Abstract
Background:
The extent of exposure to tobacco toxicants in smokers who have reduced their cigarette intake compared with smokers who are light smokers is relatively unknown. The goal of this study is to investigate the occurrence of compensatory smoking in reducers compared with light smokers by measuring toxicant exposure.
Methods:
Participants in two smoking reduction intervention studies (N = 64) were selected for comparison with a group of light smokers (N = 62) who smoked the same number of cigarettes as the reducers. A compensatory smoking score was defined (biomarker level for reducer/biomarker level for light smoker) and calculated for urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides (total NNAL), metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-I-(3-pyridyl)-1-butanone, to measure the degree of smoking compensation in reducers when compared with the light smokers.
Results:
The mean level of creatinine-adjusted total NNAL for reducers was over twice that of light smokers even when they smoked about the same number of cigarettes per day. The difference of the mean total NNAL concentrations between light smokers and reducers was highly significant (P < 0.0001). Wide variability in total NNAL concentrations was also observed in reducers, with the extent of this variability between light smokers and reducers being significantly different (P = 0.0005). The level of individual reduction was shown to be a consistent predictor of compensatory smoking (r = 0.50; adjusted Ps = 0.002), with greater cigarette reduction associated with more compensation.
Conclusions:
Compensatory smoking limits the harm reduction value of decreased smoking of cigarettes.
Introduction
Compensatory smoking refers to changes in smoking behavior to adjust for changes in nicotine levels of a tobacco product or changes in amount smoked (1-4). To maintain nicotine levels, smokers increase the extent to which each cigarette is smoked through greater inhalation of mainstream smoke (3). In large part, this compensatory smoking occurs because tobacco use behavior is considered to be primarily driven by nicotine, with users trying to maintain a specific level of nicotine in their bodies (5). Other factors, such as the sensory aspects of smoking or cigarette design, may also play a role in compensatory smoking (6).
Compensatory smoking that occurs during reduced cigarette smoking may result in less reduction in exposure to tobacco-related toxicants than is evident by the extent of cigarette reduction. In a recent study of scheduled smoking reduction using nicotine replacement therapy (7), we observed that smokers who experienced an average of 73% reduction in daily cigarette consumption only showed a 30% reduction in carcinogen uptake as measured by 4-(methylnitrosamino)-1-(3-pyridyl)-l-butanol (NNAL) and its glucuronides (total NNAL). Total NNAL is composed of metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-I-(3-pyridyl)-1-butanone. In an epidemiologic study examining the effect of cigarette reduction on lung cancer risk, smokers who reduced their mean tobacco consumption by at least 50%, from a mean of 22.2 g/d to 8.5 g/d, had an adjusted hazard ratio (HR) of 0.73 compared with heavy smokers (8). Therefore, a reduction of 62% in tobacco consumption amount was associated with only a 27% reduction in lung cancer risk. Thus, the risk reduction is markedly less with smoking reduction and similar to the extent of reduction in total NNAL observed in the study conducted by Hecht et al. (7). The purpose of the current study was to further explore this area by examining the extent of compensatory smoking in the context of smoking reduction with nicotine replacement therapy compared with a group of light smokers who smoked comparable cigarettes. Compensatory smoking was assessed by levels of total NNAL.
Materials and Methods
Study Populations.
The smoking and biomarker data from four cohorts of smokers were merged to select our two samples. Two of the cohorts, 1 and 2, were participants in clinical trials. The other two cohorts, 3 and 4, were recruited specifically for their low smoking rates.
Cohort 1 included participants from a clinical trial, the Tobacco Reduction Intervention Program study, which recruited cigarette smokers ages 18 to 70, who were interested in reducing but not quitting cigarette use in the next 30 days. This study involved reducing smoking by 25% of baseline the first 2 weeks, 50% the subsequent 2 weeks, and 75% for the final 2 weeks. Reduction was achieved through the use of nicotine replacement products (i.e., 4 mg nicotine gum and 14 and 21 mg transdermal nicotine patch), which were made available to subjects for up to 12 weeks. Subjects were asked to sustain at least a 50% reduction for the next 12 weeks. They were screened to ensure they met the following eligibility criteria: (a) smoking 15 to 45 cigarettes per day (CPD) for the past year, (b) apparent good health with no unstable medical condition, (c) no contraindications to nicotine replacement use, (d) good mental health, (e) not using other tobacco products, and (f) not pregnant or nursing. The average number of CPD was calculated from a daily diary. This study design and the results and methods for analysis of creatinine-adjusted total NNAL are described in full detail in an earlier publication (7). Levels of cotinine, a metabolite of nicotine, were also assessed but excluded from analyses because of the confounding effects from the nicotine replacement products. Only the smoking and biomarker data collected at baseline and at 6-month follow-up were analyzed for this study.
