Abstract
The Rac inhibitor EHop-016 was developed as a compound with the potential to inhibit cancer metastasis. Inhibition of the first step of metastasis, migration, is an important strategy for metastasis prevention. The small GTPase Rac acts as a pivotal binary switch that is turned “on” by guanine nucleotide exchange factors (GEFs) via a myriad of cell surface receptors, to regulate cancer cell migration, survival, and proliferation. Unlike the related GTPase Ras, Racs are not usually mutated, but overexpressed or overactivated in cancer. Therefore, a rational Rac inhibitor should block the activation of Rac by its upstream effectors, GEFs, and the Rac inhibitor NSC23766 was developed using this rationale. However, this compound is ineffective at inhibiting the elevated Rac activity of metastatic breast cancer cells. Therefore, a panel of small molecule compounds were derived from NSC23766 and screened for Rac activity inhibition in metastatic cancer cells. EHop-016 was identified as a compound that blocks the interaction of Rac with the GEF Vav in metastatic human breast cancer cells with an IC50 of ~1 μM. At higher concentrations (10 μM), EHop-016 inhibits the related Rho GTPase Cdc42, but not Rho, and also reduces cell viability. Moreover, EHop-016 inhibits the activation of the Rac downstream effector p21-activated kinase, extension of motile actin-based structures, and cell migration. Future goals are to develop EHop-016 as a therapeutic to inhibit cancer metastasis, either individually or in combination with current anticancer compounds. The next generation of EHop-016-based Rac inhibitors is also being developed.
1. INTRODUCTION
EHop-016 (N4-(9-ethyl-9H-carbazol-3-yl)-N2-(3-morpholin-4-yl-propyl)-pyrimidine-2,4-diamine) is a small molecule compound that we recently characterized as an inhibitor of the small GTPase, Rac. The relevance of developing EHop-016, and related Rac inhibitors, stems from their potential as antimetastatic cancer therapeutics.
1.1. Relevance of developing targeted therapy for cancer metastasis
Despite recent progress in early detection and improved adjuvant therapy, the prognosis for cancer patients is still limited by distant metastases [1]. During metastatic progression, malignant cancer cells migrate from the primary tumor, invade the tumor microenvironment, and enter the circulatory system to establish secondary tumors at distant sites, thus complicating accurate diagnosis and treatment [2]. In addition to the dysregulated signaling in metastatic cancer cells, their interactions with the stromal cells in the tumor microenvironment that infiltrate the tumor tissue, such as macrophages, myeloid-derived suppressor cells, T lymphocytes, and adipocytes, increase tumor-associated inflammation and invasion. Cancer and stromal cell crosstalk signaling also promotes invasion and metastasis via cytokines, growth factors, and proteases that remodel the tumor microenvironment [3]. In addition, stromal cells in specific organs such as bone and lung (e.g., neutrophils and bone marrow-derived hematopoietic immune progenitor cells) signal to the homing cancer cells to promote establishment of the premetastatic niche [4]. Therefore, a viable antimetastasis therapeutic should inhibit invasive/migratory signaling in cancer cells, as well as those of the stromal cells that recruit them to invade the vasculature and establish secondary tumors at vital organ sites.
For breast cancer, preventing metastasis is critical, because breast cancer can be cured if detected at the early stages of the disease. Nevertheless, 30% of breast cancer patients can develop stage IV metastatic disease in bone, liver, and lung with a 5-year survival rate of 20% [5–7]. Although current systemic metastatic cancer therapies are generally active at the beginning of therapy, most patients develop resistance with time [5,8]. Therefore, improved targeted and combinatorial therapeutic strategies are required to effectively combat metastatic disease.
1.1.1. Regulating the actin cytoskeleton in cancer metastasis
Intravasation, the first step of metastasis, involves migration away from the primary tumor employing mechanisms similar to those used during normal cell migration [9]. Therefore, molecules that regulate cell migration may become dysregulated during metastasis and act as metastasis promoters [10]. Cell migration and invasion are guided by actin polymerization, rearrangement of the actin cytoskeleton to extend motile structures, and the modulation of cell–cell and cell–extracellular matrix adhesions. These activities require sophisticated molecular coordination and signaling orchestrated by both cancer cells and the stromal cells in the tumor microenvironment. The Rho family small GTPases have been implicated as key regulators of the spatial and temporal activities of metastatic cancer cells, as well as stromal cells, during invasion and directed migration [11–13].
1.2. The small GTPase Rac as a central regulator of cell signaling to the actin cytoskeleton
The Rho family, of which the most-studied members are Rho, Rac, and Cdc42, is a ubiquitiously expressed and evolutionarily conserved family of Ras-related small GTP-binding proteins that regulate actin dynamics and intracellular signaling. Rho GTPases control diverse cellular functions related to cancer development, including actin cytoskeleton organization, invasion and metastasis, transcriptional regulation, cell cycle progression, apoptosis, vesicle trafficking, and cell-to-cell and cell-to-extracellular matrix adhesions. Rho GTPases are activated during signaling from cell surface receptors that regulate GTP/GDP cycling via a number of accessory proteins: Rho guanine nucleotide dissociation inhibitors, Rho guanine nucleotide exchange factors (GEFs), and Rho guanine nucleotide activating proteins (GAPs) [11,14,15].
Of the Rho family of GTPases, Rac proteins (Racs 1 and 3 expressed in nonhematopoietic cells and Rac2 in hematopoietic cells) have been specifically implicated in organization of the actin cytoskeleton into cell surface protrusions called lamellipodia that control directed cell migration during leukocyte chemotaxis [16], as well as cancer cell invasion, and thus metastasis [17–29]. The Rac homolog Cdc42 also modulates the actin cytoskeleton during migration/invasion via de novo actin polymerization and extension of motile actin structures called filopodia, and has been implicated in breast cancer malignancy [30,31].
Racs are also essential for Ras and other oncogene-mediated transformation via regulation of Ras/mitogen activated protein kinase (MAPK) signaling [32–34]. Hyperactive Rac1 and Rac3 have been implicated with increased survival, proliferation, and invasion of breast cancer, gliomas, melanomas, and leukemia [20,35–41]. Wild-type Rac1 overexpression has been associated with a range of human cancers: breast, brain, gastric, and pancreatic cancers, as well as ulcerative colitis [36,37,42–46]. Studies have also demonstrated a cancer-promoting role for the constitutively active Rac1b splice variant that is overexpressed in breast and colorectal cancers [39,47–50].
Although functionally relevant Rac1 mutations are rare, activating Rac1 mutations have been found in melanoma [51]. A “Rac1 risk allele” has also been reported from patients at risk for developing colon cancer [52]. More recently, fast cycler mutations of Rac, with transformative ability, were reported from a range of human cancer cell lines [53]. However, given their low frequency, and because these mutations were identified from cancer cell lines that have been in culture for a long period, the importance of Rac mutants in human carcinogenesis remains to be validated.
Rac and the close homolog Cdc42 are ideal therapeutic targets for metastatic cancer prevention, especially in breast cancer for a number of reasons. Rac is a key downstream effector of ErbB/epidermal growth factor receptors (EGFRs) that are often overexpressed in metastatic breast cancer [9,26]. Overexpression of human epidermal growth factor receptor 2 (HER2) in mammary epithelial cells increased Rac1 activity, implicating Rac signaling in the malignant phenotype of HER2-type breast cancer [54]. Moreover, Rac1 was recently shown to regulate breast cancer cell proliferation and estrogen receptor (ER)a levels, thus implicating Rac in modulating ER function in breast cancer [55]. Racs have also been implicated with reversal of growth factor receptor (GFR) targeted therapy resistance signaling pathways [56].
The malignant phenotype of Rac overexpression has been associated with the activity of the Rac downstream effector p21-activated kinase (PAK) [57,58]. Moreover, elevated HER2 expression in human breast cancer specimens, an indicator of poor prognosis, has been associated with PAK levels [59]. PAKs are central activators of several cancer pathways that include not only actin cytoskeletal changes during migration, but also cell adhesion, survival, and proliferation [60–62]. PAK and other downstream effectors of Racs regulate cell proliferation, survival, angiogenesis, cell polarity, epithelial to mesenchymal transition, cell–extracellular matrix adhesion, as well as migration/invasion via a number of signaling sequelae [22,26,59,62–68]. Although Rac-mediated production of reactive oxygen species (ROS) via NADPH oxidase activity is part of the innate immune response, Rac-mediated ROS production has also been shown to regulate the invasive potential of cancer cells [69–72].
