Abstract
Background
Cancer-related fatigue (CRF) is a common side effect of adjuvant therapy and becomes a chronic problem for approximately one-third of survivors. Omega-3 polyunsaturated fatty acids (O3-PUFA) demonstrated preliminary antifatigue effects in previous research, but have not been investigated in fatigued cancer survivors.
Methods
Breast cancer survivors 4–36 months posttreatment with a CRF score of 4 or more of 10 using the symptom inventory (SI) were randomly assigned to O3-PUFA (fish oil, 6 g/d), omega-6 PUFA (O6-PUFA; soybean oil, 6 g/d), or a low-dose combination of O3-/O6-PUFA (3 g/d O3-PUFA and O6-PUFA) for 6 weeks. CRF was assessed by the SI (screening question), the Brief Fatigue Inventory, and the Multidimensional Fatigue Symptom Index. Protein and mRNA levels of inflammatory and antioxidant biomarkers, along with fatty acid and lipid levels, were assessed at baseline and week 6. Statistical tests were two-sided.
Results
A total of 108 breast cancer survivors consented; 97 subjects were randomly assigned and 81 completed the trial. The SI CRF score decreased by 2.51 points at week 6 with O6-PUFA and by 0.93 points with O3-PUFA, with statistically significant between-group difference (effect size = −0.86, P < .01). Similar changes were observed for the Brief Fatigue Inventory and Multidimensional Fatigue Symptom Index but were not statistically significant. Stratified analyses showed the largest benefit was observed in those with severe baseline CRF (≥7). Compared with O3-PUFA, O6-PUFA supplementation statistically significantly decreased proinflammatory markers in the TNF-α signaling pathway.
Conclusion
Contrary to our original hypothesis, O6-PUFA statistically significantly reduced CRF compared with O3-PUFA. Further research is needed to confirm these findings and to elucidate mechanisms of action.
Cancer-related fatigue (CRF) is a multidimensional condition in persons with cancer, marked by severe exhaustion, cognitive deficits including memory loss and impaired executive function, reduced psychosocial well-being, and inability to maintain social activities (1–3). Although CRF generally resolves after about 6 months posttreatment for the majority of patients, a substantial proportion of survivors (20%–35%) continue to experience CRF 5 to 10 years after their diagnosis, which impairs quality of life and daily functioning (4–8).
Marine omega-3 polyunsaturated fatty acids (O3-PUFA; fish oil), one of the most widely used supplements in the United States, have been shown to reduce inflammation, hypertension, and hyperlipidemia (9–11). While some RCTs showed O3-PUFA supplementation improved appetite and reduced weight loss in advance cancer patients, other RCTs did not (12,13). Despite mixed effects on appetite and weight maintenance, O3-PUFA supplementation produced marked decreases in both CRF and inflammation (12,14–16). O3-PUFA supplementation is a promising candidate for the reduction of CRF in breast cancer survivors and warrants further investigation.
Omega-6 polyunsaturated fatty acids (O6-PUFA; soybean oil) supplements were selected as the control condition based on the recommendation of the Product Quality Working Group of the National Center for Complementary and Integrative Health and because soybean oil contains only small quantities of marine O3-PUFA (17). O6-PUFA have been viewed as having proinflammatory properties (18,19). Recent research has shown, however, that O6-PUFA from soybean oil have antioxidant and anti-inflammatory properties, outperforming marine O3-PUFA supplementation in reducing certain inflammatory biomarkers (20–22).
The primary aim of this phase II trial was to evaluate the preliminary efficacy (mean changes and variation) of two O3-PUFA supplementation regimens (low and high dose) vs O6-PUFA supplementation for reducing CRF in fatigued breast cancer survivors. Additionally, we examined the feasibility (adherence and adverse events) of all study arms. We also examined serum protein levels and mRNA expression of inflammatory and antioxidant biomarkers to investigate whether changes in CRF by the intervention were associated with specific biological pathways.
Patients and Methods
Study Design and Participating Sites
The University of Rochester Cancer Center National Cancer Institute Community Oncology Research Program (NCORP) Research Base conducted a nationwide, multicenter, randomized controlled trial examining the efficacy of O3-PUFA and O6-PUFA for reducing CRF in breast cancer survivors with a target enrollment of 75. This study was activated in November 2014 and closed to accrual in June 2015 with five NCORP Community Affiliates participating. Subjects were randomly assigned to one of three groups at each NCORP site and stratified by baseline CRF level (two levels: 4–6 [moderate] or ≥7 [severe] on an 11-point symptom inventory (SI) scale anchored by 0 [no fatigue] and 10 [worst possible fatigue]). Group assignment was randomly determined by a computer-generated random number table in blocks of three or six and an allocation ratio of 1:1:1. Subjects were registered via website. All study investigators, study coordinators, and subjects were blinded; only the research pharmacist had access to group assignment.
Subjects were informed of the investigational nature of the study, and informed consent was obtained before any study activity. NCORP Community Affiliates obtained the appropriate institutional review board approvals for participation in this randomized controlled trial with all sites following Good Clinical Practice guidelines.
