Key Points
Question
What are the risks of serious infection with systemic medications used for the treatment of psoriasis?
Findings
In this comparative cohort study of 107 707 patients, we found a decreased risk of serious infection among users of apremilast, etanercept, and ustekinumab when compared with methotrexate.
Meaning
Health care professionals should consider risk of infection when choosing a systemic treatment for patients with moderate-to-severe psoriasis.
Abstract
Importance
There is a need for better understanding of the comparative safety of systemic medications used in the treatment of psoriasis.
Objective
To compare the risk of serious infection associated with biologic and nonbiologic systemic medications in patients with psoriasis.
Design, Setting, and Participants
An observational cohort study was conducted using medical and outpatient pharmacy claims from 2 large US health insurance claims databases from January 1, 2003, through September 30, 2015. We included patients with a diagnosis of psoriasis who were new users of systemic medications for psoriasis.
Exposures
Prescription claims for acitretin, adalimumab, apremilast, etanercept, infliximab, methotrexate, or ustekinumab.
Main Outcomes and Measures
The primary outcome was serious infection, defined by inpatient discharge diagnosis International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Cox proportional hazards regression was used to compare rates of serious infection for each exposure (acitretin, adalimumab, apremilast, etanercept, infliximab, and ustekinumab) with the referent group (methotrexate). We used pairwise 1:1 propensity score (PS) matching to adjust for potential confounders, which were assessed during a 180-day baseline period prior to study drug initiation. Results from the 2 databases were pooled via fixed-effects analysis.
Results
The databases included 31 595 patients in the Optum Clinformatics Data Mart and 76 112 patients in Truven MarketScan who were new users of acitretin, adalimumab, apremilast, etanercept, infliximab, methotrexate, and ustekinumab. Users of acitretin, apremilast, infliximab, and methotrexate were older and had higher baseline comorbidity scores than subcutaneous biologic users (adalimumab, etanercept, and ustekinumab). The pooled PS-matched analysis yielded a decreased rate of overall serious infection in users of apremilast (hazard ratio [HR], 0.50; 95% CI, 0.26-0.94), etanercept (HR, 0.75; 95% CI, 0.61-0.93), and ustekinumab (HR, 0.65; 95% CI, 0.47-0.89) compared with methotrexate. We did not find a different rate of overall serious infection among users of acitretin, adalimumab, and infliximab compared with methotrexate. Subanalysis by type of serious infection showed a significantly increased risk of cellulitis among users of acitretin compared with methotrexate (PS-adjusted HR, 1.76; 95% CI, 1.11-2.80).
Conclusions and Relevance
Among patients with psoriasis treated with systemic medications in 2 large US claims databases, new users of apremilast, etanercept, and ustekinumab had a decreased rate of serious infection compared with methotrexate.
This cohort study compares the risk of serious infection associated with biologic and nonbiologic systemic medications in patients with psoriasis.
Introduction
Psoriasis is a common, chronic inflammatory disease of the skin.1,2 In the past decade, the treatment of psoriasis has undergone a revolution with the introduction of biologic agents.3,4,5 Despite their efficacy, adherence to biologics and systemic medications for psoriasis is low, with infection having been shown to be a leading cause of discontinuation.6,7,8 Systemic medications available in the United States and approved by the US Food and Drug Administration for moderate-to-severe and severe psoriasis include older agents such as acitretin and methotrexate, as well as novel agents including the biologics and apremilast, a small molecule inhibitor of phosphodiesterase-4. With the development of these new treatments has come a need for data on the long-term comparative safety of these different agents.9,10 Unlike other inflammatory disorders, such as rheumatoid arthritis and inflammatory bowel disease, systemic medications are more commonly used as monotherapy in the treatment of psoriasis, making it a more straightforward population in which to study adverse events.
A critical barrier to examining the risks of rare adverse events, such as serious infection, is the lack of sufficiently large sample sizes. In addition, patients with moderate to severe psoriasis may potentially be at higher risk of untoward outcomes, such as serious infection, requiring careful consideration and control for potential confounding.11 There remains a need to better understand the risk of serious infection with the use of systemic agents for psoriasis to guide optimal and cost-effective care. In this retrospective cohort study using 2 large electronic databases, we sought to compare the risk of serious infection in patients with psoriasis who were new users of 6 systemic medications (acitretin, adalimumab, apremilast, etanercept, infliximab, and ustekinumab) to new users of methotrexate using a large national cohort of commercially insured US adults.
Methods
Data
The Optum Clinformatics Data Mart (Optum) contains deidentified adjudicated medical and outpatient pharmacy claims for beneficiaries of a large commercial insurance provider in the United States, as well as patients with a Medicare supplement plan. Data available for this study covered January 1, 2004, through September 30, 2017, and approximately 68.3 million individuals. The Truven MarketScan databases (MarketScan) captures longitudinal, individual-level administrative claims data from the United States. The data available for study included the Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database, covering January 1, 2003, to January 1, 2017, which contains medical and outpatient pharmacy claims for approximately 185.3 million patients. For this study, patient data were censored on October 1, 2015, owing to transition from the use of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) in the United States. Patient informed consent was not required because the databases used contain only deidentified to protect subject confidentiality. This study was granted an exemption by the institutional review board of Brigham and Women’s Hospital.
Inclusion and Exclusion Criteria
We included patients with a diagnosis of psoriasis, defined as at least 3 ICD-9-CM codes of 696.1 on separate dates, which was found to have a high positive predictive value of identifying patients with psoriasis in 1 retrospective study.12 Patients were included if they had a prescription claim for acitretin, adalimumab, apremilast, etanercept, infliximab, ustekinumab, or methotrexate and entered the cohort on the date of the first filled prescription for one of these study drugs. Patients were required to have continuous medical and prescription coverage during the 180 days prior to and on the cohort entry date. Other biologic agents available for the treatment of psoriasis in the United States were not approved by the US Food and Drug Administration for plaque psoriasis prior to the censoring date of October 1, 2015, and thus were not included in this study.
We excluded patients with the following: (1) a claim for any study drug during the 180 days prior to the cohort entry date (thus identifying new users); (2) younger than 18 years; (3) a claim for more than 1 systemic medication for psoriasis or other immunosuppressive medications (any systemic corticosteroid, alefacept, azathioprine, cyclophosphamide, cyclosporine, efalizumab, hydroxychloroquine, leflunamide, mercaptopurine, or sulfasalazine) on the cohort entry date; (4) history of any malignancy, including lymphoma and leukemia, except nonmelanoma skin cancer, during the 180 days prior to cohort entry date; (5) history of any serious infection (as defined as the outcome measure) during the 180 days prior to cohort entry; and (6) a diagnosis code during the 180 days prior to the cohort entry date for another immune-mediated inflammatory disorder for which biologics may be used (Crohn disease, ulcerative colitis, psoriatic arthritis, systemic lupus erythematosus, systemic sclerosis, sicca syndrome, rheumatoid arthritis, Felty syndrome, juvenile chronic polyarthritis, juvenile idiopathic arthritis, ankylosing spondylitis, dermatomyositis, polymyositis, eosinophilia myalgia syndrome, other specified diffuse disease of connective tissue, or unspecified diffuse connective tissue disease); or (7) a prescription for the index study drug with days supply value of “0.” We conducted a sensitivity analysis in which we included patients with a diagnosis of psoriatic arthritis but still excluded the other previously listed inflammatory disorders.
