Abstract
The results of the RV144 vaccine clinical trial showed a correlation between high level of anti-V1V2 antibodies (Abs) and a decreased risk of acquiring HIV-1 infection. This turned the focus of HIV vaccine design to the induction of elevated levels of anti-V2 Abs to increase vaccine efficacy. In plasma samples from HIV-1 infected Cameroonian individuals, we observed broad variations in levels of anti-V2 Abs, and 6 of the 79 plasma samples tested longitudinally displayed substantial deficiency of V2 Abs. Sequence analysis of the V2 region from plasma viruses and multivariate analyses of V2 characteristics showed a significant difference in several features between V2-deficient and V2-reactive plasma Abs. These results suggest that HIV vaccine immunogens containing a shorter V2 region with fewer glycosylation sites and higher electrostatic charges can be beneficial for induction of a higher level of anti-V2 Abs and thus contribute to HIV vaccine efficacy.
Keywords: HIV-1, V1V2 region, V2 conformational antibodies, V2 linear antibodies, V2 antibody deficiency
1. Introduction
Anti-V2 antibodies (Abs) have been the subject of studies to determine their role in protection against HIV-1 infection due to the results of the Phase III RV144 vaccine trial, which showed an inverse correlation of the levels of Abs against V1V2 proteins with the risk of infection (Haynes et al., 2012; Zolla-Pazner et al., 2014).
Anti-V2 mAbs mediate several inhibitory functions including neutralization of Tier 1 viruses, phagocytosis and antibody-dependent cellular cytotoxicity (ADCC) (Corti et al., 2010; Gorny et al., 1994; Gorny et al., 2012; Liao et al., 2013; Mayr et al., 2017; Musich et al., 2017). These functions are comparable with activities of Abs specific to V3 and CD4bs, which are commonly present in HIV-1 infected individuals (Li et al., 2015; Musich et al., 2017). However, anti-V2 mAbs mediate one unique function, the inhibition of gp 120/α4β7 integrin interaction, which possibly may block the virus adhesion and consequently infection of Thl7 cells (Lertjuthaporn et al., 2018; Nakamura et al., 2012). If this function plays a role in vivo, it needs to be tested in a protection experiment. It is still possible; however, that several inhibitory activities mediated by anti-V2 Abs can work in harmony and contribute to protection against HIV-1 acquisition.
The V2 region contains at least 3 types of epitopes defined by anti-V2 monoclonal Abs (mAbs): i) V2 integrin (V2i), which is a conformation-dependent motif and includes the α4β7 integrin binding site; ii) the V2 peptide (V2p) linear epitope; and iii) the V2 quaternary (V2q) epitope, present preferentially on the trimer (Spurrier et al., 2014). The majority of anti-V2 Abs are specific for conformation-dependent V2i epitopes; a smaller amount to the linear V2p epitopes and some are specific for quaternary V2q epitopes and only present in a small proportion of HIV-infected individuals.
The frequency of anti-V2 Abs in the plasma of individuals infected with HIV-1 varies among studies, depending on the methods used and the population studied. Screening of immune plasma using V2 consensus peptides with the HxB2 sequence detected 12% and 21% positive sera from HIV-1-infected individuals (McKeating et al., 1993; Moore et al., 1993). Using V1V2 proteins expressing both conformation-dependent V2i and linear V2p epitopes, the frequency of plasma anti-V2 Abs is higher, yielding 30% and 48% in two studies (Kayman et al., 1994; McKeating et al., 1996). The prevalence of anti-V2 Abs in clade B-infected individuals was determined to be 45% when tested against a C1-V1V2 BH10 protein and 30% when screened in a competition assay with the 697 mAh specific for the conformational V2i epitope (Israel et al., 1997). The frequency of anti-V2 Abs induced by the RV144 vaccine was relatively high, given that 84% of vaccinees’ sera was reactive with the V1 V2-gp70 fusion protein and comparably with the cyclic V2 peptide (Haynes et al., 2012; Zolla-Pazner et al., 2013).
Given that only the high level of anti-V2 Abs is relevant for protection (Zolla-Pazner et al., 2014) we studied a panel of plasma samples to determine the frequency of anti-V2 Abs against different V1V2 fusion proteins and cyclic V2 peptides. Lack of anti-V2 Abs in some plasma samples was in part dependent on a weak general Ab response to HIV envelope (Env) antigens, manifested by significantly lower titers of Abs against V3, gp120, and gp41 proteins compared with plasma with reactive anti-V2 Abs. In addition, differences in the V2 region, including extended length, more glycosylation sites, lower isoelectric point, and charge may also contribute to lower immunogenicity and deficiency of anti-V2 Abs in some individuals infected with HIV-1.
2. Material and methods
2.1. Ethics Statement.
Blood samples from men and women infected with HIV-1 were received from the Medical Diagnostic Center (MDC), Yaoundé, Cameroon. Written informed consent forms were signed by all participants in the study and approved by the National Ethical Review Board in Cameroon. The study has been reviewed and approved by the Institutional Review Board of New York University School of Medicine, New York, USA.
2.2. Specimens.
The panel of 79 plasma samples from HIV infected Cameroonian individuals was randomly selected and received from the collaborative cohort shared between New York University School of Medicine, New York, NY, and the Medical Diagnostic Center (MDC), Yaoundé, Cameroon. Given that plasma samples were collected approximately 6 to 18 months after diagnosis of HIV infection, all subjects were classified as chronically infected. All donors were anti-retroviral treatment (ART) naïve according to the study questionnaire, and plasma samples were collected in 2011 and 2012 at the MDC.
