Key Points
Question
Is the combination of androgen-deprivation therapy plus docetaxel superior to androgen-deprivation therapy alone in the treatment of nonmetastatic prostate cancer in patients with increasing prostate-specific antigen levels after primary local therapy?
Findings
In this phase 3 randomized clinical trial including 254 patients, there was no significant difference between treatments for the primary outcome measure, prostate-specific antigen progression-free survival (median follow-up, 30.0 months). In addition, no significant difference was noted in the secondary outcome measures of radiologic progression-free survival and overall survival (median follow-up, 10.5 years).
Meaning
Addition of docetaxel to androgen-deprivation therapy seems to be unwarranted in patients with high-risk prostate cancer without metastases in the absence of better predictors of risk for metastatic disease.
Abstract
Importance
Androgen-deprivation therapy (ADT) plus docetaxel is the standard of care in hormone-naive metastatic prostate cancer but is of uncertain benefit in a nonmetastatic, high-risk prostate cancer setting.
Objective
To assess the benefit of ADT plus docetaxel in patients presenting with rising prostate-specific antigen (PSA) levels after primary local therapy and high-risk factors but no evidence of metastatic disease.
Design, Setting, and Participants
This open-label, phase 3, randomized superiority trial comparing ADT plus docetaxel vs ADT alone enrolled patients from 28 centers in France between June 4, 2003, and September 25, 2007; final follow-up was conducted April 12, 2017, and analysis was performed May 2 to July 31, 2017. Patients had undergone primary local therapy for prostate cancer, were experiencing rising PSA levels, and were considered to be at high risk of metastatic disease. Stratification was by prior local therapy and PSA-level doubling time (≤6 vs >6 months), and intention-to-treat analysis was used.
Interventions
Patients were randomly assigned to receive ADT (1 year) plus docetaxel, 70 mg/m2 (every 3 weeks [6 cycles]), or ADT alone (1 year).
Main Outcomes and Measures
The primary outcome was PSA progression-free survival (PSA-PFS). Secondary end points were PSA response, radiologic PFS, overall survival, safety, and quality of life.
Results
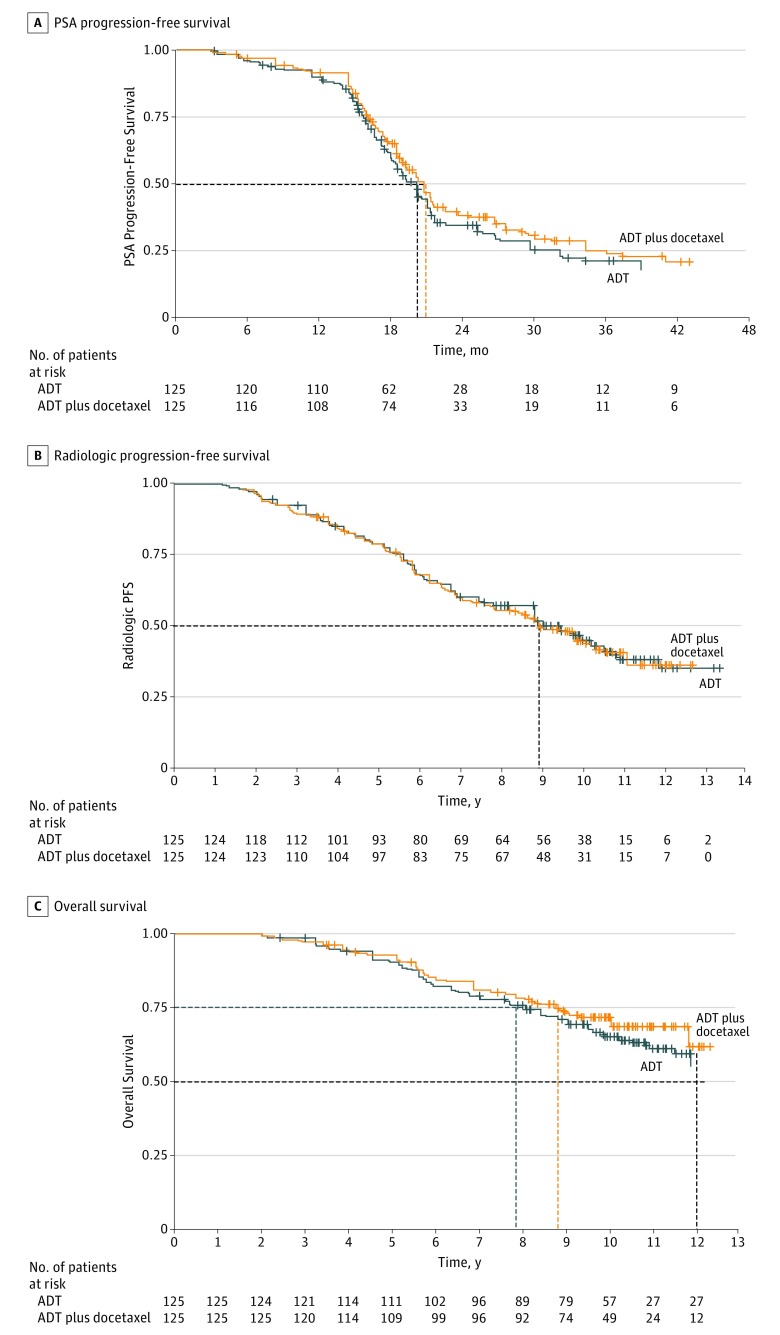
Overall, 254 patients were randomized (1:1) to the trial; median age, 64 years in the ADT plus docetaxel arm, 66 years in the ADT alone arm. At a median follow-up of 30.0 months, the median PSA-PFS was 20.3 (95% CI, 19.0-21.6) months in the ADT plus docetaxel arm vs 19.3 (95% CI, 18.2-20.8) months in the ADT alone arm (hazard ratio [HR], 0.85; 95% CI, 0.62-1.16; P = .31). At a median follow-up of 10.5 years, there was no significant between-arm difference in radiologic PFS (HR, 1.03; 95% CI, 0.74-1.43; P = .88). Overall survival data were not mature. The most common grade 3 or 4 hematologic toxic effects in the ADT plus docetaxel arm were neutropenia (60 of 125 patients [48.0%]), febrile neutropenia (10 [8.0%]), and thrombocytopenia (4 [3.0%]). There was no significant between-arm difference in overall quality of life.
