Abstract
Background
α-actinin-4 (Actinin-4 or ACTN4), originally identified as an actin-binding protein associated with the biological function of cancer cells, appears to be highly expressed in numerous human epithelial carcinomas, including breast cancer (BC). In the present study we assessed the role of serum ACTN4 as a biomarker for BC diagnosis, as well as the association between ACTN4 levels and clinicopathological features.
Material/Methods
ACTN4 expression level was measured with quantitative real-time PCR (qRT-PCR) analysis in serum specimens of 128 BC patients and 96 healthy volunteers. χ2 testing was conducted to explore the association of ACTN4 levels with clinicopathologic factors. Moreover, the diagnostic value of ACTN4 was analyzed using receiver operating characteristic (ROC) curves.
Results
Serum ACTN4 level was obviously upregulated in patients with BC compared with healthy controls (P<0.05). High ACTN4 expression was significantly associated with clinical stage (P=0.000), tumor grade (P=0.004), and lymph node status (P=0.024). However, no association was found between ACTN4 expression and age, tumor size, ER status, PR status, or HER-2 status (all P>0.05). The ROC analysis showed that the area under the curve (AUC) of ACTN4 was 0.887 (95%CI: 0.843–0.931), with sensitivity of 80.5% and specificity of 84.4%, and the cutoff value was 1.050.
Conclusions
ACTN4 in serum can serve as a clinical predictor in the diagnosis or prediction of clinical outcomes of patients with BC.
MeSH Keywords: Actinin, Breast Neoplasms, Diagnosis
Background
Breast cancer (BC) is the most common female malignancy around the world, and its high morbidity and mortality rates are mainly due to metastasis [1,2]. In China, breast cancer leads to approximately 45,000 deaths among females every year and is a major threat to human health [3]. Recently, the outcomes and quality of life of BC patients have been improved due to detection at an early stage, suitable staging, risk evaluation, clinical outcome estimation, and surveillance [4]. Clinically, this can be accomplished by evaluating biomarkers in tumor tissues and blood samples. There are some advantages of using circulating biomarkers in blood, such as being minimally-invasive and the ability to obtain multiple specimens at the same time. Unfortunately, at present, the conventional biomarkers have low diagnostic accuracy due to the advanced stages and the heterogeneity of breast cancer [5–8]. Therefore, it is of great importance to find improved molecular markers that can more accurately stratify BC patients, thus facilitating the identification of the ideal approaches of treatment for each individual patient [9,10].
Actinin-4 (gene name ACTN4) was identified in 1998 as an actin-binding protein that is closely associated with enhanced cell motility and cancer invasion and metastasis [11,12]. During the development of human cancers, the actinin-4 protein level is markedly increased in malignant phenotypes of cancer [11]. The actinin-4 protein level is closely related to the poor prognosis in human cancers, such as thyroid cancer [13], lung cancer [14,15], and salivary gland carcinoma [16]. Additionally, in lung adenocarcinoma, immunohistochemistry showed that actinin-4 protein is overexpressed and can predict the efficacy of adjuvant chemotherapy for resection [17]. However, the expression of ACTN4 and its clinical outcome in BC has not yet been investigated.
Therefore, in the present study, we examined the ACTN4 levels in blood specimens from patients with breast cancer using qRT-PCR. Additionally, we investigated the correlation between ACTN4 levels and clinicopathologic features and assessed the diagnostic values of ACTN4.
Material and Methods
Patients and sample collections
This research was authorized by the Ethics Committee of the College of Medicine, Henan University. Informed written consent from each patient and local ethics committee approval was obtained before starting data collection.
The present study was conducted on 128 female subjects aged 17–70 years. Blood was obtained from patients when diagnosed, before they received any anti-cancer therapies or surgery. Peripheral blood of 96 healthy female volunteers without any other diseases, such as tumors, autoimmune diseases, or inflammation breast cancer was obtained as a control group (age range, 18–69 years).