Twelve plasma samples collected longitudinally over a period of 8 to 60 months at the MDC were selected for further studies including 6 samples with anti-V2 deficient and 6 with anti-V2 cross-reactive Abs. The functional activity of anti-V2 Abs was not measured in these donors. One plasma sample from each of 12 subjects was used for sequencing the envelope of plasma viruses (Table 1 and Table S2).
Table 1.
Infecting HIV clades and ELISA reactivity of plasma antibodies to Env antigens of patients with and without V2 antibodies
| MDC donors | HIV-1 | V1V21 | V1V21 | V1V21 | V2pept2 | V2pept2 | V2pept2 | V2pept2,3 | V2pept2,3 | V3pept2 | gp120 | gp41 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A244 | CaseA2 | ZM109 | A244 | TH023 | Du422 | 230 | 200 | A244 | A244 | MN | ||
| AE | B | C | AE | AE | C | AG | AG | AE | AE | B | ||
| 230-1 | CRF02_AG | 3.6 | 3.5 | 2.4 | 3.6 | 2.9 | 3.5 | 3.5 | 0.8 | 3.5 | 3.2 | 3.4 |
| 6501-6 | CRF02_AG | 3.5 | 2.2 | 3.6 | 3.5 | 2.2 | 3.0 | 2.1 | 1.3 | 3.1 | 3.5 | 2.9 |
| 221-2 | CRF01_AE | 2.1 | 2.3 | 2.2 | 3.3 | 1.9 | 3.0 | 3.0 | 0.4 | 3.7 | 3.8 | 3.3 |
| 237-2 | CRF11_cpx | 2.4 | 3.3 | 2.2 | 3.6 | 1.9 | 1.4 | 0.7 | 1.6 | 3.6 | 4.0 | 3.2 |
| 001-4 | CRF18_cpx | 3.2 | 3.1 | 2.0 | 3.5 | 3.5 | 3.5 | 3.5 | 0.3 | 3.6 | 3.9 | 2.9 |
| 060-2 | CRF36_cpx | 2.6 | 3.3 | 2.5 | 2.6 | 1.1 | 1.7 | 2.3 | 0.8 | 3.6 | 3.3 | 3.5 |
| 200-2 | CRF02_AG | 0.1 | 0.2 | 0.3 | 0.3 | 0.3 | 0.5 | 0.3 | 1.4 | 2.9 | 3.2 | 3.4 |
| 122-3 | CRF01_AE | 0.5 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 | 3.6 | 3.9 | 3.5 |
| 019-3 | CRF22_01A1 | 0.2 | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 0.3 | 0.1 | 3.8 | 1.8 | 3.0 |
| 211-1 | CRF22_01A1 | 0.3 | 0.4 | 0.4 | 0.3 | 0.2 | 0.2 | 0.3 | 0.1 | 3.0 | 3.0 | 3.3 |
| 195-3 | CRF37_cpx | 0.4 | 0.2 | 0.2 | 1.0 | 0.2 | 0.5 | 0.1 | 0.1 | 3.8 | 3.4 | 3.2 |
| 014-2 | C | 0.4 | 0.5 | 0.5 | 0.4 | 0.7 | 0.3 | 0.4 | 0.2 | 1.5 | 1.7 | 2.6 |
V1V2 – V1V2 fusion protein, HIV-1 strain and subtype;
V2 and V3pept. – Biotinylated cyclic peptides;
V2 peptides with sequences from virus of donor’s plasma: MDC230-1 and MDC200-2 (this allows testing of two plasma Abs vs. autologous antigens).
Plasma samples were screened by standard ELISA at 1:100 dilution against antigens coated at 1 μg/mL; biotinylated cyclic V2 and V3 peptides were immobilized at 1 μg/mL on the streptavidin coated plates. The values bold are above cutoff (mean OD of 6 control healthy plasma samples + 3 standard deviation).
2.3. Recombinant proteins and peptides.
Seven recombinant proteins—V1V2A244-gp70 (clade AE), V1V2casc A2-gp70 (clade B), V1ΔV2A244-gp70 (V2 deleted, Table S1), V1ΔV2case A2-gp70 (V2 deleted, Table S1), gp120A244, gp41MN (clade B), and p24HXB2 (clade B) —were purchased from Immune Technology Corp., New York, NY. Biotinylated cyclic peptides —V2A244, V292TH023 (CRF01_AE), V2Du422 (clade C), V2230 (CRF02_AG, plasma virus from donor MDC230, Table S2) and V3A244 —were purchased from Biopeptide Co., Inc., San Diego, CA. One biotinylated cyclic peptide, V2200 (CRF02_AG), representing the V2 sequence of plasma viruses from donor MDC200 (Table S2), was purchased from Science Exchange, Palo Alto, CA. V1V2ZM109-1FD6 (clade C) was produced as previously described (Jiang et al., 2016).
2.4. Antibody binding assay (ELISA).
The plasma samples were screened by standard ELISA against recombinant V1V2 fusion proteins, gp120, gp41 and p24 and against biotinylated V2 and V3 peptides according to the methods in (Hessell et al., 2016; Musich et al., 2017). In short, proteins were coated directly onto Immulon 2HB plastic plates at a concentration of 1 μg/mL. After overnight incubation at 4°C, the plates were washed thrice and blocked with PBS containing 2.5% bovine serum albumin and 7.5% fetal bovine serum. Plasma at dilution 1:100 was incubated with antigens, washed and incubated with alkaline phosphatase-conjugated goat anti-human IgG (γ specific) (Southern Biotech, Birmingham, AL) followed by washing and incubation with substrate to develop color, and read at 405 nm. IgG subclasses and IgA of plasma Abs against V1V2 fusion protein were detected using mouse mAbs against IgG subclasses and goat anti-human IgA (Southern Biotech). For screenings of plasma samples against biotinylated peptides, the peptides were immobilized at a concentration of 1 μg/mL onto streptavidin-coated plates (StreptaWell plates, Roche). A positive reaction by a plasma sample at 1:100 dilution was defined as an OD of the mean + 3 standard deviations from the 6 plasma samples of healthy individuals from Cameroon.