Conclusions and Relevance
Compared with ADT alone, combined ADT plus docetaxel therapy with curative intent did not significantly improve PSA-PFS in patients with high-risk prostate cancer and rising PSA levels and no evidence of metastatic disease.
Trial Registration
French Health Products Safety Agency identifier: 030591; ClinicalTrials.gov identifier: NCT00764166
This randomized clinical trial compares the use of combined androgen-deprivation therapy plus docetaxel with androgen-deprivation therapy alone in patients with prostate cancer who develop increasing levels of prostate-specific antigen after primary local therapy.
Introduction
An estimated 15% to 30% of patients who have undergone primary therapy for localized prostate cancer (PC) will experience prostate-specific antigen (PSA) relapse within 10 years of treatment, with a median time from relapse to the development of distant metastases of 8 to 10 years.1,2,3,4 Clinicians are faced with the problem of identifying patients with rising PSA levels who are most at risk of developing metastatic disease and selecting appropriate treatment. Among factors considered to define high-risk PC are a high Gleason score (8-10), a time from primary therapy to PSA recurrence of less than 3 years, PSA velocity (>0.75 ng/mL per year [to convert to micrograms per liter, multiply by 1], and, in particular, a short PSA doubling time (DT) (<6 months).5,6,7
There is no standard of care for patients with hormone-sensitive PC with rising PSA levels after local therapy. Many clinicians initiate androgen-deprivation therapy (ADT), but only once metastatic disease has developed, and, to our knowledge, no formal clinical trials have examined the benefits of ADT in patients with biochemical relapse only.8 However, in patients at high risk with rising PSA levels after radical prostatectomy, the greater risk of developing metastases after PSA recurrence may warrant early ADT administration9,10,11,12and even more aggressive treatment than just ADT alone.13,14
The combination of ADT and docetaxel has improved outcomes over administration of ADT alone in each of 3 randomized phase 3 trials in a hormone-sensitive metastatic setting (GETUG-AFU-15,15 CHAARTED,16 STAMPEDE17). A meta-analysis of these trials noted a 9% improvement in overall survival (hazard ratio [HR], 0.77; P < .001) and a 16% reduction in failure rates (HR, 0.64; P < .001) at 4 years.18,19
According to phase 2 trials in patients with no evidence of metastases but with PSA level progression (ie, increasing above a threshold defining the progression) after primary therapy, early docetaxel administration with or without ADT is feasible and active (ie, docetaxel exhibited efficacy).20,21,22,23 However, in a nonmetastatic rather than metastatic setting, the benefit of combining docetaxel with ADT did not prove to be as clear-cut in terms of overall survival (2% absolute benefit: HR, 0.87; P = .22) in a meta-analysis of phase 3 trials (GETUG-12,24 RTOG 0521,25 STAMPEDE,17 TAX 350126) even though failure rates were significantly reduced from 30% to 22% at 4 years (HR, 0.70; P < .001). To our knowledge, no predictive factors have been identified to help select patients who might benefit from initial therapy with ADT plus docetaxel vs ADT alone.
The present randomized clinical superiority trial compared ADT plus docetaxel with ADT alone in patients with localized PC treated by radical prostatectomy and/or radiotherapy who then developed rising PSA levels. The patients had no clinical or radiographic evidence of metastatic disease but presented high-risk factors. The primary outcome was PSA progression-free survival (PSA-PFS).
Methods
Trial Design and Participants
This open-label, randomized, phase 3 superiority trial was conducted in 28 centers in France. The study enrolled patients between June 4, 2003, and September 25, 2007, final follow-up was conducted April 12, 2017, and data analysis was performed May 2 to July 31, 2017. Patients gave written informed consent; no financial compensation was provided. The study was conducted according to the Declaration of Helsinki27 and Good Clinical Practice Guidelines. Ethics approval for the initial version of the protocol was granted by the French National Ethics Committee. The trial protocol is available in Supplement 1.
Eligible patients (age ≥18 years) had histologically proven PC but no radiographic evidence of metastases, had undergone treatment for local disease (radical prostatectomy and/or radiotherapy), and were experiencing PSA recurrence. Recurrence criteria were those of the American Society for Therapeutic Radiology and Oncology (ie, 3 consecutive PSA measurements >0.2 ng/mL for patients who underwent radical prostatectomy and >1 ng/mL above the nadir for patients who received radiotherapy; modified to ≥2 ng/mL in 2006 by the American Society for Therapeutic Radiology and Oncology Phoenix Consensus).
To be eligible, patients had to present 1 or more of the following: node-positive adenocarcinoma, positive surgical margins, Gleason score 8 or higher, PSA velocity greater than 0.75 ng/mL per year, PSA-DT 6 months or less, and time to PSA recurrence 12 months or less. Further eligibility criteria were Eastern Cooperative Oncology Group performance status (ECOG-PS) 0 or 1, life expectancy 12 months or more, stage pT3 or lower, white blood cell count of 3000/μL or more (to convert to ×109 per liter, multiply by 0.001), absolute neutrophil count of 1500/μL or more (to convert to ×109 per liter, multiply by 0.001), platelets of 100 ×103/μL or more (to convert to ×109 per liter, multiply by 1), hemoglobin level of 10 g/dL or more (to convert to grams per liter, multiply by 10), creatinine level 1.5 times or lower than the reference range, alkaline phosphatase level lower than 2 times the reference range, bilirubin level lower than 1.5 times the reference range, and aminotransferase levels 1.5 times or lower than the reference range. After radical prostatectomy salvage radiotherapy was allowed in patients with positive surgical margins.