The blood samples were stored at 4°C for 1 h. After centrifuging for 5 min at 2000×g, serum was collected and centrifuged again at 12 000g for 15 min to completely remove cell debris. The serum was then aliquoted and stored separately at −80°C until used for analysis.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR) assay
Total RNA was isolated from 2 mL of serum using Trizol (Invitrogen, Carlsbad, CA) following the protocol of the manufacturer. The quality and concentration of mRNA were detected in a multi-volume spectrophotometer system (Epoch, Biotek, Winooski, VT). Real-time PCR was done in triplicate, including no-template controls. Amplification of the appropriate product was confirmed by melting curve analysis after amplification. Relative expression of ACTN4 was calculated using the comparative cycle threshold (CT) (2−ΔΔCT) method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous control to normalize the data.
Statistical analysis
All statistical analyses were carried out using the SPSS statistical package, version 20.0 (IBM, SPSS, Chicago, IL) and graphs were plotted using Origin Pro 9.0. Continuous variables are described as the mean ±SD. The χ2 test was applied to analyze the association of ACTN4 expression with clinicopathological characteristics. Receiver operating characteristic (ROC) curves were plotted and the area under the curve (AUC) was calculated. All tests were 2-sided; a P value less than 0.05 was regarded as significance.
Results
Serum ACTN4 level in breast cancer and healthy controls
The median values of serum ACTN4 levels in 96 healthy controls and 128 breast cancer patients were 0.600 and 1.335, respectively. Serum levels of ACTN4 were distinctly increased in BC patients compared with healthy individuals (1.318±0.360 vs. 0.644±0.400). Therefore, the serum levels of ACTN4 were significantly elevated in BC patients (P<0.05, Figure 1).
Figure 1.
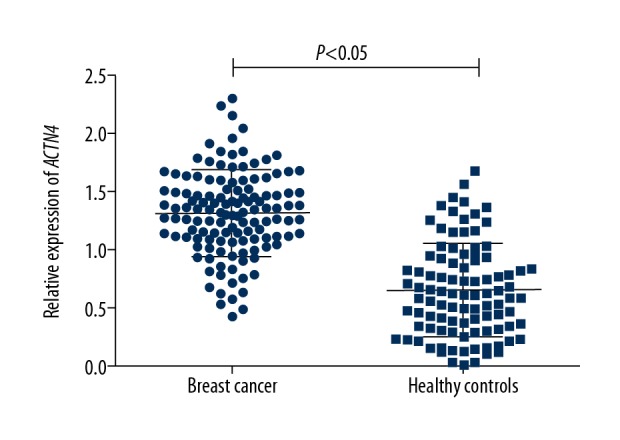
Serum ACTN4 expression levels assessed by qRT-PCR in breast cancer patients (n=128) and healthy volunteers (n=96). The ACTN4 expression levels were normalized to GAPDH. ACTN4 expression was significantly higher in patients with breast cancer than that in healthy controls. Data shown as means ±SD (P<0.05).
Correlation between ACTN4 expression and clinical parameters of BC patients
To further investigate whether the expression level of ACTN4 was correlated the clinicopathological variables, the correlation of ACTN4 expression with the clinical pathological data of the BC patients was assessed. The results revealed that ACTN4 mRNA expression was obviously related to clinical stage (P=0.000), tumor grade (P=0.004) and lymph node status (P=0.024). No relationship was found between ACTN4 and other clinicopathological parameters, including age, tumor size, ER status, PR status, and HER-2 status (all P>0.05) (Table 1).
Table 1.
Association of serum ACTN4 expression with clinicopathological characteristics of breast cancer patients.
| Characteristics | No. (n=128) | ACTN4 expression | χ2 | P values | |
|---|---|---|---|---|---|
| Low (n=49) | High (n=79) | ||||
| Age (years) | |||||
| <50 | 71 | 30 | 41 | 1.065 | 0.302 |
| ≥50 | 57 | 19 | 38 | ||
| Clinical stage | |||||
| I–II | 83 | 41 | 42 | 12.348 | 0.000 |
| III | 45 | 8 | 37 | ||
| Tumor grade | |||||
| I–II | 79 | 38 | 41 | 8.423 | 0.004 |
| III | 49 | 11 | 38 | ||
| Tumor size (cm) | |||||
| <2 | 68 | 31 | 37 | 3.278 | 0.070 |
| ≥2 | 60 | 18 | 42 | ||
| Lymph node status | |||||
| Negative | 81 | 37 | 44 | 5.110 | 0.024 |
| Positive | 47 | 12 | 35 | ||
| ER status | |||||
| Negative | 75 | 32 | 43 | 1.474 | 0.225 |
| Positive | 53 | 17 | 36 | ||
| PR status | |||||
| Negative | 89 | 39 | 50 | 3.793 | 0.051 |
| Positive | 39 | 10 | 29 | ||
| HER-2/neu status | |||||
| Negative | 86 | 37 | 49 | 2.494 | 0.114 |
| Positive | 42 | 12 | 30 | ||
ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor 2.