2.5. CD4+ T cell counts.
The CD4+ T cell counts were determined using the Guava EasyCD4 Test (Guava Technologies Inc, Hayward, CA) with 2-color direct and absolute counting of number cells per μL (Pattanapanyasat et al., 2007).
2.6. Viral load.
Viral load was determined using Abbott m2000 Real Time HIV-1 assays according to the manufacturer’s instructions (Abbott Molecular, Des Plaines, IL).
2.7. Sequencing of the virus envelope.
Plasma viruses were sequenced in the envelope protein region to analyze the sequence of V1 and V2 regions as described (Courtney et al., 2017). Briefly, viral RNA was extracted from plasma sample using the QIAamp mini kit (Qiagen Inc., Valencia, CA). Reverse transcription and nested PCR were performed using the Superscript One-Step RT-PCR system with platinum Taq polymerase (Invitrogen, Carlsbad, CA) to isolate a portion of env (gp120 + fragment of gp41), HXB2 region 6225-7817 (~1600 bp). PCR products were cloned into the pCR4 TOPO cloning vector (Life Technologies, Carlsbad, CA) and transformed into One Shot TOP 10 competent E. coli. We analyzed 3 to 9 gp120 sequences per subject. Sequence analysis and alignment was performed using DNASTAR and Clustal W (di Marzo Veronese et al., 1994).
The gp120 nucleotide sequences of 12 plasma viruses from HIV-1 infected individuals with deficient and cross-reactive anti-V2 Abs are available from GenBank with the accession numbers: MH632751 - MH632762.
2.8. Statistical analysis.
The nonparametric Mann-Whitney-Wilcoxon test was used for comparing OD, titers of plasma Abs, and composite indexes based on linear combinations of individual characteristics or features (Table 2). Each individual feature or characteristic of the V2 region (e.g., isoelectric point, charge, number of glycosylation sites) has limited capacity to distinguish the V2-deficient group from the V2-reactive group. However, composite indexes based on simple difference, sum, or linear combinations of more than 1 feature (e.g., No_AA-pI in Table 2) can separate these groups. The relationship of plasma Ab binding to different antigens was determined by Spearman correlation coefficient (r) with P values and by linear regression. Statistical analysis and graphing of the data were generated using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA).
Table 2.
Characteristics of the V2 and V1 regions in HIV-infected individual who were had V2-deficient antibody responses or cross-reactive antibody response
| Features | V2 region |
P1 | V1 region |
P1 | ||
|---|---|---|---|---|---|---|
| V2 Deficient Group | V2 Cross Group | V2 Deficient Group | V2 Cross Group | |||
| Median (min, max) | Median (min, max) | |||||
| # amino acids (AA) | 43.5 (40, 58) | 39.5 (37,41) | 0.034 | 23.5 (22, 32) | 24.5 (21, 38) | 0.572 |
| # glycosylation sites | 2 (2, 7) | 2 (0, 2) | 0.115 | 3 (2, 4) | 2.5 (1, 4) | 0.452 |
| pI | 8.47 (4.79, 9.66) | 9.25 (6.52, 9.69) | 0.108 | 4.89 (3.88, 9.37 | 4.37(3.99, 9.58) | 0.699 |
| Charge | 1 (−1, 3) | 2.5 (0, 3) | 0.161 | −1 (−3, 2) | −1.5 (−4, 2) | 0.807 |
| α-helix K168-V172 | 1.78 (1.6, 1.9) | 1.57 (1.13, 2) | 0.376 | nt | nt | nt |
| β-sheet E153-I184 | −19.82 (−22, −16.3) | −20.32 (−22.97, −18.88) | 0.699 | nt | nt | nt |
| Composite Indexes* | ||||||
| 1. AA–pI | 35.37 (31.98, 49.5) | 30.89 (27.87, 31.75) | 0.005 | 18.07 (13.63, 26.4) | 18.74 (14.42, 34.0) | 1.000 |
| 2. AA–Charge | 42 (40, 56) | 37.5 (35, 39) | 0.005 | 24.5 (21, 33) | 25.5 (22, 42) | 0.748 |
| 3. AA–pI+ Glyc. | 37.37 (33.98, 56.5) | 32.39 (27.87, 33.75) | 0.005 | 20.44 (16.63, 30.4) | 21.74 (15.42, 38) | 1.000 |
| 4. AA–pI− Glyc. | 33.37 (29.98, 42.5) | 29.39 (26.31, 29.75) | 0.005 | 16.07 (10.63, 22.4) | 15.74 (13.42, 30) | 1.000 |
| 5. AA–Charge+Glyc. | 44 (42, 63) | 39 (35, 41) | 0.005 | 27 (24, 37) | 28.5 (23, 46) | 0.936 |
| 6. AA–Charge−Glyc. | 40 (38, 49) | 36 (33, 37) | 0.005 | 22.5 (18, 29) | 22.5 (20, 38) | 0.808 |
| 7. AA–pI+helix | 37.2 (33.76, 51.4) | 32.39 (29.44, 33.7) | 0.002 | nt | nt | nt |
| 8. AA–Charge+helix | 43.82 (41.6, 57.9) | 38.99 (36.57, 40.13) | 0.002 | nt | nt | nt |
P value, V2 deficient versus cross-reactive group (non-paired tests), Wilcoxon test; nt – not tested.