Exclusion criteria were prior chemotherapy; history of another primary cancer (except basal cell carcinoma); congestive heart failure; angina (even if under control); recent acute myocardial infarction; severe lung, liver, or kidney disease; symptomatic or progressive neurologic disease; gastroduodenal ulcer; stable or unstable diabetes; and a contraindication to corticosteroid therapy. Before inclusion, all patients underwent baseline investigations including physical examination; hematologic and biochemical profile; computed tomographic scan of the upper abdomen, pelvis, and chest; and bone scan. We recorded age, clinical and/or pathologic disease stage, time from local therapy to PSA recurrence, concomitant treatments, ECOG-PS, tumor type, and histologic grade.
Interventions
Patients meeting inclusion criteria were stratified by prior therapy (radical prostatectomy vs radiotherapy) and PSA-DT (≤6 vs >6 months) and were allocated to receive either ADT alone or ADT plus docetaxel by a computer-based randomization algorithm using minimization and stratification factors (type of prior curative therapy and PSA-DT). Patients and investigators were not masked to treatment allocation. The ADT regimen was a luteinizing hormone-releasing-hormone agonist (triptorelin) injected intramuscularly (11.25 mg every 3 months for 1 year) with administration of a nonsteroidal antiandrogen (bicalutamide) during the first 3 weeks of treatment. Docetaxel was administered intravenously (70 mg/m2 on day 1 of 3-week cycles) for up to 6 cycles.
Throughout the 1-year treatment period, patients in the ADT plus docetaxel arm underwent a physical examination, conventional laboratory tests, and testosterone and PSA assays every 3 weeks during docetaxel administration, then monthly as in the ADT-alone arm. Once treatment ended, PSA levels were assayed every 3 months until progression was noted. Radiographic evaluations were recommended after 3 elevated PSA measurements. A confirmatory computed tomographic scan was performed on diagnosis of metastatic disease.
Treatment safety and patient-reported quality of life were assessed before each treatment cycle using NCI-CTC (National Cancer Institute–Common Terminology Criteria) criteria, version 3.0, and the EORTC-QLQ-C30 (European Organisation for Research and Treatment of Cancer–Quality of Life) questionnaire, respectively.
Outcome Assessment
The primary outcome was PSA-PFS. Progression-free survival progression was defined as a 50% or more relative increase above the nadir accompanied by an absolute PSA increase defined in a sensitivity analysis (increase varied from 0.2 to 2.0 ng/mL in 0.1-ng/mL increments), with confirmation by 2 further measurements at 3-week intervals. Secondary outcomes included PSA response, radiologic PFS (rPFS) (time from randomization to the first detection of distant metastasis or death from any cause, whichever came first), overall survival (time from randomization to death from any cause), safety, and quality of life. A complete PSA response was defined as a 50% or more decline in PSA and an absolute PSA level of 0.1 ng/mL or lower. A partial PSA response was defined as a 50% or more decline in PSA and an absolute PSA level greater than 0.1 ng/mL.
Statistical Analysis
Sample size calculations were based on the hypothesis that the 3-year PSA-PFS rate in the ADT plus docetaxel arm would be superior to that in the ADT arm by 15% (81% vs 66%). This higher percentage corresponds to median progression times of 9.8 vs 5.0 months (HR, 0.507) as calculated using an exponential event distribution. A total of 69.6 PSA progressions (25.3 and 44.3, respectively) and 252 patients (126/arm) were needed for a 2-sided type I error of P = .05 and 80% power, 2-year accrual period, 3-year follow-up, and an anticipated 10% loss to follow-up.
The PSA-PFS, rPFS, and overall survival adjusted for stratification factors were estimated using the Kaplan-Meyer method on the intention-to-treat population. Treatment arms were compared using HRs. Inverse censoring was used to calculate median follow-up time for PSA-PFS, rPFS, and overall survival. A backward-selection Cox proportional hazards regression model adjusted for stratification factors was used to investigate predictors of PSA-PFS and rPFS (P ≤ .20). Statistical significance was set at an unpaired, 2-sided level of α = .05. The value of PSA-PFS in predicting 10-year rPFS was assessed separately for ADT and ADT plus docetaxel, using dynamic landmark analysis comparing patients with and without PSA progression at various times between 3 and 36 months.
The safety analysis included all patients who received at least 1 docetaxel cycle or at least 1 subcutaneous ADT injection and had undergone at least 1 safety assessment. Quality-of-life analysis used linear and nonlinear mixed models with continuous and discrete time points from baseline to 12 months. Time to an absolute 25% worsening of global health status/quality-of-life score was calculated using a Cox proportional hazards regression model adjusted for stratification factors. We used SAS, version 9.4 software (SAS Institute Inc), for analysis.
Results
Participants and Treatment
Between June 4, 2003, and September 25, 2007, we recruited 277 high-risk, hormone-naive patients with PSA relapse. Overall, 254 individuals met eligibility criteria. One patient per arm withdrew consent and 1 patient per arm was lost to follow-up (Figure 1). Treatment arms were well balanced, with similar percentages displaying at least 4 high-risk factors at inclusion (Table). Overall, 119 of 125 patients (95.2%) received all 6 docetaxel cycles. The median docetaxel dose per cycle was 132 mg (range, 70-170 mg); dose intensity was 99.4% of the target.
Figure 1. CONSORT Diagram.
ITT indicates intention-to-treat.