The diagnostic value of serum ACTN4 expression in BC
Receiver operating characteristic (ROC) curve analysis was used to estimate the predictive power and diagnostic accuracy of serum ACTN4 levels for BC. The results showed that the area under the curve (AUC) was 0.887, with a sensitivity of 80.5% and specificity of 84.4% (Figure 2). The cutoff value for ACTN4 expression level was 1.050. These outcomes indicated that ACTN4 can be used to distinguish BC patients from healthy controls.
Figure 2.
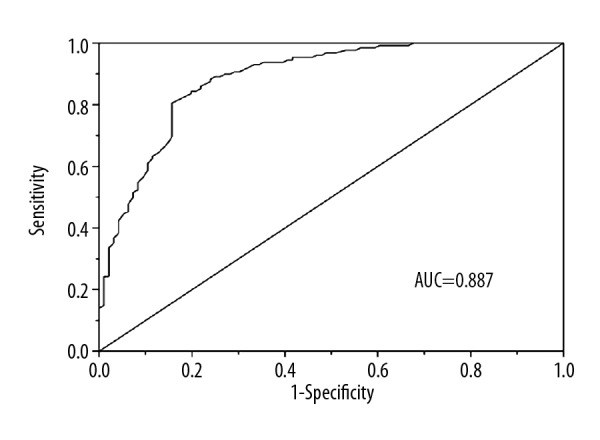
ROC analysis for evaluation of the accuracy of serum ACTN4 to discriminate patients with breast cancer from healthy controls. ROC – receiver operating characteristic; AUC – area under the curve.
Discussion
Breast cancer is the second most common cause of malignancy-associated death, after lung cancer, in women worldwide. BC is a heterogeneous disease because of its complicated etiology, including both genetic and environmental factors, such as family history, genetic defect, and immune system abnormalities [18,19]. BC comprises multiple entities associated with distinctive histological and biological features, clinical presentations, and behaviors and responses to therapy. From the molecular point of view, breast cancer can be divided into 5 intrinsic molecular subtypes [20,21]. The low survival rate shows the need to identify new diagnostic biomarkers for improving the therapeutic response and prognosis in BC patients to rationalize treatment decisions.
Alpha-actinins (ACTNs) are ubiquitously expressed proteins known to be cross-linked with filamentous actin (F-actin) to maintain cytoskeletal integrity and to control cell movement [22]. Four ACTN family members, numbered 1–4, are present in humans and are highly conserved in other mammals [11,23–25]. ACTN4, an actin-bundling protein encoded by ACTN4 gene, is widely expressed in a number of tissues, most notably glomerular podocytes [26]. ACTN4 has 1 actin-binding domain at the N-terminus, and ACTN4 monomers can form a homodimer by binding in the opposite direction to form a dumbbell-shaped structure [27]. The protein overexpression of ACTN4 in cancer cells stimulates dynamic remodelling of the actin cytoskeleton, which is why ACTN4-overexpressing cancer cells have metastatic potential [12]. Okamoto et al. showed that ACTN4 is expressed in small-cell lung cancer (NSCLC) and is obviously related to distant metastasis [16]. Miura et al. found that ACTN4 is a promising predictive indicator for the efficacy of adjuvant chemotherapy in NSCLC [17]. In salivary gland carcinoma, ACTN4 expression level was increased and might be a contribute to the poor overall survival of patients [18]. Therefore, it is important to explore the role of ACTN4 in BC.