Subject Participants
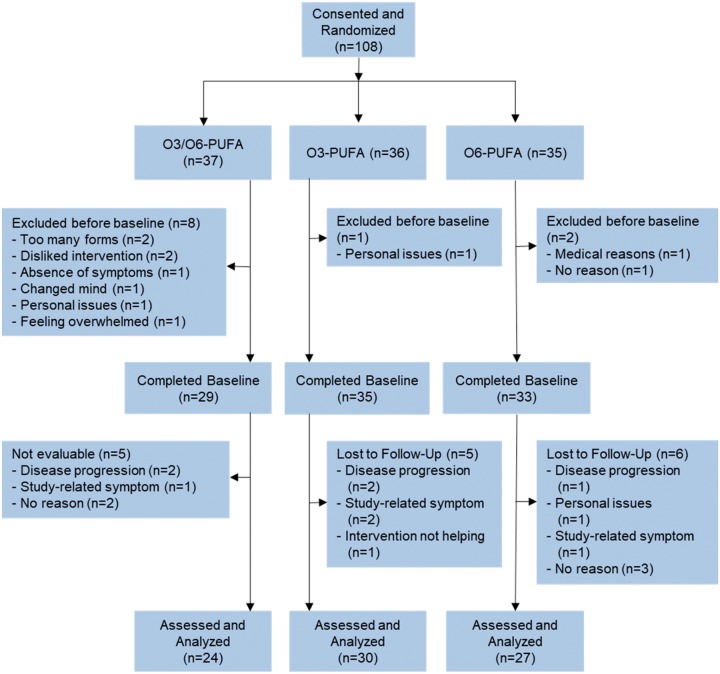
Breast cancer survivors were recruited by clinical research coordinators through the use of direct contact during regularly scheduled oncologic visits. Eligibility criteria included (1) a confirmed diagnosis of breast cancer (stage 0–III), (2) completed postadjuvant treatment within the past 4–36 months (ongoing hormonal therapy was allowed), (3) CRF, as indicated by a response of 4 or more on an 11-point SI scale anchored by “0” = no fatigue and “10” = as bad as you can imagine, and (4) female sex, 18 years of age or older. Subjects were excluded if they (1) used O3-PUFA supplementation within the previous 12 weeks, (2) were taking anticoagulant medication, (3) had an allergy to fish/soybean products, or (4) had a confirmed diagnosis known to cause severe fatigue. The CONSORT diagram (Figure 1) shows the flow of subjects through recruitment and participation.
Figure 1.
CONSORT diagram. PUFA = polyunsaturated fatty acid.
Trial Design
This randomized, double-blind, multicenter trial compared (1) high-dose O3-PUFA supplementation (3.3 g/d of DHA plus EPA from six 1-g capsules) to (2) low-dose Omega-3 and Omega-6 supplementation (O3/O6-PUFA; 1.65 g/d of DHA and EPA from three 1-g O3-PUFA capsules and three 1-gram O6-PUFA capsules) to (3) high-dose O6-PUFA supplementation (six 1-g O6-PUFA capsules) for 6 weeks. A trial period of 6 weeks was chosen because it is (1) long enough to elicit a biological response from the supplementation (2,23,24) long enough for CRF to change (25), but (3) also short enough to encourage enrollment and minimize burden. The O3-PUFA study agent was comprised solely of fish oil, and the O6-PUFA study agent was comprised solely of soybean oil; all study agents were supplied by Nordic Naturals (Watsonville, CA). Each O3-PUFA capsule contained 325 mg of EPA and 225 of DHA. Both O3-PUFA and O6-PUFA capsules were similar in color and contained a blend of natural lemon and rosemary extract to blind subjects to smell and taste. An 8-hour fasting blood draw was performed at baseline, week 3, and week 6 for all subjects and collected six tubes of blood (two serum, two RNA, one plasma, one DNA). Serum and plasma tubes first sat upright for 30 minutes at room temperature. The tubes were then centrifuged for 15 minutes and the supernatant was then gently aliquoted into microfuge tubes, which were stored at −20°C or −80°C until they were shipped to the University of Rochester Cancer Center NCORP Research Base, where they were stored at −80°C until testing. The RNA (Paxgene) tubes were rocked 10 times, stored upright for a minimum or 2 hours and a maximum of 24 hours at room temperature, and placed in a −20°C for a minimum of 72 hours. After 72 hours, the RNA tubes were transferred to a −80°C freezer until they were shipped to the research base, where they was stored at −80°C before testing. The DNA tube was rocked 10 times and placed upright in a −20°C freezer for a minimum of 24 hours, after which it was transferred to a −80°C freezer until shipping to the research base, where it remained at −80°C until testing.
Outcome Measures
Three measures of CRF were used for this study: (1) single-item fatigue question on the SI (adapted from the M.D. Anderson Symptom Inventory (26), which is an 11-point scale anchored by 0 = no fatigue and 10 = as bad as you can imagine); (2) the Brief Fatigue Inventory (BFI), a psychometrically validated, patient-reported, nine-item instrument; (27) and (3) the Multidimensional Fatigue Symptom Inventory (MFSI) short form, a validated, patient-reported 30-item instrument (28). The single-item SI fatigue question was the screening question, and the BFI and MFSI were the primary outcomes.
We also examined single-item questions from the BFI (eg, current fatigue level, average fatigue level, and worst fatigue level) as secondary outcomes. For these measures, higher scores indicate greater CRF. Lastly, we also used daytime drowsiness, a single item from the SI, an 11-point scale anchored by 0 = no drowsiness and 10 = as bad as you can imagine.
Demographic, Medical, and Compliance Data
Clinical data were collected by clinical research coordinators from medical charts, and demographic information was collected by using study-specific forms completed by subjects. Compliance with the supplementation regimen was monitored through a pill count. Subjects also completed forms that collected information on side effects of the supplementation regimen.
Biomarkers
Levels of inflammatory proteins (CRP, IFN-γ, IL-6, IL-10, Prostaglandin E Synthase 2 [PTGES2], TNF-α, TNFR1, and TNFR2), an oxidation protein (Superoxide Dismutase 2 [SOD2]), long-chain fatty acids, and lipids were assessed. mRNA quantification was also performed using microarrays for prespecified mRNA targets (CRP, IFN-γ, IL-6, IL-10, TNF-α, TNFR1, TNFR2, and SOD2) and whole-genome mRNA sequencing (mRNASeq) for unspecified targets. Details are provided in the Supplementary Methods (available online).
Statistical Considerations
The primary aim of the study was to obtain preliminary efficacy effects (mean changes, SDs) of O3-PUFA vs O6-PUFA for reducing CRF in breast cancer survivors at week 6, as measured by the SI fatigue question, BFI, and MFSI total score. We originally planned a total accrual of 75 subjects, with an assumed 20% dropout rate, resulting in 60 evaluable subjects. Accrual was extremely rapid, and 108 subjects consented by the accrual cut-off date.
Secondary analyses examined single-item BFI questions and the SI daytime drowsiness question at week 6, including analyses of CRF outcomes that were stratified by baseline CRF level. Additional secondary analyses assessed adherence and side effects by arm assignment. Lastly, we analyzed the change in serum biomarkers (both protein and RNA levels) by intervention arm from baseline to week 6. Because the distributions of IL-6, SOD2, and CRP protein levels were skewed, these values were −2 log-transformed to provide increased normality and were used in all statistical analyses.