Table. Baseline Characteristics of Eligible Patients Who Received Allocated Intervention.
| Characteristic | ADT Plus Docetaxel (n = 125) | ADT (n = 125) |
|---|---|---|
| Age, median (IQR), y | 64 (58-70) | 66 (61-71) |
| ECOG-PS, No. (%) | ||
| 0 | 119 (95.2) | 116 (92.8) |
| 1 | 4 (3.2) | 8 (6.4) |
| Stage T3/T4, No. (%) | 73 (58.4) | 74 (59.2) |
| Prior radical prostatectomy, No. (%) | 90 (72.0) | 92 (73.6) |
| Prior radiotherapy ± ADT, No. (%) | 35 (28.0) | 33 (26.4) |
| Salvage radiotherapy, No. (%) | 54 (43.2) | 56 (44.8) |
| Baseline measurements, median (IQR) | ||
| PSA level, ng/mL | 2.6 (1.0-6.2) | 2.9 (1.0-6.0) |
| PSA-DT, mo | 5.8 (3.2-8.4) | 5.8 (3.7-9.1) |
| PSA velocity, ng/mL per year | 1.4 (1.0-1.0) | 1.4 (1.0-1.0) |
| Testosterone, ng/dL | 420.5 (334.1-636.5) | 414.7 (371.5-699.8) |
| Time RP/RT to PSA progression, mo | 30 (17.0-51.5) | 27 (16.5-43.7) |
| High-risk factors at inclusion, No. (%) | ||
| Gleason score ≥8 | 41 (32.8) | 36 (28.8) |
| PSA-DT ≤6 mo | 67 (53.6) | 67 (53.6) |
| PSA velocity >0.75 ng/mL per year | 106 (84.8) | 101 (80.8) |
| Positive surgical margins | 45 (36.0) | 49 (39.2) |
| Node positive | 4 (3.2) | 6 (4.8) |
| Median time RP/RT to PSA progression ≤12 mo | 19 (15.2) | 21 (16.8) |
| At least 4 high-risk factors | 13 (10.4) | 17 (13.6) |
Abbreviations: ADT, androgen-deprivation therapy; DT, doubling time; ECOG-PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
SI conversion: To convert PSA to micrograms per liter, multiply by 1; testosterone to nanomoles per liters, multiply by 0.0347.
PSA Progression-Free Survival
In a scheduled sensitivity analysis in which the absolute PSA increase-defining progression varied from 0.2 to 2.0 ng/mL in 0.1-ng/mL increments, the HR never reached significance (eFigure 1 in Supplement 2). A 0.2-ng/mL increase was chosen to define PSA progression. Data analysis cutoff level for PSA-PFS was January 11, 2011 (median follow-up, 30.0 months). A total of 79 patients (63.2%) in the ADT plus docetaxel arm and 81 patients (64.8%) in the ADT-alone arm experienced progression. Median PSA-PFS was 20.3 months (95% CI, 19.0-21.6 months) in the ADT plus docetaxel arm vs 19.3 months (95% CI, 18.2-20.8 months) in the ADT-alone arm (HR, 0.85; 95% CI, 0.62-1.16; P = .31) (Figure 2A). To allow for any confounding effect of a PSA flare, PSA progression was also assessed after the postrandomization exclusion period of 12 weeks; no effect on the results was demonstrated.
Figure 2. Survival.
A, Prostate-specific antigen (PSA) progression-free survival in 79 patients receiving androgen-deprivation therapy (ADT) plus docetaxel and 81 receiving ADT alone. Median follow-up, 30.0 months (ADT plus docetaxel, 20.3 [95% CI, 19.0-21.6]; ADT alone: 19.3 months [95% CI, 18.2-20.8]); hazard ratio [HR], 0.85 (95% CI, 0.62-1.16); P = .31. B, Radiologic progression-free survival (including deaths from cancer) in 72 patients receiving ADT plus docetaxel and 69 receiving ADT alone. Median follow-up for ADT plus docetaxel, 8.9 years (7.3-10.2); ADT alone, 9.0 years (range, 7.3-10.4); HR 1.03 (95% CI, 0.74-1.43); P = .88. C, Overall all-cause survival in 40 patients receiving ADT plus docetaxel and 46 receiving ADT alone. Median follow-up, 10.5 years; 25th percentile, ADT plus docetaxel, 8.7 years (6.7-10.2); ADT alone, 7.8 years (6.2-9.3); 12-year survival rate, ADT plus docetaxel, 60% (not reached, 70%); ADT alone, 55% (NR, 65%); HR, 0.86 (95% CI, 0.56-1.31); P = .49. Dashed lines indicate 50% (median) and 75% (25th percentile).
PSA Response Rates
All patients experienced a 50% or more PSA level decline from baseline, with 116 (93.0%) experiencing a 50% or more PSA level decline in the ADT plus docetaxel arm and 116 (92.8%) in the ADT-alone arm at 12 weeks. A complete PSA response was observed in 91 of 125 patients (72.8%) who received ADT plus docetaxel vs 81 of 125 patients (64.8%) who received ADT alone. Of the 125 patients in each treatment group, partial PSA response rates at 12 weeks were shown in 25 (20.0%) and 40 (32.0%) in the ADT plus docetaxel and ADT-alone arms, respectively.
Radiologic Progression-Free and Overall Survival
Median follow-up time for rPFS and overall survival was 10.5 years. Median time to radiologic progression adjusted for stratification factors was 8.9 (7.3-10.2) years for ADT plus docetaxel and 9.0 (7.3-10.4) years for ADT alone. There was no significant between-arm difference in rPFS (HR, 1.03; 95% CI, 0.74-1.43; P = .88) (Figure 2B).
There were 40 all-cause deaths (32.0%) in the ADT plus docetaxel arm and 46 all-cause deaths (36.8%) in the ADT-alone arm. The 25th percentiles were 8.7 (95% CI, 6.7-10.2) and 7.8 (95% CI, 6.2-9.3) years, respectively (HR, 0.86; 95% CI, 0.56-1.31; P = .49) (Figure 2C). Median overall survival was not reached.
Quality of Life
Baseline mean (SD) global health status/quality-of-life score was 81.5% (14.4%) for ADT plus docetaxel and 80.9% (17.7%) for ADT alone (P = .84). At the 3-month visit, the score had fallen to 70.6% for ADT plus docetaxel and 75.6% for ADT alone (P = .04). At 6 months, the scores fell , in the ADT plus docetaxel and ADT alone arms, respectively (66.9% vs 73.9%, P = .09), but recovered to reach closer between-arm values at 9 and then 12 months (eFigure 2A in Supplement 2). Time to a 25% decrease in quality of life from baseline was shorter in the ADT plus docetaxel (median, 5.4 months) than ADT- alone arm (median, 16.3 months).