In our research, the expression of ACTN4 was detected in 128 BC patients and 96 healthy controls, and it was demonstrated that ACTN4 strongly differentiates BC patients from healthy controls. A highly significant increased expression of serum ACTN4 was found in BC patients compared with that in healthy individuals. Moreover, receiver operating curve (ROC) analysis indicated that the AUC of ACTN4 was 0.887 and the sensitivity and specificity at the optimal cutoff were 80.5% and 84.4%, respectively, indicating that it might be a promising biomarker for diagnosing BC. Hsu et al. reported that ACTN4 is part of the BC cell motile apparatus and is highly expressed in the nucleus, suggesting a central role in BC tumorigenesis [28]. Liu et al. also revealed that depletion of ACTN1 or ACTN4 suppresses the migration of BC cells [29], consistent with our results.
We also analyzed the serum expression levels of ACTN4 in relation to the different clinical pathologic characteristics in BC patients. The results showed that serum ACTN4 expression was related with clinical stage, tumor grade, and lymph node status. However, there were no significant relationships between serum ACTN4 expression and other features, including age, tumor size, ER status, PR status, and HER-2 status.
Several shortcomings in the present study should be acknowledged. Firstly, the sample size was relatively small, which might have reduced the statistical power of our results. Secondly, the study aimed to explore a biomarker for non-invasive detection of BC; therefore, the expression of ACTN4 protein in BC tissues and normal tissues was not detected by immunohistochemistry or Western blot methods. In addition, the present study was a primary study to explore the clinical significance of serum ACTN4 for early diagnosis of BC. We found that the overexpression of ACTN4 might contribute to aggressive progression of BC, but the molecular mechanisms underlying the oncogenic function of ACTN4 in BC remain poorly defined. Therefore, further in vitro and in vivo experiments should be designed to address these issues.
Conclusions
In summary, our data show that ACTN4 expression levels are upregulated in BC patients. Moreover, the ROC results show the high diagnostic accuracy of ACTN4. The present findings suggest that serum ACTN4 is a potentially useful biomarker for BC detection. Further research with larger samples involving validation and optimizing improvement should be conducted to confirm our results.
Footnotes
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Geng SQ, Alexandrou AT, Li JJ. Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett. 2014;349:1–7. doi: 10.1016/j.canlet.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Xiao L, Ren G. Experiences of social support among Chinese women with breast cancer: A qualitative analysis using a framework approach. Med Sci Monit. 2018;24:574–81. doi: 10.12659/MSM.908458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braden AM, Stankowski RV, Engel JM, Onitilo AA. Breast cancer biomarkers: Risk assessment, diagnosis, prognosis, prediction of treatment efficacy and toxicity, and recurrence. Curr Pharm Des. 2014;20:4879–98. doi: 10.2174/1381612819666131125145517. [DOI] [PubMed] [Google Scholar]
- 5.Fejzić H, Mujagić S, Azabagić S, Burina M. Tumor marker CA 15-3 in breast cancer patients. Acta Med Acad. 2015;44:39–46. doi: 10.5644/ama2006-124.125. [DOI] [PubMed] [Google Scholar]
- 6.Stötzer OJ, Lehner J, Fersching-Gierlich D, et al. Diagnostic relevance of plasma DNA and DNA integrity for breast cancer. Tumour Biol. 2014;35:1183–91. doi: 10.1007/s13277-013-1158-4. [DOI] [PubMed] [Google Scholar]
- 7.Stieber P, Nagel D, Blankenburg I, et al. Diagnostic efficacy of CA 15-3 and CEA in the early detection of metastatic breast cancer-A retrospective analysis of kinetics on 743 breast cancer patients. Clin Chim Acta. 2015;448:228–31. doi: 10.1016/j.cca.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Qin Y, Zhang J, et al. Nipple discharge of CA15-3, CA125, CEA and TSGF as a new biomarker panel for breast cancer. Int J Mol Sci. 2014;15:9546–65. doi: 10.3390/ijms15069546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Machiraju R, Huang K. Breast cancer patient stratification using a molecular regularized consensus clustering method. Methods. 