Abstract
Background:
In 2014, an outbreak of Enterovirus D68 (EV-D68) was recorded as the largest in the United States with cases confirmed in 49 states. Intravenous immune globulin (IVIG) has been used to treat enterovirus infections in neonates and is an accepted replacement therapy for immunodeficient patients.
Objectives:
This study aimed to detect the presence of neutralizing antibodies to EV-D68 viruses from the 2014 outbreak in commercially available IVIG products.
Study Design:
Commercially available lots of IVIG preparations were obtained from five different manufacturers (2–10 preparations per manufacturer) and tested for neutralizing antibodies against the prototype EV-D68 virus and three EV-D68 isolates representing strains circulating during the 2014 outbreak.
Results:
All lots of IVIG tested were positive for EV-D68 neutralizing antibodies, with high titers ranging from 9.5 log2 to 17.5 log2, and with comparable median titers to all four EV-D68 viruses.
Conclusions:
Amino acid sequence differences in the regions of the predicted antigenic sites on the viral capsid may explain some of the differences in neutralization among the different strains. The neutralization titers suggests that the 2014 outbreak EV-D68 viruses share some antigenic sites with the prototype virus and also present some unique antigenic sites distinct from the prototype. However, the commercial IVIG lots tested all contained high levels of neutralizing antibodies against EV-D68.
Keywords: Enterovirus D68, intravenous immunoglobulin, antibody titers
1. Background
Enterovirus D68 (EV-D68) belongs to the Enterovirus genus (Picornaviridae family) of non-enveloped, positive sense, single-stranded RNA viruses that commonly infect humans. Initially isolated from children with pneumonia and bronchiolitis in 1962, EV-D68 has been the cause of isolated clusters of acute respiratory disease in children in the United States from 1989 to 2010[1, 2]. The recent 2014 outbreak represents the largest and most widespread EV-D68 outbreak to date with 1,153 laboratory-confirmed cases since August [3]. Intravenous immune globulin (IVIG) is commonly used in passive immunization or replacement immunotherapies in enterovirus infections in neonates and immunocompromised patients, respectively [4–6]. The neutralizing capacity of commercial IVIG products against EV-D68 is unknown.
2. Objective
To detect the presence of neutralizing antibodies to EV-D68 viruses from the 2014 outbreak in commercially available IVIG products.
3. Study Design
3.1. Intravenous Immune globulin
Multiple lots of IVIG products were obtained from five manufacturers/suppliers in the US and Europe (designated Manufacturers A-E). All lots tested for EV-D68 neutralizing antibodies were 10% protein preparations. Products were stored at 4°C until tested, as recommended by the manufacturers.
3.2. EV-D68 strains
Four EV-D68 strains were selected for microneutralization assays: Fermon (Genbank AY426531.1), 14–18949 (KM851227.1), 14–18952 (KM851230.1), and 14–18953 (KM851231.1). Each virus was grown on confluent human rhabdomyosarcoma (RD; ATCC#: CCL-136) cell monolayers in 150 cm2 flasks (Corning; Corning, NY) at 33°C, 5% CO2 until viral cytopathic effect (CPE) was observed. To harvest virus, flasks were frozen and thawed three times and the supernatant was collected and clarified by centrifugation (30 minutes, 15,000 x g). Virus titer was determined by limiting dilution assay. Each dilution was added to 20 wells in a 96-well plate and combined with 7,500 RD cells/well. After incubation for 6 days at 33°C and 5% CO2, the number of wells CPE-positive was counted for each dilution series using an inverted light microscope. The titer (50% cell culture infectious dose; CCID50) for each virus was calculated using the Spearman-Kärber formula [7].
3.3. EV-D68 microneutralization assay
Briefly, IVIG products were heat-inactivated for 30 minutes at 56°C then diluted 1:4 in minimum essential medium (MEM) (Life Technologies; Grand Island, NY) containing 2% fetal bovine serum (FBS) (Atlanta Biologicals; Flowery Branch, GA). After cooling to room temperature, diluted products were transferred in triplicate to the first row of one 96-well plate for each EV-D68 strain, and then diluted 2-fold, from 1:8 to 1:262,144. For each strain, 80–100 CCID50 were combined with the diluted sera in a 96-well, white, opaque-bottom plate and incubated at 33°C for 3 hours before addition of 7,500 RD cells/well. After incubation for 6 days at 33°C and 5% CO2, neutralization was assessed by using a cell viability luminescence kit according to the manufacturer’s instructions (ATPlite; PerkinElmer; Waltham, MA). Luminescence for each assay plate was read on a Victor X4 Multimode Reader (PerkinElmer). Neutralization titers were estimated by the Spearman-Kärber method [7] and expressed in log2 form (e.g., 5 is a titer of 1:32) [7]. Each run contained a reference positive and negative antiserum, horse anti-EV-D68 (Fermon) and rabbit anti-EV-D70 (J670/71), respectively, in addition to a cell control plate and back titration plate for each EV-D68 virus.