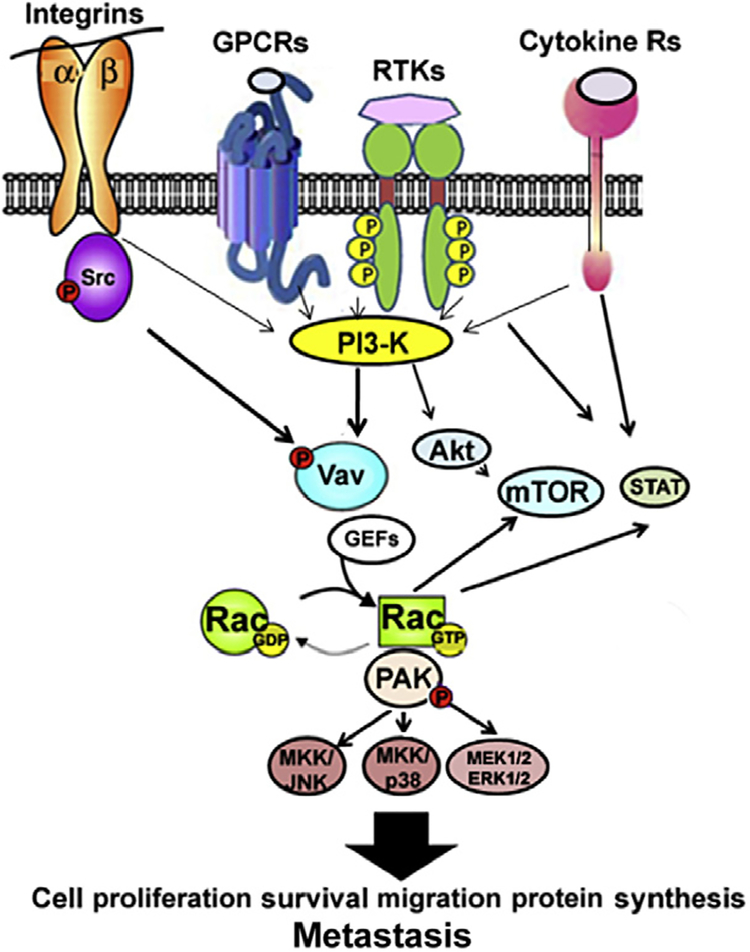
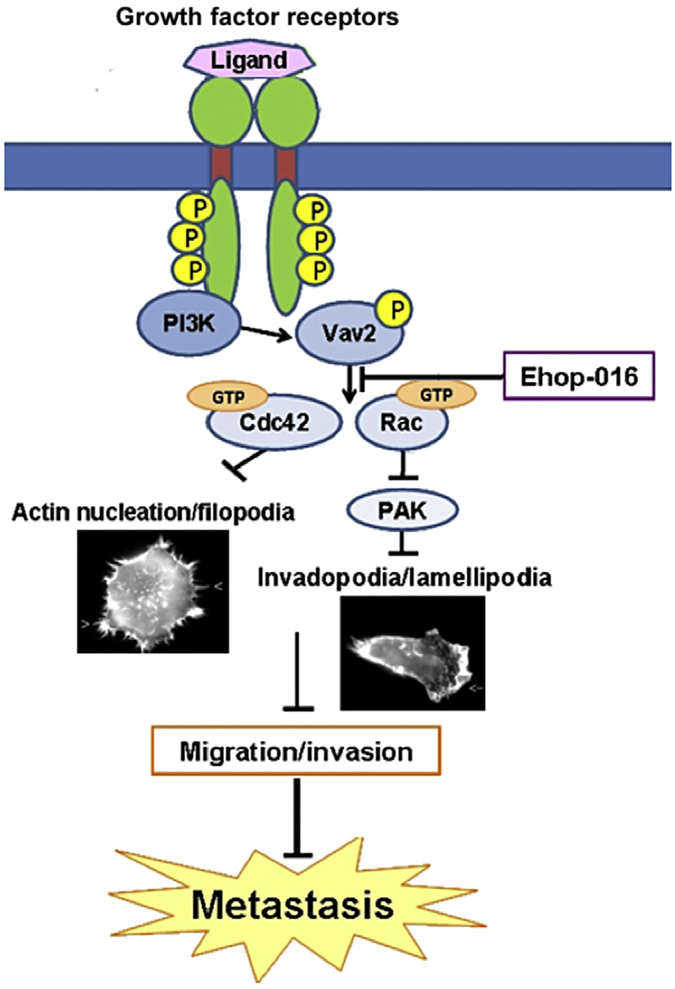
Rac action is implicated with cancer progression and acquisition of therapy resistance via multiple pathways that include signal transduction to Rac. GEFs from integrin, G protein-coupled receptors (GPCR), GFR/receptor tyrosine kinases (RTK), and cytokine/janus kinase/signal transducer and activator of transcription (STAT) receptors. These cell surface receptors regulate a myriad of cancer-promoting signal cascades that have also been implicated with Rac/PAK activity. These pathways include: phosphoinositide 3-kinase (PI3-K)/Akt/mammalian target of rapamycin (mTOR); MAPKs: extracellular regulated kinase, jun kinase (JNK), and p38 MAPK; protein kinas C ε, and STATs (Fig. 6.1) [22,26,34,61–67,73–80]. Recent studies have implicated Rac activity in mTOR signaling, where Rac regulates both mTOR complex 1 (mTORC1) and mTORC2 activation during cancer cell migration, cell size control, epithelial to mesenchymal transition, and metastasis [66–68]. Since mTOR signaling has emerged as a central regulator of cancer progression and acquisition of therapy resistance [81], these studies suggest a key role for Rac in regulation of cancer malignancy.
Figure 6.1.
Simplified scheme of the major signaling pathways of Rac activation in human cancer. Cell surface integrin receptors, G protein coupled receptors (GPCR), receptor tyrosine kinases (RTK), cytokine receptors, and nonreceptor tyrosine kinases activate Ras/mitogen activated protein kinase (MAPK), phosphoinositide 3-kinases (PI3-K), and signal transducers and activator of transcription (STAT) pathways. PI3-K phosphorylates phosphatidyl inositol biphosphate (PIP2) to form the signaling intermediate PIP3 that recruits phospholipid-dependent kinase and Akt/protein kinase B. Akt activates the mammalian target of rapamycin (mTOR), a central regulator of protein synthesis and metabolism. PI3-K signaling also modulates Vav and other Rac.GEFs such as p-REX that exchange the GDP on Rac for GTP, thus activating Rac. Rac action is implicated with signaling to PAK, mTOR complex1 (mTORC1), mTORC2, MAPKs: extracellular regulated kinases (ERK1/2), p38 MAPK, Jun kinase (JNK), and STATs to control cancer progression to metastasis.
Because the malignant phenotype of Rac is closely associated with activation of its direct downstream effectors PAKs [57,58], much effort has been focused on the development of PAK inhibitors as anticancer therapeutics [82–84]. However, in addition to PAK, Racs have multiple downstream effectors, such as WASP family verprolin-homologous protein (WAVE) and mammalian-enabled (Mena)/vasodilator-stimulated phosphoprotein, that contribute to cancer [85,86]. Therefore, targeting Rac activation is a more viable approach for the development of anticancer drugs [87].
1.3. Rac.GEFs in cancer metastasis
So far, over 60 potential Rac.GEFs have been identified [88–90]. A large subset of the Rho.GEFs is characterized by a Dbl homology (DH) domain, followed by a pleckstrin homology domain that forms the structural basis for the guananine nucleotide exchange activity [91]. DH domains are present in a number of proto-oncogenes such as T-cell invasion and metastasis gene product (Tiam-1), Trio, Vav, and PIP3-dependent Rac exchanger 1 (p-Rex1) that have been implicated in cancer progression [24,26,92–97].
1.3.1. Vav/Rac signaling in cancer metastasis
Of the Rho GEFs, Vav is of note due to its importance in both hematopoietic (Vav1) and nonhematopoietic (Vav2, 3) signaling to activate Rac2 (in immune cells) and Rac1 and Rac3 in cancer cells. Vav isoforms have also been implicated in tumor growth, angiogenesis, and metastasis in a number of cancers including breast cancer [24,93–95,98–105], as well as immune and stromal cell signaling relevant for recruitment of cancer cells in the tumor microenvironment [106–108]. Although Vav2 can act as a GEF for RhoA, Rac1, and Cdc42 in vitro, its transforming activity has been ascribed primarily to its ability to activate Rac1 [93]. Therefore, cell surface receptor-activated or oncogenic Vav has been shown to regulate tumor progression, invasion, and angiogenesis via Rac-regulated activation of downstream cancer-promoting molecules such as PI3K, PAK, p38 MAPK, and JNK [93–95,105,109–113] (Fig. 6.1). Moreover, Rac and Cdc42 have been shown to be necessary for Vav-induced cell transformation, migration, and metastasis [93,112,114–120]. Vav/Rac signaling is also significant during the development of breast cancer therapy resistance [54,121–123]. Recent studies have shown that Vav2 and Vav3 regulate a lung metastasis-specific gene signature in breast cancer cells, thus implicating Vav/Rac signaling in the control of specific steps of metastasis to the lung at the transcription level [24,98]. Significantly, the current literature on cell signaling during cancer metastasis strongly implicates Vav/Rac signaling in promoting cancer malignancy both via signaling in cancer cells as well as in stromal and immune cells in the tumor microenvironment. Thus, our recent characterization of EHop-016 as a specific inhibitor of the Vav/Rac interaction has direct implications for its further development as an anticancer metastasis therapeutic [124].
2. DEVELOPMENT OF Rac INHIBITORS TO IMPEDE METASTATIC CANCER PROGRESSION
Using the rationale that a structure–function-based design to block the signaling step of Rac activation by GEFs is a viable strategy for inhibiting Rac functions, Gao et al. [125] identified a small chemical compound from the National Cancer Institute chemical database, NSC23766, as a Racspecific inhibitor. This compound fits into a surface groove of Rac1 known to be critical for GEF specification, and was shown to inhibit Rac1 binding and activation by a subset of Rac-specific GEFs, that is, Trio and T-cell invasion and metastasis gene product (Tiam1), in a dose-dependent manner. The interaction between NSC23766 and Rac is specific and does not affect Cdc42 or RhoA binding or activation by their respective GEFs, interaction of Rac1 and GAPs, or other downstream effectors [126,127]. NSC23766 has been used to demonstrate the significance of Rac activity in cancer cell proliferation, migration, invasion, metastasis, and therapy resistance, as well as platelet aggregation and hematopoietic cell migration [122,126,128–134]. However, the high effective concentrations (IC50>75 μM) of NSC23766 limit its use as a therapeutic agent [125].
Other known Rac inhibitors, including NSC23766 derivatives, have IC50s of 10–50 μM [135,136]. Virtual screening of NSC23766 in a ZINC database identified improved structures with IC50s from 12 to 57 μM [135]. EHT 1864, a recently described inhibitor that selectively blocks the interaction of Rac with its downstream effectors, is also effective at 10–50 μM and is, therefore, more useful than NSC23766 at inhibiting a number of Rac functions [136,137]. Accordingly, EHT 1864 has been successfully used to demonstrate the role of Rac1 in growth and inflammatory responses in human endothelial cells, medulloblastoma migration, ER expression and spread of breast cancer, platelet activation, and lymphoma development [22,55,138–141].
Selective inhibitors for the Rac1B GTPase, which is an alternatively spliced constitutively active form Rac1, have also been designed with specificity for Rac1B inhibition over Rac1 or Cdc42 [142]. However, the Rac1B-specific inhibitors are not universally applicable to inhibit invasive cancers with overactive Rac or Cdc42.
2.1. Design and synthesis of NSC23766 derivatives
A rational Rac inhibitor should be effective at physiologically relevant concentrations (in the nM range), be specific to Rac activation by a range of Rac.GEFs, have high solubility and bioavailability, and be nontoxic in cell and animal models. To determine the effective concentration of NSC23766 in invasive cancer cells, we first tested the effect of this compound on the Rac activity of a highly metastatic human cancer cell line, MDA-MB-435. Using metastatic variants of this MDA-MB-435 cell line, we previously reported that hyperactive Rac is associated with high invasive and metastatic efficiency [36]. In MDA-MB-435 cells, the IC50 for Rac activity inhibition by NSC23766 is as high as ~100 μM [124,143]. Therefore, we used NSC23766 as a lead structure for the synthesis of new derivatives with the potential for improved Rac1 inhibitory activity.