Outcomes
The primary outcome was serious infection requiring hospitalization, using previously validated ICD-9-CM codes with an overall positive predictive value of 90.2%.13 Serious infection was defined as a primary inpatient diagnosis code for pneumonia, meningitis/encephalitis, bacteremia/sepsis, cellulitis/soft-tissue infection, endocarditis, pyelonephritis, and septic arthritis/osteoarthritis (eTable 1 in the Supplement). We also conducted secondary analyses examining each type of serious infection separately.
Statistical Analyses
Separate analyses were performed first for each database. The primary analysis used an as-treated analysis in which follow-up began 1 day after the cohort entry date (first claim of medication) and continued until the first occurrence of 1 of the following: the outcome of serious infection, treatment discontinuation (defined as 120 days from last medication dispensing, allowing 1-day grace periods between dispensings), death, crossover of exposure group or addition of drug from the other exposure group, disenrollment, or the date of October 1, 2015. We performed sensitivity analyses using a grace period of 30 days.
To compare the likelihood of serious infection between each treatment group (acitretin, adalimumab, apremilast, etanercept, infliximab, and ustekinumab) and the referent group of methotrexate users, we used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% confidence intervals, with the outcome as the dependent variable and the treatment as the independent variable. We used pairwise 1:1 propensity score (PS) matching to adjust for differences in measured patient characteristics between groups. The following covariates were included in the PS model: age, sex, combined comorbidity score,14 depression, obesity, smoking, region, plan type, insurance type (commercial vs Medicare), influenza vaccination, lipid screening, use of nursing home, occurrence of emergency department visit, occurrence of inpatient hospitalization, occurrence of any laboratory tests, and prior use of medications (antibiotics, angiotensin-converting enzyme inhibitors, antidepressants, anticoagulants, angiotensin II receptor blockers, aspirin/dipyridamole, β-blockers, calcium channels blockers, diabetes medications, nonselective anti-inflammatory drugs, other antiplatelet agents, other hypertension drugs, statins, and other lipid-lowering medications). Covariates were assessed during the 180-day baseline period prior to the cohort entry date. In subgroup analyses, we examined both sex and age category as effect modifiers.
Results from the Optum and MarketScan analyses were pooled using a fixed-effects analysis. The pooled effect estimate is a weighted mean of the study-specific estimates, with the inverse variance of the estimates used as the weight. A traditional meta-analysis assumes that the studies to be pooled are independent. This assumption may be violated if the studies were conducted in databases with overlapping patient populations, which we estimated to be approximately 20%. This was based on an estimate that the Truven MarketScan database included approximately 20% of individuals with employer-sponsored commercial health insurance in the United States during the study period. In the event of overlap, the variance of the pooled effect estimate may be underestimated. To account for sample overlap in this analysis, the variance of the pooled effect was reestimated based on this assumption.15,16 We performed a sensitivity analysis excluding those patients with nursing home stays, as well as excluding those with missing sex.
We additionally performed a post hoc analysis using adalimumab as the reference group, based on this being the largest cohort of biologic users in our study, as well as our finding of a similar risk of serious infection among users of adalimumab compared with methotrexate in our primary analysis. In this post hoc analysis, we additionally censored for addition of methotrexate during the follow-up period because this is the most common medication to combine with other systemic agents for psoriasis.
All analyses were conducted with the Aetion Evidence Generation Platform using the Comparative Safety and Meta-Analysis applications, version 3.8, with supplemental statistical computations estimated using R, version 3.4.2 (The R Foundation). All P values were 2-sided, and the P value level of significance was .05. This study followed the STROBE guidelines for reporting.17
Results
We identified 31 595 patients in Optum and 76 112 patients in MarketScan who were new users of acitretin, adalimumab, apremilast, etanercept, infliximab, methotrexate, and ustekinumab (eTables 2 and 3 in the Supplement). Baseline characteristics for patients in Optum and MarketScan were similar (Table 1). At treatment initiation, users of acitretin, apremilast, infliximab, and methotrexate were older and had higher baseline comorbidity scores than subcutaneous biologic users (adalimumab, etanercept, and ustekinumab). Within the apremilast cohort, a higher proportion of patients were censored because of reaching the date of October 1, 2015 (ICD-10-CM transition date), compared with other medications. Additionally, within the infliximab cohort, a higher proportion of patients were censored owing to crossover to the reference group (ie, exposure to methotrexate) (eTable 4 in the Supplement). The matched populations across for all comparison cohorts are shown in eTable 5 in the Supplement.
Table 1. Baseline Characteristics by Medication Cohort for Optum Clinformatics Data Mart and Truven MarketScan.
| Variable | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Reference Group: MTX Users (Compared With Adalimumab)a | Adalimumab Users | Acitretin Users | Apremilast Users | Etanercept Users | Infliximab Users | Ustekinumab Users | Total Across Medications | |
| Optum | ||||||||
| Patients, y, No. | 8470 | 7181 | 2726 | 1623 | 7102 | 408 | 4085 | 31 595 |
| Age, y | ||||||||