Exploratory Analyses
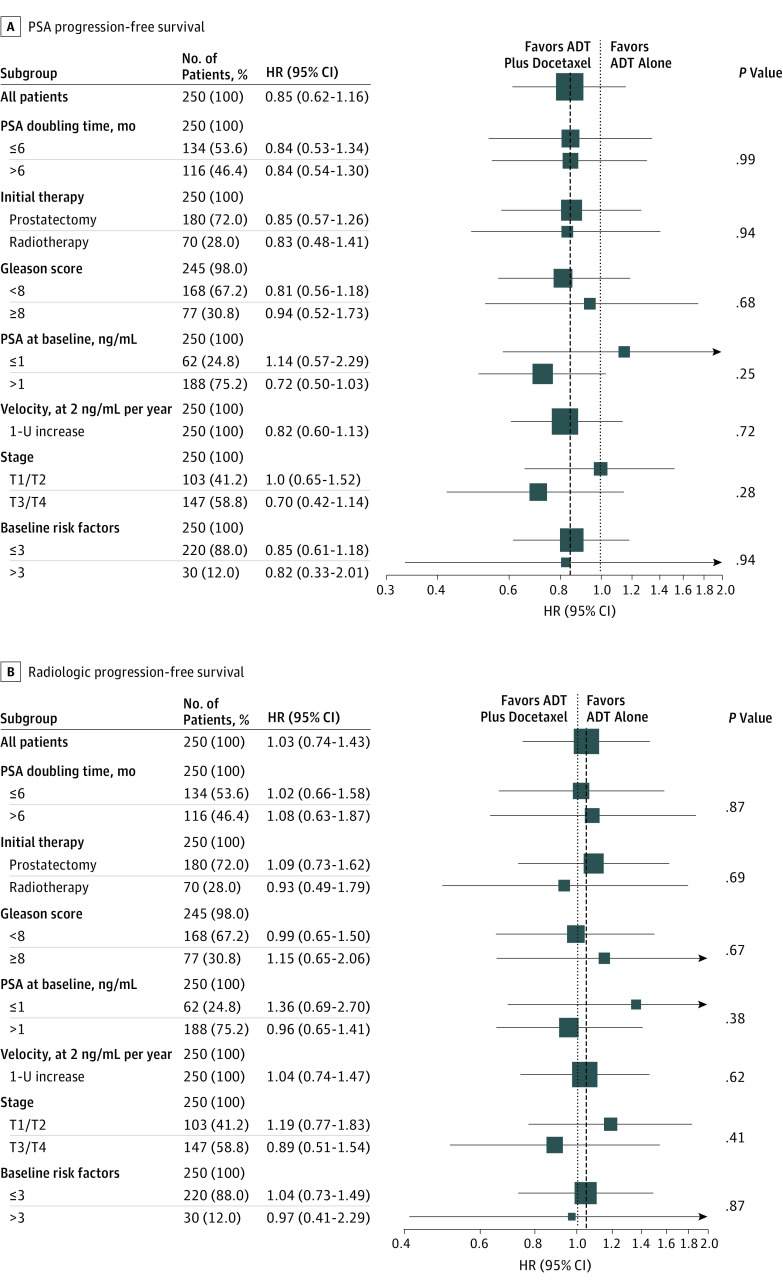
In adjusted subgroup analyses based on baseline attributes, the HR was in favor of treatment with ADT plus docetaxel over ADT alone for PSA-PFS for all attributes (Figure 3A). However, no baseline attributes revealed any treatment effect for rPFS (Figure 3B). No evidence of heterogeneity across subsets defined by baseline attributes was found.
Figure 3. Subgroup Analysis.
A, Prostate-specific antigen (PSA) progression-free survival. Progression defined by 1-ng/mL PSA cutoff level. B, Radiologic progression-free survival. The P value refers to the interaction between therapy arm and subgroup variable. To convert the PSA level to micrograms per liter, multipy by 1. ADT indicates androgen-deprivation therapy; HR, hazard ratio.
Median rPFS was significantly longer in patients with 3 or less rather than more than 3 high-risk factors at baseline (9.7 [95% CI, 8.7-10.5] vs 6.1 [95% CI, 2.9-8.4] months; HR, 0.54; 95% CI, 0.34-0.86; P = .008) (eFigure 3A in Supplement 2), and no interaction was found with treatment arm (eFigure 3B in Supplement 2).