2.1.1. Synthesis of NSC23766 derivatives
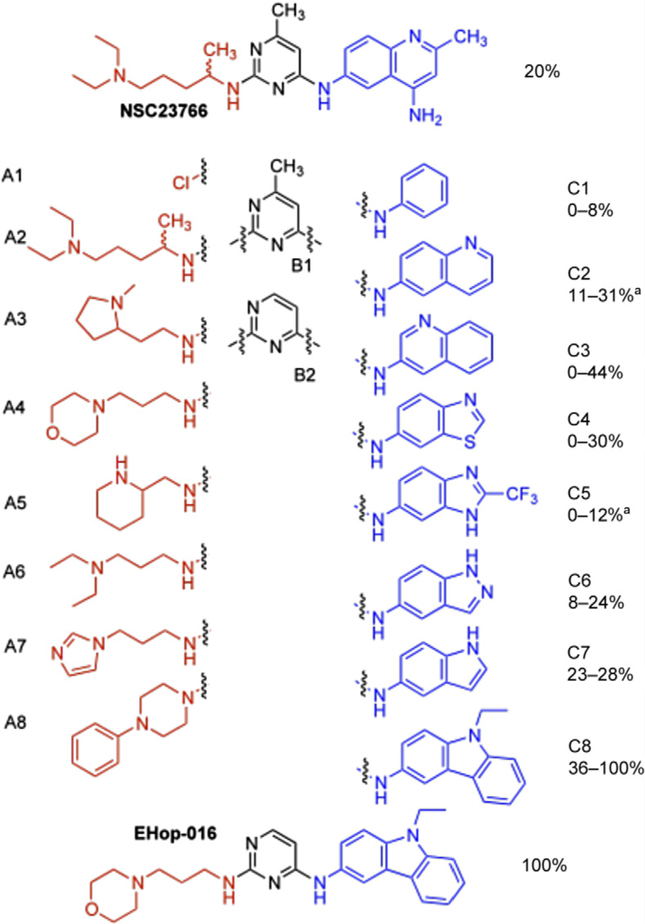
As described in our strategy for the development of Rac1 inhibitors with increased potency, we utilized NSC23766 as a template in which the three major chemical building blocks were substituted with commercially available heteroarylamines, dichloropyrimidines, and primary or secondary aliphatic amines with tail-end amino-substituents [143]. The central building block of NSC23766, the pyrimidine core (B1–B2) that binds a critical tryptophan residue (Trp56) of Rac1 [125], was maintained in our design (Fig. 6.2). The second group of building blocks (C1–C8) consisted of heterobicyclic arylamino groups that were connected to the 4-position of the pyrimidine ring, thereby mimicking the substituted aminoquinoline group of NSC23766. The third group of building blocks contained primary or secondary aliphatic amine with a tail-end amino group (A1–A8), mimicking the substituent on the 2-position of the pyrimidine ring of NSC23766. The new NSC23766 derivatives were prepared via a facile two-step synthesis by reacting dichloropyrimidines (B1–B2) with (hetero)-arylamines (C1–C8) to provide a 3:1 to 5:1 mixture of the 4-substituted and 2-substituted products. After silica gel chromatography, the pure 4-substituted product was reacted with amines (A2–A8) to produce the desired novel derivatives (Fig. 6.2). Detailed synthetic methods are described in Ref. [143]. Out of the 128 possible combinations, 32 compounds were randomly selected for synthesis and subsequently screened for activity.
Figure 6.2.
Representation of the building blocks (A,B,C) utilized to prepare a small library of NSC23766 derivatives. Indicated in italics are the percentages of Rac inhibition of a group of compounds containing units C1–C8.
2.1.2. Initial screening of novel Rac inhibitors
To screen for the relative efficiency of the new NSC23766 derivatives, we used The G-LISA Rac Activation Assay (Cytoskeleton, Inc., Denver, CO), as described in Ref. [124,143]. This assay detects the activated (GTP bound) Rac1, 2, and 3 isoforms from cell lysates by their specific interaction with the Cdc42 and Rac interactive binding domain (CRIB) from PAK [144].
In the highly metastatic MDA-MB-435 cells, NSC23766 inhibited Rac with an IC50 of 95 μM. Therefore, we compared the effect of the 32 new compounds at 50 μM, following a 24-h incubation in MDA-MB-435 cells, in the presence of serum. At this concentration, NSC23766 inhibits Rac by 20% compared to vehicle controls. In Fig. 6.2, the range of Rac inhibitory activities for compounds with the C1–C8 building blocks is provided. Each range represents at least two compounds with these fragments that in addition have different building blocks selected from B1 to B2 and A1 to A8. In the series of compounds with C2 and C4 blocks, the most active compounds are approximately 50% more active than NSC23766 (30–31% versus 20% Rac inhibition, respectively). In addition, the most active compound in the series with C3 is two times more active than NSC23766 (44% versus 20% Rac inhibition). Significantly, all of the four compounds in the series with the carbazole fragment C8 were found to inhibit Rac at a higher efficiency than NSC23766. Initially, the Rac inhibitory potential of these compounds was thought to result partially from interference of cytotoxicity and (or) inhibition of Rac-induced effects on cell viability. Nevertheless, upon lowering the assay concentration of the most active compound (EHop-016) to noncytotoxic levels, this compound was indeed found to inhibit Rac with an IC50 ~1 mM [124].
Since the preliminary screening experiments indicated that derivatives with fragment C8 were cytotoxic, we initially focused our attention on the compounds with C2, C3, or C4 building blocks. The most active compounds of these series inhibited Rac approximately 1.5–2 times more potently than NSC23766, and demonstrated higher efficiency at reducing cell spreading, extension of lamellopodia, and directed migration toward serum at 50 μM concentrations [143]. In addition, although Rac is only inhibited by 30–44% at this concentration, cell migration was reduced by 80–90%. Therefore, it may be hypothesized that partial inhibition of Rac, or localization at different compartments, is sufficient to produce a dramatic effect on Rac-regulated cell functions. Nevertheless, preliminary data also demonstrated that at least one of the compounds inhibits Cdc42 to a similar extent as Rac. Thus, Cdc42 inhibition may also contribute to the effects of NSC23766 derivatives on cell functions relevant for metastasis. Although selectivity for specific GTPases is important for the development of biochemical probes, for therapeutic purposes, it will be more advantageous to develop dual inhibitors, where dual Rac–Cdc42 inhibitors are expected to be synergistic and interfere with multiple steps in the metastatic process. Consequently, among the future goals in our laboratories is the development of prospective dual Rac–Cdc42 inhibitors.
2.2. Identification of EHop-016 as a Rac inhibitor
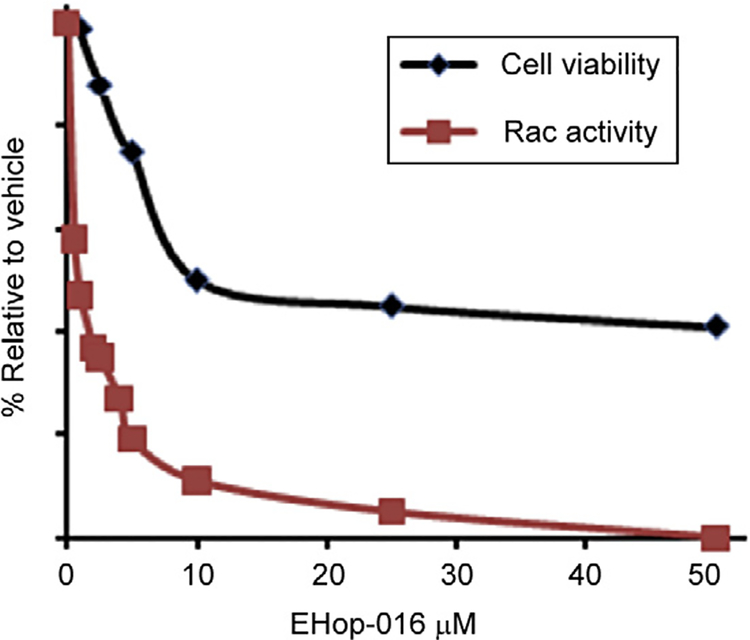
From the screening results described above, EHop-016 appeared to demonstrate complete inhibition of Rac1 at a concentration of 50 μM. As shown in Fig. 6.3, the decreased Rac activity in response to 50 μM EHop-016 may at least be partially due to the reduced MDA-MB-435 cell viability (~40%) at this concentration. Therefore, we subsequently determined the Rac inhibitory activity of EHop-016 at concentrations at which EHop-016 was not cytotoxic (<5 μM). Serendipitously, EHop-016 specifically inhibited Rac activity at these lower concentrations, and the IC50 for Rac inhibition in this cell line was subsequently determined to be ~1 μM [124]. In conclusion, we identified EHop-016 as a novel inhibitor of Rac, at 100-fold more potency than the parent compound NSC23766 and 10–50-fold more effective than other available Rac inhibitors [135,137,145].
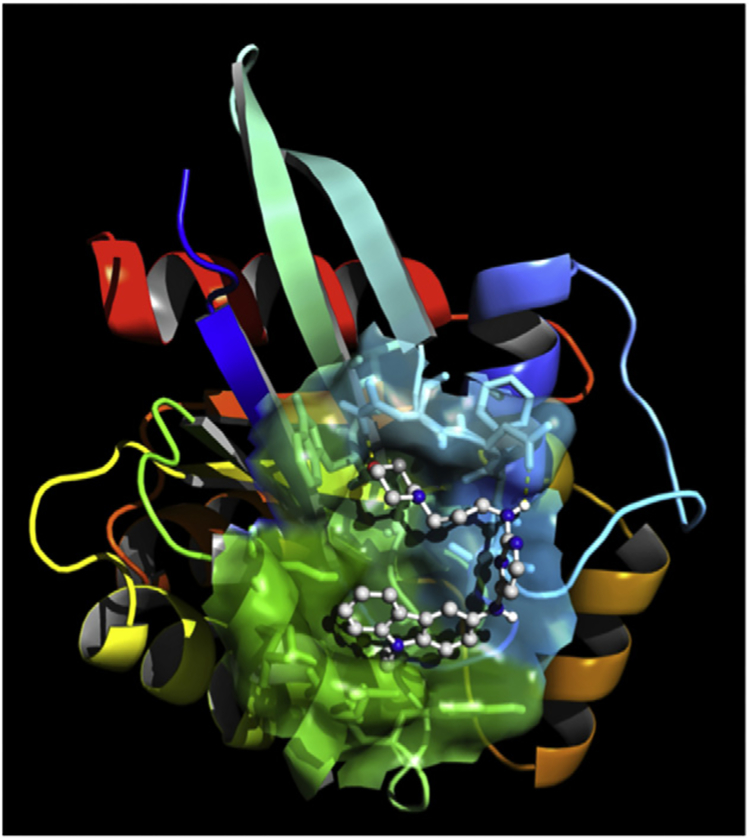
Figure 6.3.
EHop-016 docked into the GEF-binding pocket of Rac1. AutodockTools were used to prepare the GEF-interacting region of Rac and EHop-016 for docking, centered on the original position of NSC23766 on the crystal structure of the GEF-interacting surface of Rac. In this configuration, Ehop-016 interacts with residues that have been shown to interact with the Rac guanine nucleotide exchange factor Vav.