| Mean (SD) | 53.69 (15.48) | 46.10 (12.97) | 52.31 (13.89) | 51.37 (13.77) | 45.45 (12.99) | 50.20 (14.59) | 46.50 (12.84) | NAb |
| Categories | ||||||||
| 18-54 | 4387 (51.8) | 5255 (73.2) | 1506 (55.2) | 944 (58.2) | 5332 (75.1) | 253 (62.0) | 2974 (72.8) | 20 651 (65.4) |
| 55-64 | 1892 (22.3) | 1389 (19.3) | 759 (27.8) | 417 (25.7) | 1304 (18.4) | 85 (20.8) | 815 (20.0) | 6661 (22.1) |
| 65-74 | 1484 (17.5) | 449 (6.3) | 324 (11.9) | 201 (12.4) | 372 (5.2) | 49 (12.0) | 236 (5.8) | 3115 (9.9) |
| ≥75 | 707 (8.3) | 88 (1.2) | 137 (5.0) | 61 (3.8) | 94 (1.3) | 21 (5.1) | 60 (1.5) | 1168 (3.7) |
| Sex | ||||||||
| Male | 4043 (47.7) | 4061 (56.6) | 1582 (58.0) | 820 (50.5) | 3903 (55.0) | 202 (49.5) | 2302 (56.4) | 16 913 (53.5) |
| Female | 4426 (52.3) | 3120 (43.4) | 1144 (42.0) | 803 (49.5) | 3199 (45.0) | 206 (50.5) | 1781 (43.6) | 14 679 (46.5) |
| Unknown | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.0) | 3 (0.0) |
| Obese | 526 (6.2) | 372 (5.2) | 137 (5.0) | 87 (5.4) | 336 (4.7) | 27 (6.6) | 251 (6.1) | 1736 (5.5) |
| Smokers | 477 (5.6) | 326 (4.5) | 148 (5.4) | 57 (3.5) | 332 (4.7) | 24 (5.9) | 184 (4.5) | 1548 (4.9) |
| Depression | 191 (2.3) | 178 (2.5) | 45 (1.7) | 27 (1.7) | 176 (2.5) | 13 (3.2) | 76 (1.9) | 706 (2.2) |
| Combined comorbidity score, mean (SD) | 0.14 (1.03) | 0.07 (0.79) | 0.14 (1.08) | 0.14 (0.85) | 0.07 (0.81) | 0.23 (1.13) | 0.08 (0.78) | NAb |
| Region | ||||||||
| Northeast | 771 (9.1) | 602 (8.4) | 305 (11.2) | 209 (12.9) | 662 (9.3) | 34 (8.3) | 423 (10.4) | 3006 (9.5) |
| Midwest | 2140 (25.3) | 1771 (24.7) | 582 (21.3) | 380 (23.4) | 1659 (23.4) | 137 (33.6) | 975 (23.9) | 7644 (24.2) |
| South | 3719 (43.9) | 3726 (51.9) | 1388 (50.9) | 706 (43.5) | 3708 (52.2) | 195 (47.8) | 2027 (49.6) | 15 469 (49.0) |
| West | 1813 (21.4) | 1075 (15.0) | 447 (16.4) | 326 (20.1) | 1064 (15.0) | 42 (10.3) | 656 (16.1) | 5423 (17.2) |
| Other | 27 (0.3) | 7 (0.1) | 4 (0.1) | 2 (0.1) | 9 (0.1) | 0 (0.0) | 4 (0.1) | 53 (0.17) |
| Commercial/Medicare | ||||||||
| Commercial | 6304 (74.4) | 6740 (93.9) | 2371 (87.0) | 1389 (85.6) | 6720 (94.6) | 351 (86.0) | 3807 (93.2) | 27 682 (87.6) |
| Medicare | 2166 (25.6) | 441 (6.1) | 355 (13.0) | 234 (14.4) | 382 (5.4) | 57 (14.0) | 278 (6.8) | 3913 (12.4) |
| Nursing home resident | 33 (0.4) | 4 (0.1) | 8 (0.3) | 4 (0.2) | 4 (0.1) | 3 (0.7) | 6 (0.1) | 62 (0.2) |
| ≥1 Outpatient office visit | 8155 (96.3) | 7065 (98.4) | 2658 (97.5) | 1605 (98.9) | 6961 (98.0) | 402 (98.5) | 4026 (98.6) | 30 872 (97.7) |
| ≥1 Emergency department visit | 806 (9.5) | 551 (7.7) | 236 (8.7) | 131 (8.1) | 482 (6.8) | 45 (11.0) | 314 (7.7) | 2565 (8.1) |
| ≥1 Inpatient hospitalization | 416 (4.9) | 241 (3.4) | 125 (4.6) | 63 (3.9) | 315 (4.4) | 32 (7.8) | 115 (2.8) | 1307 (4.1) |
| ≥1 Laboratory test | 6473 (76.4) | 5794 (80.7) | 2045 (75.0) | 1142 (70.4) | 5457 (76.8) | 364 (89.2) | 3244 (79.4) | 24 519 (77.6) |
| Truven MarketScan | ||||||||
| Patients, No. | 20 609 | 17 912 | 7456 | 4476 | 16 791 | 1027 | 7841 | 76 112 |
| Age, y | ||||||||
| Mean (SD) | 50.62 (13.82) | 46.20 (12.89) | 53.00 (12.99) | 50.52 (12.84) | 46.65 (13.27) | 48.18 (12.47) | 46.89 (12.86) | NAb |
| Categories | ||||||||
| 18-54 | 12 100 (58.7) | 12 848 (71.7) | 3822 (51.3) | 2631 (58.8) | 11 790 (70.2) | 691 (67.3) | 5502 (70.2) | 49 384 (64.9) |
| 55-64 | 5819 (28.2) | 4064 (22.7) | 2505 (33.6) | 1373 (30.7) | 3800 (22.6) | 252 (24.5) | 1821 (23.2) | 19 634 (25.8) |
| 65-74 | 1791 (8.7) | 767 (4.3) | 773 (10.4) | 359 (8.0) | 894 (5.3) | 62 (6.0) | 404 (5.2) | 5050 (6.6) |
| ≥75 | 899 (4.4) | 233 (1.3) | 356 (4.8) | 113 (2.5) | 307 (1.8) | 22 (2.1) | 114 (1.5) | 2044 (2.7) |
| Sex | ||||||||
| Male | 9604 (46.6) | 9903 (55.3) | 4222 (56.6) | 2176 (48.6) | 9101 (54.2) | 474 (46.2) | 4303 (54.9) | 39 783 (52.3) |
| Female | 11 005 (53.4) | 8009 (44.7) | 3234 (43.4) | 2300 (51.4) | 7690 (45.8) | 553 (53.8) | 3538 (45.1) | 36 329 (47.7) |
| Obese | 843 (4.1) | 746 (4.2) | 256 (3.4) | 245 (5.5) | 607 (3.6) | 59 (5.7) | 379 (4.8) | 3135 (4.1) |
| Smokers | 812 (3.9) | 711 (4.0) | 284 (3.8) | 169 (3.8) | 536 (3.2) | 50 (4.9) | 357 (4.6) | 2919 (3.8) |
| Depression | 987 (4.8) | 879 (4.9) | 319 (4.3) | 141 (3.2) | 825 (4.9) | 65 (6.3) | 364 (4.6) | 3580 (4.7) |
| Combined comorbidity score, mean (SD) | 0.08 (0.82) | 0.05 (0.72) | 0.08 (0.91) | 0.08 (0.81) | 0.06 (0.72) | 0.18 (1.04) | 0.05 (0.77) | NAb |
| Region | ||||||||
| Northeast | 3088 (15.0) | 2588 (14.4) | 1323 (17.7) | 994 (22.2) | 2428 (14.5) | 158 (15.4) | 1494 (19.1) | 12 073 (15.9) |
| North Central | 4940 (24.0) | 4266 (23.8) | 1611 (21.6) | 986 (22.0) | 4210 (25.1) | 244 (23.8) | 1595 (20.3) | 17 852 (23.5) |
| South | 8478 (41.1) | 8210 (45.8) | 3022 (40.5) | 1928 (43.1) | 7251 (43.2) | 531 (51.7) | 3560 (45.4) | 32 980 (43.3) |
| West | 3777 (18.3) | 2547 (14.2) | 1420 (19.0) | 554 (12.4) | 2576 (15.3) | 87 (8.5) | 1016 (13.0) | 11 977 (15.7) |
| Unknown | 326 (1.6) | 301 (1.7) | 80 (1.1) | 14 (0.3) | 326 (1.9) | 7 (0.7) | 176 (2.2) | 1230 (1.6) |
| Commercial/Medicare | ||||||||
| Commercial | 17 872 (86.7) | 16 896 (94.3) | 6297 (84.5) | 3995 (89.3) | 15 563 (92.7) | 935 (91.0) | 7299 (93.1) | 68 857 (90.5) |
| Medicare | 2737 (13.3) | 1016 (5.7) | 1159 (15.5) | 481 (10.7) | 1228 (7.3) | 92 (9.0) | 542 (6.9) | 7255 (9.5) |
| Nursing home resident | 26 (0.1) | 9 (0.1) | 9 (0.1) | 5 (0.1) | 7 (0.0) | 2 (0.2) | 9 (0.1) | 67 (0.1) |
| ≥1 Outpatient office visit | 19 848 (96.3) | 17 644 (98.5) | 7261 (97.4) | 4427 (98.9) | 16 417 (97.8) | 1018 (99.1) | 7735 (98.6) | 74 350 (97.7) |
| ≥1 Emergency department visit | 2256 (10.9) | 1721 (9.6) | 717 (9.6) | 473 (10.6) | 1676 (10.0) | 148 (14.4) | 754 (9.6) | 7745 (10.2) |
| ≥1 Inpatient hospitalization | 886 (4.3) | 636 (3.6) | 334 (4.5) | 192 (4.3) | 706 (4.2) | 69 (6.7) | 259 (3.3) | 3082 (4.0) |
| ≥1 Laboratory test | 15 896 (77.1) | 15 016 (83.8) | 5465 (73.3) | 3264 (72.9) | 13 033 (77.6) | 907 (88.3) | 6332 (80.8) | 59 913 (78.7) |
Abbreviations: MTX, methotrexate; NA, not available.