2014;67:304–12. doi: 10.1016/j.ymeth.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy PG, Chan SM, Ng V, et al. Risk stratification of patients with early breast cancer. Clin Breast Cancer. 2014;14:68–73. doi: 10.1016/j.clbc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Honda K. The biological role of actinin-4 (ACTN4) in malignant phenotypes of cancer. Cell Biosci. 2015;5:41. doi: 10.1186/s13578-015-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DG, Robinson DN. The fifth sense: Mechanosensory regulation of alpha-actinin-4 and its relevance for cancer metastasis. Semin Cell Dev Biol. 2017;71:68–74. doi: 10.1016/j.semcdb.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka N, Yamashita T, Yamamoto S, et al. Histological growth pattern of and alpha-actinin-4 expression in thyroid cancer. Anticancer Res. 2014;34:3157–63. [PubMed] [Google Scholar]
- 14.Okamoto N, Suzuki H, Kawahara K, et al. The alternatively spliced actinin-4 variant as a prognostic marker for metastasis in small-cell lung cancer. Anticancer Res. 2015;35:1663–67. [PubMed] [Google Scholar]
- 15.Miura N, Kamita M, Kakuya T, et al. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget. 2016;7:33165–78. doi: 10.18632/oncotarget.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watabe Y, Mori T, Yoshimoto S, et al. Copy number increase of ACTN4 is a prognostic indicator in salivary gland carcinoma. Cancer Med. 2014;3:613–22. doi: 10.1002/cam4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiraishi H, Fujiwara Y, Kakuya T, et al. Actinin-4 protein overexpression as a predictive biomarker in adjuvant chemotherapy for resected lung adenocarcinoma. Biomark Med. 2017;11(9):721–31. doi: 10.2217/bmm-2017-0150. [DOI] [PubMed] [Google Scholar]
- 18.Zhang N, Zuo L, Zheng H, et al. Increased expression of CD81 in breast cancer tissue is associated with reduced patient prognosis and increased cell migration and proliferation in MDA-MB-231 and MDA-MB-435S human breast cancer cell lines in vitro. Med Sci Monit. 2018;24:5739–47. doi: 10.12659/MSM.911612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Q, Gan L, Chen YY, et al. Percent body fat change in chinese women after adjuvant chemotherapy for breast cancer. Med Sci Monit. 2018;24:5988–95. doi: 10.12659/MSM.911423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Panis C, Pizzatti L, Herrera AC, et al. Label-free proteomic analysis of breast cancer molecular subtypes. J Proteome Res. 2014;13:4752–72. doi: 10.1021/pr500676x. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro Ede A, Jr, Pinotsis N, Ghisleni A, et al. The structure and regulation of human muscle α-actinin. Cell. 2014;159:1447–60. doi: 10.1016/j.cell.2014.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng D, DuMontier C, Pollak MR. The role of alpha-actinin-4 in human kidney disease. Cell Biosci. 2015;5:44. doi: 10.1186/s13578-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Li XW, Wang X, et al. Alpha-asctinin-4 is a possible target protein for aristolochic acid i in human kidney cells in vitro. Am J Chin Med. 2016;44:291–304. doi: 10.1142/S0192415X16500178. [DOI] [PubMed] [Google Scholar]
- 25.Ogneva IV, Biryukov NS, Leinsoo TA, Larina IM. Possible role of non-muscle alpha-actinins in muscle cell mechanosensitivity. PLoS One. 2014;9:e96395. doi: 10.1371/journal.pone.0096395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Hsu KS, Lim JH, et al. α-Actinin 4 potentiates nuclear factor κ-light-chain-enhancer of activated B-cell (NF-κB) activity in podocytes independent of its cytoplasmic actin binding function. J Biol Chem. 2015;290:338–49. doi: 10.1074/jbc.M114.597260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shams H, Golji J, Garakani K, Mofrad MR. Dynamic regulation of α-Actinin’s calponin homology domains on F-actin. Biophys J. 2016;110:1444–55. doi: 10.1016/j.bpj.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu KS, Kao HY. Alpha-actinin 4 and tumorigenesis of breast cancer. Vitam Horm. 2013;93:323–51. doi: 10.1016/B978-0-12-416673-8.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Zhan Y, Tu Y, et al. PDZ and LIM domain protein 1(PDLIM1)/CLP36 promotes breast cancer cell migration, invasion and metastasis through interaction with α-actinin. Oncogene. 2015;34:1300–11. doi: 10.1038/onc.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]