Figure 6.2 demonstrates that all compounds containing C8 (four derivatives were tested) were more potent Rac inhibitors than the parent compound, indicating that this carbazole group is key to increased Rac inhibitory activity. To explain the marked difference in inhibition potency between the carbazole unit and the other arylamine fragments C1–C7, molecular docking studies were conducted utilizing the crystal structure of the Rac–NSC23766 complex [126,146] as a template. Figure 6.3 demonstrates that the energetically most favorable position of EHop-016 in Rac1 is in the area around Trp56, which has been shown to be critical for binding of Rac to its GEFs, similar to the region of NSC23766 interaction. However, the crystal structure of the Rac effector region and NSC23766 demonstrates that NSC23766 is stretched linearly on the surface of Rac [125,132]. Our calculations with EHop-016 in the same Rac effector region indicate a bent U-shape that places the carbazole group (C8) of EHop-016 (or its analogs) with relatively lipophilic residues in a more efficient configuration. In addition, unit A4 of EHop-016 provides additional binding energy via hydrogen bonding with Asn39 [124].
In light of our finding that EHop-016 inhibits the Vav2–Rac interaction [124], it is notable that Ehop-016 interacts with residues Thr35, Val36, Asn39, Ala59, and Tyr64, which have been demonstrated to be relevant for binding of the closely related GEF Vav1 to Rac1. Attempts to further elucidate binding interactions via cocrystallization of EHop-016 with Rac are ongoing.
2.3. Functional characterization of EHop-016
2.3.1. EHop-016 inhibits Rac activity at 0.5–5 μM and Cdc42 at concentrations >5 μM
As shown in Fig. 6.4, the 100% inhibition of Rac activity in response to 50 μM EHop-016 may partially be due to the reduced MDA-MB-435 cell viability (~40%) at this concentration. We subsequently determined that in this highly metastatic HER2 overexpressing cancer cell line, EHop-016 inhibited Rac activity at low physiologically relevant concentrations (<5 μM) with an IC50 of ~1 μM [124]. Therefore, EHop-016 is 100-fold more potent than the parent compound NSC23766 and 10–50-fold more effective than other available Rac inhibitors [135,137,145]. EHop-016 also inhibited the Rac activity of the HER2 (–) (triple negative) metastatic breast cancer cell line MDA-MB-231; however, the IC50 for Rac inhibition by EHop-016 in this cell line was higher (~3 μM) [124]. Additionally, we have determined that the MDA-MB-435 cell line expresses three to four times more Vav2 than MDA-MB-231 cells (data not shown). Therefore, the increased EHop-016 potency in the MDA-MB-435 cells may indicate that EHop-016 action depends on inhibition of HER2 and (or) Vav signaling.
Figure 6.4.
Effect of EHop-016 on cell viability and Rac activity. MDA-MB-435 cells were treated with vehicle (0.1% DMSO), or varying concentrations of EHop-016 (0–10 μM) for 24 h. Cell viability was measured using the MTT cell survival and proliferation kit (Millipore, Inc). Rac activity was measured from cell lysates by a G-LISA Rac1 Activation Assay (Cytoskeleton, Inc., Denver, CO). The mean values SEM (N = 3) are presented relative to vehicle (100%). All data points for Rac activity > 0.5 μM EHop-016, and for viability at >5.0 μM EHop-016, were statistically significant compared to vehicle controls (p≤0.05).
2.3.2. EHop-016 reduces cell viability at concentrations >5 μM
At concentrations <5 μM, EHop-016 is specific for Rac, and has no effect on Rho or the close homolog Cdc42. However, at concentrations >5 μM, EHop-016 inhibits Cdc42 activity with a 75% inhibition at 10 μM [124]. Interestingly, MDA-MB-435 cell viability was not significantly affected at the concentrations that inhibited Rac activity (<5 μM), while significant effects on cell viability were observed at EHop-016 concentrations that also inhibit Cdc42 activity (Fig. 6.4), suggesting that the reduced cell viability in response to EHop-016 may be due to inhibition of Cdc42 activity or a combination of reduced Rac and Cdc42 activities.
2.3.3. EHop-016 inhibits the interaction of Vav2 and Rac
To identify upstream effectors of Rac that are inhibited by EHop-016, MDA-MB-435 cell lysates were incubated with glutathione beads coupled to a GST-tagged nucleotide-free form of Rac1, Rac1(G15A), with a high affinity for Rac GEFs. Vav2 specifically associated with Rac1(G15A), while neither Tiam-1 or Trio-bound Rac1(G15A) from MDA-MB-435 cells in serum [124]. EHop-016 (4 μM) inhibited the association of Vav2 with Rac1 (G15A) by 50% (Fig. 6.5). Since 4 μM EHop-016 results in ~100% inhibition of Rac activity [124], EHop-016 may also inhibit the interaction of GEFs that have yet to be identified. Moreover, EHop-016 at >40 μM inhibited the interaction of a purified active domain of Tiam-1 with Rac1(G15A) ([124], demonstrating that at high concentrations, EHop-016 may inhibit other GEFs.
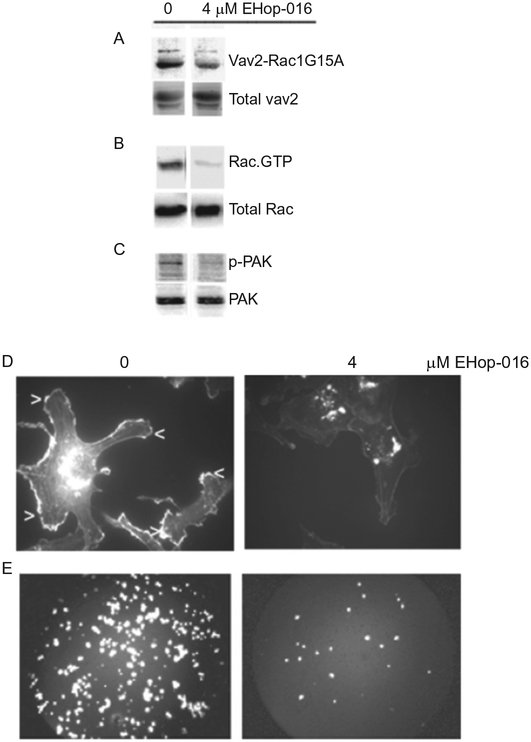
Figure 6.5.
Effect of EHop-016 on Rac activation and downstream activities in highly metastatic MDA-MB-435 cells. (A) GST-Rac1(G15A) (mutant form of Rac that selectively binds active GEFs) beads were preincubated with vehicle (0), or 4 μM EHop-016 prior to incubation with MDA-MB-435 cell lysates. A representative western blot (N = 3) immunostained for Vav2 is shown. Top row, pulldown; bottom row, total cell lysate. (B) MDA-MB-435 cells treated with vehicle (0) or EHop-016 (4 μM) for 24 h were lysed and subjected to a pulldown assay for Rac.GTP using a GST-CRIB domain of PAK and western blotted with a pan Rac antibody (Rac1,2,3). Representative western blot (N = 3) is shown. Top row, pulldown; bottom row, total cell lysate. (C) MDA-MB-435 cells were treated with vehicle (0) or 4 μM EHop-016 for 24 h and the cells lysed and western blotted for active P-thr 423 PAK (upper band) or total PAK (lower band). Representative western blot (N = 3) is shown. (D) MDA-MB-435 metastatic breast cancer cells were treated with vehicle or EHop-016 at 4 μM for 24 h. Cells were fixed, permeabilized, and stained with Rhodamine phalloidin to visualize F-actin. Representative micrographs are shown. Arrows indicate lamellipodia. (E) MDA-MB-435 cells treated with vehicle (0) or EHop-016 (4 μM) for 24 h were subjected to a Transwell migration assay. The cells that migrated to the underside of the membrane of the top well (through 8 μM diameter pores) were stained with propidium iodide and quantified. Representative micrographs of cells that migrated for each treatment are shown.
Vav is a GEF for RhoA, Rac1, and Cdc42; however, Vav-mediated cell transformation has been ascribed mainly to its ability to activate Rac1 [93]. At higher concentrations (≥10 μM), EHop-016 also inhibits Cdc42, but not RhoA, indicating a potential inhibition of Cdc42 activity either via blocking the interaction of Cdc42 with Vav or alternative GEFs. Taken together, our results agree with the reports of Rac1 and Cdc42 mediating the constitutively active Vav2 phenotype, with Rho A playing an antagonistic role [147].
2.3.4. EHop-016 inhibits Rac signaling to PAK, the actin cytoskeleton, and cancer cell migration
The downstream effector of Rac, PAK, is a key regulator of the Rac-mediated actin cytoskeletal changes that direct forward migration during cancer invasion, as well as cell adhesion, survival, and proliferation [60–62]. Therefore, we determined the effect of EHop-016 on PAK activity. As shown in Fig. 6.5, 4 μM EHop-016 reduced PAK activity by ~80%. We also reported that at similar concentrations, EHop-016 significantly reduced the extension of Rac-directed motile actin structures called lamellipodia (~70%) in both MDA-MB-435 (Fig. 6.5) and MDA-MB-231 cells [124]. Since leading edge lamellipodia are important for directed migration, we tested the effect of EHop-016 on cell migration by a Transwell assay. At the concentrations of EHop-016 that inhibit Rac activity, EHop-016 significantly reduced directed cell migration by ~60% (Fig. 6.5) Therefore, inhibition of Vav interaction with Rac and Cdc42 by EHop-016 results in reduced metatstaic cancer cell viability, lamellipodia extension, and cell migration, implicating EHop-016 as a viable antimetastatic cancer therapeutic.
2.3.5. Summary
The working model that the Rac inhibitor EHop-016 can impede breast cancer metastasis is based on our data that EHop-016 inhibits the interaction of Vav with Rac, PAK activity, and decreases invasive actin structures and migration [124] (Fig. 6.6). Inhibition of the Vav/Rac interaction may not only impede PAK signaling to the actin cytoskeleton, it also has the potential to block effects of PAK on cell survival and proliferation [60–62]. Moreover, our data suggest that EHop-016 may block GEFs other than Vav. These may be oncogenic GEFs, such as p-REX that is regulated via GPCR-mediated activation of PI3-K that has been implicated in Rac-mediated cell migration/invasion, tumorigenesis, and metastasis [23,25].
Figure 6.6.
Effects of EHop-016 on Rac signaling. EHop-016 inhibits the Vav2/Rac interaction, Rac and PAK activities, lamellipodia formation, and metastatic cancer cell migration. Thus, EHop-016 holds promise as an anticancer metastatic agent.