Composite indexes are based on difference or sum or linear combinations of more than one features; 1. # AA–pI; 2. # AA–Charge; 3. # AA–pI+ # glycosylation sites; 4. # AA–pI − # glycosylation sites; 5. # AA–Charge+# glycosylation sites; 6. # AA–Charge−# glycosylation sites; 7. # AA–pI+α-helix propensity; 8. # AA–Charge+α-helix propensity.
3. Results
3.1. Frequency of plasma Abs against V2 and control antigens.
A panel of 79 plasma samples was screened at a 1:100 dilution by ELISA against three V1V2 fusion proteins and five biotinylated cyclic V2 peptides. Given that anti-V1 Abs are sequence-specific (He et al., 2002) and do not bind to proteins/peptides with heterologous V1 sequences, plasma samples can be tested against V1V2 fusion proteins with heterologous sequence to detect specific Abs against the V2 region. To confirm that the anti-V1 Abs would not bind to heterologous V1V2 fusion proteins, we screened all plasma samples against two fusion proteins with the major parts of V2 deleted: V1ΔV2A244-gp70 and V1ΔV2CaseA2-gp70 (Table S1). None of the 79 plasma samples reacted with these two V1ΔV2 proteins, confirming that binding of plasma to heterologous V1V2 fusion proteins usually detects Abs specific for V2.
The frequency of plasma with anti-V2 Abs varied depending on the antigen sequence. The highest percentage of plasma samples reacted with V1V2case A2-gp70 (clade B) at 85%, while 80% reacted with V1V2A244-gp70 (CRF02_ AE), and 53% of plasma Abs bound to V1V2ZM109-1FD6 (clade C) fusion proteins (Fig. 1A). The frequency of anti-V2 peptide Abs was lower, ranging from 72% to 40% for plasma binding to V2A244, V292TH023 (CRF02_ AE), V2Du422 (clade C), V2230 and V2200 (clade AG) (Fig. 1A).
Fig 1. Reactivity of plasma Abs against V2 antigens and control proteins.
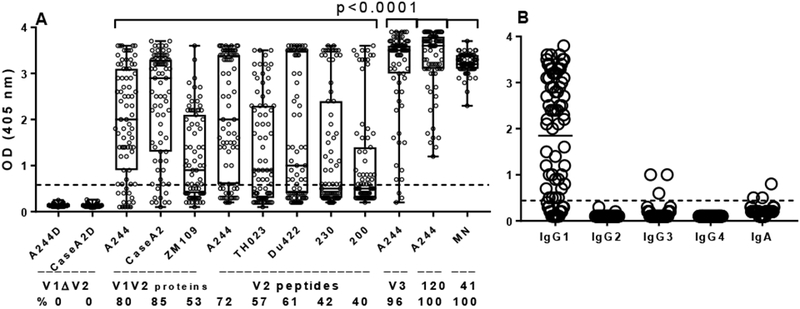
(A) All 79 plasma samples from Cameroonian HIV-1 infected individuals were tested at 1:100 dilutions by ELISA against proteins coated at 1 μg/mL, Antigens included: two V1V2-gp70 fusion proteins with V2 deleted (V1ΔV2) with sequences from A244 (CRF01_ AE) and CaseA2 (clade B); three V1V2 fusion proteins: V1V2A244-gp70, V1V2Case A2-gp70 and V1V2ZM109-1FD6 (clade C); five biotinylated cyclic V2 peptides with sequences from A244 and TH023 (CRF01_AE), Du422 (clade C), 230 and 200 (CRF02_AG); one biotinylated cyclic V3A244 peptide, gp120A244 and gp41MN. Percentage of plasma samples with specific anti-V2 Abs is shown below each antigen. Statistical significance between Abs against V2 and control antigens was determined by nonparametric Mann-Whitney test. The shape of the distribution is shown by a box with the ends of the box representing the 5th and 95th percentile, and the median marked by a horizontal line inside the box. (B) IgG subclasses and IgA of plasma Abs binding to V1V2A244-gp70 fusion protein.
All 79 plasma samples reacted by ELISA with gp120A244 and gp41MN proteins, while 76 (96%) bound to cyclic V3A244 peptide, most of the latter reaching the saturation level at OD405 3.5 to 3.9 (Fig. 1A). The IgG subclasses and IgA of plasma Abs, tested separately against V1V2A244-gp70, showed the dominance of IgG1 Abs, whereas IgG3 and IgA were only detected in 3 plasma samples each, and IgG2 and IgG4 binding was not detectable (Fig. 1B). The results showed a broad range of ODs for anti-V1V2 fusion proteins and anti-V2 peptides Abs, from close to 0 to 3.9, while the OD range for anti-gp120 was from l.2 to 3.9 and for anti-gp4l from 2.l to 3.7 OD (all positive). This indicates a weaker immunogenicity of the V2 region compared with gp120 and gp4l.
3.2. Cross-reactivity of plasma anti-V2 Abs.
The majority of 79 plasma samples at 1:100 dilutions (92%) were cross-reactive against 8 V2 antigens including 3 V1V2 fusion proteins and 5 cyclic V2 peptides representing sequences from HIV-1 subtypes AE, AG, B and C. Eleven plasma samples (14%) reacted with all 8 V2 antigens, while 62 plasma samples (78%) reacted with several (l to 7) V2 antigens (Fig. 2A, 2B). Six plasma samples (8%) did not react with any of the 8 V2 antigens (Fig. 2A, 2B); however, still reacted with cyclic V3A244 peptide, gp120A244 and gp4lMN (Fig. 1A, 3). Higher cross-reactivity of plasma Abs with V2 antigens depends on higher level of anti-V2 plasma Abs measured by ELISA. The number of reactive V2 antigens correlated with the level (OD) of anti-V2 Abs against all 8 V2 antigens; the highest level of plasma anti-V2 Abs reacted with the larger number of V2 antigens (Fig. 2A, 2B).
