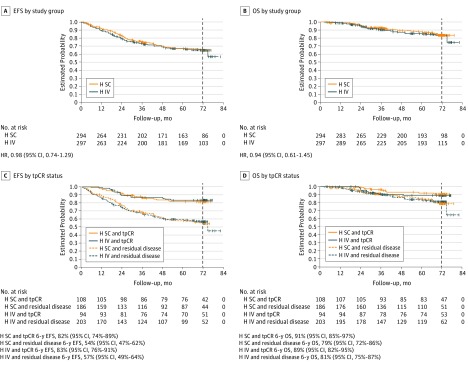
Event-free survival among patients treated with subcutaneous trastuzumab was almost identical with that observed among patients treated with intravenous trastuzumab (6-year EFS rates, 65% in both study groups; HR, 0.98; 95% CI, 0.74-1.29; Figure, A). Overall survival was also similar among patients who received subcutaneous trastuzumab and those who received intravenous trastuzumab (6-year OS rates, 84% in both study groups; HR, 0.94; 95% CI, 0.61-1.45; Figure, B).
Figure. Event-Free Survival (EFS) and Overall Survival (OS) in the Intention-to-Treat Population.
The vertical dashed line in each panel indicates the 6-year cutoff for this final analysis. H IV indicates intravenous trastuzumab; HR, hazard ratio; H SC, subcutaneous trastuzumab; and tpCR, total pathologic complete response.
tpCR and Long-term Efficacy
Event-free survival was similar among patients treated with subcutaneous trastuzumab and those treated with intravenous trastuzumab who achieved a tpCR (HR, 1.10; 95% CI, 0.56-2.16) and those with residual disease after neoadjuvant treatment (HR, 1.01; 95% CI, 0.74-1.37). In both treatment groups, EFS was longer for patients achieving a tpCR compared with those with residual disease (Figure, C). Six-year OS rates were also higher among patients who achieved tpCR compared with those with residual disease (Figure, D).
Given the similarity of the associations between tpCR and EFS within each study group, the groups were pooled for further exploratory analyses. The EFS benefit for patients achieving tpCR compared with patients with residual disease after neoadjuvant treatment was evident both for patients with hormone receptor–positive and hormone receptor–negative disease (Table 1). Similar results were seen for patients by estrogen receptor status alone.
Table 1. Six-Year EFS Rates According to Total Pathologic Complete Response and Hormone Receptor Status.
| Hormone Receptor Status | tpCR (n = 202) | Residual Disease After Neoadjuvant Treatment (n = 389)a | HR (95% CI) | ||
|---|---|---|---|---|---|
| Patients, No. | 6-y EFS, %b | Patients, No. | 6-y EFS, %b | ||
| Overall | 202 | 82 | 389 | 56 | 0.33 (0.23-0.47) |
| ER-positive | 71 | 88 | 231 | 61 | 0.24 (0.12-0.50) |
| ER-negative | 131 | 79 | 158 | 48 | 0.32 (0.20-0.49) |
| ER-positive and/or PgR-positive | 75 | 88 | 243 | 59 | 0.25 (0.13-0.49) |
| ER-positive and PgR-positive | 49 | 85 | 170 | 64 | 0.35 (0.16-0.76) |
| ER-positive and PgR-negative | 22 | 95 | 61 | 52 | 0.07 (0.01-0.55) |
| ER-negative and PgR-positive | 4 | 75 | 12 | 30 | 0.27 (0.03-2.17) |
| ER-negative and PgR-negative | 126 | 79 | 144 | 50 | 0.33 (0.21-0.52) |
| Unknown | 1 | NA | 2 | NA | NA |
Abbreviations: EFS, event-free survival; ER, estrogen receptor; HR, hazard ratio; NA, not available; PgR, progesterone receptor; tpCR, total pathologic complete response.
Includes patients withdrawn before surgery.
Event-free survival rates were estimated using the Kaplan-Meier method.
Long-term Safety
Overall rates of AEs (290 of 297 [97.6%] vs 282 of 298 [94.6%]), grade 3 or higher AEs (158 of 297 [53.2%] vs 160 of 298 [53.7%]), and serious AEs (65 of 297 [21.9%] vs 45 of 298 [15.1%]) were comparable between the subcutaneous and intravenous trastuzumab treatment groups (Table 2). The incidence of cardiac AEs was low and was similar for patients treated with subcutaneous trastuzumab and patients treated with intravenous trastuzumab (44 of 297 [14.8%] vs 42 of 298 [14.1%]; Table 2), even for patients in the lowest body-weight quartile (<59 kg).
Table 2. Adverse Events During the Overall Study Period by Body-Weight Quartilea.
| Adverse Event | Patients, No. (%) | |
|---|---|---|
| Subcutaneous Trastuzumab (n = 297) | Intravenous Trastuzumab (n = 298) | |
| Any | 290 (97.6) | 282 (94.6) |
| Grade ≥3 | 158 (53.2) | 160 (53.7) |
| Serious | 65 (21.9) | 45 (15.1) |
| Cardiac, kg | 44 (14.8) | 42 (14.1) |
| <59 | 6 (8.5) | 9 (11.7) |
| ≥59 to <68 | 8 (11.4) | 9 (10.7) |
| ≥68 to <79 | 15 (21.1) | 7 (10.0) |
| ≥79 kg | 15 (17.6) | 17 (25.4) |
| LVEF decline (≥10% points from baseline to <50%)b | 11 (3.8) | 12 (4.2) |
Abbreviation: LVEF, left ventricular ejection fraction.