Cohort 2 included participants from a clinical trial, the Reduction of Smoking in Cardiac Patients study. This study included cigarette smokers ages 18 to 80 with at least one diagnosis of heart disease who were interested in reducing smoking but not quitting in the next 30 days. The study design involved randomly assigning smokers with cardiovascular disease to a smoking reduction intervention using nicotine replacement treatment or usual care (e.g., advice and brief assistance for smoking cessation). Eligibility criteria included the following: (a) smoking ≥ 15 CPD; (b) having at least one of the following diagnoses: coronary artery disease, arrhythmia, congestive heart failure, peripheral vascular disease, or history of cerebrovascular disease; (c) no unstable angina in the past 2 weeks; (d) no unstable mental health or substance use diagnoses; and (e) no contraindications to nicotine replacement therapy (including pregnancy or intention to become pregnant). The average number of CPD was calculated from recall of the smoking level in the past week. This cohort was merged with cohort 1, and only the smoking and biomarker data at baseline and at 6-month follow-up were analyzed.
As the comparison group, low-level smokers were recruited from the Minneapolis-Saint Paul metropolitan area (Adult Cross-sectional Study, cohort 3) and the Minneapolis Veterans Affairs Medical Center (Low Level Smoking Study, cohort 4). Eligibility criteria for cohort 3 were the same as for cohort 1; however, participants had to smoke <15 CPD at a stable level for a minimum of 1 year. Eligibility criteria for cohort 4 were the same as for cohort 2; however, participants also had to smoke <15 CPD at a stable level for a minimum of 1 year. These smokers were seen one to two times (corresponding to baseline data collected for the clinical trials) to obtain a history and biomarker specimens. For subjects who came in twice in the baseline phase (cohorts 1 and 3), data values were averaged.
The Institutional Review Board of the University of Minnesota (Minneapolis, MN) approved the study protocols. Written consent was obtained from all volunteers at the orientation visit, where detailed information was given about the study protocols.
Study Samples.
The reducers consisted of 64 participants in clinical trials: cohorts 1 (n = 48) and 2 (n = 16). Reducers were selected from cohorts 1 and 2 using on these two criteria: (a) they reduced their CPD at least 40% from enrollment to 6-month follow-up and (b) at 6 months, they smoked no more than 10 CPD (the maximum number of cigarettes observed from sample 1). The light smokers sample consisted of 62 smokers from cohorts 3 (n = 55) and 4 (n = 7). The aim here was to create two study samples with similar smoking levels.
Measure of Smoking Compensation.
At any point in time, the rate of tobacco biomarker exposure can be calculated as an exposure rate: M/C. In this formula, a marker of smoking exposure (e.g., total NNAL) is denoted as M and daily cigarette consumption (cigarettes per day) as C. This rate defines amount or quantity of a biomarker per cigarette smoked (e.g., creatinine-adjusted total NNAL per cigarette). Change in the exposure rate from one time to another or from one subgroup to another can be used to index compensation. Compensatory smoking reflects increased uptake of mainstream smoke relative to the number of cigarettes smoked. Compensatory smoking may be evaluated as a compensation rate difference, M1/C1 – M0/C0, where 0 refers to baseline and 1 to a time period assessed during cigarette reduction. Alternatively, compensatory smoking may be appraised as a compensation rate ratio (CRR; refs. 9,10)
Compensatory smoking has, in fact, been defined in several ways and measured by several different statistics (1, 3, 4, 11), but all these methods are primarily different functions of the above exposure rate. The common weakness of these measures of compensatory smoking is that exposure rate implicitly assumes a linear and proportional relationship between M and C. In reality, the relationship between M and C may be depicted as a sigmoid curve. We adopted a modified version of the CRR statistics to describe the level of compensatory smoking, where we fixed a common value for C and defined CRR as the ratio M1/M0. Therefore, CRR was calculated by comparing the extent of biomarker exposure in smokers who had reduced (M1) versus smokers who were persistent light smokers (M0) rather than the extent of change in biomarkers per cigarette within a smoker overtime.
Results
Comparison of Study Samples.
Light smokers had an average age of 47.7 years (range, 19-75), were 53% female, and smoked a mean of 5.6 CPD (range, 1-10). Reducers had an average age of 51.2 years (range, 20-73), were 39% female, and smoked a mean of 26.0 CPD (range, 15-50). All subjects in the second group reduced their smoking by at least 40% and, on the average, smoked 5.0 CPD at 6 months after enrollment (range, 1-10). The differences in age (P = 0.11) and sex (P = 0.11) between the groups, although rather small and only near significant, were adjusted in the comparison of mean total NNAL.