Clinical and sociodemographic variables were evaluated with two-sided (α = 0.05) t tests for continuous variables and χ2 tests for categorical variables to assess differences between treatment arms. Analysis of covariance (ANCOVA) models, with arm as the main factor, corresponding baseline levels as the covariate, and arm by baseline interaction, were used to evaluate treatment effects on the CRF outcomes. If the interaction term was found to be statistically nonsignificant (P ≥ .10), it was removed from ANCOVA models. Estimated within-group effects from ANCOVA models were expressed as mean difference from baseline to week 6. Additionally, effect sizes (ES) were calculated by dividing the mean change by the baseline SD of the study population. To determine whether changes were clinically significant, we defined the minimal clinically important difference (MCID) as outcomes having an ES of at least 0.3 or −0.3 or less based on the recommendation for patient-reported outcomes in cancer patients (29,30). The intent-to-treat principle was followed, because multiple imputation was used on any subjects who had missing data. A sensitivity analysis showed multiple imputation estimates that accounted for missing data were very similar to the complete case analysis.
Mixed-effects ANCOVA was used to assess all serum proteins and mRNA targets. The multiplicity adjustment was based on a false discovery rate of 0.1 using the Benjamini-Hochberg step-up method (31).
Results
A total of 108 female breast cancer survivors consented and 97 were randomly assigned to one of three arms (Figure 1), with 81 providing fully evaluable data. There was no difference in subject characteristics or NCORP sites between subjects who did and did not complete the trial. Twenty-six percent of subjects did not provide fully evaluable data, which is in line with similar clinical trials conducted in the NCORP network (32,33). Among subjects who withdrew, 40% withdrew before the baseline assessment or any study procedures. Table 1 shows the baseline characteristics of the study group by arm. There were no statistically significant differences in any of the baseline characteristics across all of the groups. Overall, the majority of subjects were postmenopausal, hormone receptor positive, white, and an average of 20 months postdiagnosis.
Table 1.
Participant demographics and characteristics*
| Characteristic | O6-PUFA | O3/O6-PUFA | O3-PUFA | P |
|---|---|---|---|---|
| Menopausal status | ||||
| Pre | 12.1% | 17.2% | 8.6% | |
| Post | 87.9% | 82.8% | 91.4% | .58 |
| Age, y | 58.0 | 60.7 | 60.4 | .54 |
| Time from diagnosis to study, months | 21.1 | 22.4 | 19.1 | .34 |
| BMI, kg/m2 | 32.7 | 30.2 | 32.4 | .35 |
| KPS | 89.7 | 91.4 | 92.0 | .35 |
| Stage | ||||
| 0 | 3.1% | 7.1% | 2.9% | |
| I | 37.5% | 39.3% | 50.0% | |
| II | 40.6% | 50.0% | 35.3% | |
| III | 18.8% | 3.6% | 11.8% | .51 |
| ER status | ||||
| Positive | 81.8% | 86.2% | 80.0% | |
| Negative | 18.2% | 13.8% | 20.0% | .80 |
| PR status | ||||
| Positive | 75.8% | 79.3% | 57.1% | |
| Negative | 24.2% | 20.7% | 42.9% | .11 |
| HER2 status | ||||
| Positive | 21.9% | 11.1% | 24.2% | |
| Negative | 78.1% | 88.9% | 75.8% | .41 |
| Current hormonal therapy | ||||
| Yes | 72.7% | 75.9% | 80.0% | |
| No | 27.3% | 24.1% | 20.0% | .36 |
| Race | ||||
| White | 100.0% | 93.1% | 88.6% | |
| Non-white | 0.0% | 6.9% | 11.4% | .15 |
BMI = body mass index; KPS = Karnovsky performance status; ER = estrogen receptor; PR = progesterone receptor.
CRF at Week 6
The mean SI CRF level decreased statistically significantly from baseline to week 6 by 2.51 points (ES = 1.48) in the O6-PUFA group, 2.14 points (ES = 1.11) in the O3/O6-PUFA group, and 0.93 (ES = 0.62) points in the O3-PUFA group (Table 2). Similar changes were noted for the SI daytime drowsiness level: the largest decrease was found in the O6-PUFA group (ES = 0., P < .01), followed by the O3/O6-PUFA group (ES = 0.54, P < .01) and the O3-PUFA group (ES = 0.30, P = .26). All three groups experienced a statistically significant improvement in BFI total score, with the O6-PUFA group (ES = 1.34, P < .01) having the greatest improvement. Similar trends were observed for the MFSI and single-item BFI questions.
Table 2.