Data on body-weight quartiles are based on the subcutaneous trastuzumab group (<59 kg, n = 71; ≥59 to <68 kg, n = 70; ≥68 to <79 kg, n = 71; ≥79 kg, n = 85) and the intravenous trastuzumab group (<59 kg, n = 77; ≥59 to <68 kg, n = 84; ≥68 to <79 kg, n = 70; ≥79 kg, n = 67).
Data on LVEF decline are based on 291 patients in the subcutaneous trastuzumab group and 288 patients in the intravenous trastuzumab group with LVEF values at baseline and at least 1 postbaseline time point.
Discussion
To our knowledge, the HannaH trial is the largest randomized clinical trial investigating the use of subcutaneous trastuzumab and intravenous trastuzumab for patients with ERBB2-positive EBC. Event-free survival and OS results after 6 years of follow-up continue to support the noninferiority of subcutaneous trastuzumab to intravenous trastuzumab observed in the primary analysis.5 Results for EFS were consistent with those observed in the Neoadjuvant Herceptin (NOAH) trial of intravenous trastuzumab.11
Rates of tpCR were similar among patients receiving subcutaneous trastuzumab and those receiving intravenous trastuzumab; achieving tpCR was associated with longer EFS and OS in both study groups, consistent with previous analyses of the HannaH trial6 and with long-term efficacy in other studies, such as the CTNeoBC (Collaborative Trials in Neoadjuvant Breast Cancer) study,12 and in line with the relevant US Food and Drug Administration guidance.13 This final analysis of the HannaH trial supports the use of tpCR as a surrogate end point for long-term efficacy outcomes in similar settings. The overall and cardiac safety profiles of subcutaneous trastuzumab at 6 years’ follow-up remains consistent with that of intravenous trastuzumab and with the safety profile of subcutaneous trastuzumab observed in the SafeHer and PrefHer studies.8,9
Although the overall treatment landscape for EBC has recently changed after the publication of results from APHINITY,14 KATHERINE (Trastuzumab Emtansine [T-DM1; Kadcyla] vs Trastuzumab [Herceptin] as Adjuvant Therapy in Patients With HER2-Positive Early-Stage Breast Cancer With Residual Invasive Disease After Receiving Neoadjuvant Chemotherapy and Trastuzumab),15 and ExteNET (Extended Adjuvant Treatment of Breast Cancer With Neratinib),16 trastuzumab-based therapies remain a standard of care for patients with ERBB2-positive EBC, thus highlighting the relevance of these long-term follow-up results for patients who are not considered to have a high risk of recurrence and therefore do not qualify for the treatment regimens investigated in these trials.
Limitations
The limitations of the study include the small and unbalanced sample sizes used in the subgroup analyses. In addition, the exploratory subgroup analyses were not preplanned.
Conclusions
This final analysis of the phase 3 HannaH trial further supports the long-term comparability of the efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab, consistent with previous reports from this study.5,6,7
Trial Protocol
eFigure. CONSORT Diagram
Data Sharing Statement
References
- 1.Pivot X, Gligorov J, Müller V, et al. ; PrefHer Study Group . Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):-. doi: 10.1016/S1470-2045(13)70383-8 [DOI] [PubMed] [Google Scholar]
- 2.De Cock E, Pivot X, Hauser N, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5(3):389-397. doi: 10.1002/cam4.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53(2):192-201. doi: 10.1177/0091270012436560 [DOI] [PubMed] [Google Scholar]
- 4.Pivot X, Gligorov J, Müller V, et al. ; PrefHer Study Group . Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979-1987. doi: 10.1093/annonc/mdu364 [DOI] [PubMed] [Google Scholar]
- 5.Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869-878. doi: 10.1016/S1470-2045(12)70329-7 [DOI] [PubMed] [Google Scholar]
- 6.Jackisch C, Hegg R, Stroyakovskiy D, et al. HannaH phase III randomised study: association of total pathological complete response with event-free survival in HER2-positive early breast cancer treated with neoadjuvant–adjuvant trastuzumab after 2 years of treatment-free follow-up. Eur J Cancer. 2016;62:62-75. doi: 10.1016/j.ejca.2016.03.087 [DOI] [PubMed] [Google Scholar]
- 7.Jackisch C, Kim SB, Semiglazov V, et al. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26(2):320-325. doi: 10.1093/annonc/mdu524 [DOI] [PubMed] [Google Scholar]
- 8.Gligorov J, Ataseven B, Verrill M, et al. ; SafeHer Study Group . Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2–positive early breast cancer: SafeHer phase III study’s primary analysis of 2573 patients. Eur J Cancer. 2017;82:237-246. doi: 10.1016/j.ejca.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 9.Pivot X, Verma S, Fallowfield L, et al. ; PrefHer Study Group . Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2-positive early breast cancer: final analysis of the randomised, two-cohort PrefHer study. Eur J Cancer. 2017;86:82-90. doi: 10.1016/j.ejca.2017.08.019 [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640-647. doi: 10.1016/S1470-2045(14)70080-4 [DOI] [PubMed] [Google Scholar]
- 12.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration, Center for Drug Evaluation and Research Guidance for industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. Published October 2014. Accessed November, 2018.
- 14.von Minckwitz G, Procter M, de Azambuja E, et al. ; APHINITY Steering Committee and Investigators . Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-131. doi: 10.1056/NEJMoa1703643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi:10.1056/NEJMoa1814017 doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 16.Martin M, Holmes FA, Ejlertsen B, et al. ; ExteNET Study Group . Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700. doi: 10.1016/S1470-2045(17)30717-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. CONSORT Diagram
Data Sharing Statement