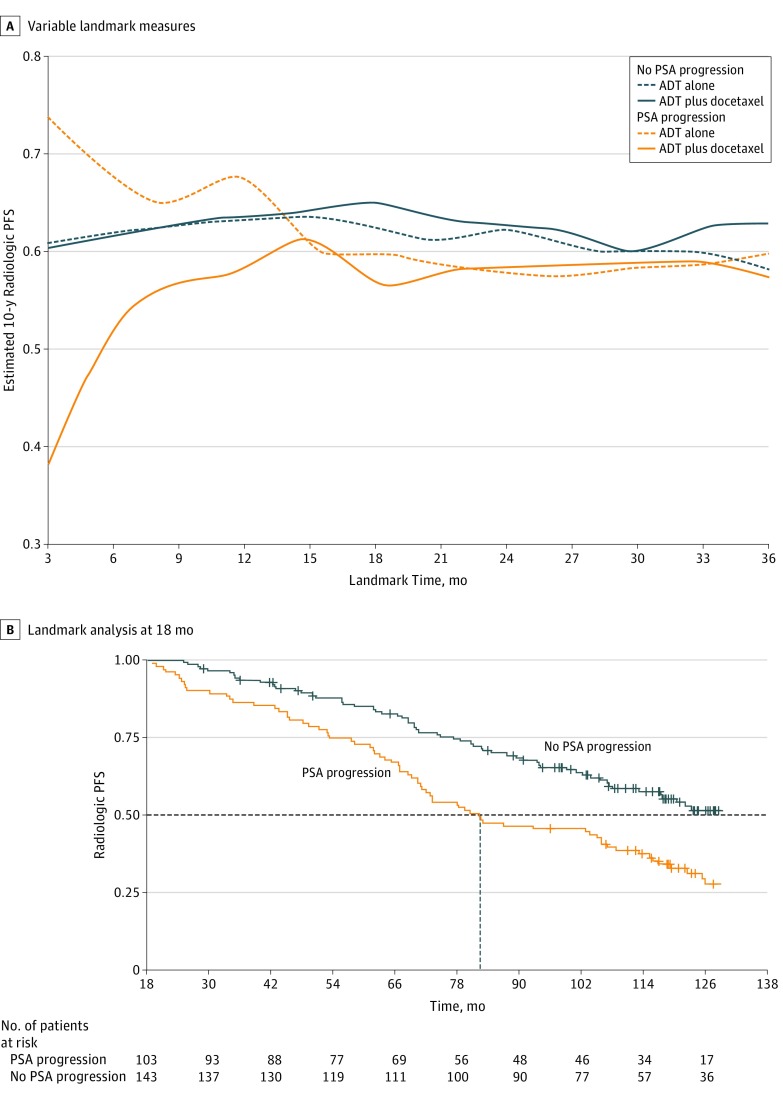
Estimated mean 10-year probability of metastatic progression in patients with no detected PSA progression at landmark times between 3 and 36 months after randomization did not differ significantly between treatment arms. However, in patients with PSA progression, the probability of metastatic progression was higher in patients with earlier PSA progression for ADT plus docetaxel, but not for ADT alone: PSA progression within 6 months after randomization was associated with a 10-year rPFS of 0.52 in the ADT plus docetaxel arm but 0.67 in the ADT-alone arm (Figure 4A). In the ADT plus docetaxel arm, estimated outcome was worse the sooner PSA had progressed. In an 18-month landmark analysis, rPFS was significantly worse in patients with than without PSA progression (log-rank P < .001) (Figure 4B). The PSA-PFS at a 12-month landmark time was predictive of overall survival (log-rank P < .001).
Figure 4. Estimated 10-Year Radiologic Progression-Free Survival (PFS) According to Occurrence of Prostate-Specific Antigen (PSA) Progression.
A, At different landmark times (ADT-alone arm vs ADT plus docetaxel arm) for no PSA progression and PSA progression. B, Landmark analysis at 18 months post randomization in ADT-alone arm vs ADT plus docetaxel arm. The dashed horizontal line indicates the median value. Log rank P < .001.
Local radical prostatectomy rather than radiotherapy predicted a better PSA-PFS (HR, 0.29; 95% CI, 0.21-0.42; P < .001). A PSA-DT of 6 months or less vs more than 6 months predicted a worse rPFS (HR, 1.73; 95% CI, 1.33-2.45; P = .002). No factor reached significance in predicting overall survival.
Safety
Adverse events (eTable in Supplement 2) prompted 4 withdrawals in the ADT plus docetaxel arm and 1 in the ADT-alone arm. The most common grade 3 or 4 hematologic adverse events (>1% incidence) in patients in the ADT plus docetaxel arm who had received at least 1 docetaxel cycle and had undergone 1 tolerance assessment were neutropenia (60 of 125 patients [48.0%]), febrile neutropenia (10 [8.0%]), and thrombocytopenia (4 [3.0%]). The incidence of grade 3 or 4 nonhematologic adverse events was less than 1% for all toxic effects except hair loss (5 [4.0%]). There were no treatment-related deaths.
Discussion
This phase 3 trial comparing treatment with ADT plus docetaxel vs ADT alone in patients at high risk for increasing PSA levels after primary local treatment of nonmetastatic PC found no significant between-arm difference in the primary outcome of PSA-PFS after a median follow-up of 30.0 months. This lack of significance occurred despite a lower risk of PSA progression (approximately 15%) observed on the addition of docetaxel for most patient subgroups. Prior radical prostatectomy rather than radiotherapy predicted a better PSA-PFS possibly because patients who receive radiotherapy tend to have less-advanced disease.6 After a median follow-up of 10.5 years, there was no significant improvement in rPFS on docetaxel addition to ADT. The rPFS was significantly better in patients with longer (>6 months) than shorter PSA-DT and in patients with fewer (≤3) high-risk factors, independent of docetaxel administration, indicating that, in this patient population, risk factors overrode any treatment effect.
In the landmark analyses,28 time to PSA progression affected the estimated mean 10-year probability of metastatic progression in line with published observations.29,30 Overall survival data were not mature.
Few trials have tested docetaxel addition to standard of care in a nonmetastatic setting. To our knowledge, only the phase 3 TAX 3503 study recruited a patient population similar to ours (rising PSA levels and short PSA-DTs after radical prostatectomy).31 At a median follow-up of 31.5 months, docetaxel addition yielded a statistical trend toward improved PFS (HR, 0.79; 95% CI, 1.0-1.60; P = .04) but only marginal clinical benefit. The study was terminated early.