Fig 2. Binding of plasma antibodies to V2 antigens correlates with the level of anti-V2 antibodies; correlates between anti-V2 antibodies and with binding to gp120/gp41.
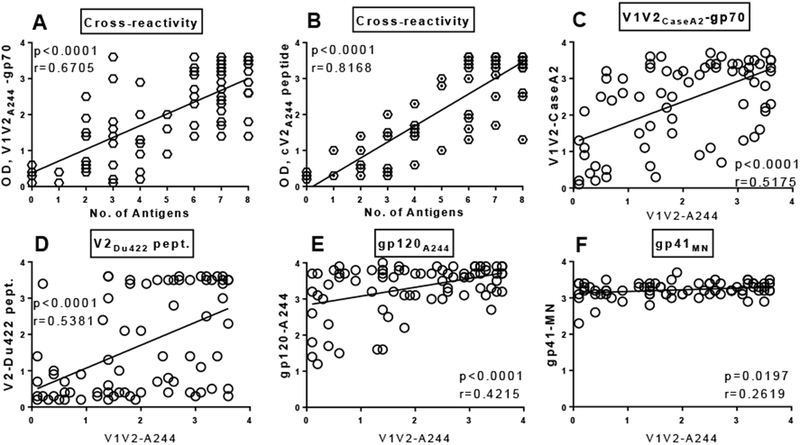
(A) Cross-reactivity (No. of V2 antigens reactive) correlates with the level (OD) of plasma anti-V1V2A244-gp70 Abs; (B) and with anti-cyclic V2A244 peptide. (C) Anti-V1V2A244 Abs correlate with anti-V1V2CaseA2-gp70; (D) and with binding to V2Du422 peptide. (E) Anti-V1V2A244 Abs correlate with binding to gp120A244; (F) and with binding to gp41MN. Plasma samples were diluted 1:100 and tested by ELISA against V1V2 fusion proteins and V2 peptides coated onto plates at 1 μg/ml. Correlation was determined by non-parametric Spearman correlation coefficient (r) and by linear regression with P values.
Fig 3. Titers of anti-V2 and control Abs in sequential plasma samples.

(A) Six donors with lack of anti-V2 Abs over 11 to 54 months of observation; (B) six donors with cross-reactive anti-V2 Abs over 28 to 60 months; (C) Titers of plasma Abs against 5 HIV-1 antigens were compared between the non-V2-reactive (crossed circles) and cross-reactive anti-V2 Abs (open circles). The 50% titers of plasma Abs were determined by ELISA at five serial 10-fold dilutions starting from 1:100 and tested against V1V2A244-gp70 (CRF01_AE) protein, biotinylated cyclic V3A244 peptide, gp120A244, gp41MN (clade B), and p24HXB2 (clade B) proteins. The antigens were coated or immobilized onto plate at 1 μg/mL. Statistical significance was determined by nonparametric Mann-Whitney test.
Given that plasma sample reactivity is usually tested against heterologous V2 sequences, we also included cyclic V2 peptides with sequences derived from two blood donors, MDC230 and MDC200, to test the reactivity to autologous linear V2 epitopes (Table 1). The results showed strong binding of plasma MDC230-1 (V2-reactive group) to the autologous V2230 peptide, while plasma MDC200-2 (V2-deficient group) bound weakly to the autologous V2200 peptide but not to other heterologous V2 antigens (Table 1).
3.3. Selection of V2 deficient and V2 cross-reactive plasma samples
The V2 deficient panel of 6 plasma samples needed for further studies comprised of 4 plasma without detectable anti-V2 Abs and 2 plasma samples with a low level (low OD) of Abs against one of eight V2 antigens (Table 1). The second panel of 6 plasma samples displayed cross-reactive anti-V2 Abs reacting with all 8 V2 antigens (Table 1). Both panels were selected based on their reactivity to 8 V2 antigens and availability of longitudinally collected plasma samples.
The 50% titers for Abs against V1V2A244-gp70, cyclic V3A244 peptide, gp120A244, and gp41MN were significantly higher (P<0.0001) in the group of V2 cross-reactive Abs compared to those with V2-deficitent Abs (Fig. 3C). The CD4+ T cell counts, viral loads and level of anti-p24 Abs were comparable in two tested groups (data not shown).
Glycoprotein 120 from 12 plasma viruses (6 each from the V2-deficient and V2-reactive groups) was sequenced, and virus subtypes were identified (Table 1). All but one virus (clade C) were HIV-1 circulating recombinant forms (CRF), which dominate in Cameroon according to recent analysis (Courtney et al., 2016; Nanfack et al., 2017). The identified subtypes in this study were as follows: CRF02_AG (3 plasma samples), CRF01_AE (2), CRF22_01A1 (2), CRF11_cpx, CRF18_cpx, CRF36_cpx, and CRF37_cpx (Table 1).
3.4. Longitudinal studies of plasma anti-V2 Abs.
To determine whether the deficiency of V2 Abs is temporary or stable, we analyzed plasma samples from six V2 Ab-deficient donors over 11 to 54 months of observation and from six V2 Ab-reactive donors over 28 to 60 months of observation (Fig. 3A, 3B). These sequential samples were titrated and screened against five Env antigens: V1V2A244-gp70, cyclic V3A244 peptide, gp120A244, gp41MN, and p24HXB2 (Fig. 3A, 3B). Titers of anti-V2A244 Abs in the V2-deficient group were undetectable (<1:100 dilution) (Fig. 3A) and designated as a 50% binding titer of 100. Longitudinal analyses confirmed that lack of V2 Abs was constant over 11 to 54 months of observation. Similarly, Abs to four other antigens (V3, gp120, gp41, p24) were detected at relatively constant levels, albeit at intermediate to high titers; these results show an exclusive, constant lack of Ab response to V2 (Fig. 3A). The panel of sequential plasma samples collected over 28 to 60 months from six volunteers with reactive V2 Abs displayed relatively constant levels of all five types of Abs, including anti-V2 Abs (Fig. 3B).