Within-group changes for CRF measures
| Baseline |
Follow-up* |
Within-group difference* |
|||||
|---|---|---|---|---|---|---|---|
| Measure | No. | Mean (SE) | No. | Mean (SE) | Mean (SE) | Effect size | P |
| Symptom inventory: fatigue | |||||||
| Omega-6 | 33 | 6.46 (0.32) | 27 | 3.72 (0.42) | −2.51 (0.42) | 1.48 | <.001 |
| Omega-3/omega-6 | 29 | 5.97 (0.34) | 24 | 4.10 (0.45) | −2.14 (0.45) | 1.11 | <.001 |
| Omega-3 | 35 | 6.57 (0.31) | 30 | 5.31 (0.40) | −0.93 (0.40) | 0.62 | .017 |
| Symptom inventory: drowsiness | |||||||
| Omega-6 | 33 | 5.18 (0.46) | 27 | 2.86 (0.40) | −1.98 (0.40) | 0.83 | .001 |
| Omega-3/omega-6 | 29 | 4.90 (0.51) | 24 | 3.34 (0.43) | −1.50 (0.43) | 0.54 | <.001 |
| Omega-3 | 35 | 5.03 (0.50) | 30 | 4.29 (0.38) | −0.55 (0.38) | 0.30 | .255 |
| BFI total | |||||||
| Omega-6 | 33 | 5.47 (0.30) | 27 | 2.99 (0.33) | −2.18 (0.33) | 1.34 | <.001 |
| Omega-3/omega-6 | 29 | 4.91 (0.32) | 24 | 3.66 (0.35) | −1.50 (0.35) | 0.78 | <.001 |
| Omega-3 | 35 | 5.31 (0.33) | 30 | 3.68 (0.31) | −1.48 (0.31) | 0.87 | <.001 |
| BFI-1: fatigue now | |||||||
| Omega-6 | 33 | 6.03 (0.33) | 27 | 3.77 (0.42) | −2.11 (0.42) | 1.24 | <.001 |
| Omega-3/omega-6 | 29 | 5.38 (0.33) | 24 | 4.96 (0.46) | −0.92 (0.46) | 0.46 | .265 |
| Omega-3 | 35 | 6.49 (0.29) | 30 | 4.54 (0.41) | −1.34 (0.41) | 0.89 | <.001 |
| BFI-2: usual fatigue | |||||||
| Omega-6 | 33 | 5.91 (0.28) | 27 | 4.46 (0.36) | −1.42 (0.36) | 0.98 | .005 |
| Omega-3/omega-6 | 29 | 5.72 (0.30) | 24 | 4.67 (0.38) | −1.20 (0.38) | 0.72 | .011 |
| Omega-3 | 35 | 6.09 (0.29) | 30 | 4.95 (0.35) | −0.93 (0.35) | 0.54 | <.001 |
| BFI-3: worst fatigue | |||||||
| Omega-6 | 33 | 7.24 (0.29) | 27 | 5.32 (0.39) | −1.87 (0.39) | 1.26 | .001 |
| Omega-3/omega-6 | 29 | 6.97 (0.28) | 24 | 6.14 (0.42) | −1.04 (0.42) | 0.62 | .026 |
| Omega-3 | 35 | 7.49 (0.26) | 30 | 5.77 (0.37) | −1.42 (0.37) | 0.99 | <.001 |
| MFSI-SF total | |||||||
| Omega-6 | 33 | 27.55 (2.78) | 27 | 10.94 (2.58) | −13.44 (2.58) | 0.85 | <.001 |
| Omega-3/omega-6 | 29 | 20.14 (2.79) | 24 | 11.03 (2.78) | −13.36 (2.78) | 0.84 | <.001 |
| Omega-3 | 35 | 28.17 (2.67) | 30 | 13.93 (2.45) | −10.45 (2.45) | 0.67 | <.001 |
Adjusted for corresponding baseline values. BFI = Brief Fatigue Inventory; CRF = cancer-related fatigue; MFSI = Multidimensional Fatigue Symptom Inventory; SE = Standard Error.
Table 3 shows between-group comparisons for CRF outcomes, and Supplementary Figure S1 (available online) shows these comparisons using box plots with 95% confidence intervals. The O6-PUFA group had a statistically significant reduction in SI CRF level compared with the O3-PUFA group (ES = −0.86, P < .01) and the O3/O6-PUFA group (ES = −0.20, P = .048). Similar results were observed for the SI daytime drowsiness level. The O6-PUFA group had a reduction in BFI total score compared with the O3-PUFA group (ES = −0.39, P = .13) and the O3/O6-PUFA group (ES = −0.86, P = .17), but the differences were not statistically significant. Supplementary Table S1 (available online) displays the changes in CRF outcomes stratified by baseline CRF level (moderate or severe). Overall, the biggest effects of the O6-PUFA intervention were noted in those with severe baseline CRF. Among those with severe baseline CRF, subjects in the O6-PUFA group demonstrated large, statistically significant improvements in SI CRF level (ES = −1.19, P = .01), SI daytime drowsiness level (ES = −0.71, P < .01), and BFI total score (ES = −0.80, P = .04) compared with the O3-PUFA group.
Table 3.
Between-group changes for CRF measures
| Between-group difference* |
||||
|---|---|---|---|---|
| Measure | Mean difference (SE) | Effect size | 95% CI | P |
| Symptom inventory: fatigue | ||||
| Omega-6 vs omega-3 | −1.58 (0.58) | −0.86 | (−1.49 to −0.23) | <.01 |
| Omega-6 vs omega-3/omega-6 | −0.38 (0.61) | −0.20 | (−1.30 to −0.01) | .05 |
| Symptom inventory: drowsiness | ||||
| Omega-6 vs omega-3 | −1.43 (0.56) | −0.52 | (−0.93 to −0.12) | .01 |
| Omega-6 vs omega-3/omega-6 | −0.48 (0.59) | −0.18 | (−0.60 to 0.25) | .41 |
| BFI total | ||||
| Omega-6 vs omega-3 | −0.69 (0.46) | −0.39 | (−0.89 to 0.12) | .13 |
| Omega-6 vs omega-3/omega-6 | −0.68 (0.48) | −0.38 | (−0.91 to 0.16) | .17 |
| BFI-1: fatigue now | ||||
| Omega-6 vs omega-3 | −0.77 (0.59) | −0.42 | (−1.07 to 0.22) | .19 |
| Omega-6 vs omega-3/omega-6 | −1.19 (0.62) | −0.65 | (−1.33 to 0.03) | .06 |
| BFI-2: usual fatigue | ||||
| Omega-6 vs omega-3 | −0.49 (0.50) | −0.30 | (−0.90 to 0.31) | .33 |
| Omega-6 vs omega-3/omega-6 | −0.21 (0.53) | −0.13 | (−0.76 to 0.51) | .60 |
| BFI-3: worst fatigue | ||||
| Omega-6 vs omega-3 | −0.45 (0.54) | −0.29 | (−0.97 to 0.39) | .40 |
| Omega-6 vs omega-3/omega-6 | −0.83 (0.57) | −0.52 | (−1.24 to 0.20) | .15 |
| MFSI-SI total | ||||
| Omega-6 vs omega-3 | −2.99 (3.55) | −0.19 | (−0.54 to 0.22) | .40 |
| Omega-6 vs omega-3/omega-6 | −0.08 (3.81) | −0.01 | (−0.41 to 0.40) | .98 |
*Adjusted for corresponding baseline values. BFI = Brief Fatigue Inventory; CI = confidence interval; CRF = cancer-related fatigue; MFSI = Multidimensional Fatigue Symptom Inventory; SE = Standard Error.