A meta-analysis of 4 trials of docetaxel plus standard of care totaling 2348 men with locally advanced disease (GETUG-12,24 RTOG 0521,25 STAMPEDE,17 TAX 350126) concluded that docetaxel improved failure-free survival significantly (HR, 0.70; 95% CI, 0.61-0.81; P < .001) and reduced overall failure significantly (by 8%) at 4 years.18 GETUG-12 and STAMPEDE significantly favored the addition of docetaxel (HR for 8-year relapse-free survival, 0.71; 95% CI, 0.54-0.94; P = .02; and HR for failure-free survival, 0.60; 95% CI, 0.45-0.80; P = .283 × 10−3, respectively).17,24 However, in the RTOG 0521 trial, the difference in 5-year disease-free survival rate with the docetaxel addition barely reached significance (HR, 0.76; 95% CI, 0.57-1.00; P = .05).25 TAX 3501 was terminated prematurely because it was underpowered.2 All of these trials and ours differ in several aspects: definition of high-risk disease, timing of therapy (rising PSA levels after local therapy vs adjuvant setting), type of prior therapy (radical prostatectomy or radiotherapy), stratification factors, chosen end point, docetaxel dose (70 vs 75 mg/m2),24 number of docetaxel cycles (from 4 to 10),24,31 and concomitant treatments (prednisone, prednisolone, prednisone plus estramustine17,24,25). Both prednisone and estramustine can initiate or enhance clinically relevant PSA responses.32
In our trial, as in the STAMPEDE,17 CHAARTED,15 and GETUG-1514 trials, ADT plus docetaxel was associated with a slightly higher incidence of febrile neutropenia than in the TAX 327 trial,33 possibly because of lower hepatic docetaxel clearance in men who do not vs those who do undergo castration.34 Systematic prophylaxis with granulocyte colony-stimulating factor is thus recommended in all hormone-naive patients from cycle 1. The ADT plus docetaxel combination had no significant effect on quality of life during the first 12 months of treatment; this finding is in line with observations in the GETUG-12 trial, which, however, administered only 4 cycles of docetaxel therapy.24
Limitations
Our study has limitations. First, the clinical relevance of PSA-PFS as an end point is disputed, with preference being given to end points such as PC-specific mortality, metastasis-free survival, and time to metastasis.35,36 However, PSA-PFS was an appropriate choice within our study setting and time frame because rPFS depends on any subsequent PSA-relapse management (eg, reintroduction of intermittent or continuous ADT, administration of new drugs) and regular PSA sampling for landmark analyses would have been more difficult to obtain as main end point. Disease progression usually occurs after at least 1 rising PSA level recurrence as evidenced by the superimposable survival curves of our study (end point PSA-PFS) and of TAX 350331 (end point PSA-PFS with rPFS). Overall survival was an ambitious secondary end point because we have to wait a very long time to have a sufficient number of events. A second limitation is that our trial could detect only a large effect. At the time of the study design, a 50% reduction in PSA-relapse risk was considered an acceptable objective, but this proved to be an overestimation.
An additional limitation could be the open-label design, but we believe that the primary end point chosen was unlikely to threaten study validity. The median number of PSA evaluations and the incidence of longer intervals between evaluations were similar in both arms. Prostate-specific antigen is a measure not thwarted by subjective assessment (although not measured in a central laboratory) and the PSA-based end point was clearly defined. Central review of imaging, however, might have lessened any underestimation of metastases.
Proposals for future studies are stratification of patients on their number of poor prognosis factors in addition to prior therapy and PSA-DT, as well as better definition of high risk (eg, using genomic markers such as circulating tumor cells and cell-free DNA).37,38
Conclusions
In this dedicated, phase 3 comparison of ADT plus docetaxel with ADT alone in patients with high-risk, nonmetastatic PC, docetaxel addition yielded a 15% benefit in time to PSA progression, but statistical significance was not reached for the primary end point of PSA-PFS. The effect on PSA progression did not translate into a benefit in terms of time to onset of metastatic disease. Overall survival data were not mature. It follows that docetaxel may not be as suitable in a high-risk setting as in a metastatic setting. Significant improvements in metastasis-free survival and a reduction in PC deaths in high-risk, nonmetastatic, castration-resistant PC have recently been reported with new potent amides blocking the androgen-receptor pathway.39
Trial Protocol
eTable 1. Adverse Events
eFigure 1. Sensitivity of PSA Progression-Free Survival Hazard Ratio
eFigure 2. Global Health Status/Quality of Life Score
eFigure 3. Radiological Progression-Free Survival
Data Sharing Statement
References
- 1.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591-1597. doi: 10.1001/jama.281.17.1591 [DOI] [PubMed] [Google Scholar]
- 2.Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170(5):1872-1876. doi: 10.1097/01.ju.0000091876.13656.2e [DOI] [PubMed] [Google Scholar]
- 3.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109(1):32-39. doi: 10.1111/j.1464-410X.2011.10422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertsen PC, Hanley JA, Penson DF, et al. Validation of increasing prostate specific antigen as a predictor of PC death after treatment of localized PC with surgery or radiation. J Urol. 2004;171(6, pt 1):2221-2225. [DOI] [PubMed] [Google Scholar]
- 5.Jhaveri FM, Klein EA. How to explore the patient with a rising PSA after radical prostatectomy: defining local versus systemic failure. Semin Urol Oncol. 1999;17(3):130-134. [PubMed] [Google Scholar]
- 6.Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D’Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23(28):6992-6998. doi: 10.1200/JCO.2005.01.2906 [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25(13):1765-1771. doi: 10.1200/JCO.2006.08.0572 [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Eisenberger M, D’Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22(3):537-556. doi: 10.1200/JCO.2004.07.099 [DOI] [PubMed] [Google Scholar]
- 9.van den Bergh RC, van Casteren NJ, van den Broeck T, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence after local curative treatment: a systematic review. Eur Urol. 2016;69(5):802-820. doi: 10.1016/j.eururo.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 10.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171(3):1141-1147. doi: 10.1097/01.ju.0000113794.34810.d0 [DOI] [PubMed] [Google Scholar]