Comparison of Total NNAL.
The descriptive statistics for mean (SD) total NNAL for both study samples are listed in Table 1.
Table 1.
Descriptive statistics for mean (SD) total NNAL in light smokers and reducers
| Sample | n | Total NNAL (pmol/mg creatinine) |
||
|---|---|---|---|---|
| Mean | SD | 95% CI | ||
| Light smokers | 62 | 0.85 | 0.80 | 0.65-1.05 |
| Reducers | 64 | 2.07 | 1.25 | 1.76-2.38 |
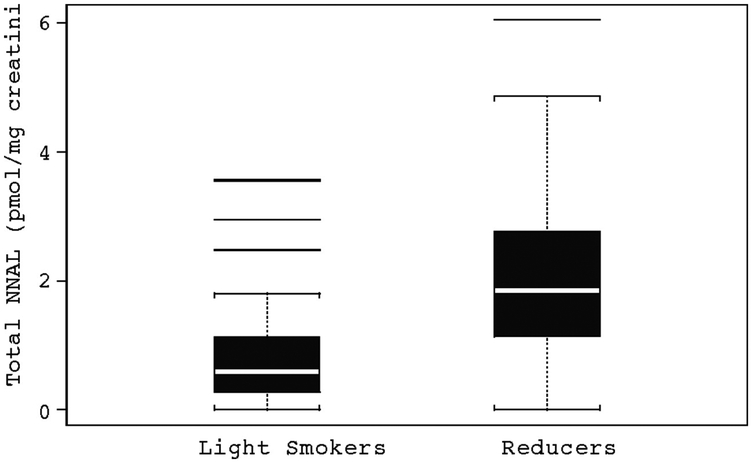
A box plot is shown in Fig. 1.
Figure 1.
Variability in total NNAL (pmol/mg creatinine) in light smokers and reducers.
The major aim here was to compare the average total NNAL level for light smokers versus the average total NNAL level for reducers measured at 6 months when both groups have similar levels of smoking.
The data showed that the two study samples corresponded to very different levels of variation. The ratio of sample SD, reducers divided by light smokers, was 1.25 / 0.80 = 1.56. The difference was highly statistically significant (F = 2.46; P = 0.0005). More importantly, the mean level of total NNAL for reducers was much higher than that of light smokers. The ratio of sample means in reducers divided by light smokers was 2.07 / 0.85 = 2.43. We did a statistical comparison of means with the two-sample t test using the Satterthwaite method to account for lack of homogeneity of variance. The difference was highly statistically significant (t = 6.54; P < 0.0001).
Because subjects in the two study samples had somewhat different age and sex composition, we adjusted our comparison of mean total NNAL for possible confounding effects of age and sex. Using individual total NNAL level as the dependent variable, a multiple regression analysis was done on three independent variables: sample membership, age, and sex. The adjusted difference (in total NNAL adjusted for age and sex) was also highly significant; instead of a difference 2.07 – 0.85 = 1.22 pmol/mg creatinine, the adjusted difference (slope of the sample membership indicator) was 1.27 pmol/mg creatinine (t = 6.57; P < 0.0001).
Investigation of Compensatory Smoking.
For each subject in the sample of reducers, the subgroup of light smokers having the same CPD as the reducers at 6 months was identified. The average total NNAL measured from each reducer at 6 months (M1) was divided by average total NNAL of his/her CPD-matched subgroup of light smokers (M0) to obtain his/her individual CRR. The mean of CRRs for subgroups of reducers with the same CPD was calculated and is shown in Table 2; the average CRR ranged from 94% to 841%. The vast majority was >100% and the fewer the CPD, the higher the compensation index.
Table 2.