Results presented for methotrexate users were obtained from the adalimumab comparative analysis.
Groups were not completely mutually exclusive, and thus a pooled standard deviation could not be calculated for the mean.
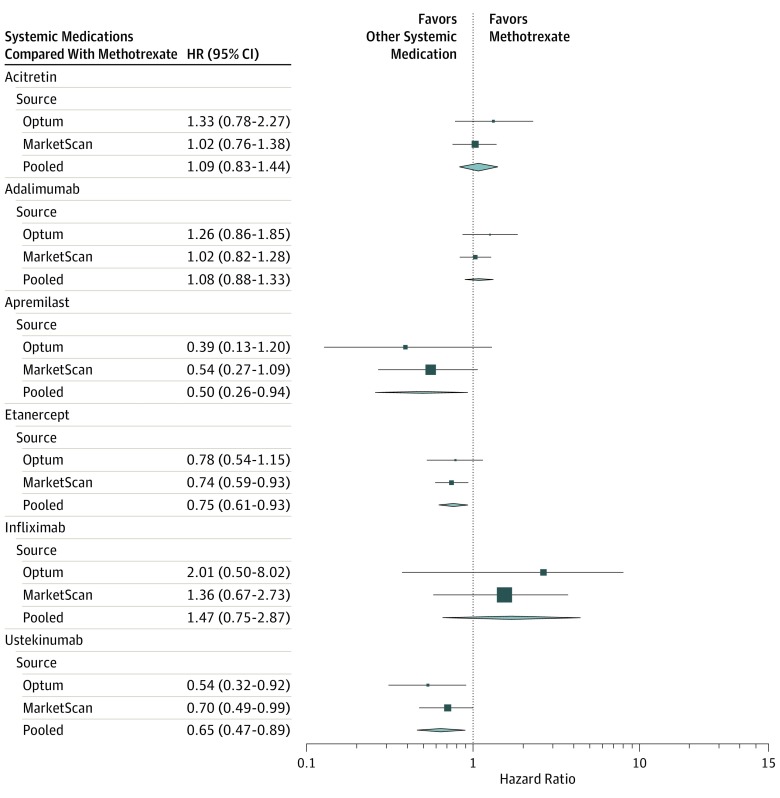
The unadjusted and PS-adjusted HRs for the rates of overall serious infection by medication compared with methotrexate for Optum and MarketScan are presented in Table 2. Hazard ratios for subtypes of serious infection for each database are presented in eTables 6 and 7 in the Supplement. For both the Optum and MarketScan databases, there was a decreased risk of serious infection among new users of ustekinumab compared with methotrexate (PS-adjusted HRs, 0.54; 95% CI, 0.32-0.92 and 0.70; 95% CI, 0.49-0.99, respectively) (Table 2). For all systemic medications, the most common types of serious infection (in order of frequency) were cellulitis, pneumonia, and bacteremia/sepsis (Table 3). The analysis of pooled PS-adjusted HRs across the Optum and MarketScan databases adjusted for overlap (Table 3 and Figure) showed a statistically significant decreased rate of overall serious infection in users of apremilast (HR, 0.50; 95% CI, 0.26-0.94), etanercept (HR, 0.75; 95% CI, 0.61-0.93), and ustekinumab (HR, 0.65; 95% CI, 0.47-0.89) compared with methotrexate. We did not find a materially different rate of overall serious infection among users of acitretin, adalimumab, and infliximab compared with methotrexate. Subanalyses by type of serious infection (Table 3) showed an increased rate of cellulitis among acitretin users (PS-adjusted HR, 1.76; 95% CI, 1.11-2.80), as well as decreased rates of bacteremia/sepsis among etanercept users (PS-adjusted HR, 0.51; 95% CI, 0.32-0.82) and pneumonia among ustekinumab users (PS-adjusted HR, 0.53; 95% CI, 0.32-0.88) compared with methotrexate users. Sensitivity analyses including patients with psoriatic arthritis in Optum (eTable 8 in the Supplement) and excluding patients with missing sex and nursing home visits showed similar results to the primary analysis. We found no evidence of effect modification by sex or age category (eTable 9 in the Supplement).
Table 2. Overall Serious Infections in Patients With Psoriasis Receiving Systemic Medications Compared With Methotrexate for Optum Clinformatics Data Mart and Truven MarketScan .