3. FUTURE DIRECTIONS
3.1. EHop-016 in metastatic cancer therapy
Future directions include the identification of the role of Vav and other GEFs that may be inhibited by EHop-016, and a comprehensive analysis of all of the molecular mechanisms that are inhibited by EHop-016. As shown in Fig. 6.1, EHop-016 may impede cancer progression via multiple mechanisms as a result of the specific inhibition of the interaction of Vav with Racs and Cdc42, as well as other Rho.GEFs that will be identified in future studies. Vav/Rac signaling is critical for normal immune function [148], and EHop-016 administration for long periods may have adverse effects on the immune system. In preliminary studies, we determined that administration of EHop-016 (10 mg/kg BW) by weekly oral gavage to athymice nude mice for ~2 months did not affect average mouse weight (see supplementary data [124]). Although athymic nude mice lack T lymphocytes, they possess B cells, macrophages, and neutrophils, whose activities are modulated by Vav1/Rac2 signaling [149–152]. Since macrophages and neutrophils within the breast tumor microenvironment promote cancer cell invasion [3,153,154], inhibition of Vav/Rac signaling in immune cells may in fact enhance the antimetastatic effects of EHop-016. Therefore, the importance of Vav/Rac signaling in both hematopoietic and nonhematopoietic cell signaling offers the potential for targeted delivery of EHop-016 to impede both cancer cell and stromal/immune cell crosstalk signaling and invasion in the tumor microenvironment.
3.2. EHop-016 as a chemosensitization agent
Our initial characterization of EHop-016 was conducted with two human metastatic breast cancer cell lines: the ER (–) HER2 (++) MDA-MB-435 high metastatic cells and the ERα (–) MDA-MB-231 þþlowthat metastatic cells do not express a functional HER2. The marked inhibitory effects of EHop-016 on the MDA-MB-435 cells that have high intrinsic Rac and Vav activities [36,124] suggest that HER2 signaling to Vav and Rac, or oncogenic Vav activity of the MDA-MB-435 cell line is inhibited by EHop-016.
EGFR1 and HER2 are often upregulated in metastatic breast cancers and HER2 amplification is a prognostic factor for metastatic breast cancer [155,156]. Such overexpression of HER2 occurs in 30% of breast cancer patients and leads to increased metastasis and reduced disease-free survival [157–159]. Intriguingly, a number of studies have linked HER2 signaling with Rac activity in metastatic cancer. Rac activity is associated with trastuzumab resistance and inhibition of Rac has been shown to reverse trastuzumab resistance [122,123]. Moreover, overexpression of HER2 in mammary epithelial cells increased Rac1 activity [54]. HER2 expression has also been correlated with the activity of the Rac downstream effector PAK in human breast cancer specimens [59].
The current therapies for HER2 type breast cancer are the monoclonal antibody trastuzumab (Herceptin) and the tyrosine kinase inhibitor lapatinib [160]. Unfortunately, patients often present with intrinsic or acquired resistance to these anti-EGFR therapeutics [161,162]. Trastuzumab resistance has been attributed to many factors, including bypass signaling through other receptors and activation of downstream signaling pathways independent of HER2 [157,163]. Therefore, a viable strategy to overcome resistance to HER2-directed therapy is combinatorial therapies targeting HER2, as well as downstream resistance pathways [164].
Elevated downstream signaling in trastuzumab-resistant breast cancers has been attributed to MAPK and PI3-K/Akt/mTOR signaling pathways [121,163,165–168]. As discussed in Section 1, PI3-K signaling activates Rac GEFs, such as Vav, p-REX-1, and Tiam-1, that have been implicated in increased metastatic properties and survival of HER-2 overexpressing cells [114,115,117] (Fig. 6.1). Moreover, receptor-activated or oncogenic Vavs regulate tumor progression, invasion, and angiogenesis via Rac-mediated activation of PAK, p38 MAPK, and JNK [109–113].
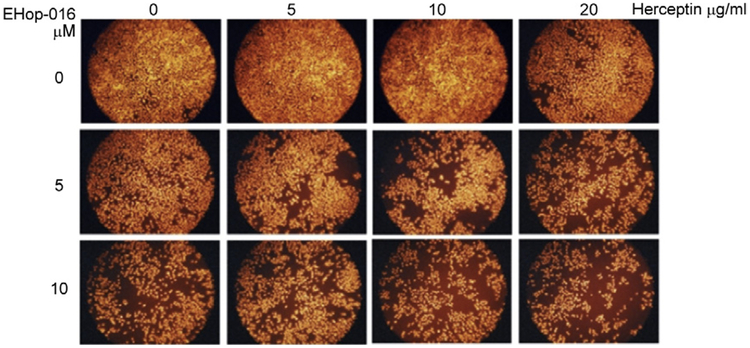
Our initial studies to test the potential of EHop-016 to sensitize the trastuzumab-resistant MDA-MB-435 cell line to trastuzumab therapy are promising. As shown in Fig. 6.2A, following a 24-h treatment, trastuzumab did not affect MDA-MB-435 cell viability at concentrations as high as 20 μg/ml. As previously reported by us [124] and shown in Fig. 6.3, MDA-MB-435 cell numbers decreased ~20% and 50%, respectively, in response to 5 and 10 μM EHop-016. Interestingly, MDA-MB-435 cell viability decreased to a higher extent, that is, ~40% and 80%, respectively, when 5 or 10 μM of EHop-016 was combined with 5 or 10 μg/ml trastuzumab (Fig. 6.7). These data indicate a synergistic effect of EHop-016 and trastuzumab on inhibition of MDA-MB-435 cell viability.
Figure 6.7.
Effect of EHop-016 and trastuzumab on MDA-MB-435 cell viability. Trastuzumab-resistant MDA-MB-435 cells were treated with 0, 5, 10 μM EHop-016 (rows) or 0, 5, 10 μg/ml trastuzumab (columns) for 24 h, fixed and stained with propidium iodide. Representative micrographs from duplicates are shown.
A recent paradigm shift in effective aggressive breast cancer therapy is to mitigate the high prevalence of intrinsic and acquired resistance to singleagent regimens by dual anti-HER2 therapy of trastuzumab and other inhibitors [169,170]. Therefore, our characterization of EHop-016 offers a timely alternative to effective combinatorial therapy. We are currently testing the pharmacodynamics, pharmacokinetics, toxicity, and efficiency of EHop-016 in mouse models.
3.3. Development of next-generation inhibitors
EHop-016 fits some of the criteria for a specific inhibitor of metastasis by demonstrating an IC50 of 1 μM and being specific for the activation of Rac by Vav. Moreover, EHop-016 does not affect cell viability at <10 μM, and is relatively soluble in aqueous solutions. However, the micromolar effective concentrations of EHop-016 may prove to be physiologically insufficient for development as a viable cancer therapeutic. Therefore, plans are underway to develop the next generation of EHop-016 compounds that are expected to be effective at lower (nanomloar) concentrations with a tighter and more energetically favorable binding at the GEF interaction site of Rac.
ACKNOWLEDGMENTS
We thank the following members of the Dharmawardhane laboratory who characterized the efficacy of EHop-016 in metastatic cancer cells: Linette Castillo-Pichardo, Ph.D.; Alina De La Mota-Peynado; Tessa Humphries-Bickley; and Brendaliz Montalvo-Ortiz, Ph.D. This work was supported by DoD/US Army BCRPW81XWH-0701-0330 and NIH/NIGMS SC3GM084824-02S1 (to S. Dharmawardhane), RCMI G12RR03051 to UPR MSC, and RCMI G12RR03035 to Universidad Central del Caribe, PR.
REFERENCES
- [1].Riethdorf S, Pantel K, Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization, Pathobiology 75 (2008) 140–148. [DOI] [PubMed] [Google Scholar]
- [2].Woodhouse EC, Chuaqui RF, Liotta LA, General mechanisms of metastasis, Cancer 80 (1997) 1529–1537. [DOI] [PubMed] [Google Scholar]
- [3].Condeelis J, Pollard JW, Macrophages: obligate partners for tumor cell migration, invasion, and metastasis, Cell 124 (2006) 263–266. [DOI] [PubMed] [Google Scholar]
- [4].Smith HA, Kang Y, The metastasis-promoting roles of tumor-associated immune cells, J. Mol. Med 91 (4) (2013) 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN, Overview of resistance to systemic therapy in patients with breast cancer, Adv. Exp. Med. Biol 608 (2007) 1–22. [DOI] [PubMed] [Google Scholar]
- [6].Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J, Genes that mediate breast cancer metastasis to lung, Nature 436 (2005) 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mundy GR, Metastasis to bone: causes, consequences and therapeutic opportunities, Nat. Rev. Cancer 2 (2002) 584–593. [DOI] [PubMed] [Google Scholar]
- [8].Gligorov J, Lotz JP, Optimal treatment strategies in postmenopausal women with hormone-receptor-positive and HER2-negative metastatic breast cancer, Breast Cancer Res. Treat 112 (2008) 53–66. [DOI] [PubMed] [Google Scholar]
- [9].Condeelis J, Singer RH, Segall JE, The great escape: when cancer cells hijack the genes for chemotaxis and motility, Annu. Rev. Cell Dev. Biol 21 (2005) 695–718. [DOI] [PubMed] [Google Scholar]
- [10].Steeg PS, Metastasis suppressors alter the signal transduction of cancer cells, Nat. Rev. Cancer 3 (2003) 55–63. [DOI] [PubMed] [Google Scholar]
- [11].Vega FM, Ridley AJ, Rho GTPases in cancer cell biology, FEBS Lett 582 (2008) 2093–2101. [DOI] [PubMed] [Google Scholar]
- [12].Ridley AJ, Regulation of macrophage adhesion and migration by Rho GTP-binding proteins, J. Microsc 231 (2008) 518–523. [DOI] [PubMed] [Google Scholar]
- [13].Heasman SJ, Ridley AJ, Mammalian Rho GTPases: new insights into their functions from in vivo studies, Nat. Rev. Mol. Cell Biol 9 (2008) 690–701. [DOI] [PubMed] [Google Scholar]
- [14].Ridley AJ, Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking, Trends Cell Biol 16 (2006) 522–529. [DOI] [PubMed] [Google Scholar]
- [15].Rathinam R, Berrier A, Alahari SK, Role of Rho GTPases and their regulators in cancer progression, Front. Biosci 17 (2012) 2561–2571. [DOI] [PubMed] [Google Scholar]
- [16].Dharmawardhane S, Bokoch GM, Rho GTPases and leukocyte cytoskeletal regulation, Curr. Opin. Hematol 4 (1997) 12–18. [DOI] [PubMed] [Google Scholar]
- [17].Hall A, Rho GTPases and the control of cell behaviour, Biochem. Soc. Trans 33 (2005) 891–895. [DOI] [PubMed] [Google Scholar]
- [18].Lin M, van Golen KL, Rho-regulatory proteins in breast cancer cell motility and invasion, Breast Cancer Res. Treat 84 (2004) 49–60. [DOI] [PubMed] [Google Scholar]
- [19].Kleer CG, Griffith KA, Sabel MS, Gallagher G, van Golen KL, Wu ZF, Merajver SD, RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast, Breast Cancer Res. Treat 93 (2005) 101–110. [DOI] [PubMed] [Google Scholar]
- [20].Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR,Symons M, Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion, Oncogene 24 (2005) 7821–7829. [DOI] [PubMed] [Google Scholar]
- [21].Burbelo P, Wellstein A, Pestell RG, Altered Rho GTPase signaling pathways in breast cancer cells, Breast Cancer Res. Treat 84 (2004) 43–48. [DOI] [PubMed] [Google Scholar]