Serum Protein and mRNA Analysis
The changes in inflammatory and antioxidant biomarkers for both protein and mRNA levels are shown in Table 4 with Supplementary Figure S2 (available online) showing the box plots with 95% confidence interval for selected biomarkers. Subjects in the O6-PUFA group had within-group decreases in CRP, TNF-α, and IFN-γ with an increase in IL-6, although these changes were not statistically significant. For between-group protein changes, the O6-PUFA group had a statistically nonsignificant decrease in TNF-α and TNFR1, with a statistically significant decrease (P = .048) in TNF-α and a statistically nonsignificant decrease (P = .11) in TFNR1 mRNA expression. Additionally, IL-10 activity was stable in the O6-PUFA group whereas it decreased in the other two groups (P = .31). Compared with O6-PUFA, the O3-PUFA group had a statistically significant decrease in IL-6, PTGES2, and IFNγ. Compared with the O6-PUFA group, the O3-PUFA group also had a statistically significant increase in SOD2 protein levels. However, changes in SOD2 mRNA expression were similar between the O6-PUFA and the O3-PUFA group (P = .18).
Table 4.
Changes in protein and mRNA expression of inflammatory and antioxidant biomarkers
| Between-group difference* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| All subjects |
Moderate CRF (4–6 on 0–10 scale) at baseline |
Severe CRF (7–10 on 0–10 scale) at baseline |
|||||||
| Measure | Cohen's d | Mean difference (SE) | Effect size | 95% CI | P | Mean difference (SE) | Effect size | 95% CI | P |
| Symptom inventory: fatigue | |||||||||
| Omega-6 vs omega-3 | −0.86 | −0.84 (0.84) | −0.60 | (−1.83 to 0.62) | .32 | −2.21 (0.83) | −1.19 | (−2.10 to −0.29) | .01 |
| Omega-6 vs omega-3/omega-6 | −0.20 | −0.36 (0.85) | −0.26 | (−1.51 to 0.98) | .67 | −0.28 (0.91) | −0.15 | (−1.15 to 0.84) | .76 |
| Symptom inventory: drowsiness | |||||||||
| Omega-6 vs omega-3 | −0.52 | −0.85 (0.86) | −0.37 | (−1.18 to 0.39) | .33 | −2.14 (0.56) | −0.71 | (−1.19 to −0.24) | <.01 |
| Omega-6 vs omega-3/omega-6 | −0.18 | −0.18 (0.86) | −0.08 | (−0.85 to 0.69) | .84 | −1.09 (0.59) | −0.36 | (−0.88 to 0.16) | .17 |
| BFI total | |||||||||
| Omega-6 vs omega-3 | −0.39 | −0.01 (0.73) | −0.01 | (−0.99 to 0.97) | .98 | −1.30 (0.59) | −0.80 | (−1.54 to −0.06) | .04 |
| Omega-6 vs omega-3/omega-6 | −0.38 | −0.36 (0.74) | −0.24 | (−1.23 to 0.75) | .63 | −0.96 (0.67) | −0.59 | (−1.42 to 0.24) | .17 |
| BFI-1: fatigue now | |||||||||
| Omega-6 vs omega-3 | −0.42 | −0.21 (0.85) | −0.13 | (−1.18 to 0.93) | .81 | −1.38 (0.82) | −0.88 | (−1.94 to 0.18) | .10 |
| Omega-6 vs omega-3/omega-6 | −0.65 | −1.20 (0.84) | −0.73 | (−1.77 to 0.31) | .16 | −1.22 (0.93) | −0.78 | (−1.98 to 0.42) | .20 |
| BFI-2: usual fatigue | |||||||||
| Omega-6 vs omega-3 | −0.30 | −0.021 (0.81) | −0.02 | (−1.24 to 1.21) | .98 | −0.83 (0.64) | −0.59 | (−1.50 to 0.33) | .19 |
| Omega-6 vs omega-3/omega-6 | −0.13 | −0.569 (0.82) | −0.42 | (−1.65 to 0.81) | .49 | 0.28 (0.70) | 0.20 | (−0.80 to 1.19) | .69 |
| BFI-3: worst fatigue | |||||||||
| Omega-6 vs omega-3 | −0.29 | −0.05 (0.89) | −0.29 | (−0.97 to 0.39) | .95 | −0.99 (0.68) | −0.71 | (−1.74 to 0.29) | .15 |
| Omega-6 vs omega-3/omega-6 | −0.52 | −1.29 (0.88) | −0.52 | (−1.24 to 0.20) | .15 | −0.47 (0.75) | −0.35 | (−1.47 to 0.78) | .54 |
| MFSI-SF total | |||||||||
| Omega-6 vs omega-3 | −0.19 | 1.16 (5.83) | 0.10 | (−0.91 to 1.11) | .84 | −6.71 (4.38) | −0.37 | (−0.86 to 0.12) | .13 |
| Omega-6 vs omega-3/omega-6 | −0.01 | 2.54 (5.98) | 0.22 | (−0.82 to 1.25) | .67 | −3.07 (5.17) | −0.17 | (−0.75 to 0.41) | .56 |
*Adjusted for corresponding baseline values. BFI = Brief Fatigue Inventory; CI = confidence interval; CRF = cancer-related fatigue; MFSI = Multidimensional Fatigue Symptom Inventory; SE = Standard Error .