| Exposure | Reference Group (MTX) | Exposure Group | Unadjusted Rate Difference (95% CI) | Unadjusted HR (95% CI) | P Value | PS-Adjusted HR (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, No. | Events, No.a | Incidence Rateb | Patients, No. | Events, No. | Incidence Rate | ||||||
| Acitretin | |||||||||||
| Optum | 9048 | 127 | 14.46 | 2726 | 30 | 14.44 | −0.02 (−5.76 to 5.73) | 1.00 (0.67 to 1.50) | .99 | 1.33 (0.78 to 2.27) | .29 |
| MarketScan | 21 935 | 258 | 12.11 | 7456 | 81 | 13.68 | 1.57 (−1.75 to 4.90) | 1.14 (0.89 to 1.46) | .31 | 1.02 (0.76 to 1.38) | .87 |
| Adalimumab | |||||||||||
| Optum | 8470 | 112 | 14.24 | 7181 | 79 | 10.33 | −3.91 (−7.39 to −0.42) | 0.73 (0.55 to 0.98) | .03 | 1.26 (0.86 to 1.85) | .23 |
| MarketScan | 20 609 | 229 | 12.14 | 17 912 | 197 | 9.75 | −2.39 (−4.47 to −0.31) | 0.80 (0.66 to 0.97) | .02 | 1.02 (0.82 to 1.28) | .84 |
| Apremilast | |||||||||||
| Optum | 9383 | 134 | 14.62 | 1623 | 4 | 4.36 | −10.27 (−15.20 to −5.33) | 0.30 (0.11 to 0.80) | .02 | 0.39 (0.13 to 1.20) | .10 |
| MarketScan | 22 987 | 288 | 12.72 | 4476 | 11 | 5.35 | −7.37 (−10.86 to −3.88) | 0.45 (0.25 to 0.82) | .008 | 0.54 (0.27 to 1.09) | .09 |
| Etanercept | |||||||||||
| Optum | 8475 | 118 | 14.98 | 7102 | 68 | 8.05 | −6.94 (−10.25 to −3.63) | 0.54 (0.40 to 0.72) | <.001 | 0.78 (0.54 to 1.15) | .21 |
| MarketScan | 20 878 | 224 | 11.6 | 16 791 | 168 | 7.66 | −3.95 (−5.86 to −2.03) | 0.66 (0.54 to 0.80) | <.001 | 0.74 (0.59 to 0.93) | .01 |
| Infliximab | |||||||||||
| Optum | 9378 | 132 | 14.58 | 408 | 6 | 17.67 | 3.09 (−11.27 to 17.45) | 1.22 (0.54 to 2.76) | .62 | 2.01 (0.50 to 8.02) | .32 |
| MarketScan | 22 642 | 253 | 11.68 | 1027 | 20 | 17.08 | 5.41 (−2.22 to 13.03) | 1.44 (0.91 to 2.27) | .12 | 1.36 (0.67 to 2.73) | .39 |
| Ustekinumab | |||||||||||
| Optum | 9167 | 133 | 14.95 | 4085 | 21 | 5.57 | −9.37 (−12.86 to −5.89) | 0.38 (0.24 to 0.61) | <.001 | 0.54 (0.32 to 0.92) | .02 |
| MarketScan | 22 556 | 280 | 12.7 | 7841 | 56 | 7.06 | −5.63 (−8.01 to −3.26) | 0.56 (0.42 to 0.75) | <.001 | 0.70 (0.49 to 0.99) | .05 |
Abbreviations: HR, hazard ratio; MTX, methotrexate; PS, propensity score.
The sum of events in the subanalyses of serious infections may be greater than the number of events in the overall serious infection analysis if a patient experienced more than 1 serious infectious event.
Incident rate in 1000 person-years.
Table 3. Analysis of Pooled Hazard Risk of Serious Infection in Patients With Psoriasis Receiving Systemic Medications Compared With Methotrexate.
| Exposure | Reference Group (MTX), No. | Exposure Group, No. | Pooled HR (95% CI)b | P Value for Test for Overall Effect | ||
|---|---|---|---|---|---|---|
| Pooled Patients | Pooled Eventsa | Pooled Patients | Events | |||
| Acitretin | ||||||
| Overall serious infection | 30 983 | 385 | 10 182 | 111 | 1.09 (0.83-1.44) | .55 |
| Bacteremia/sepsis | 88 | 23 | 0.93 (0.51-1.70) | .82 | ||
| Cellulitis/soft-tissue infection | 110 | 53 | 1.76 (1.11-2.80) | .02 | ||
| Endocarditis | 0 | 0 | NAc | NAc | ||
| Meningitis/encephalitis | 8 | 1 | NAc | NAc | ||
| Pneumonia | 168 | 36 | 0.85 (0.54-1.35) | .50 | ||
| Pyelonephritis | 12 | 2 | NAc | NAc | ||
| Septic arthritis/osteomyelitis | 12 | 2 | 1.46 (0.18-11.56) | .73 | ||
| Adalimumab | ||||||
| Overall serious infection | 29 079 | 341 | 25 093 | 276 | 1.08 (0.88-1.33) | .47 |
| Bacteremia/sepsis | 78 | 55 | 1.06 (0.66-1.68) | .82 | ||
| Cellulitis/soft-tissue infection | 97 | 117 | 1.34 (0.95-1.89) | .10 | ||
| Endocarditis | 0 | 0 | NAc | NAc | ||
| Meningitis/encephalitis | 6 | 2 | 0.78 (0.10-6.28) | .83 | ||
| Pneumonia | 151 | 98 | 0.94 (0.68-1.31) | .72 | ||
| Pyelonephritis | 9 | 8 | 1.11 (0.27-4.51) | .89 | ||
| Septic arthritis/osteomyelitis | 13 | 8 | 0.73 (0.25-2.19) | .58 | ||
| Apremilast | ||||||
| Overall serious infection | 32 370 | 422 | 6099 | 15 | 0.50 (0.26-0.94) | .03 |
| Bacteremia/sepsis | 94 | 5 | 0.87 (0.26-2.90) | .83 | ||
| Cellulitis/soft-tissue infection | 123 | 6 | 0.51 (0.18-1.42) | .20 | ||
| Endocarditis | 0 | 1 | NAc | NAc | ||
| Meningitis/encephalitis | 8 | 0 | NAc | NAc | ||
| Pneumonia | 186 | 5 | 0.42 (0.14-1.22) | .12 | ||
| Pyelonephritis | 12 | 0 | NAc | NAc | ||
| Septic arthritis/osteomyelitis | 13 | 0 | NAc | NAc | ||
| Etanercept | ||||||
| Overall serious infection | 29 353 | 342 | 23 893 | 236 | 0.75 (0.61-0.93) | .01 |
| Bacteremia/sepsis | 78 | 37 | 0.51 (0.32-0.82) | .01 | ||
| Cellulitis/soft-tissue infection | 93 | 106 | 1.16 (0.82-1.65) | .41 | ||
| Endocarditis | 0 | 3 | NAc | NAc | ||
| Meningitis/encephalitis | 8 | 0 | NAc | NAc | ||
| Pneumonia | 151 | 98 | 0.94 (0.68-1.31) | .72 | ||
| Pyelonephritis | 9 | 6 | 0.68 (0.20-2.34) | .55 | ||
| Septic arthritis/osteomyelitis | 10 | 13 | 1.61 (0.36-7.16) | .54 | ||
| Infliximab | ||||||
| Overall serious infection | 32 020 | 385 | 1435 | 26 | 1.47 (0.75-2.87) | .26 |
| Bacteremia/sepsis | 89 | 4 | 1.30 (0.19-8.63) | .80 | ||
| Cellulitis/soft-tissue infection | 107 | 10 | 1.76 (0.55-5.63) | .35 | ||
| Endocarditis | 0 | 0 | NAc | NAc | ||
| Meningitis/encephalitis | 7 | 1 | NAc | NAc | ||
| Pneumonia | 170 | 8 | 0.80 (0.29-2.24) | .68 | ||
| Pyelonephritis | 9 | 6 | 0.68 (0.20-2.34) | .55 | ||
| Septic arthritis/osteomyelitis | 12 | 1 | NAc | NAc | ||
| Ustekinumab | ||||||
| Overall serious infection | 31 723 | 413 | 11 926 | 77 | 0.65 (0.47-0.89) | .01 |
| Bacteremia/sepsis | 92 | 16 | 0.83 (0.39-1.73) | .64 | ||
| Cellulitis/soft-tissue infection | 121 | 31 | 0.87 (0.51-1.48) | .60 | ||
| Endocarditis | 0 | 0 | NAc | NAc | ||
| Meningitis/encephalitis | 8 | 0 | NAc | NAc | ||
| Pneumonia | 181 | 28 | 0.53 (0.32-0.88) | .01 | ||
| Pyelonephritis | 12 | 3 | 1.32 (0.20-8.78) | .79 | ||
| Septic arthritis/osteomyelitis | 13 | 2 | 0.51 (0.08-3.52) | .50 | ||
Abbreviations: HR, hazard ratio; MTX, methotrexate; NA, not applicable.