3.4. Statistical Analysis
One-way ANOVA with Dunn’s Multiple Comparison test was used for comparisons of neutralization titers between strains and IVIG products (GraphPad Prism 5, GraphPad Software; La Jolla, CA).
3.5. Protein sequence alignment of EV-D68 capsids
Protein alignments of full capsids were performed using MegAlign (v12.1, DNASTAR, Inc., Madison, WI). Putative antigenic sites were identified based on known rhinovirus 14 (RV-14) sites [8].
4. Results
Neutralization titers for the control reference antiserum indicate that the assay is specific to EV-D68 viruses, with undetectable neutralization titers for the EV-D70 antiserum, another species D enterovirus related to EV-D68 (Figure 1). The neutralizing titer for the control EV-D68 antiserum was higher against the homologous antigen, Fermon (17.5 log2), compared to titers against the 2014 EV-D68 viruses, 14–18949 (13.5 log2), 14–18952 (13.83 log2) and 14–18953 (9.83 log2). Only the titer difference between Fermon and 14–18953 was statistically significant (p < 0.05).
Figure 1.

Enterovirus D68 (EV-D68) antisera recognizes homologous EV-D68 virus and 2014 outbreak strains. Antisera raised against the Fermon strain and EV-D70 were used to determine the specificity of the serology assay. Median titers are shown for three replicate assay runs. ULD = upper limit of detection, LLD = lower limit of detection; * p > 0.05 for difference in median titers, with Fermon as the reference.
To detect and quantify EV-D68 antibody in commercial IVIG preparations, we measured neutralizing antibodies to Fermon and the three representative strains from the 2014 outbreak (Figure 2). The small number of preparations from manufacturers C, D, and E limited the statistical analysis of differences between these sources of IVIG. We observed high titers of EV-D68 neutralizing antibodies in all IVIG lots against Fermon (median = 13.83 log2), 14–18949 (12.17 log2), 14–18952 (14.17 log2), and 14–18953 (13.17 log2). Median titers for each EV-D68 strain were consistent amongst all IVIG manufacturers (Figure 2). Neutralization titers differences were statistically significant for 14–18949 between manufacturers A and C, for 14–18952 between manufacturers A and B and A, and C, and for 14–18953 between manufacturers C and D); however, these differences are likely not clinically significant [5].
Figure 2.
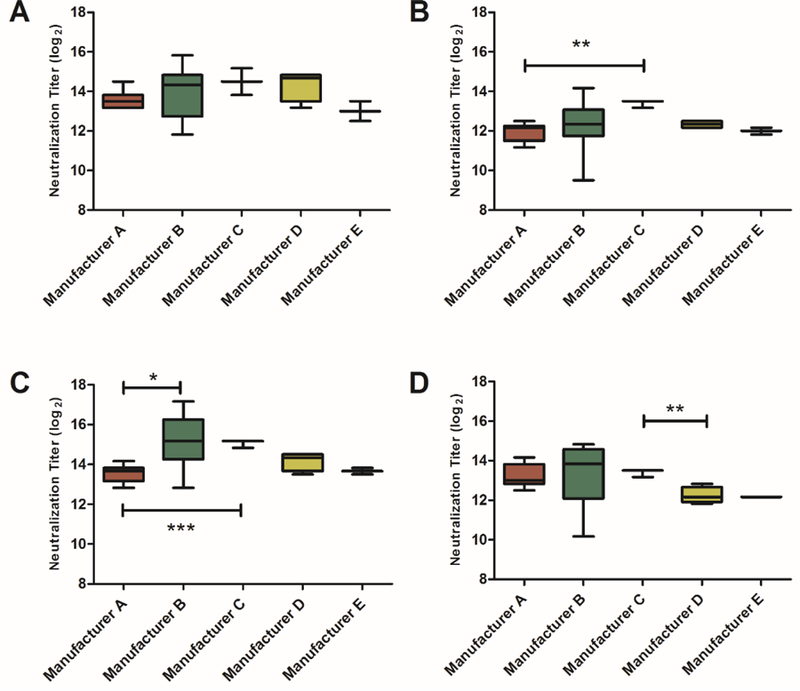
Commercial IVIG lots neutralize EV-D68 strains with equivalent efficiencies. IVIG products were tested for neutralizing antibodies against the EV-D68 strains Fermon (A) and three clinical isolates 14–18949 (B), 14–18952 (C), and 14–18953 (D). For each manufacturer, median neutralization titers are indicated in box plots with horizontal line with 5–95% confidence intervals. (A, n=10; B, n=10; C, n=3; D, n=4, E, n=2). Data represents results from a single assay run. *p < 0.05, ** p < 0.01, *** p < 0.001 for difference in median titers.