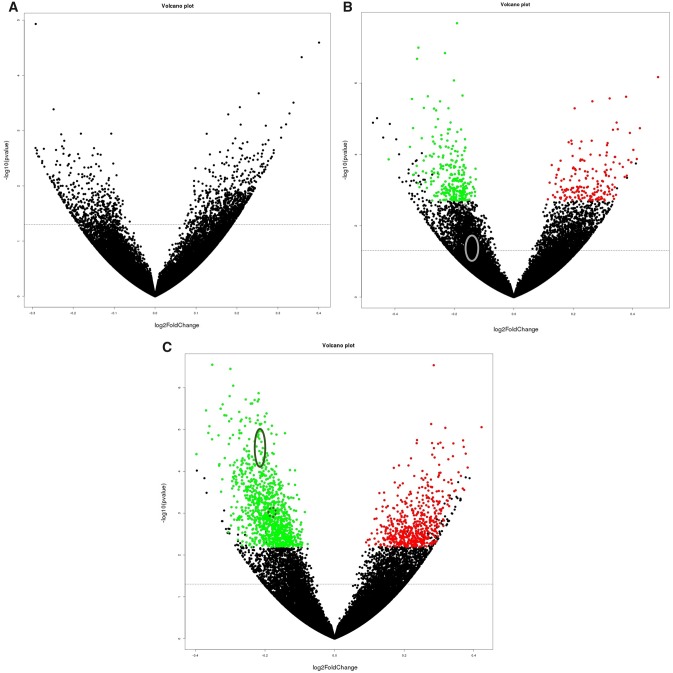
mRNASeq was used to evaluate changes in expression of 39 265 transcripts for each study arm (Figure 2, A–C). After adjusting for a false discovery rate, no statistically significant differences in mRNA expression were noted in the O3/O6-PUFA group, 441 (268 down-regulated and 173 up-regulated) statistically significant differences were noted for the O3-PUFA group, and 1618 (1053 down-regulated and 565 up-regulated) statistically significant differences were found in the O6-PUFA group. In the O6-PUFA group, multiple transcripts were down-regulated within the TNF and related inflammatory pathways (TNFRSF1A, TNFRSF1B, TNFAIP2, and NFKB2), whereas none of those changes were noted in the O3-PUFA group (Figure 2, B and C).
Figure 2.
The transcriptome of whole blood cells from breast cancer survivors within each of the three study groups (A: Omega-3 polyunsaturated fatty acids/omega-6 polyunsaturated fatty acids [O3/O6-PUFA]), B: Omega-3 polyunsaturated fatty acids (O3-PUFA), and C: O6-PUFA) was compared at the end of the study to baseline. The x-axis specifies the fold-changes (base 2 log-transformed) and the y-axis specifies the negative of the base 10 logarithm of the P values. Red and green dots represent transcripts expressed at statistically significantly (P < .05) higher (A: O3/06-PUFA n = 0; B: O3-PUFA n = 173; C: O6-PUFA n = 565) or lower (A: O3/06-PUFA n = 0; B: O3-PUFA n = 268; C: O6-PUFA n = 1053) levels at the end of the study relative to baseline, respectively. The solid circles on B and C denote the area for TNFRSF1A, TNFRSF1B, and NFKB2, and the dotted circles denote the area for TNFAIP2.
Supplementary Table S2 (available online) shows changes in serum fatty acids and lipids by group. Large changes were found for DHA and EPA in the O3-PUFA and O3/O6-PUFA groups with no change in the O6-PUFA group. The mean O6-PUFA:O3-PUFA ratio for the O3-PUFA group fell from 16.4 at baseline to 5.1 at follow-up, the ratio for the O3/O6-PUFA group decreased from 15.9 to 7.3, and the ratio for the O6-PUFA group remained relatively unchanged from 13.8 to 14.6. Arachidonic acid levels decreased across all groups with the O3-PUFA experiencing the largest reduction. Linolenic acid levels statistically significantly increased for the O6-PUFA and O3/O6-PUFA groups compared with the O3-PUFA group (P = .04).
Compliance and Adverse Events
Compliance with the intervention was excellent, with 84.5% of all subjects taking 90% of their instructed supplements, as measured by final pill count. Additionally, there was no difference between groups regarding compliance. Compliance was confirmed with the serum fatty acid analysis, which showed large increases in DHA and EPA for the O3-PUFA and O3/O6-PUFA groups. No increase in DHA and EPA was noted for the O6-PUFA group, indicating a lack of contamination across the groups.
Adverse events were monitored and collected continuously throughout the study (Supplementary Table S3, available online). The most common adverse events were burping, upset stomach, nausea, gas/bloating, diarrhea, and uneven heartbeats. Although adverse events were collected primarily for descriptive purposes, there does not appear to be an imbalance of adverse events across the groups.
Discussion
In this multicenter, randomized controlled trial, we found O6-PUFA supplementation statistically significantly reduced CRF levels compared with O3-PUFA, contrary to our original hypothesis. Analyses from within-group data showed that CRF statistically significantly decreased across all three study arms, with the largest ES in the O6-PUFA group. Although this study was not statistically powered to determine efficacy, between-group analyses found O6-PUFA statistically significantly reduced CRF levels compared with O3-PUFA, with ES ranging from −0.29 to −0.86, with corroboration between instruments. There is some variation in the ES estimates between the different CRF outcomes and this may be related to the construct of the instruments, because the SI is a single-item question whereas the MFSI-SF is composed of 30 questions. Despite the difference in ES, they all point in the same direction, consistently indicating CRF reductions for the O6-PUFA group. Stratified analyses revealed the greatest effect on CRF occurred in subjects with severe baseline CRF (≥7 on a 0–10 scale). Improvements in CRF in the O6-PUFA group were clinically significant across multiple CRF outcomes, based on our predefined MCID. Stratified analyses demonstrated clinically significant changes were observed in those with severe baseline CRF, whereas CRF changes in those with moderate baseline CRF (4–6 on 0–10 scale) mostly failed to meet our MCID. Compliance did not appear to be an issue, because serum EPA and DHA levels rose in a dose-dependent manner in the two arms containing O3-PUFA and approximately 85% of all subjects in all arms took at least 90% of the prescribed supplements.
Although the reduction in CRF in the O6-PUFA group was unexpected, biological data from our study supports this result. Whereas CRF has been well characterized, its biological underpinnings remain unclear (34). Inflammation has emerged as a likely contributing biological factor for CRF, because clinical research shows a link between an increased inflammatory state and higher CRF levels (35–38). In this trial, O6-PUFA supplementation statistically significantly reduced TNF-α mRNA expression. A similar, statistically nonsignificant decrease in proinflammatory serum markers TNF-α and CRP for the O6 group were noted. Additionally, mRNASeq analyses also showed inflammatory transcripts involving the TNF-α signaling pathway were significantly down-regulated in the O6-PUFA group, whereas similar changes were not observed in the other groups. Although O6 PUFA are generally regarded as proinflammatory, our finding that O6-PUFA supplementation reduced markers of inflammation has been shown by others (39–41). O6-PUFA supplementation reduced TNF-α and CRP levels, and O3-PUFA supplementation significantly reduced the levels of inflammatory markers IFNγ, IL-6, and PTGES2. It is possible that CRF is most closely related to the TNF-α pathway (42), because it is a major mediator of cancer-related inflammation and increased levels are associated with high levels of CRF (43–45). Further research is needed to fully elucidate the role of inflammation in regard to CRF and dietary fats.