- [22].Katz E, Sims AH, Sproul D, Caldwell H, Dixon MJ, Meehan RR, Harrison DJ, Targeting of Rac GTPases blocks the spread of intact human breast cancer, Oncotarget 3 (2012) 608–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bustelo XR, Intratumoral stages of metastatic cells: a synthesis of ontogeny, Rho/Rac GTPases, epithelial-mesenchymal transitions, and more, Bioessays 34 (2012) 748–759. [DOI] [PubMed] [Google Scholar]
- [24].Barrio-Real L, Kazanietz MG, Rho GEFs and cancer: linking gene expression and metastatic dissemination, Sci. Signal 5 (2012) e43. [DOI] [PubMed] [Google Scholar]
- [25].Parri M, Chiarugi P, Rac and Rho GTPases in cancer cell motility control, Cell Commun. Signaling 8 (2010) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Soledad SM, Kazanietz MG, Rac signaling in breast cancer: a tale of GEFs and GAPs, Cell. Signal 24 (2011) 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A, The diverse roles of Rac signaling in tumorigenesis, Cell Cycle 10 (2011) 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bustelo XR, Sauzeau V, Berenjeno IM, GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo, Bioessays 29 (2007) 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pai SY, Kim C, Williams DA, Rac GTPases in human diseases, Dis. Markers 29 (2010) 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shi HY, Stafford LJ, Liu Z, Liu M, Zhang M, Maspin controls mammary tumor cell migration through inhibiting Rac1 and Cdc42, but not the RhoA GTPase, Cell Motil. Cytoskeleton 64 (May 2007) 338–346. [DOI] [PubMed] [Google Scholar]
- [31].Hirsch DS, Wu WJ, Cdc42: an effector and regulator of ErbB1 as a strategic target in breast cancer therapy, Expert Rev. Anticancer Ther 7 (2007) 147–157. [DOI] [PubMed] [Google Scholar]
- [32].Qiu RG, Chen J, Kirn D, McCormick F, Symons M, An essential role for Rac in Ras transformation, Nature 374 (1995) 457–459. [DOI] [PubMed] [Google Scholar]
- [33].Renshaw MW, Lea-Chou E, Wang JY, Rac is required for v-Abl tyrosine kinase to activate mitogenesis, Curr. Biol 6 (1996) 76–83. [DOI] [PubMed] [Google Scholar]
- [34].Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S, Mei Q,Karlsson R, Chrostek-Grashoff A, Brakebusch C, Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo, Oncogene 29 (2010) 3362–3373. [DOI] [PubMed] [Google Scholar]
- [35].Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF, Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells, Neoplasia 9 (2007) 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF, Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells, Breast Cancer Res 7 (2005) R965–R974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG, Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway, Proc. Natl. Acad. Sci. U.S.A 97 (2000) 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, Zhang B, Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein, Mol. Cancer Ther 9 (2010) 1657–1668. [DOI] [PubMed] [Google Scholar]
- [39].Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E, Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b, Oncogene 19 (2000) 3013–3020. [DOI] [PubMed] [Google Scholar]
- [40].Walf-Vorderwulbecke V, de Boer J, Horton SJ, van Amerongen R, Proost N, Berns A, Williams O, Frat2 mediates the oncogenic activation of Rac by MLL fusions, Blood 120 (2012) 4819–4828. [DOI] [PubMed] [Google Scholar]
- [41].Dalton LE, Kamarashev J, Barinaga-Rementeria Ramirez I, White G, Malliri A,Hurlstone A, Constitutive Rac activation is not sufficient to initiate melanocyte neoplasia but accelerates malignant progression, J. Invest. Dermatol 133 (2013) 1572–1581. [DOI] [PubMed] [Google Scholar]
- [42].Wertheimer E, Kazanietz MG, Rac1 takes center stage in pancreatic cancer and ulcerative colitis: quantity matters, Gastroenterology 141 (2011) 427–430. [DOI] [PubMed] [Google Scholar]
- [43].Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, Siveke JT, Early requirement of Rac1 in a mouse model of pancreatic cancer, Gas-troenterology 141 (2011) 719–730. [DOI] [PubMed] [Google Scholar]
- [44].Morris CM, Haataja L, McDonald M, Gough S, Markie D, Groffen J, Heisterkamp N, The small GTPase RAC3 gene is located within chromosome band 17q25.3 outside and telomeric of a region commonly deleted in breast and ovarian tumours, Cytogenet. Cell Genet 89 (2000) 18–23. [DOI] [PubMed] [Google Scholar]
- [45].Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, Fan D, Expression of seven main Rho family members in gastric carcinoma, Biochem. Biophys. Res. Commun 315 (2004) 686–691. [DOI] [PubMed] [Google Scholar]
- [46].Hwang SL, Chang JH, Cheng TS, Sy WD, Lieu AS, Lin CL, Lee KS, Howng SL, Hong YR, Expression of Rac3 in human brain tumors, J. Clin. Neurosci 12 (2005) 571–574. [DOI] [PubMed] [Google Scholar]
- [47].Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ, Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation, Oncogene 23 (2004) 9369–9380. [DOI] [PubMed] [Google Scholar]
- [48].Stallings-Mann M, Radisky D, Matrix metalloproteinase-induced malignancy in mammary epithelial cells, Cells Tissues Organs 185 (2007) 104–110. [DOI] [PubMed] [Google Scholar]
- [49].Jordan P, Brazao R, Boavida MG, Gespach C, Chastre E, Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors, Oncogene 18 (1999) 6835–6839. [DOI] [PubMed] [Google Scholar]
- [50].Matos P, Jordan P, Increased Rac1b expression sustains colorectal tumor cell survival, Mol. Cancer Res 6 (2008) 1178–1184. [DOI] [PubMed] [Google Scholar]
- [51].Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, StemkeHale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DS, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L, A landscape of driver mutations in melanoma, Cell 150 (2012) 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Muise AM, Walters T, Xu W, Shen-Tu G, Guo CH, Fattouh R, Lam GY, Wolters VM, Bennitz J, van Limbergen J, Renbaum P, Kasirer Y, Ngan BY, Turner D, Denson LA, Sherman PM, Duerr RH, Cho J, Lees CW, Satsangi J, Wilson DC, Paterson AD, Griffiths AM, Glogauer M, Silverberg MS, Brumell JH, Single nucleotide polymorphisms that increase expression of the guanosine triphosphatase RAC1 are associated with ulcerative colitis, Gastroenterology 14 (2011) 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kawazu M, Ueno T, Kontani K, Ogita Y, Ando M, Fukumura K, Yamato A, Soda M, Takeuchi K, Miki Y, Yamaguchi H, Yasuda T, Naoe T, Yamashita Y, Katada T, Choi YL, Mano H, Transforming mutations of RAC guanosine triphosphatases in human cancers, Proc. Natl. Acad. Sci. U.S.A 110 (2013) 3029–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ueda Y, Wang S, Dumont N, Yi JY, Koh Y, Arteaga CL, Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility, J. Biol. Chem 279 (2004) 24505–24513. [DOI] [PubMed] [Google Scholar]
- [55].Rosenblatt AE, Garcia MI, Lyons L, Xie Y, Maiorino C, Desire L, Slingerland J, Burnstein KL, Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer, Endocr. Relat. Cancer 18 (2011) 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vu T, Claret FX, Trastuzumab: updated mechanisms of action and resistance in breast cancer, Front Oncol 2 (2012) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Whale A, Hashim FN, Fram S, Jones GE, Wells CM, Signalling to cancer cell invasion through PAK family kinases, Front. Biosci 16 (2011) 849–864. [DOI] [PubMed] [Google Scholar]
- [58].Dummler B, Ohshiro K, Kumar R, Field J, Pak protein kinases and their role in cancer, Cancer Metastasis Rev 28 (2009) 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, Chernoff J, A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells, Oncogene 29 (2010) 5839–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ye DZ, Field J, PAK signaling in cancer, Cell Logist 2 (2012) 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Menges CW, Sementino E, Talarchek J, Xu J, Chernoff J, Peterson JR, Testa JR, Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways, Mol. Cancer Res 10 (2012) 1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rias-Romero LE, Chernoff J, p21-Activated kinases in Erbb2-positive breast cancer: a new therapeutic target? Small Gtpases 1 (2010) 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R, Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase, J. Biol. Chem 273 (1998) 28238–28246. [DOI] [PubMed] [Google Scholar]
- [64].Brumby AM, Goulding KR, Schlosser T, Loi S, Galea R, Khoo P, Bolden JE, Aigaki T, Humbert PO, Richardson HE, Identification of novel Ras-cooperating oncogenes in Drosophila melanogaster: a RhoGEF/Rho-family/JNK pathway is a central driver of tumorigenesis, Genetics 188 (2011) 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Samaga R, Saez-Rodriguez J, Alexopoulos LG, Sorger PK, Klamt S, The logic of EGFR/ErbB signaling: theoretical properties and analysis of high-throughput data, PLoS Comput. Biol 5 (2009) e1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Saci A, Cantley LC, Carpenter CL, Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size, Mol. Cell 42 (2011) 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM, mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways, Cancer Res 71 (2011) 3246–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bracho-Valdes I, Moreno-Alvarez P, Valencia-Martinez I, Robles-Molina E, Chavez-Vargas L, Vazquez-Prado J, mTORC1- and mTORC2-interacting proteins keep their multifunctional partners focused, IUBMB Life 63 (2011) 880–898. [DOI] [PubMed] [Google Scholar]
- [69].Bokoch GM, Diebold B, Kim JS, Gianni D, Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases, Antioxid. Redox Signal 11 (2009) 2429–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Park SJ, Kim YT, Jeon YJ, Antioxidant dieckol downregulates the Rac1/ROS signaling pathway and inhibits Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma cells, Mol. Cells 33 (2012) 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nieborowska-Skorska M, Kopinski PK, Ray R, Hoser G, Ngaba D, Flis S, Cramer K, Reddy MM, Koptyra M, Penserga T, Glodkowska-Mrowka E, Bolton E, Holyoake TL, Eaves CJ, Cerny-Reiterer S, Valent P, Hochhaus A, Hughes TP, van der Kuip H, Sattler M, Wiktor-Jedrzejczak W, Richardson C, Dorrance A, Stoklosa T, Williams DA, Skorski T, Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors, Blood 119 (2012) 4253–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wu WS, The signaling mechanism of ROS in tumor progression, Cancer Metastasis Rev 25 (2006) 695–705. [DOI] [PubMed] [Google Scholar]