Average CRRs and their variations in light smokers and reducers
| CPD | Light smokers (n) | Reducers (n) | Mean total NNAL (pmol/mg creatinine) |
Average CRR | |
|---|---|---|---|---|---|
| Light smokers | Reducers (at 6 mo) | ||||
| 1 | 7 | 9 | 0.3256 | 1.4151 | 4.35 |
| 2 | 7 | 11 | 0.2963 | 2.4921 | 8.41 |
| 3 | 8 | 6 | 0.4524 | 2.2360 | 4.94 |
| 4 | 2 | 6 | 1.9449 | 1.8355 | 0.94 |
| 5 | 9 | 4 | 0.7133 | 2.2130 | 3.10 |
| 6 | 2 | 7 | 0.9594 | 1.9220 | 2.00 |
| 7 | 8 | 5 | 1.0064 | 3.1562 | 3.14 |
| 8 | 4 | 2 | 0.9369 | 0.9126 | 0.97 |
| 9 | 3 | 2 | 1.3497 | 3.0076 | 2.23 |
| 10 | 12 | 12 | 1.3918 | 1.8173 | 1.31 |
Using individual CRR index level as the dependent variable, a multiple regression analysis was done on three dependent variables: age, sex, and the individual smoking reduction percentage (from baseline CPD to 6-month CPD). Age and sex had no significant effect (P > 0.05). The effect of reduction percentage was significant (t = 3.61; P = 0.0006); the greater the reduction, the more compensation occurred. In addition, wide variability in compensation scores continued to be observed; a scatter diagram is shown in Fig. 2. The simple coefficient of correlation was r = 0.50 (n = 64).
Figure 2.
CRR versus smoking reduction percentage.
Discussion
The results of this study point to three main conclusions. First, the extent of tobacco toxicant exposure in former heavy smokers who significantly reduced their smoking is higher than those who were consistent light smokers. Given the same amount of smoking, the amount of total NNAL exposure per cigarette in reducers was two to three times higher than existing light smokers. Furthermore, the results showed that the greater the reduction in smoking, the greater the amount of compensatory smoking that occurred. That is, smokers who reduced their smoking to one to three CPD experienced a 4- to 8-fold increased exposure to 4-(methylnitrosamino)-I-(3-pyridyl)-1-butanone per cigarette compared with light smokers.
In general, these findings are concordant with the results from epidemiologic studies that have compared morbidity and mortality risk among smokers who have reduced their smoking by at least 50% with continuous light smokers. For example, one study found that the amount of risk for lung cancer among reducers compared with continuous heavy smokers [HR, 0.73; 95% confidence interval (95% Cl), 0.54-0.98] was −1.7 times that of light smokers (HR, 0.44; 95% Cl, 0.35-0.56), although the mean number of cigarettes was equivalent if not slightly higher among light smokers (8). Another study that examined the effects of cigarette reductions of ≥50% in decreasing hospitalizations for chronic obstructive pulmonary disease showed no significant decrease in risk for hospitalization among reducers compared with continuous heavy smokers (HR, 0.93; 95% CI, 0.73-1.18) but a significantly lower risk in continuous light smokers compared with heavy smokers (HR, 0.59; 95% CI, 0.51-0.70; ref. 12). For fatal and nonfatal myocardial infarction, these investigators also found that reducers did not experience a significant risk reduction (HR, 1.15; 95% CI, 0.94-1.40) but a modest risk reduction was observed for light smokers (HR, 0.86; 95% CI, 0.70-0.98; ref. 13). These differences in risk across disease states between reducers and light smokers were attributed by these authors to compensatory smoking. It should be emphasized that, even with lower levels of disease risk among continuous light smokers, a very low rate of smoking still confers increased risk compared with nonsmokers and quitters (14, 15).
Second, this new method of calculating compensatory smoking has an advantage over prior methods, which have relied on using baseline measures of smoke exposure (i.e., biomarkers per cigarette) as a point of comparison. Compensatory smoking observed in previous studies may be influenced by a “nonlinear relationship” between the amount of tobacco consumption and the level of tobacco toxin exposure (total NNAL; ref. 16) such that a small reduction in cigarette smoking by heavy smokers could lead to an increase in exposure rate. In this study, this confounding effect was overcome by not using baseline measures of markers but by using a control group of light smokers who have a comparable level of cigarette smoking. By ruling out the confounding effect of the nonlinear relationship between tobacco consumption and tobacco toxin exposure, we can conclusively attribute the increased toxicant exposure in reducers compared with light smokers to smoking compensation.
Third, there is no suggestion that sex has an effect on the extent of compensatory smoking. These results, on the surface, seem contradictory to previous studies, which have shown that women have more difficulty discriminating across different doses of nicotine compared with men (17), show less nicotine regulation (18, 19), and are less sensitive to the withdrawal symptom relief of nicotine (20). However, our findings do not rule out the hypothesis that compensatory smoking in women may be more a function of compensating for the sensory aspects of smoking compared with compensating for reduced levels of nicotine.