3.5. Correlation between Ab binding to V2 and other Env antigens.
To determine if the deficit of anti-V2 Abs is related to the general Ab response to HIV-1 Env proteins, we analyzed the relationships of the ELISA binding activities (OD405) between plasma Abs against V1V2A244-gp70 protein and other antigens using linear regression and the Spearman correlation coefficient (r ) (Fig. 2C-F). There was a strong correlation (P<0.0001) between a level (OD) of Abs against V1V2A244-gp70 versus two other V1V2 fusion proteins and two cyclic V2 peptides, as well as gp120A244 and gp41MN (p=0.0197) (Fig. 2C-F). Thus, correlation of low level of Abs against V2 and low level of Abs against gp120/gp41 suggest that a deficiency of anti-V2 Abs is related to a generally weaker Ab response to HIV-1 Env antigens in a subset of individuals infected with HIV-1.
3.6. Characteristics of the V2 and V1 regions.
We sequenced gp120 of the viruses in plasma of 12 selected patients to analyze the V2 and V1 regions in the V2-deficient vs V2-reactive groups (Table 1), including properties that may have some influence on the immunogenicity of V2 (Table S3). None of the sequenced plasma viruses were transmitted/founder (T/F) sequences because plasma samples were collected 6 to 18 months after HIV diagnosis. From each plasma virus, 3 to 9 gp120 sequences were produced and used to generate consensus V2 sequences for each of 12 subjects (Table 2S). We analyzed the amino acid (AA) length, number of putative N-linked glycosylation sites, isoelectric point (pI), and charge of the V2 and V1 regions (Table 2). In the V2 region, AA length was significantly longer in the V2-deficient vs V2-reactive group (P=0.0338), while the number of glycosylation sites was higher, and pI and charge were lower, but not significantly different in the V2-deficient vs the V2-reactive group, respectively (Table 2). In contrast to these data, the V1 region displayed statistically comparable all four features (Table 2).
We also analyzed the β-sheet propensity for the central conserved segment (El 53 to 1184) and the α-helix propensity (K168 to V172) of the V2 region. The β-sheet propensity correlates with neutralization sensitivity of the virus (Totrov, 2014) and may suggest a different immunogenicity profile, i.e., preferential presentation of different epitopes. The a-helix propensity is an estimate of the helical conformation, which is recognized by anti-V2 peptide mAbs such as CH58 (Liao et al., 2013) and putatively induces anti-V2p Abs; lower propensity suggest a higher tendency to form a helical structure. The two parameters were comparable between the V2-deficient and V2-reactive groups (Table 2, Table S3). Thus, the V2 consensus sequences from the V2-deficient compared with the V2-reactive group were slightly longer, with one additional N-linked glycosylation site on average and a tendency to a lower pI and electrostatic charge in the V2-deficient group.
3.7. Multivariable analysis.
Given that the majority of features characterizing the V2 region, except for length, are not significantly different between the V2-deficient vs V2-reactive groups, we tested whether the combination of multiple features could distinguish these two groups (Table 2). We defined composite indexes by combining 2 or 3 features to determine if the difference between the V2-deficient and V2-reactive groups, in the V2 and V1 regions, is significant, using the nonparametric Wilcoxon test. The difference between “No. AA” and “pI” and the difference between “No. AA” and “Charge” completely separated the V2-deficient from V2-reactive groups. That is, for each of the composite indexes, the maximum value (Max) in the V2-reactive group was lower than the minimum value (Min) in the V2-deficient group. Despite the small sample sizes, the lack of overlap in the values of each composite index between the two groups is statistically significant (P=0.005) (Table 2). Similarly, combining three features (“the sum of No.AA and No. glycosylation sites minus pI”, “the sum of No. AA and No. glycosylation sites minus charge,” and “the sum of No. AA and α-helix minus pI or charge” also separated the V2-deficient and V2-reactive groups; the non-overlapping index values between the groups are statistically significant (P=0.005 and 0.002, respectively (Table 2). This analysis provides evidence that multivariate predictions are more powerful than any single V2 variable for distinguishing V2-deficient from V2-reactive patient groups (Table 2). Analysis of the V1 region, as a control, did not show any significant differences between the two groups (Table 2).
4. Discussion
The present study analyzed frequency of anti-V2 Abs in plasma samples derived from 79 Cameroonian volunteers chronically infected with HIV-1. Given that some samples were negative for anti-V2 Abs, we focused on characterization of the V2 region of these individuals’ viruses to look for characteristics that might explain the deficiency of anti-V2 response.
Analysis of V2 sequences revealed that the V2 region of viruses from donors with V2-deficient versus V2-reactive Abs was significantly longer (P=0.0303), with one additional N-linked glycosylation site, on average. Usually, an increased number of N-linked glycosylation sites accompanies a longer V2 region (Owens et al., 2007; Sagar et al., 2006; van Gils et al., 2011). There is a correlation between the length and number of glycosylation sites, as has been observed in 41 pseudo-typed viruses in which neutralization-sensitive viruses to anti-V2 mAbs displayed shorter length and fewer glycosylation sites (Gorny et al., 2012). Given that additional glycosylation sites in the V2 region confer resistance to neutralization by V2 mAbs, this may also have an impact on immunogenicity, resulting in weaker or lack of Ab response, particularly in subjects with poor humoral immune response to infecting virus.