- [73].Costa C, Germena G, Hirsch E, Dissection of the interplay between class I PI3Ks and Rac signaling in phagocytic functions, Sci. World J 10 (2010) 1826–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yang HW, Shin MG, Lee S, Kim JR, Park WS, Cho KH, Meyer T, Do HW, Cooperative activation of PI3K by Ras and Rho family small GTPases, Mol. Cell 47 (2012) 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kim EK, Yun SJ, Ha JM, Kim YW, Jin IH, Yun J, Shin HK, Song SH, Kim JH, Lee JS, Kim CD, Bae SS, Selective activation of Akt1 by mammalian target of rapamycin complex 2 regulates cancer cell migration, invasion, and metastasis, Oncogene 30 (2011) 2954–2963. [DOI] [PubMed] [Google Scholar]
- [76].Caino MC, Lopez-Haber C, Kissil JL, Kazanietz MG, Non-small cell lung carcinoma cell motility, rac activation and metastatic dissemination are mediated by protein kinase C epsilon, PLoS One 7 (2012) e31714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aksamitiene E, Achanta S, Kolch W, Kholodenko BN, Hoek JB, Kiyatkin A, Prolactin-stimulated activation of ERK1/2 mitogen-activated protein kinases is controlled by PI3-kinase/Rac/PAK signaling pathway in breast cancer cells, Cell. Signal 23 (2011) 1794–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ramgolam VS, DeGregorio SD, Rao GK, Collinge M, Subaran SS,Markovic-Plese S, Pardi R, Bender JR, T cell LFA-1 engagement induces HuR-dependent cytokine mRNA stabilization through a Vav-1, Rac1/2, p38MAPK and MKK3 signaling cascade, PLoS One 5 (2010) e14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang Y, Rivera Rosado LA, Moon SY, Zhang B, Silencing of D4-GDI inhibits growth and invasive behavior in MDA-MB-231 cells by activation of Rac-dependent p38 and JNK signaling, J. Biol. Chem 284 (2009) 12956–12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Arulanandam R, Geletu M, Feracci H, Raptis L, Activated Rac1 requires gp130 for Stat3 activation, cell proliferation and migration, Exp. Cell Res 316 (2010) 875–886. [DOI] [PubMed] [Google Scholar]
- [81].Laplante M, Sabatini DM, mTOR signaling in growth control and disease, Cell 149 (2012) 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kichina JV, Goc A, Al-Husein B, Somanath PR, Kandel ES, PAK1 as a therapeutic target, Expert Opin. Ther. Targets 14 (2010) 703–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yi C, Maksimoska J, Marmorstein R, Kissil JL, Development of small-molecule inhibitors of the group I p21-activated kinases, emerging therapeutic targets in cancer, Biochem. Pharmacol 80 (2010) 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O’Brien T, Vucic D, Harris AL, Belvin M, Friedman LS, Blackwood EM, Koeppen H, Hoeflich KP, Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells, Proc. Natl. Acad. Sci. U.S.A 108 (2011) 7177–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bishop AL, Hall A, Rho GTPases and their effector proteins, Biochem. J 348 (2000) 241–255. [PMC free article] [PubMed] [Google Scholar]
- [86].Baranwal S, Alahari SK, Rho GTPase effector functions in tumor cell invasion and metastasis, Curr. Drug Targets 12 (2011) 1194–1201. [DOI] [PubMed] [Google Scholar]
- [87].Deacon SW, Peterson JR, Chemical inhibition through conformational stabilization of Rho GTPase effectors, Handb. Exp. Pharmacol 186 (2008) 431–460. [DOI] [PubMed] [Google Scholar]
- [88].Rossman KL, Der CJ, Sondek J, GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors, Nat. Rev. Mol. Cell Biol 6 (2005) 167–180. [DOI] [PubMed] [Google Scholar]
- [89].Schmidt A, Hall A, Guanine nucleotide exchange factors for Rho GTPases: turning on the switch, Genes Dev 16 (2002) 1587–1609. [DOI] [PubMed] [Google Scholar]
- [90].Bernards A, GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila, Biochim. Biophys. Acta 1603 (2003) 47–82. [DOI] [PubMed] [Google Scholar]
- [91].Hoffman GR, Cerione RA, Signaling to the Rho GTPases: networking with the DH domain, FEBS Lett 513 (2002) 85–91. [DOI] [PubMed] [Google Scholar]
- [92].Adams HC III., Chen R, Liu Z, Whitehead IP, Regulation of breast cancer cell motility by T-cell lymphoma invasion and metastasis-inducing protein, Breast Cancer Res 12 (2010) R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Palmby TR, Abe K, Karnoub AE, Der CJ, Vav transformation requires activation of multiple GTPases and regulation of gene expression, Mol. Cancer Res 2 (2004) 702–711. [PubMed] [Google Scholar]
- [94].Miller SL, DeMaria JE, Freier DO, Riegel AM, Clevenger CV, Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor, Mol. Endocrinol 19 (2005) 939–949. [DOI] [PubMed] [Google Scholar]
- [95].Minard ME, Kim LS, Price JE, Gallick GE, The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression, Breast Cancer Res. Treat 84 (2004) 21–32. [DOI] [PubMed] [Google Scholar]
- [96].Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, Luo J, Benovic JL, Klein-Szanto A, Yagi H, Gutkind JS, Parsons RE, Kazanietz MG, Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer, Mol. Cell 40 (2010) 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Montero JC, Seoane S, Ocana A, Pandiella A, P-Rex1 participates in Neuregulin-ErbB signal transduction and its expression correlates with patient outcome in breast cancer, Oncogene 30 (2011) 1059–1071. [DOI] [PubMed] [Google Scholar]
- [98].Citterio C, Menacho-Marquez M, Garcia-Escudero R, Larive RM, Barreiro O,Sanchez-Madrid F, Paramio JM, Bustelo XR, The rho exchange factors vav2 and vav3 control a lung metastasis-specific transcriptional program in breast cancer cells, Sci. Signal 5 (2012) ra71. [DOI] [PubMed] [Google Scholar]
- [99].Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan-Ramu N, Katzav S, The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas, J. Pathol 199 (2003) 526–533. [DOI] [PubMed] [Google Scholar]
- [100].Denicola G, Tuveson DA, VAV1: a new target in pancreatic cancer? Cancer Biol. Ther 4 (2005) 509–511. [DOI] [PubMed] [Google Scholar]
- [101].Lazer G, Idelchuk Y, Schapira V, Pikarsky E, Katzav S, The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis, J. Pathol 219 (2009) 25–34. [DOI] [PubMed] [Google Scholar]
- [102].Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J, Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion, Cancer Res 66 (2006) 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lin KT, Gong J, Li CF, Jang TH, Chen WL, Chen HJ, Wang LH, Vav3-Rac1 signaling regulates prostate cancer metastasis with elevated Vav3 expression correlating with prostate cancer progression and posttreatment recurrence, Cancer Res 72 (2012) 3000–3009. [DOI] [PubMed] [Google Scholar]
- [104].Valderrama F, Thevapala S, Ridley AJ, Radixin regulates cell migration and cell-cell adhesion through Rac1, J. Cell Sci 125 (2012) 3310–3319. [DOI] [PubMed] [Google Scholar]
- [105].Sastry SK, Rajfur Z, Liu BP, Cote JF, Tremblay ML, Burridge K, PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP, J. Biol. Chem 281 (2006) 11627–11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ilan L, Katzav S, Human Vav1 expression in hematopoietic and cancer cell lines is regulated by c-Myb and by CpG methylation, PLoS One 7 (2012) e29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Groysman M, Hornstein I, Alcover A, Katzav S, Vav1 and Ly-GDI two regulators of Rho GTPases, function cooperatively as signal transducers in T cell antigen receptor-induced pathways, J. Biol. Chem 277 (2002) 50121–50130. [DOI] [PubMed] [Google Scholar]
- [108].Oberley MJ, Wang DS, Yang DT, Vav1 in hematologic neoplasms, a mini review, Am. J. Blood Res 2 (2012) 1–8. [PMC free article] [PubMed] [Google Scholar]
- [109].Brantley-Sieders DM, Zhuang G, Vaught D, Freeman T, Hwang Y, Hicks D, Chen J, Host deficiency in Vav2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo, Mol. Cancer Res 7 (2009) 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Garrett TA, Van Buul JD, Burridge K, VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2, Exp. Cell Res 313 (2007) 3285–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Liu BP, Burridge K, Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins, Mol. Cell Biol 20 (2000) 7160–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Patel V, Rosenfeldt HM, Lyons R, Servitja JM, Bustelo XR, Siroff M, Gutkind JS, Persistent activation of Rac1 in squamous carcinomas of the head and neck: evidence for an EGFR/Vav2 signaling axis involved in cell invasion, Carcinogenesis 28 (2007) 1145–1152. [DOI] [PubMed] [Google Scholar]
- [113].Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS, Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src, J. Biol. Chem 278 (2003) 34339–34346. [DOI] [PubMed] [Google Scholar]
- [114].Yang C, Klein EA, Assoian RK, Kazanietz MG, Heregulin beta1 promotes breast cancer cell proliferation through Rac/ERK-dependent induction of cyclin D1 and p21Cip1, Biochem. J 410 (2008) 167–175. [DOI] [PubMed] [Google Scholar]
- [115].De Laurentiis A, Pardo OE, Palamidessi A, Jackson SP, Schoenwaelder SM, Reichmann E, Scita G, Arcaro A, The catalytic class I(A) PI3K isoforms play divergent roles in breast cancer cell migration, Cell. Signal 23 (2010) 529–541. [DOI] [PubMed] [Google Scholar]
- [116].Sachdev P, Zeng L, Wang LH, Distinct role of phosphatidylinositol 3-kinase and Rho family GTPases in Vav3-induced cell transformation, cell motility, and morphological changes, J. Biol. Chem 277 (2002) 17638–17648. [DOI] [PubMed] [Google Scholar]
- [117].Wang SE, Shin I, Wu FY, Friedman DB, Arteaga CL, HER2/Neu (ErbB2) signaling to Rac1-Pak1 is temporally and spatially modulated by transforming growth factor beta, Cancer Res 66 (2006) 9591–9600. [DOI] [PubMed] [Google Scholar]
- [118].Zeng L, Sachdev P, Yan L, Chan JL, Trenkle T, McClelland M, Welsh J, Wang LH, Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation, Mol. Cell Biol 20 (2000) 9212–9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Marcoux N, Vuori K, EGF receptor mediates adhesion-dependent activation of the Rac GTPase: a role for phosphatidylinositol 3-kinase and Vav2, Oncogene 22 (2003) 6100–6106. [DOI] [PubMed] [Google Scholar]
- [120].Wilsbacher JL, Moores SL, Brugge JS, An active form of Vav1 induces migration of mammary epithelial cells by stimulating secretion of an epidermal growth factor receptor ligand, Cell Commun. Signal 4 (2006) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ, HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment, Breast Cancer Res. Treat 122 (2010) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhao Y, Wang Z, Jiang Y, Yang C, Inactivation of Rac1 reduces Trastuzumab resistance in PTEN deficient and insulin-like growth factor I receptor overexpressing human breast cancer SKBR3 cells, Cancer Lett 313 (2011) 54–63. [DOI] [PubMed] [Google Scholar]