As a caveat, this study did not take into account potential differences in brand and type of cigarettes smoked (regular, light, or ultralight) or exposure to secondhand smoke. That is, it is possible that reducers tended to smoke cigarettes that are associated with higher tar exposure and/or to be exposed to greater amounts of secondhand smoke, accounting for the higher total NNAL in reducers. Using existing data, in a post hoc analysis, no significant effects were found for types of cigarettes on relative exposure to total NNAL when matching for number of cigarettes (P = 0.93) or when looking within the light smokers or reducers (P = 0.98 and 0.61, respectively). Furthermore, a previous study has also shown no differences in levels of toxicant exposure across types of cigarettes (21). With regards to the issue of secondhand smoke, the contribution of secondhand smoke to the levels of toxicant exposure observed from cigarette smoking in reducers compared with light smokers is likely to be minimal (22).
In summary, the results of this study show that smokers who reduce cigarette smoking experience greater toxicant exposure than those who are persistent light smokers even when matching for number of cigarettes smoked at the time of assessment. Therefore, simple emphasis on total cigarette reduction belies the effects of compensation on maintaining potentially toxic or carcinogenic levels of chemicals in smokers who reduce their smoking. These results are consistent with the epidemiologic studies for disease risk, showing higher risks for reducers than light smokers. To date, the absolute threshold levels of toxicant exposure that leads to “significant” reduction in tobacco-related illnesses remain unknown and are likely to vary depending on the physiologic vulnerabilities of the smoker. Therefore, smoking cessation should continue to be the message as the end goal for intervention.
Acknowledgments
Grant support: NIH grant P50-DA013333.
References
- 1.McMorrow MJ, Foxx RM. Nicotine’s role in smoking: an analysis of nicotine regulation. Psychol Bull 1983;93:302–27. [PubMed] [Google Scholar]
- 2.National Cancer Institute. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine Smoking and Tobacco Control Monograph No 13. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2001. [Google Scholar]
- 3.Scherer G Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145:1–20. [DOI] [PubMed] [Google Scholar]
- 4.Stephen AM, Frost C, Thompson S, Wald NJ. Estimating extent of compensatory smoking In: Wald NJ, Froggatt P, editors. Nicotine, smoking, and the low tar programme. Oxford: Oxford University Press; 1989. [Google Scholar]
- 5.U.S. Department of Health and Human Services. The health consequences of smoking: nicotine addiction A report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1988. [Google Scholar]
- 6.Hatsukami DK, Slade J, Benowitz NL, et al. Reducing tobacco harm: research challenges and issues. Nicotine Tob Res 2002;4 Suppl 2:S89–101. [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS, Murphy SE, Carmella SG, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst 2004;96:107–15. [DOI] [PubMed] [Google Scholar]
- 8.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA 2005;294:1505–10. [DOI] [PubMed] [Google Scholar]
- 9.Fant RV, Schuh KJ, Stitzer ML. Response to smoking as a function of prior smoking amounts. Psychopharmacology (Berl) 1995;119:385–90. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Stable EJ, Herrera B, Jacob P III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA 1998;280:152–6. [DOI] [PubMed] [Google Scholar]
- 11.Harris JE. Incomplete compensation does not imply reduced harm: yields of 40 smoke toxicants per milligram nicotine in regular filter versus low-tar cigarettes in the 1999 Massachusetts Benchmark Study. Nicotine Tob Res 2004;6:797–807. [DOI] [PubMed] [Google Scholar]
- 12.Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax 2002;57:967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godtfredsen NS, Osler M, Vestbo J, Andersen I, Prescott E Smoking reduction, smoking cessation, and incidence of fatal and non-fatal myocardial infarction in Denmark 1976-1998: a pooled cohort study. J Epidemiol Community Health 2003;57:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control 2005;14:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godtfredsen NS, Holst C, Prescott E, Vestbo J, Osler M. Smoking reduction, smoking cessation, and mortality: a 16-year follow-up of 19,732 men and women from The Copenhagen Centre for Prospective Population Studies. Am J Epidemiol 2002;156:994–1001. [DOI] [PubMed] [Google Scholar]
- 16.Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev 2005;14:2963–8. [DOI] [PubMed] [Google Scholar]
- 17.Perkins KA. Individual variability in responses to nicotine. Behav Genet 1995;25:119–32. [DOI] [PubMed] [Google Scholar]
- 18.Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol 1996;4:166–77. [Google Scholar]
- 19.Woodward M, Tunstall-Pedoe H. Self-titration of nicotine: evidence from the Scottish Heart Health Study. Addiction 1993;88:821–30. [DOI] [PubMed] [Google Scholar]
- 20.Hatsukami D, Skoog K, Allen S, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Exp Clin Psychopharmacol 1995;3:163–73. [Google Scholar]
- 21.Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev 2005;14:693–8. [DOI] [PubMed] [Google Scholar]
- 22.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control 2004;13:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]