The sum of events in the subanalyses of serious infections may be greater than the number of events in the overall serious infection analysis if a patient experienced more than 1 serious infectious event.
Adjusted for 20% overlap of patients between United Optum Clinformatics Data Mart and Truven MarketScan.
Pooled HRs not calculated when HR not calculated in either Optum or MarketScan owing to zero events in exposure or reference groups.
Figure. Pooled Analysis of Overall Serious Infections in Patients With Psoriasis on Systemic Treatments Compared With Methotrexate.
Propensity-score adjusted hazard ratios (HRs) and 95% confidence intervals are presented. Pooled HR via analysis was adjusted for 20% overlap of patients between Optum Clinformatics Data Mart (Optum) and Truven MarketScan (MarketScan).
We performed a post hoc analysis using adalimumab as the reference group (Table 4). Censoring reasons and subanalyses by type of serious infection are presented in eTables 10 and 11 in the Supplement, respectively. In the pooled analysis adjusted for overlap, we found a significantly decreased risk of serious infection among new users of apremilast (PS-adjusted HR, 0.31; 95% CI, 0.15, 0.65) as well as etanercept (PS-adjusted HR, 0.76; 95% CI, 0.61-0.94). The analysis adjusted for overlap yielded an HR of 0.70 for ustekinumab (95% CI, 0.50-1.00) compared with adalimumab. Infliximab showed a significantly increased rate of serious infection compared with adalimumab (PS-adjusted HR, 1.92; 95% CI, 1.01-3.62).
Table 4. Analysis of Overall Serious Infections in Patients With Psoriasis Receiving Systemic Medications Compared With Adalimumab.
| Exposure | Reference Group (Adalimumab) | Exposure Group | Unadjusted Rate Difference (95% CI) | Unadjusted HR (95% CI) | P Value | PS-Adjusted HR (95% CI) | P Value | Pooled HR (95% CI)b | P Valuec | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, No. | Events, No. | Incidence Ratea | Patients, No. | Events, No. | Incidence Rate | ||||||||
| Acitretin | |||||||||||||
| Optum | 8477 | 82 | 9.92 | 2835 | 29 | 14.12 | 4.21 (−1.36 to 9.78) | 1.38 (0.90 to 2.12) | .14 | 1.18 (0.69 to 2.00) | .54 | 1.06 (0.79 to 1.43) | .71 |
| MarketScan | 21 759 | 206 | 9.32 | 7883 | 78 | 13.01 | 3.69 (0.54 to 6.85) | 1.36 (1.05 to 1.77) | .02 | 1.02 (0.74 to 1.41) | .91 | ||
| Apremilast | |||||||||||||
| Optum | 8730 | 89 | 10.27 | 1573 | 2 | 2.34 | −7.93 (−11.81 to −4.05) | 0.21 (0.05 to 0.84) | .03 | 0.17 (0.04 to 0.74) | .02 | 0.31 (0.15 to 0.65) | .002 |
| MarketScan | 22 655 | 230 | 9.77 | 4344 | 8 | 4.21 | −5.57 (−8.74 to −2.39) | 0.39 (0.19 to 0.80) | .01 | 0.37 (0.17 to 0.80) | .01 | ||
| Etanercept | |||||||||||||
| Optum | 6008 | 54 | 9.9 | 7698 | 67 | 7.93 | −1.97 (−5.22 to 1.29) | 0.82 (0.57 to 1.18) | .28 | 0.65 (0.43 to 0.98) | .04 | 0.76 (0.61 to 0.94) | .01 |
| MarketScan | 16 979 | 148 | 9.04 | 18 719 | 176 | 7.77 | −1.27 (−3.12 to 0.59) | 0.87 (0.69 to 1.08) | .20 | 0.79 (0.63 to 1.01) | .06 | ||
| Methotrexate | |||||||||||||
| Optum | 7181 | 79 | 10.33 | 8470 | 112 | 14.42 | 3.91 (0.42 to 7.39) | 1.37 (1.03 to 1.83) | .03 | 0.77 (0.53 to 1.12) | .17 | 0.93 (0.75 to 1.14) | .51 |
| MarketScan | 17 912 | 197 | 9.75 | 20 609 | 229 | 12.14 | 2.39 (0.31 to 4.47) | 1.25 (1.03 to 1.51) | .02 | 0.99 (0.79 to 1.24) | .94 | ||
| Infliximab | |||||||||||||
| Optum | 8839 | 87 | 9.96 | 458 | 6 | 20.77 | 10.81 (−5.94 to 27.56) | 2.03 (0.88 to 4.65) | .09 | 2.49 (0.62 to 10.04) | .20 | 1.92 (1.01 to 3.62) | .04 |
| MarketScan | 22 588 | 222 | 9.51 | 1332 | 22 | 20.11 | 10.60 (2.11 to 19.10) | 2.13 (1.38 to 3.31) | <.001 | 1.81 (0.93 to 3.49) | .08 | ||
| Ustekinumab | |||||||||||||
| Optum | 8590 | 86 | 10.01 | 3254 | 18 | 5.53 | −4.48 (−8.00 to −0.96) | 0.62 (0.37 to 1.04) | .07 | 0.73 (0.40 to 1.33) | .31 | 0.70 (0.49 to 1.00) | .05 |
| MarketScan | 22 352 | 220 | 9.62 | 6028 | 41 | 7.04 | −2.58 (−5.08 to −0.08) | 0.72 (0.52 to 1.01) | .06 | 0.69 (0.47 to 1.03) | .07 | ||
Abbreviations: HR, hazard ratio; PS, propensity score.
Incident rate in 1000 person-years.
Pooled HR adjusted for 20% overlap.
P values correspond to unadjusted pooled HR.
Discussion
In this large observational comparative cohort study including 107 707 patients with psoriasis who were new users of systemic medications, we found a significantly decreased rate of serious infection with apremilast, etanercept, and ustekinumab compared with methotrexate. Our findings were consistent in both databases that were used.
There have been several observational studies that have analyzed the risk of serious infection with biologic agents compared with nonbiologic systemic medications with widely differing results. One large observational study in the United States found a small increased risk of serious infection for biologic users compared with nonbiologic users (adjusted HR, 1.31; 95% CI, 1.02-1.68), but did not analyze biologics separately. Almost all other observational studies examining the risk of serious infections with these agents have used data from registries, both in the United States and Europe.18,19,20,21,22 A registry study of systemic treatments for psoriasis in Spain18 found a significantly increased risk of serious infection compared with methotrexate for etanercept and infliximab but no difference in risk for adalimumab and ustekinumab.18 Two registry studies21,23 in the United Kingdom and Republic of Ireland found no significant increased risk of serious infection for adalimumab, etanercept, or ustekinumab compared with nonbiologic systemic therapies or methotrexate alone but nearly a 3-fold increased risk of serious infection with the use of infliximab compared with methotrexate.