- 11.Shipley WU, Desilvio M, Pilepich MV, et al. Early initiation of salvage hormone therapy influences survival in patients who failed initial radiation for locally advanced prostate cancer: a secondary analysis of RTOG protocol 86-10. Int J Radiat Oncol Biol Phys. 2006;64(4):1162-1167. doi: 10.1016/j.ijrobp.2005.09.039 [DOI] [PubMed] [Google Scholar]
- 12.Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17(6):727-737. doi: 10.1016/S1470-2045(16)00107-8 [DOI] [PubMed] [Google Scholar]
- 13.Graff JN, Alumkal JJ, Beer TM. Should chemotherapy be used in nonmetastatic PC? JAMA Oncol. 2017;3(1):11-12. doi: 10.1001/jamaoncol.2016.3623 [DOI] [PubMed] [Google Scholar]
- 14.Yu EY, Lin DW. Avoiding undertreatment of aggressive prostate cancer by early use of chemotherapy. JAMA Oncol. 2017;3(1):13-14. doi: 10.1001/jamaoncol.2016.3634 [DOI] [PubMed] [Google Scholar]
- 15.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158. doi: 10.1016/S1470-2045(12)70560-0 [DOI] [PubMed] [Google Scholar]
- 16.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive PC. N Engl J Med. 2015;373(8):737-746. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vale CL, Burdett S, Rydzewska LHM, et al. ; STOpCaP Steering Group . Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243-256. doi: 10.1016/S1470-2045(15)00489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botrel TE, Clark O, Lima Pompeo AC, et al. Efficacy and safety of combined androgen deprivation therapy (ADT) and docetaxel compared with ADT alone for metastatic hormone-naive PC: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0157660. doi: 10.1371/journal.pone.0157660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain A, Dawson N, Amin P, et al. Docetaxel followed by hormone therapy in men experiencing prostate-specific antigen after primary local treatments for PC. J Clin Oncol. 2005;23(12):2789-2796. doi: 10.1200/JCO.2005.07.152 [DOI] [PubMed] [Google Scholar]
- 21.Goodin S, Medina P, Capanna T, et al. Effect of docetaxel in patients with hormone-dependent prostate-specific antigen progression after local therapy for prostate cancer. J Clin Oncol. 2005;23(15):3352-3357. doi: 10.1200/JCO.2005.11.111 [DOI] [PubMed] [Google Scholar]
- 22.Taplin ME, Xie W, Bubley GJ, et al. Docetaxel, estramustine, and 15-month androgen deprivation for men with prostate-specific antigen progression after definitive local therapy for prostate cancer. J Clin Oncol. 2006;24(34):5408-5413. doi: 10.1200/JCO.2006.06.6589 [DOI] [PubMed] [Google Scholar]
- 23.Hainsworth JD, Meluch AA, Spigel DR, Yost K, Meng C, Greco FA. Weekly docetaxel/estramustine phosphate in patients with increasing serum prostate-specific antigen levels after primary treatment for prostate cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Clin Genitourin Cancer. 2006;4(4):287-292. doi: 10.3816/CGC.2006.n.009 [DOI] [PubMed] [Google Scholar]
- 24.Fizazi K, Faivre L, Lesaunier F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16(7):787-794. doi: 10.1016/S1470-2045(15)00011-X [DOI] [PubMed] [Google Scholar]
- 25.Sandler HM, Hu C, Rosenthal SA, et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk PC (RTOG 0521) [abstract]. J Clin Oncol. 2015;33(suppl):LBA5002. [Google Scholar]
- 26.Schweizer MT, Huang P, Kattan MW, et al. Adjuvant leuprolide with or without docetaxel in patients with high-risk prostate cancer after radical prostatectomy (TAX-3501): important lessons for future trials. Cancer. 2013;119(20):3610-3618. doi: 10.1002/cncr.28270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 28.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710-719. doi: 10.1200/JCO.1983.1.11.710 [DOI] [PubMed] [Google Scholar]
- 29.Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2011;108(3):378-385. doi: 10.1111/j.1464-410X.2010.09878.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harshman LC, Chen YH, Liu G, et al. ; ECOG-ACRIN 3805 Investigators . Seven-month prostate-specific antigen is prognostic in metastatic hormone-sensitive PC treated with androgen deprivation with or without docetaxel. J Clin Oncol. 2018;36(4):376-382. doi: 10.1200/JCO.2017.75.3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris MJ, Hilden P, Gleave M, et al. Efficacy analysis of a phase III study of androgen deprivation therapy (ADT) +/− docetaxel (D) for men with biochemical relapse (BCR) after prostatectomy [abstract]. J Clin Oncol. 2015;33(15)(suppl):5011. [Google Scholar]
- 32.Eymard JC, Priou F, Zannetti A, et al. Randomized phase II study of docetaxel plus estramustine and single-agent docetaxel in patients with metastatic hormone-refractory prostate cancer. Ann Oncol. 2007;18(6):1064-1070. doi: 10.1093/annonc/mdm083 [DOI] [PubMed] [Google Scholar]
- 33.Tannock IF, de Wit R, Berry WR, et al. ; TAX 327 Investigators . Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512. [DOI] [PubMed] [Google Scholar]
- 34.Franke RM, Carducci MA, Rudek MA, Baker SD, Sparreboom A. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol. 2010;28(30):4562-4567. doi: 10.1200/JCO.2010.30.7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney C, Nakabayashi M, Regan M, et al. ; ICECaP Working Group . The development of intermediate clinical endpoints in cancer of the prostate (ICECaP). J Natl Cancer Inst. 2015;107(12):djv261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie W, Regan MM, Buyse M, et al. ; ICECaP Working Group . Metastasis-free survival is a strong surrogate of overall survival in localized PC. J Clin Oncol. 2017;35(27):3097-3104. doi: 10.1200/JCO.2017.73.9987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilson C, Chowdhury S, Parmar MKB, Sydes MR; STAMPEDE Investigators . Incorporating biomarker stratification into STAMPEDE: an adaptive multi-arm, multi-stage trial platform. Clin Oncol (R Coll Radiol). 2017;29(12):778-786. doi: 10.1016/j.clon.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terada N, Akamatsu S, Kobayashi T, Inoue T, Ogawa O, Antonarakis ES. Prognostic and predictive biomarkers in prostate cancer: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(8):565-573. doi: 10.1177/1758834017719215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford ED, Schellhammer PF, McLeod DG, et al. Androgen receptor–targeted treatments for prostate cancer: 35 years’ progress with antiandrogens. J Urol. 2018;200(5):956-966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Adverse Events
eFigure 1. Sensitivity of PSA Progression-Free Survival Hazard Ratio
eFigure 2. Global Health Status/Quality of Life Score
eFigure 3. Radiological Progression-Free Survival
Data Sharing Statement