- [123].Dokmanovic M, Hirsch DS, Shen Y, Wu WJ, Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer, Mol. Cancer Ther 8 (2009) 1557–1569. [DOI] [PubMed] [Google Scholar]
- [124].Montalvo-Ortiz BL, Castillo-Pichardo L, Hernandez E, Humphries-Bickley T, De LM-P, Cubano LA, Vlaar CP, Dharmawardhane S, Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase, J. Biol. Chem 287 (2012) 13228–13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y, Rational design and characterization of a Rac GTPase-specific small molecule inhibitor, Proc. Natl. Acad. Sci. U.S.A 101 (2004) 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nassar N, Cancelas J, Zheng J, Williams DA, Zheng Y, Structure-function based design of small molecule inhibitors targeting Rho family GTPases, Curr. Top. Med. Chem 6 (2006) 1109–1116. [DOI] [PubMed] [Google Scholar]
- [127].Gao Y, Xing J, Streuli M, Leto TL, Zheng Y, Trp(56) of rac1 specifies interaction with a subset of guanine nucleotide exchange factors, J. Biol. Chem 276 (2001) 47530–47541. [DOI] [PubMed] [Google Scholar]
- [128].Gastonguay A, Berg T, Hauser AD, Schuld N, Lorimer E, Williams CL, The role of Rac1 in the regulation of NF-kB activity, cell proliferation, and cell migration in non-small cell lung carcinoma, Cancer Biol. Ther 13 (2012) 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Mizukawa B, Wei J, Shrestha M, Wunderlich M, Chou FS, Griesinger A, Harris CE, Kumar AR, Zheng Y, Williams DA, Mulloy JC, Inhibition of Rac GTPase signaling and downstream prosurvival Bcl-2 proteins as combination targeted therapy in MLL-AF9 leukemia, Blood 118 (2011) 5235–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chen QY, Xu LQ, Jiao DM, Yao QH, Wang YY, Hu HZ, Wu YQ, Song J, Yan J, Wu LJ, Silencing of Rac1 modifies lung cancer cell migration, invasion and actin cytoskeleton rearrangements and enhances chemosensitivity to antitumor drugs, Int. J. Mol. Med 28 (2011) 769–776. [DOI] [PubMed] [Google Scholar]
- [131].Hamalukic M, Huelsenbeck J, Schad A, Wirtz S, Kaina B, Fritz G, Rac1-regulated endothelial radiation response stimulates extravasation and metastasis that can be blocked by HMG-CoA reductase inhibitors, PLoS One 6 (2011) e26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y, Rational design and applications of a Rac GTPase-specific small molecule inhibitor, Methods Enzymol 406 (2006) 554–565. [DOI] [PubMed] [Google Scholar]
- [133].Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, Neviani P, Druker BJ, Setchell KD, Zheng Y, Harris CE, Williams DA, Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease, Cancer Cell 12 (2007) 467–478. [DOI] [PubMed] [Google Scholar]
- [134].Binker MG, Binker-Cosen AA, Gaisano HY, Cosen-Binker LI, Inhibition of Rac1 decreases the severity of pancreatitis and pancreatitis-associated lung injury in mice, Exp. Physiol 93 (2008) 1091–1103. [DOI] [PubMed] [Google Scholar]
- [135].Ferri N, Corsini A, Bottino P, Clerici F, Contini A, Virtual screening approach for the identification of new Rac1 inhibitors, J. Med. Chem 52 (2009) 4087–4090. [DOI] [PubMed] [Google Scholar]
- [136].Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ, Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases, J. Biol. Chem 282 (2007) 35666–35678. [DOI] [PubMed] [Google Scholar]
- [137].Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ, Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases, Methods Enzymol 439 (2008) 111–129. [DOI] [PubMed] [Google Scholar]
- [138].Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM, Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells, Circ. Res 106 (2010) 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Davare MA, Saneyoshi T, Soderling TR, Calmodulin-kinases regulate basal and estrogen stimulated medulloblastoma migration via Rac1, J. Neurooncol 104 (2011) 65–82. [DOI] [PubMed] [Google Scholar]
- [140].Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Desire L, Leblond B, Andre P, Conley PB, Bergmeier W, Rap1-Rac1 circuits potentiate platelet activation, Arterioscler. Thromb. Vasc. Biol 32 (2012) 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Colomba A, Giuriato S, Dejean E, Thornber K, Delsol G, Tronchere H, Meggetto F, Payrastre B, Gaits-Iacovoni F, Inhibition of Rac controls NPM-ALK-dependent lymphoma development and dissemination, Blood Cancer J 1 (2011) e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Beausoleil E, Chauvignac C, Taverne T, Lacombe S, Pognante L, Leblond B, Pallares D, Oliveira CD, Bachelot F, Carton R, Peillon H, Coutadeur S, Picard V, Lambeng N, Desire L, Schweighoffer F, Structure-activity relationship of isoform selective inhibitors of Rac1/1b GTPase nucleotide binding, Bioorg. Med. Chem. Lett 19 (2009) 5594–5598. [DOI] [PubMed] [Google Scholar]
- [143].Hernandez E, De LM-P, Dharmawardhane S, Vlaar CP, Novel inhibitors of Rac1 in metastatic breast cancer, P. R. Health Sci. J 29 (2010) 348–356. [PubMed] [Google Scholar]
- [144].Benard V, Bokoch GM, Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods, Methods Enzymol 345 (2002) 349–359. [DOI] [PubMed] [Google Scholar]
- [145].Surviladze Z, Waller A, Wu Y, Romero E, Edwards BS, Wandinger-Ness A, Sklar LA, Identification of a small GTPase inhibitor using a high-throughput flow cytometry bead-based multiplex assay, J. Biomol. Screen 15 (2010) 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zheng Y, Nassar N, Skowronek KR. U.S.Patent No. 17,826,982, (2010).
- [147].Duan L, Chen G, Virmani S, Ying G, Raja SM, Chung BM, Rainey MA, Dimri M, Ortega-Cava CF, Zhao X, Clubb RJ, Tu C, Reddi AL, Naramura M, Band V, Band H, Distinct roles for Rho versus Rac/Cdc42 GTPases downstream of Vav2 in regulating mammary epithelial acinar architecture, J. Biol. Chem 285 (2010) 1555–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Turner M, Billadeau DD, VAV proteins as signal integrators for multi-subunit immune-recognition receptors, Nat. Rev. Immunol 2 (2002) 476–486. [DOI] [PubMed] [Google Scholar]
- [149].Malhotra S, Kovats S, Zhang W, Coggeshall KM, Vav and Rac activation in B cell antigen receptor endocytosis involves Vav recruitment to the adapter protein LAB,J. Biol. Chem 284 (2009) 36202–36212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Bhavsar PJ, Vigorito E, Turner M, Ridley AJ, Vav GEFs regulate macrophage morphology and adhesion-induced Rac and Rho activation, Exp. Cell Res 315 (2009) 3345–3358. [DOI] [PubMed] [Google Scholar]
- [151].Ming W, Li S, Billadeau DD, Quilliam LA, Dinauer MC, The Rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity, Mol. Cell Biol 27 (2007) 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kim C, Marchal CC, Penninger J, Dinauer MC, The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions, J. Immunol 171 (2003) 4425–4430. [DOI] [PubMed] [Google Scholar]
- [153].Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J, Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors, Cancer Res 67 (2007) 2649–2656. [DOI] [PubMed] [Google Scholar]
- [154].Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L, Li JJ, Chen YB, Wang DD, Pan QZ, Li XD, Xia JC, The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection, PLoS One 7 (2012) e33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Chan SK, Hill ME, Gullick WJ, The role of the epidermal growth factor receptor in breast cancer, J. Mammary Gland Biol. Neoplasia 11 (2006) 3–11. [DOI] [PubMed] [Google Scholar]
- [156].Arteaga CL, Truica CI, Challenges in the development of anti-epidermal growth factor receptor therapies in breast cancer, Semin. Oncol 31 (2004) 3–8. [DOI] [PubMed] [Google Scholar]
- [157].Bender LM, Nahta R, Her2 cross talk and therapeutic resistance in breast cancer, Front. Biosci 13 (2008) 3906–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Menard S, Fortis S, Castiglioni F, Agresti R, Balsari A, HER2 as a prognostic factor in breast cancer, Oncology 61 (2001) 67–72. [DOI] [PubMed] [Google Scholar]
- [159].Nahta R, Shabaya S, Ozbay T, Rowe DL , Personalizing HER2-targeted therapy in metastatic breast cancer beyond HER2 status: what we have learned from clinical specimens, Curr. Pharmacogenomics Pers. Med 7 (2009) 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Jeyakumar A, Younis T, Trastuzumab for HER2-positive metastatic breast cancer: clinical and economic considerations, Clin. Med. Insights Oncol 6 (2012) 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lantz E, Cunningham I, Higa GM, Targeting HER2 in breast cancer: overview of long-term experience, Int. J. Womens Health 1 (2010) 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Garrett JT, Arteaga CL, Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications, Cancer Biol. Ther 11 (2011) 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wilken JA, Maihle NJ, Primary trastuzumab resistance: new tricks for an old drug, Ann. N. Y. Acad. Sci 1210 (2010) 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Rexer BN, Arteaga CL, Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications, Crit. Rev. Oncog 17 (2012) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wang L, Zhang Q, Zhang J, Sun S, Guo H, Jia Z, Wang B, Shao Z, Wang Z, Hu X, PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib, BMC Cancer 11 (2011) 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Normanno N, De LA, Maiello MR, Campiglio M, Napolitano M, Mancino M, Carotenuto A, Viglietto G, Menard S, The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib, J. Cell. Physiol 207 (2006) 420–427. [DOI] [PubMed] [Google Scholar]
- [167].Brunner-Kubath C, Shabbir W, Saferding V, Wagner R, Singer CF, Valent P, Berger W, Marian B, Zielinski CC, Grusch M, Grunt TW, The PI3 kinase/mTOR blocker NVP-BEZ235 overrides resistance against irreversible ErbB inhibitors in breast cancer cells, Breast Cancer Res. Treat 29 (2010) 387–400. [DOI] [PubMed] [Google Scholar]
- [168].Hynes NE, MacDonald G, ErbB receptors and signaling pathways in cancer, Curr. Opin. Cell Biol 21 (2009) 177–184. [DOI] [PubMed] [Google Scholar]
- [169].Konecny GE, Emerging strategies for the dual inhibition of HER2-positive breast cancer, Curr. Opin. Obstet. Gynecol 25 (2013) 55–65. [DOI] [PubMed] [Google Scholar]
- [170].Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, Guardino E, Song C, Tong B, Ng V, Chu YW, Perez EA, Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer, J. Clin. Oncol 31 (2013) 1157–1163. [DOI] [PubMed] [Google Scholar]