The results of our study are most consistent with data from the largest registry study, Psoriasis Longitudinal Assessment and Registry (PSOLAR), which includes 11 466 patients receiving biologics and nonbiologic systemic agents for psoriasis in the United States. The authors found exposure to infliximab and adalimumab were independently associated with the risk of serious infection but not for ustekinumab or etanercept. However, the main analysis of this study included prevalent users of systemic medications for psoriasis, who may have a different risk profile than incident users because patients who stay receive treatment for a longer time may be less susceptible to infectious or other adverse outcomes.24,25
To our knowledge, this is the largest study to date comparing the risk of serious infection among different systemic medications for psoriasis and adequately powered to provide estimates for individual systemic medications. We suspect that the decreased risk of serious infection among users of etanercept compared with methotrexate, but not the other 2 anti–tumor necrosis factor agents (adalimumab and infliximab), may be owing to a dosing effect, in that etanercept is considered to be the least effective in treating psoriasis and likewise potentially the least immunsuppressive,26 and infliximab is one of the most effective in the initial few months of treatment and potentially more immunosuppressive.27 We found an increased rate of serious infection among users of infliximab compared with adalimumab (pooled PS-adjusted HR, 1.92; 1.01-3.62) but not in our primary analysis compared with methotrexate (pooled PS-adjusted HR, 1.47; 95% CI, 0.75-2.87). This may have been owing to increased power in the post hoc analysis, which had a higher number of infliximab users in the pooled analysis (n = 1790) compared with our primary analysis (n = 1435) (Table 4; eTable 11 in the Supplement). The comparatively small sample sizes in these cohorts may have decreased the efficacy of matching, and residual confounding could have also potentially led to this discrepancy. Infliximab, owing to its nonhuman (chimeric) structure, carries higher risk of inducing neutralizing antibodies, and unlike other biologics used in psoriasis, it is common clinical practice to prescribe methotrexate concomitantly. There was substantial crossover and censoring among infliximab users owing to the addition of methotrexate, and although we attempted to control for this, it may have affected our results.
Ustekinumab, a biologic that blocks interleukin (IL)-12 and IL-23, was found to have a significantly decreased risk of infection compared with methotrexate. We also found a numerically decreased risk, albeit statistically not significant, when compared with adalimumab in our pooled analyses (HR, 0.70; 95% CI, 0.49-1.00). Despite ustekinumab’s higher efficacy in treating psoriasis compared with anti–tumor necrosis factor and other nonbiologic medications,27,28 this finding is suggestive that ustekinumab is less broadly immunosuppressive owing to its differing mechanism of action. Given its mechanism of therapeutic action, which does not directly target inflammatory cytokines, not surprisingly, apremilast use was associated with a significantly decreased risk of serious infection compared with both methotrexate and adalimumab. However, it also tends to be one of the least effective systemic treatments.29 Interestingly, although we found no increased risk of overall serious infection in users of acitretin compared with methotrexate, in our subanalyses, we found a significantly increased risk of cellulitis in acitretin users (Table 3). Prior studies have shown that oral retinoid therapy increases the rate of colonization by Staphylococcus aureus and the fragility of skin, which may explain this finding.30,31,32,33
Our study has several important strengths. To ensure comparability of our systemic medication user cohorts, we used a new-user design. We reduced the likelihood of outcome misclassification using validated measures for serious infection13 and used pharmacy claims with dispensing and refill records to identify exposures to medication. We adjusted for potential confounders by PS matching. Propensity score matching permits adjustment for a large number of potential confounders, which is particularly important in this study owing to the potential for confounding by indication; ie, patients being prescribed different medications for psoriasis may have different comorbidities or other characteristics that may affect the risk of serious infection. Compared with prior studies using data from clinical trials and registries, the results of this study are generalizable to real-world practice in the United States.
Limitations
There are some limitations to note. Infliximab is administered intravenously and is often not dispensed or coded as an outpatient prescription. Thus, we were likely not able to identify many users of this medication in our study. Other limitations are inherent to using claims-based data. We had no access to data on baseline psoriasis severity, which may affect the risk of serious infection and could have confounded the results.34 As with all studies relying on administrative claims, data are subject to coding errors. However, it is unlikely that coding errors were differential among medication groups.
Conclusions
In conclusion, we found significant differences in the risk of serious infection across different systemic treatments for psoriasis. This information should be considered when prescribing therapies for individual patients, as well as future treatment algorithms. Validation studies using ICD-10-CM codes to identify adverse events such as serious infection will be needed in the future to investigate the comparative safety of newer systemic agents used for psoriasis.
eTable 1. ICD-CM-9 Codes for Serious Infection and Associated Positive Predictive Values (PPV)
eTable 2. Patient Flow Chart for Optum Clinformatics Data Mart
eTable 3. Patient Flow Chart for Truven MarketScan
eTable 4. Censoring Reasons for Medication Cohorts for Optum Clinformatics Data Mart and Truven MarketScan for Systemic Medications Compared With Methotrexate
eTable 5. Matched Cohorts for Systemic Medications Compared With Methotrexate in Optum Clinformatics Data Mart and Truven MarketScan
eTable 6. Overall and Subtypes of Serious Infections in Patient With Psoriasis on Systemic Medications Compared With methotrexate in Optum Clinformatics Data Mart
eTable 7. Overall and Subtypes of Serious Infections in Patients With Psoriasis on Systemic Medications Compared With Methotrexate in Truven MarketScan
eTable 8. Overall Serious Infections in Patients With Psoriasis and Psoriatic Arthritis on Systemic Medications Compared With Methotrexate in Optum© Clinformatics® Data Mart
eTable 9. Overall Risk of Serious Infection Compared With Methotrexate in Patients With Psoriasis by Age and Gender in Optum Clinformatics Data Mart
eTable 10. Censoring Reasons For Medication Cohorts For Optum Clinformatics Data Mart and Truven MarketScan for Systemic Medications Compared With Adalimumab
eTable 11. Meta-analysis of Pooled Hazard Risk of Serious Infection in Patients With Psoriasis on Systemic Medications Compared With Adalimumab
References
- 1.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136-139. [DOI] [PubMed] [Google Scholar]
- 2.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512-516. doi: 10.1016/j.jaad.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 3.Baddley JW, Winthrop KL, Chen L, et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety Assessment of Biologic ThERapy (SABER) study. Ann Rheum Dis. 2014;73(11):1942-1948. doi: 10.1136/annrheumdis-2013-203407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331-2339. doi: 10.1001/jama.2011.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winthrop KL, Baddley JW, Chen L, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA. 2013;309(9):887-895. doi: 10.1001/jama.2013.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632-2640. doi: 10.1038/jid.2015.208 [DOI] [PubMed] [Google Scholar]