The charge and pI of the V2 region were lower in the V2-deficient versus V2-reactive panel of sequences (Table 2). Both these factors also play a role in anti-V3 mAbs; the pI of the VH CDR3 was significantly lower for human anti-V3 mAbs neutralizing Tier 2 and 3 pseudo-typed viruses vs non-neutralizing mAbs (Li et al., 2015). Although the results do not correspond to the phenomenon of failed Ab response to the V2 region, they underline a functional role of electrostatic interactions between Ab and virus and, possibly, in immunologic recognition as well.
For most features, changes in the V2 region were minimal for the V2-deficient and V2-reactive groups, although the length was significantly longer in the V2-deficient group. However, multivariable analysis including combination of 2 or 3 features completely separated the two groups and resulted in a highly significant difference between V2-deficient and V2-reactive groups (Table 2). Thus, we conclude that the combination of these small differences in multiple features may be responsible for the lower immunogenicity of the V2 region resulting in lack of anti-V2 Abs in patients with significantly lower Ab response to the Env proteins. All 4 features—length, number of N-glycosylation sites, pI, and charge—were no different in the V1 region between the subjects with V2-deficient and V2-reactive Abs (Table 2).
The low response to HIV Env antigens in the V2 deficient group of 6 patients could be influenced by host genetics (Watson et al., 2017). However, this is a topic for separate studies with potential to understand this observed disparity in anti-HIV antibody response.
These results can be useful to design the protein boosts for HIV vaccine development. Given that only a high level of anti-V2 Abs was correlated with reduction of HIV-1 infection (Zolla-Pazner et al., 2014) it would be critical for HIV vaccine to induce such a level of anti-V2 Abs. The HIV vaccine usually contains gp120, trimeric gpl40 or SOSIP as protein boosts and the envelope sequence should be selected with a shorter V2 region, with less glycosylation sites and higher pI and charge to enhance induction of anti-V2 Abs in vaccines.
In conclusion, our results show a broad range of binding activity of plasma Abs against V1V2 fusion proteins and V2 peptides including six plasma samples with lack of anti-V2 Abs. A strong correlation between anti-V2 and anti-gp120/gp41 Abs suggests that lack of anti-V2 Abs depends in part on the general ability of patients to mount Ab responses to HIV-1 proteins. Sequence analysis of the V2 region from plasma viruses in the V2-deficient Ab group suggest that HIV vaccine immunogens containing V2 segments with fewer glycosylation sites and higher electrostatic charge may be advantageous in inducing higher titers of anti-V2 Abs.
Supplementary Material
Acknowledgments
The authors thank the individuals with HIV-1 infection for their donation of blood samples for this study, which were collected at the Medical Diagnostic Center, Yaoundé, Cameroon. We would like to thank Dr. Michelle Ryndak for reviewing the manuscript. The study was supported by the National Institutes of Health [grant numbers AII12546 (MKG) and AI100151 (SZP/XPK)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare have no conflict of interest.
References
- Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, Willems B, Zekveld MJ, Dreja H, O’Sullivan E, Pade C, Orkin C, Jeffs SA, Montefiori DC, Davis D, Weissenhorn W, McKnight A, Heeney JL, Sallusto F, Sattentau QJ, Weiss RA, Lanzavecchia A, 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PloS one 5, e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney CR, Agyingi L, Fokou A, Christie S, Asaah B, Meli J, Ngai J, Hewlett I, Nyambi PN, 2016. Monitoring HIV-1 Group M Subtypes in Yaoundé, Cameroon Reveals Broad Genetic Diversity and a Novel CRF02_AG/F2 Infection. AIDS Res. Hum. Retroviruses 32, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney CR, Mayr L, Nanfack AJ, Banin AN, Tuen M, Pan R, Jiang X, Kong XP, Kirkpatrick AR, Bruno D, Martens CA, Sykora L, Porcella SF, Redd AD, Quinn TC, Nyambi PN, Durr R, 2017. Contrasting antibody responses to intrasubtype superinfection with CRF02_AG. PloS one 12, e0173705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Marzo Veronese F, Willis AE, Boyer-Thompson C, Appella E, Perham RN, 1994. Structural mimicry and enhanced immunogenicity of peptide epitopes displayed on filamentous bacteriophage. The V3 loop of HIV-1 gp120. J Mol Biol 243, 167–172. [DOI] [PubMed] [Google Scholar]
- Gorny MK, Moore JP, Conley AJ, Karwowska S, Sodroski J, Williams C, Burda S, Boots LJ, Zolla-Pazner S, 1994. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of HIV-1. J. Virol. 68, 8312–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Pan R, Williams C, Wang XH, Volsky B, O’Neal T, Spurrier B, Sampson JM, Li L, Seaman MS, Kong XP, Zolla-Pazner S, 2012. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from human immunodeficiency virus type 1-infected individuals. Virology 427, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH, 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366, 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Honnen WJ, Krachmarov CP, Burkhart M, Kayman SC, Corvalan J, Pinter A, 2002. Efficient isolation of novel human monoclonal antibodies with neutralizing activity against HIV-1 from transgenic mice expressing human Ig loci. J Immunol 169, 595–605. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, McBurney S, Pandey S, Sutton W, Liu L, Li L, Totrov M, Zolla-Pazner S, Haigwood NL, Gorny MK, 2016. Induction of neutralizing antibodies in rhesus macaques using V3 mimotope peptides. Vaccine 34, 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel ZR, Gorny MK, Palmer C, McKeating JA, Zolla-Pazner S, 1997. Prevalence of a V2 epitope in clade B primary isolates and its recognition by sera from HIV-1-infected individuals. AIDS 11, 128–130. [PubMed] [Google Scholar]