Putative neutralizing antibody epitopes for EV-D68-Fermon have been proposed based on structural homology with RV-14 [8]. Putative antigenic sites for each EV-D68 strain were mapped by multiple sequence alignments of the capsid proteins (Figure 3). Three residues in the RV-14 antigenic site Nim-II did not correspond to any residues in EV-D68 according to structural alignments of the capsids [8]. In general, the residue identities of for each antigenic site were highly conserved between the 2014 clinical isolates and Fermon. However, there is sequence diversity in the residues flanking the putative antigenic sites, suggesting that other residues are likely involved in antibody binding and neutralization.
Figure 3.
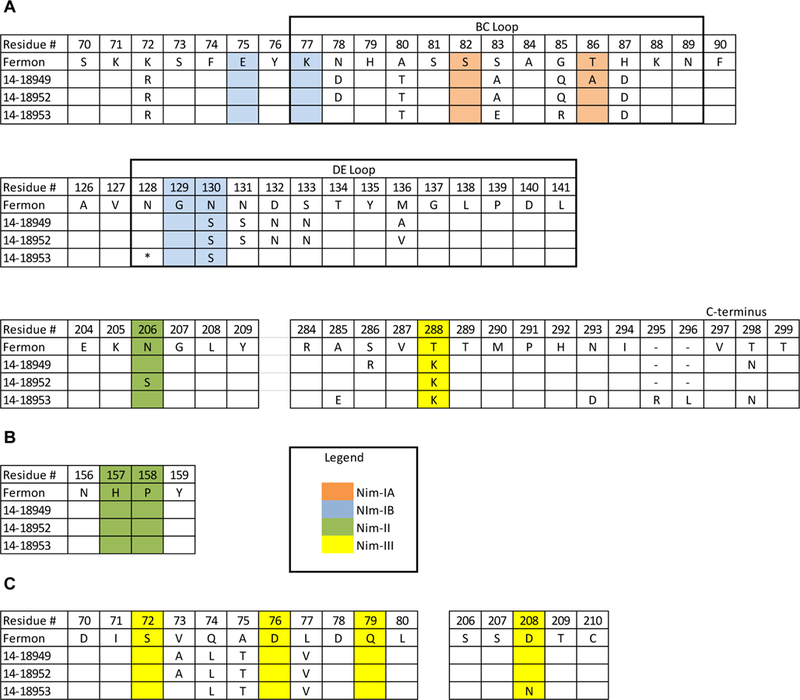
Protein sequence alignment of putative antigenic sites for EV-D68 viruses used in microneutralization assay. Alignments of EV-D68 capsid proteins VP1 (A), VP2 (B), and VP3 (C); only amino acid residues that differ from Fermon virus are shown. Residue number for each amino acid chain is based on the predicted cleavage sites from Liu et al [8]. Putative antigenic sites based on RV-14 are shaded as follows: NIm-IA (orange), Nim-IB (blue), Nim-II (green), and Nim-III (yellow).
* denotes an amino acid deletion at residue 140 in 14–18953.
- denotes a gap in the sequence alignment due to a two-amino-acid insertion in 14–18953.
5. Discussion
From mid-August through December of 2014, there were over 1,100 cases of respiratory illness confirmed as EV-D68-positive, in 49 states and the District of Columbia, making the 2014 outbreak the largest and most widespread to date [3]. IVIG is an important therapy for treatment of patients with primary antibody deficiencies and for passive immunization of neonates at increased risk of enterovirus infections. IVIG has also been used as a therapeutic in respiratory infections such as influenza and respiratory syncytial virus [9–11]. The effectiveness of IVIG treatments for enterovirus infections depends on the presence and level of anti-EV neutralizing antibodies specific for the infecting serotype [5, 12, 13].
Differences in neutralization titers for the control antiserum amongst the EV-D68 viruses suggest subtle antigenic variation between the 2014 outbreak viruses and the prototype virus. The putative antigenic sites for the four EV-D68 viruses, predicted on the Fermon crystal structure and known RV-14 antigenic sites [8, 14], demonstrated a high degree of conservation. However, diversity in the regions flanking the predicted sites suggest that other residues likely contribute to the neutralizing epitopes of EV-D68. Variability in these regions is consistent with differences in neutralization between the Fermon and 2014 strains. Additional work is required to confirm the identity of the antigenic sites and to characterize other antigenic determinants of the EV-D68 virus capsids.
Seroprevalence to EV-D68 in the general population is currently unknown. Testing of sera from Finnish women at the end of the first trimester of pregnancy, collected in 1983, 1993 and 2002, indicated 100% seropositivity to the Fermon virus in this population[15]. This is consistent with our observation that all lots of commercial IVIG tested contained high titers of neutralizing antibodies against the prototype EV-D68 and three 2014 outbreak viruses. Variations between lots and manufacturers of IVIG could be attributed to the number and the geographic location of donors used for production [16–19]. Data on the effectiveness of IVIG treatment of enterovirus infections [5] suggests that the lots of IVIG tested in this study have sufficiently high neutralization titers to be an effective prophylactic treatment in patients with primary antibody deficiencies.