- 7.Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol. 2016;74(6):1057-1065.e4. doi: 10.1016/j.jaad.2016.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dommasch ED, Lee MP, Joyce CJ, Garry EM, Gagne JJ. Drug utilization patterns and adherence in patients on systemic medications for the treatment of psoriasis: a retrospective, comparative cohort study. J Am Acad Dermatol. 2018;79(6):1061-1068.e1. doi: 10.1016/j.jaad.2018.06.053 [DOI] [PubMed] [Google Scholar]
- 9.Carretero G, Ferrandiz C, Dauden E, et al. ; BIOBADADERM Study Group . Risk of adverse events in psoriasis patients receiving classic systemic drugs and biologics in a 5-year observational study of clinical practice: 2008-2013 results of the Biobadaderm registry. J Eur Acad Dermatol Venereol. 2015;29(1):156-163. doi: 10.1111/jdv.12492 [DOI] [PubMed] [Google Scholar]
- 10.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64(6):1035-1050. doi: 10.1016/j.jaad.2010.09.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586-592. doi: 10.1111/j.1365-2133.2010.09941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Icen M, Crowson CS, McEvoy MT, Gabriel SE, Maradit Kremers H. Potential misclassification of patients with psoriasis in electronic databases. J Am Acad Dermatol. 2008;59(6):981-985. doi: 10.1016/j.jaad.2008.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiese AD, Griffin MR, Stein CM, et al. Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open. 2018;8(6):e020857. doi: 10.1136/bmjopen-2017-020857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munder T, Brütsch O, Leonhart R, Gerger H, Barth J. Researcher allegiance in psychotherapy outcome research: an overview of reviews. Clin Psychol Rev. 2013;33(4):501-511. doi: 10.1016/j.cpr.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 16.Bom PRD. Accounting for sample overlap in meta-analysis. http://www.stiftung.at/wp-content/uploads/2015/04/BomPaper_Oct_2014.pdf. Accessed January 7, 2019. [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 18.Dávila-Seijo P, Dauden E, Descalzo MA, et al. ; BIOBADADERM Study Group . Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM Registry. J Invest Dermatol. 2017;137(2):313-321. doi: 10.1016/j.jid.2016.08.034 [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Doval I, Cohen AD, Cazzaniga S, et al. ; Psonet Network . Risk of serious infections, cutaneous bacterial infections, and granulomatous infections in patients with psoriasis treated with anti-tumor necrosis factor agents versus classic therapies: prospective meta-analysis of Psonet registries. J Am Acad Dermatol. 2017;76(2):299-308.e16. doi: 10.1016/j.jaad.2016.07.039 [DOI] [PubMed] [Google Scholar]
- 20.Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and registry (psolar). JAMA Dermatol. 2015;151(9):961-969. doi: 10.1001/jamadermatol.2015.0718 [DOI] [PubMed] [Google Scholar]
- 21.Yiu ZZN, Exton LS, Jabbar-Lopez Z, et al. Risk of serious infections in patients with psoriasis on biologic therapies: a systematic review and meta-analysis. J Invest Dermatol. 2016;136(8):1584-1591. doi: 10.1016/j.jid.2016.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yiu ZZN, Smith CH, Ashcroft DM, et al. ; BADBIR Study Group . Risk of serious infection in patients with psoriasis receiving biologic therapies: a prospective cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2018;138(3):534-541. doi: 10.1016/j.jid.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yiu ZZN, Ashcroft DM, Evans I, et al. ; BADBIR Study Group . Infliximab is associated with an increased risk of serious infection in patients with psoriasis in the U.K. and Republic of Ireland: results from the British Association of Dermatologists Biologic Interventions Register (BADBIR). Br J Dermatol. 2019;180(2):329-337. doi: 10.1111/bjd.17036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14(7):706-714. [PubMed] [Google Scholar]
- 25.Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ; British Society for Rheumatology Biologics Register Control Centre Consortium; British Society for Rheumatology Biologics Register . Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56(9):2896-2904. doi: 10.1002/art.22808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2008;159(3):513-526. doi: 10.1111/j.1365-2133.2008.08732.x [DOI] [PubMed] [Google Scholar]
- 27.Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74(5):851-61.e4. doi: 10.1016/j.jaad.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 28.Griffiths CEM, Strober BE, van de Kerkhof P, et al. ; ACCEPT Study Group . Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118-128. doi: 10.1056/NEJMoa0810652 [DOI] [PubMed] [Google Scholar]
- 29.Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507-517. doi: 10.1111/jdv.14015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lianou P, Bassaris H, Vlachodimitropoulos D, Tsambaos D. Acitretin induces an increased adherence of S aureus to epithelial cells. Acta Derm Venereol. 1989;69(4):330-332. [PubMed] [Google Scholar]
- 31.Williams RE, Doherty VR, Perkins W, Aitchison TC, Mackie RM. Staphylococcus aureus and intra-nasal mupirocin in patients receiving isotretinoin for acne. Br J Dermatol. 1992;126(4):362-366. doi: 10.1111/j.1365-2133.1992.tb00679.x [DOI] [PubMed] [Google Scholar]
- 32.Graham ML II, Corey R, Califf R, Phillips H. Isotretinoin and Staphylococcus aureus infection: a possible association. Arch Dermatol. 1986;122(7):815-817. doi: 10.1001/archderm.1986.01660190093024 [DOI] [PubMed] [Google Scholar]
- 33.Flemetakis AC, Tsambaos DG. Effects of synthetic retinoids on the growth of bacteria and their susceptibility to antibiotics. J Chemother. 1989;1(6):374-376. doi: 10.1080/1120009X.1989.11738926 [DOI] [PubMed] [Google Scholar]
- 34.Takeshita J, Shin DB, Ogdie A, Gelfand JM. Risk of serious infection, opportunistic infection, and herpes zoster among patients with psoriasis in the United Kingdom. J Invest Dermatol. 2018;138(8):1726-1735. doi: 10.1016/j.jid.2018.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-CM-9 Codes for Serious Infection and Associated Positive Predictive Values (PPV)
eTable 2. Patient Flow Chart for Optum Clinformatics Data Mart
eTable 3. Patient Flow Chart for Truven MarketScan
eTable 4. Censoring Reasons for Medication Cohorts for Optum Clinformatics Data Mart and Truven MarketScan for Systemic Medications Compared With Methotrexate
eTable 5. Matched Cohorts for Systemic Medications Compared With Methotrexate in Optum Clinformatics Data Mart and Truven MarketScan
eTable 6. Overall and Subtypes of Serious Infections in Patient With Psoriasis on Systemic Medications Compared With methotrexate in Optum Clinformatics Data Mart
eTable 7. Overall and Subtypes of Serious Infections in Patients With Psoriasis on Systemic Medications Compared With Methotrexate in Truven MarketScan
eTable 8. Overall Serious Infections in Patients With Psoriasis and Psoriatic Arthritis on Systemic Medications Compared With Methotrexate in Optum© Clinformatics® Data Mart
eTable 9. Overall Risk of Serious Infection Compared With Methotrexate in Patients With Psoriasis by Age and Gender in Optum Clinformatics Data Mart
eTable 10. Censoring Reasons For Medication Cohorts For Optum Clinformatics Data Mart and Truven MarketScan for Systemic Medications Compared With Adalimumab
eTable 11. Meta-analysis of Pooled Hazard Risk of Serious Infection in Patients With Psoriasis on Systemic Medications Compared With Adalimumab