It is also possible that fats in soybean oil other than O6 may play a role in reducing CRF. Although soybean oil is rich in O6-PUFA (50%–57%), it also contains a relatively high amount of Omega-9 monounsaturated fatty acids (O9-MUFA: 18%–29%) (46). Trials of supplementation with O9-MUFA demonstrated increased physical activity, improved resting energy expenditure, and reduced levels of inflammation (47,48). It is possible that O9-MUFA play a role in the reduction in CRF, and future studies should look to tease apart the specific effects of various dietary fatty acids.
Consumption of soy products remains a controversial topic for women, because it has been hypothesized that high amounts of soy increase the risk of incident and recurrent breast cancer, chiefly due to an abundance of isoflavones that exert estrogen-like effects under certain conditions (49–51). Evidence shows that moderate intake of soy products does not increase the risk of breast cancer recurrence and may actually reduce the risk of recurrence (52,53). Soy products have also been shown to reduce side effects, including CRF, in breast cancer survivors (54). Current evidence suggests consumption of soy products, including soybean oil, is safe for breast cancer patients and survivors.
There are limitations that should be considered when interpreting the results of this study. Although this was a nationwide, multi-center trial, only five sites were used, and this may limit the generalizability of the results. Furthermore, generalizability is limited because the majority of subjects were white, postmenopausal, and early stage (stage 0/I/II) female breast cancer survivors. For this study, we chose to use supplements as opposed to increasing dietary intake of fish to increase marine O3-PUFA levels. It is possible the use of supplements may not be ideal in terms of absorption and metabolism. The American Dietetic Association recommends fish consumption over supplements to increase O3-PUFA levels, because supplements are metabolized differently and consumption of the O3-PUFA supplements may impair the metabolism of other fatty acids (55). Additionally, due to the wide variability in measurement coupled with the smaller sample size, biomarker data should be interpreted with caution. Lastly, approximately 25% (11% before randomization and 14% after randomization) of subjects failed to provide fully evaluable data. Although there was no difference between those who did and did not complete the study, efforts are needed to improve retention rates in symptom management clinical trials. Because a significant number of subjects withdrew from the study, both before and after randomization, a run-in period may be advisable to increase compliance and reduce withdrawals. Furthermore, the largest reductions in CRF were in those with severe baseline CRF, and regression to the mean cannot be ruled out as a reason for this reduction.
Contrary to our original hypothesis, O6-PUFA supplementation reduced CRF compared with O3-PUFA supplementation in fatigued breast cancer survivors. Although O6-PUFA are ubiquitous in Western diets, supplementation with 6 g/d of O6-PUFA reduced proinflammatory markers and produced a greater systemic biological change than equal amounts of O3-PUFA, as shown by mRNASeq. Further trials are necessary to replicate the findings from our phase II trial and confirm the mechanisms of action. The identification and utilization of a placebo for O6-PUFA supplements that has a very limited biological response, such as Olestra (56), which is not absorbed or digested, will be particularly important for future studies. Currently, few therapies are available for the treatment of CRF, and the limited therapies that are effective, such as exercise, often have low rates of uptake and compliance. Clearly, new therapies that are both effective and acceptable to cancer patients are needed.
Funding
This project was supported by NIH Grants R03-CA175599, UG1-CA189961, K07-CA168911, and R25-CA102618.
Notes
Affiliations of authors: Department of Surgery, University of Rochester Medical Center (URMC), Rochester, NY (LJP, JEI, KMM, CEH, CSK, EC, PJL, MCJ); Cancer Research Consortium of West Michigan, Grand Rapids, MI (GDAP); Department of Medicine (SGM) and Department of Radiation Oncology (SLK), URMC, Rochester, NY; Dayton Clinical Oncology Program, Dayton, OH (SC).
The authors declare no potential conflicts of interest.
A special thank you to Nordic Naturals, Inc. for supplying all study agents. We thank the participants of this study and all staff at the University of Rochester Cancer Center NCORP Research Base and our NCORP affiliate sites that recruited and observed participants. We also thank the staff of the Cancer Control Behavioral Medicine Research Unit and the Cancer Control and Psychoneuroimmunology Lab. All RNA analyses were performed by the Functional Genomics Core, directed by John Ashton, PhD. Finally, we sincerely thank Susan Rosenthal, MD, and Amber Kleckner, PhD, for their critical review of this manuscript.
Clinical Trials Registration Number: NCT02352779.