Acknowledgements
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of CDC and other contributing agencies.
We thank the Plasma Protein Therapeutics Association for providing the five intravenous immune globulin products.
Funding
This work was supported by federal appropriations to CDC from the Emerging Infections line item.
Footnotes
Conflict of Interest
None to declare.
Competing Interests
None to declare.
Ethical approval
None. All experiments used commercially available products.
References
- [1].Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. The Journal of general virology 2004. September;85(Pt 9):2577–84. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease C, Prevention. Clusters of acute respiratory illness associated with human enterovirus 68--Asia, Europe, and United States, 2008–2010. MMWR Morbidity and mortality weekly report 2011. September 30;60(38):1301–4. [PubMed] [Google Scholar]
- [3].Centers for Disease C, Prevention http://www.cdc.gov/non-polio-enterovirus/outbreaks/EV-D68-outbreaks.html. 2014 3/9/2015.
- [4].Arora R, Kaplan M, Nelson M. Enterovirus-specific IgG in intravenous immunoglobulin preparations. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2011. June;106(6):544–5. [DOI] [PubMed] [Google Scholar]
- [5].Abzug MJ, Keyserling HL, Lee ML, Levin MJ, Rotbart HA. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 1995. May;20(5):1201–6. [DOI] [PubMed] [Google Scholar]
- [6].Pasic S, Jankovic B, Abinun M, Kanjuh B. Intravenous immunoglobulin prophylaxis in an echovirus 6 and echovirus 4 nursery outbreak. The Pediatric infectious disease journal 1997. July;16(7):718–20. [DOI] [PubMed] [Google Scholar]
- [7].Kärber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Archiv für Experimentalische Pathologie und Pharmakologie 1931;162:480–3. [Google Scholar]
- [8].Liu Y, Sheng J, Fokine A, Meng G, Shin WH, Long F, et al. Virus structure. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015. January 2;347(6217):71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jegaskanda S, Vandenberg K, Laurie KL, Loh L, Kramski M, Winnall WR, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. The Journal of infectious diseases 2014. December 1;210(11):1811–22. [DOI] [PubMed] [Google Scholar]
- [10].Hung IF, To KK, Lee CK, Lee KL, Yan WW, Chan K, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013. August;144(2):464–73. [DOI] [PubMed] [Google Scholar]
- [11].Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics 1997. January;99(1):93–9. [DOI] [PubMed] [Google Scholar]
- [12].Planitzer CB, Farcet MR, Schiff RI, Ochs HD, Kreil TR. Neutralization of different echovirus serotypes by individual lots of intravenous immunoglobulin. Journal of medical virology 2011. February;83(2):305–10. [DOI] [PubMed] [Google Scholar]
- [13].Wu CY, Wang HC, Wang KT, Weng SC, Chang WH, Shih DY, et al. Neutralization of five subgenotypes of Enterovirus 71 by Taiwanese human plasma and Taiwanese plasma derived intravenous immunoglobulin. Biologicals : journal of the International Association of Biological Standardization 2013. May;41(3):154–7. [DOI] [PubMed] [Google Scholar]
- [14].Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. Journal of virology 1986. January;57(1):246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smura T, Ylipaasto P, Klemola P, Kaijalainen S, Kyllonen L, Sordi V, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. Journal of medical virology 2010. November;82(11):1940–9. [DOI] [PubMed] [Google Scholar]
- [16].Simon HU, Spath PJ. IVIG--mechanisms of action. Allergy 2003. July;58(7):543–52. [DOI] [PubMed] [Google Scholar]
- [17].Norrby-Teglund A, Basma H, Andersson J, McGeer A, Low DE, Kotb M. Varying titers of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G: implications for therapeutic efficacy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 1998. March;26(3):631–8. [DOI] [PubMed] [Google Scholar]
- [18].Lamari F, Anastassiou ED, Tsegenidis T, Dimitracopoulos G, Karamanos NK. An enzyme immunoassay to determine the levels of specific antibodies toward bacterial surface antigens in human immunoglobulin preparations and blood serum. Journal of pharmaceutical and biomedical analysis 1999. September;20(6):913–20. [DOI] [PubMed] [Google Scholar]
- [19].Matejtschuk P, Chidwick K, Prince A, More JE, Goldblatt D. A direct comparison of the antigen-specific antibody profiles of intravenous immunoglobulins derived from US and UK donor plasma. Vox sanguinis 2002. July;83(1):17–22. [DOI] [PubMed] [Google Scholar]