- Jiang X, Totrov M, Li W, Sampson JM, Williams C, Lu H, Wu X, Lu S, Wang S, Zolla-Pazner S, Kong XP, 2016. Rationally Designed Immunogens Targeting HIV-1 gp120 V1V2 Induce Distinct Conformation-Specific Antibody Responses in Rabbits. J. Virol. 90, 11007–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayman SC, Wu Z, Revesz K, Chen H, Kopelman R, Pinter A, 1994. Presentation of native epitopes in the V1/V2 and V3 regions of human immunodeficiency virus type 1 gp120 by fusion glycoproteins containing isolated gp120 domains. J. Virol. 68, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertjuthaporn S, Cicala C, Van Ryk D, Liu M, Yolitz J, Wei D, Nawaz F, Doyle A, Horowitch B, Park C, Lu S, Lou Y, Wang S, Pan R, Jiang X, Villinger F, Byrareddy SN, Santangelo PJ, Morris L, Wibmer CK, Biris K, Mason RD, Gorman J, Hiatt J, Martinelli E, Roederer M, Fujikawa D, Gorini G, Franchini G, Arakelyan A, Ansari AA, Pattanapanyasat K, Kong XP, Fauci AS, Arthos J, 2018. Select gp120 V2 domain specific antibodies derived from HIV and SIV infection and vaccination inhibit gp120 binding to alpha4beta7. PLoS pathogens 14, e1007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang XH, Williams C, Volsky B, Steczko O, Seaman MS, Luthra K, Nyambi P, Nadas A, Giudicelli V, Lefranc MP, Zolla-Pazner S, Gorny MK, 2015. A broad range of mutations in HIV-1 neutralizing human monoclonal antibodies specific for V2, V3, and the CD4 binding site. Mol. Immunol. 66, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF, 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38, 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr LM, Decoville T, Schmidt S, Laumond G, Klingler J, Ducloy C, Bahram S, Zolla-Pazner S, Moog C, 2017. Non-neutralizing Antibodies Targeting the V1V2 Domain of HIV Exhibit Strong Antibody-Dependent Cell-mediated Cytotoxic Activity. Sci Rep 7, 12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating JA, Shotton C, Cordell J, Graham S, Balfe P, Sullivan N, Charles M, Page M, Bolmstedt A, Olofsson S, Kayman SC, Wu Z, Pinter A, Dean C, Sodroski J, Weiss RA, 1993. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J. Virol. 67, 4932–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating JA, Shotton C, Jeffs S, Palmer C, Hammond A, Lewis J, Oliver K, May J, Balfe P, 1996. Immunogenicity of full length and truncated forms of the human immunodeficiency virus type I envelope glycoprotein. Immunol Letters 51, 101–105. [DOI] [PubMed] [Google Scholar]
- Moore JP, Sattentau QJ, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon SW, Fung MS, Traincard F, Pinkus M, al., e., 1993. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J. Virol. 67, 6136–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, Gorny MK, 2017. Monoclonal Antibodies Specific for the V2, V3, CD4-Binding Site, and gp41 of HIV-1 Mediate Phagocytosis in a Dose-Dependent Manner. J. Virol. 91, e02325–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura GR, Fonseca DP, O’Rourke SM, Vollrath AL, Berman PW, 2012. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of alpha4beta7 binding. PloS one 7, e39045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanfack AJ, Redd AD, Bimela JS, Ncham G, Achem E, Banin AN, Kirkpatrick AR, Porcella SF, Agyingi LA, Meli J, Colizzi V, Nadas A, Gorny MK, Nyambi PN, Quinn TC, Duerr R, 2017. Multimethod Longitudinal HIV Drug Resistance Analysis in Antiretroviral-Therapy-Naive Patients. J Clin Microbiol 55, 2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GP, Winges KM, Ritchie AM, Edwards S, Burgoon MP, Lehnhoff L, Nielsen K, Corboy J, Gilden DH, Bennett JL, 2007. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J Immunol 179, 6343–6351. [DOI] [PubMed] [Google Scholar]
- Pattanapanyasat K, Phuang-Ngern Y, Lerdwana S, Wasinrapee P, Sakulploy N, Noulsri E, Thepthai C, McNicholl JM, 2007. Evaluation of a single-platform microcapillary flow cytometer for enumeration of absolute CD4+ T-lymphocyte counts in HIV-1 infected Thai patients. Cytometry B Clin Cytom 72, 387–396. [DOI] [PubMed] [Google Scholar]
- Sagar M, Wu X, Lee S, Overbaugh J, 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. Journal of virology 80, 9586–9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrier B, Sampson J, Gorny MK, Zolla-Pazner S, Kong XP, 2014. Functional implications of the binding mode of a human conformation-dependent V2 monoclonal antibody against HIV. J. Virol. 88, 4100–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totrov M, 2014. Estimated secondary structure propensities within V1/V2 region of HIV gp120 are an important global antibody neutralization sensitivity determinant. PloS one 9, e94002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils MJ, Bunnik EM, Boeser-Nunnink BD, Burger JA, Terlouw-Klein M, Verwer N, Schuitemaker H, 2011. Longer V1V2 Region with Increased Number of Potential N-Linked Glycosylation Sites in the HIV-1 Envelope Glycoprotein Protects against HIV-Specific Neutralizing Antibodies. J. Virol. 85, 6986–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CT, Glanville J, Marasco WA, 2017. The Individual and Population Genetics of Antibody Immunity. Trends Immunol 38, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, deCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O’Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD, 2014. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PloS one 9, e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, Berman P, Shen X, Andrews C, O’Connell RJ, Ngauy V, Nitayaphan S, de Souza M, Korber B, Koup R, Bailer RT, Mascola JR, Pinter A, Montefiori D, Haynes BF, Robb ML, Rerks-Ngarm S, Michael NL, Gilbert PB, Kim JH, 2013. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PloS one 8, e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