Supplementary Material
References
- 1. Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21(7):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrow GR. Cancer-related fatigue: causes, consequences, and management. Oncologist. 2007;12(suppl 1):1–3. [DOI] [PubMed] [Google Scholar]
- 3. Morrow GR, Shelke AR, Roscoe JA, et al. Management of cancer-related fatigue. Cancer Invest. 2005;23(3):229–239. [DOI] [PubMed] [Google Scholar]
- 4. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758. [DOI] [PubMed] [Google Scholar]
- 5. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4–10. [DOI] [PubMed] [Google Scholar]
- 6. Schmitz KH, Speck RM, Rye SA, et al. Prevalence of breast cancer treatment sequelae over 6 years of follow-up: the Pulling Through Study. Cancer. 2012;118(8 suppl):2217–2225. [DOI] [PubMed] [Google Scholar]
- 7. Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. [DOI] [PubMed] [Google Scholar]
- 8. Mallinson T, Cella D, Cashy J, et al. Giving meaning to measure: linking self-reported fatigue and function to performance of everyday activities. J Pain Symptom Manage. 2006;31(3):229–241. [DOI] [PubMed] [Google Scholar]
- 9. Kastelein JJ, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8(1):94–106. [DOI] [PubMed] [Google Scholar]
- 10. Jain AP, Aggarwal KK, Zhang PY.. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19(3):441–445. [PubMed] [Google Scholar]
- 11. Lee YH, Bae SC, Song GG.. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012;43(5):356–362. [DOI] [PubMed] [Google Scholar]
- 12. Cerchietti LC, Navigante AH, Castro MA.. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59(1):14–20. [DOI] [PubMed] [Google Scholar]
- 13. Murphy RA, Yeung E, Mazurak VC, et al. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br J Cancer. 2011;105(10):1469.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruera E, Strasser F, Palmer JL, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol. 2003;21(1):129–134. [DOI] [PubMed] [Google Scholar]
- 15. Burns CP, Halabi S, Clamon G, et al. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia. Cancer. 2004;101(2):370–378. [DOI] [PubMed] [Google Scholar]
- 16. Persson C, Glimelius B, Ronnelid J, et al. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition. 2005;21(2):170–178. [DOI] [PubMed] [Google Scholar]
- 17. Vargas ML, Almario RU, Buchan W, et al. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism. 2011;60(12):1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patterson E, Wall R, Fitzgerald GF, et al. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379. [DOI] [PubMed] [Google Scholar]
- 20. Bersch-Ferreira AC, Sampaio GR, Gehringer MO, et al. Association between polyunsaturated fatty acids and inflammatory markers in patients in secondary prevention of cardiovascular disease. Nutrition. 2017;37(5):30–36. [DOI] [PubMed] [Google Scholar]
- 21. Wohlers M, Xavier RA, Oyama LM, et al. Effect of fish or soybean oil-rich diets on bradykinin, kallikrein, nitric oxide, leptin, corticosterone and macrophages in carrageenan stimulated rats. Inflammation. 2005;29(2–3):81–89. [DOI] [PubMed] [Google Scholar]
- 22. Silveira VLF, Limãos EA, Nunes DW.. Participation of the adrenal gland in the anti-inflammatory effect of polyunsaturated diets. Mediators Inflamm. 1995;4(5):359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Long H, Yang H, Lin Y, et al. Fish oil-supplemented parenteral nutrition in patients following esophageal cancer surgery: effect on inflammation and immune function. Nutr Cancer. 2013;65(1):71–75. [DOI] [PubMed] [Google Scholar]
- 24. Damsbo-Svendsen S, Ronsholdt MD, Lauritzen L.. Fish oil-supplementation increases appetite in healthy adults. A randomized controlled cross-over trial. Appetite. 2013;66(7):62–66. [DOI] [PubMed] [Google Scholar]
- 25. Mustian KM, Peppone L, Darling TV, et al. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol. 2009;7(5):158–167. [PMC free article] [PubMed] [Google Scholar]
- 26. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 27. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 28. Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Revicki DA, Cella D, Hays RD, et al. Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes. 2006;4(9):70–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lipscomb J, Gotay CC, Snyder C.. Outcomes Assessment in Cancer. Cambridge, UK; New York: Cambridge University Press; 2005. [Google Scholar]
- 31. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57(1):289–300. [Google Scholar]
- 32. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gewandter JS, Mohile SG, Heckler CE, et al. A phase III randomized, placebo-controlled study of topical amitriptyline and ketamine for chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study of 462 cancer survivors. Support Care Cancer. 2014;22(7):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(suppl 1):22–34. [DOI] [PubMed] [Google Scholar]
- 35. Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bower JE, Ganz PA, Aziz N, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. [DOI] [PubMed] [Google Scholar]
- 37. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–2766. [DOI] [PubMed] [Google Scholar]
- 38. Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Navarro-Xavier RA, de Barros KV, de Andrade IS, et al. Protective effect of soybean oil- or fish oil-rich diets on allergic airway inflammation. J Inflamm Res. 2016;9(9):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barros KV, Xavier RA, Abreu GG, et al. Soybean and fish oil mixture increases IL-10, protects against DNA damage and decreases colonic inflammation in rats with dextran sulfate sodium (DSS) colitis. Lipids Health Dis. 2010;9(1):68.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vieira de Barros K, Gomes de Abreu G, Xavier RA, et al. Effects of a high fat or a balanced omega 3/omega 6 diet on cytokines levels and DNA damage in experimental colitis. Nutrition. 2011;27(2):221–226. [DOI] [PubMed] [Google Scholar]
- 42. Wu Y, Zhou BP.. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102(4):639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang X, Lin Y.. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29(11):1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jim HS, Park JY, Permuth-Wey J, et al. Genetic predictors of fatigue in prostate cancer patients treated with androgen deprivation therapy: preliminary findings. Brain Behav Immun. 2012;26(7):1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Firestone D. Physical and Chemical Characteristics of Oils, Fats, and Waxes. Urbana, IL: AOCS Press; 2013. [Google Scholar]
- 47. Dumas JA, Bunn JY, Nickerson J, et al. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism. 2016;65(10):1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kien CL, Bunn JY, Tompkins CL, et al. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am J Clin Nutr. 2013;97(4):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Messina MJ, Loprinzi CL.. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131(11 suppl):3095S–3108S. [DOI] [PubMed] [Google Scholar]
- 50. Messina M, McCaskill-Stevens W, Lampe JW.. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98(18):1275–1284. [DOI] [PubMed] [Google Scholar]
- 51. Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302(22):2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caan BJ, Natarajan L, Parker BA, et al. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Prev Biomarkers 2011;20(5):854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang FF, Haslam DE, Terry MB, et al. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: the Breast Cancer Family Registry. Cancer. 2017;123(11):2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nomura SJO, Hwang Y-T, Gomez SL, et al. Dietary intake of soy and cruciferous vegetables and treatment-related symptoms in Chinese-American and non-Hispanic White breast cancer survivors. Breast Cancer Res Treat. 2017; doi:10.1007/s10549-017-4578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kris-Etherton PM, Hill AM.. n-3 fatty acids: food or supplements? J Am Diet Assoc. 2008;108(7):1125–1130. [DOI] [PubMed] [Google Scholar]
- 56. Jandacek RJ. Review of the effects of dilution of dietary energy with olestra on energy intake. Physiol Behav. 2012;105(5):1124–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.